Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Examination of urine is one of the oldest medical tests, used by Samarians, Babylonians, Egyptians, Indians, and Greeks in their traditional medicine. It was not until after Papanicolaou and Marshall published the first article in 1945 that urine cytology was used to detect urothelial carcinoma. Subsequently, Koss, Melamed, and colleagues characterized urine cytology and histology in 1960. Numerous classification systems have been introduced, and those before 2013 are nicely reviewed by Owens et al.
The greatest contemporary issues with urine cytology are low sensitivity detection of low-grade cancer, poor interobserver agreement (especially with atypia), and lack of standardized diagnostic criteria. Recent efforts described in this chapter offer great promise in resolving these concerns.
This chapter discusses the spectrum of cytologic abnormalities in voided urine samples and washings to allow comparison with biopsy findings described in Chapters 5 and 6 , and presents classifications published after the last edition of this text. The clinically significant and common problem of hematuria is also addressed from the perspective of the cytopathologist.
Cytologic examination of the urine sediment is of value in the diagnosis of a wide variety of benign and malignant diseases of the bladder, urethra, ureter, and kidney. The chief indications for the use of cytology in disorders of the urinary tract include:
Screening and diagnosis of carcinoma in situ and high-grade carcinoma
Follow-up of patients with atypical cytology evaluation or urothelial tumor, regardless of grade
Monitoring of patients with urothelial tumor undergoing or after treatment, including active surveillance
Evaluation of hematuria, including separation of kidney (upper tract disease) and nonkidney (lower tract) causes
The sources of urologic cytology specimens include voided urine, catheterized urine, bladder washing (barbotage), brushing, ureteral and renal pelvic brushing and washing, and neobladder urine from an ileal conduit or colonic pouch. Initial morning first-void urine includes exfoliated cells, debris, and impurities that have collected in the urinary tract and urethral opening during the night, and may optimize yield of potential pathogens such as human papillomavirus. Ureteral washings and other instrumented specimens require caution because they may produce artifactually clustered urothelium.
The specimen source is critically important for diagnosis. For example, upper urinary tract washings were superior to voided samples in detection of upper tract high-grade carcinoma (90% versus 50% yield, respectively). Similarly, urinary diversion cytology specimens from patients who undergo radical cystectomy are often submitted for screening for recurrent urothelial carcinoma, and those with carcinoma (2% to 6% incidence rate) revealed scant, well-preserved urothelial cells either alone or in clusters with high-grade features, including eccentrically located, enlarged, hyperchromatic nuclei; irregular nuclear borders; and high nuclear-to-cytoplasmic (N/C) ratios, often with an inflammatory background. The sensitivity, specificity, positive predictive value, and negative predictive value of cytology for high-grade carcinoma in diversion remnants were 82%, 97%, 75%, and 98%, respectively; 21% of patients with atypia were eventually diagnosed with carcinoma. In another study, bladder washing specimens were more predictive of high-grade cancer than voided urine specimens.
Adequacy is a reflection of how representative the specimen is based chiefly on cellularity, although the presence of obscuring elements is also important. Regardless of the specimen type (voided urine or instrumented), an unsatisfactory or inadequate specimen is one that is poorly cellular, completely obscured, or predominantly degenerate. Obscuring elements include neutrophils, lubricants, other foreign debris, crystals, bacteria, squames, and spermatozoa. Conversely, according to one group, if there are any atypical cells, regardless of the overall cellularity, this represents a satisfactory specimen. Brief exposure to contrast agents does not influence adequacy; thus, contrast washings of the urinary tract can be sent for cytologic diagnosis if fixed within a short time.
Inadequate voided specimens were defined by Bastacky et al. and the Papanicolaou Society of Cytopathology as those that contained fewer than 15 intermediate or basal urothelial cells, obscuring blood or inflammation, or poor cellular preservation. Prather et al. defined adequacy as the presence of a total of 2600 cells or 20 urothelial cells in 10 consecutive high-power fields (hpf) in instrumented urine specimens processed using the ThinPrep method. The Paris System 2013 included a category of unsatisfactory/nondiagnostic, but provided no qualifications for different specimen sources (voided, instrumented) and preparation types.
Adequacy is based on multiple features, including: (1) volume, (2) collection method, (3) processing method, (4) underlying patient condition, (5) operator-dependent factors, and (6) logistic factors. Volume is an important determinant of adequacy of voided urine specimens. Adequacy increased linearly for each increment of urine volume submitted to the laboratory up to 30 mL, after which the correlation was nonlinear, and low-volume specimens were less likely to harbor suspicious or malignant cells. Collection methods are also significantly associated with specimen adequacy and cellularity. Comparison of voided urine and bladder washings revealed concordance in more than 99% of cases, indicating equivalence of these collection techniques. Conversely, in a series of patients with biopsy-proven low-grade carcinoma on follow-up, 56% of urine cytology specimens demonstrated atypical features, including the presence of tissue fragments, cytoplasmic tails, and eccentric and enlarged nuclei, and these features were significantly more common in washings than in voided urine specimens.
Contemporary processing methods include conventional cytospin, Meiers improved filter method, ThinPrep, and SurePath methods ( Table 7.1 ); direct smear has been largely abandoned. The cytospin method was superior to direct smear, Thin Prep, and SurePath in a comparative study of voided urine specimens; the rate of unsatisfactory preparations was quite low (0.30%), and the overall sensitivity, specificity, and positive and negative predictive values for urothelial carcinoma were 0.72, 0.92, 0.97, and 0.46, respectively. Cytospin was also superior to ThinPrep for evaluation of nuclear detail and background material in specimens with nonurothelial malignancies. Conversely, Kim and Kim found similar sensitivity and specificity for cytospin and ThinPrep in a large series of bladder washings (61% versus 60% and 95% versus 95%, respectively). Metaanalysis showed that there was similar sensitivity with cytospin and liquid-based cytology methods. Using the Paris System 2013, cytospin and ThinPrep had similar sensitivity and specificity for the diagnosis of suspicious or high-grade urothelial carcinoma. The filter method was superior to cytospin by decreasing the number of false-negative cases because of higher cellularity and improved detection of atypical or malignant epithelial cells. ThinPrep increased the number of nonatypical urothelial cell clusters compared with cytospin (13% to 21% versus 7%, respectively).
| Method | Description |
|---|---|
| Cytospin smear (two-step centrifugation/fixation method) | Urine samples are sedimented at 700 g for 10 minutes. The supernatant is removed to within approximately 1-2 mL of the cell pellet, and the pellet is then resuspended and rinsed with 10 mL of hypotonic solution (0.075 M potassium chloride) for 10 minutes. The cells are resedimented at 600 g for 10 minutes, and the supernatant is removed to within 0.5 mL of the pellet. The pellet is then gently vortexed and resuspended in 10 mL 3:1 methanol/glacial acetic acid fixative. Fixed specimens are left at room temperature for 30 minutes. The urinary cells are then sedimented at 600 g for 5 minutes, aspirated, and transferred to a 2-mL microfuge tube. The final cell pellet is left in approximately 100-500 μL of residual methanol/glacial acetic acid fixative, depending on the size of the cell pellet. A total of 10 μL of cell sediment is placed on the slide, and the specimen is allowed to dry. |
| Meiers improved filter method | Urine samples are fixed in ethanol and drawn up into a 60-mL syringe threaded with a Luer lock tip. An 8.0-μM filter mounted in a filter holder is subsequently attached to the syringe tip. The urine sample is then pushed gently through the filter until complete. The membrane filter is placed on a positively charged glass slide. Gauze is placed over the membrane and slight pressure applied with the palm of the hand to transfer the cell filtrate. The cell filtrate is placed on a slide in a manner similar to Cytospin. The membrane is discarded and the filter holder was deposited in a 10% bleach solution overnight until next use. |
| ThinPrep (Hologic, Bedford, MA) | ThinPrep test is performed with a proprietary automated liquid-based monolayer cell preparation system. Urine samples are immersed in a buffered preservative solution, transferred to a bowl, and a cylinder with a filtration membrane is then placed in the bowl to ensure that the cells are homogeneously distributed. Using negative pressure, the erythrocytes and mucus penetrate the filtration membrane, leaving only the filtration membranes for the diagnostic procedure. This maneuver is repeated until an appropriate number of cells (2000-50,000) is collected. Thereafter the cylinder is removed from the bowl; cells left on the filtration membrane are attached to the slide and then fixed in 95% alcohol. |
| SurePath (BD Diagnostics, Burlington, NC) | The SurePath test is performed with a proprietary liquid-based monolayer cell preparation system density gradient-based cell enrichment. Urine samples are immersed in ethanolic preservative solution and a device is placed into the vial to ensure that cells are homogenously distributed. A polysaccharide-based density gradient reagent is used to filter debris, centrifuged, resuspended, and centrifuged again. The PrepStain processor creates and stains the slides. |
The patient’s underlying condition and his or her indication for cytologic evaluation influences specimen adequacy. Increased cellularity is observed in specimens from patients with cancer, calculi, or infection compared with those with only hematuria or irritative voiding symptoms. Other adequacy factors include level of hydration, micturition before specimen acquisition, the use of diuretics or other medications, the presence of obstructive conditions such as benign prostatic hyperplasia, with consequent reduction of bladder capacity, and medical problems resulting in oliguria.
Operator-dependent factors refer to expertise of the examiner and the potential for human bias and error. Logistic factors that influence adequacy include length of time from collection to processing, container leakage with potential drying artifacts, and many others.
Several reporting and classification systems for urine cytology have been published, each of which has relative strengths and weaknesses. Unlike cervical cytology, there has not been widespread acceptance and use of any single reporting system for urine cytology studies. Thus, terminology and criteria for urine cytology reporting are not uniform among pathologists. The major diagnostic categories that we use at our laboratory are presented in Table 7.2 .
| Bostwick Laboratories System (2018) a | Paris System for Reporting Urine Cytology (2013) | International Consultation on Urologic Disease–European Association of Urology (2015) | Recommended Diagnostic Response c |
|---|---|---|---|
| Nondiagnostic | Nondiagnostic | Nondiagnostic | Repeat within 3 months |
| Negative | Negative for high-grade urothelial carcinoma | Negative for epithelial cell abnormality | Clinical follow-up as needed |
| Atypical | Atypical urothelial cells | Atypical urothelial cells of undetermined significance | Clinical follow-up; consider ancillary tests |
| Suspicious | Low-grade urothelial neoplasm d | Low-grade urothelial carcinoma | Cystoscopy and biopsy |
| Suspicious for high-grade urothelial carcinoma | Atypical urothelial cells, cannot rule out high-grade urothelial carcinoma | Cystoscopy and biopsy | |
| Malignant cells present | High-grade urothelial carcinoma | High-grade urothelial carcinoma | Cystoscopy and biopsy |
| Other (specify) | Others: primary and secondary malignancies and miscellaneous lesions | Other (specify) | Cystoscopy and biopsy, depending on specificity of findings |
a Expanded terminology: These are the templated words that appear on the Bostwick Laboratories’ reports. Nontumor-associated cytology:
Normal cells/negative for malignant cells
Inflammatory changes: specific type or nonspecific
Tumor-associated cytology:
Rare single cells and clusters of mildly to moderately atypical urothelial cells; this may represent a reactive process, but neoplasm should be considered; clinical correlation is indicated
Rare highly atypical urothelial cells suspicious for neoplasm; reactive process cannot be excluded; repeat study and/or further investigation may be of value
Severely atypical urothelial cells highly suspicious for neoplasm; clinical correlation is recommended
Malignant cells present most suggestive of urothelial carcinoma
Malignant cells present (specify squamous cell carcinoma, adenocarcinoma, prostatic adenocarcinoma, renal cell carcinoma, other)
Malignant cells present, not otherwise specified
d In the Paris System, the presence of fibrovascular cores is rare and is the only instance in which the diagnosis of low-grade urothelial neoplasm in instrumented urine can be made. Low-grade urothelial neoplasm should be used sparingly and in conjunction with the negative category to clarify the absence of high-grade carcinoma in the Paris System. In the Bostwick Laboratories classification, the presence of fibrovascular cores is considered suspicious.
Recently two international consensus conferences published their classifications: the Paris System 2013 and the International Consultation on Urologic Disease–European Association of Urology 2015 ( Table 7.2 ). Both were based on expert consensus by small, self-selected academic groups with minimal input from other cytopathologists, urologists, oncologists, or others.
It should be noted that evidence-based guidelines have supplanted such expert panels and simple consensus conference-based conclusions, and are now considered to be the contemporary standard for defining the practice of medicine; thus it is surprising that these recent efforts failed to abide by even the most basic tenets of evidence-based medicine, instead resorting to “biology by democracy.” All proper methods of systematic review and guideline generation share certain core concepts, including careful selection of the guideline topic, thorough structured review of the evidence with grading and synthesis, creation of recommendations, consultation and peer review, dissemination and implementation, revision, and updating. According to the U.S. Agency for Healthcare Research and Quality, three key principles are required for successful conduct of systematic reviews: (1) the review must be relevant and timely, focusing on the most important issues and the optimal time to initiate a review; (2) the review must be objective and scientifically rigorous, free from conflicts of interest; and (3) the review must include public participation and transparency to ensure confidence and credibility, and provide for accountability. Thus the recent cytopathology consensus statements should be considered below the standards of current practice of evidence-based medicine. Nonetheless, any efforts to create standardized terminology are laudable and generate renewed interest in refinement of diagnostic criteria, continuing the work of the Papanicolaou Society at creation of uniformity in cytopathology practice.
In Paris System 2013, the recommended diagnostic words are also problematic. The words “negative for high-grade urothelial carcinoma” on a report could easily be mistyped or misinterpreted by the transcriptionist, cytopathologist, or urologist if the word negative is overlooked while the word carcinoma registers, potentially resulting in serious consequences for the patient. Reasonable alternatives include “negative for high-grade malignancy,” “negative for high-grade neoplasia,” and “no definite evidence of malignancy.”
The Paris System 2013 focused chiefly on accuracy of identification of high-grade carcinoma, requiring five criteria for a definitive diagnosis: at least 5 malignant cells (10 cells for upper tract cancer), elevated N/C ratio (≥ 0.7), markedly atypical nuclear borders, moderate to severe hyperchromasia, and coarse chromatin ( Tables 7.2 and 7.3 ). However, malignant specimens often contain degenerative changes, and this may limit the number of diagnostic cells; consequently, about half of positive cases failed to fulfill this criterion in a report from Johns Hopkins University. Furthermore less than 20% of cells present in positive specimens fulfilled all five of the criteria. The second most restrictive criterion, N/C ratio ≥ 0.7, was present in only 78% of positive specimens. Nonetheless, the Paris System 2013 upgraded about 40% of indeterminate specimens and did not change the frequency of diagnosis of high-grade carcinoma.
| Category | Nuclear-to-Cytoplasmic Ratio (Feature 1) |
Nuclear Chromasia (Feature 2) |
Chromatinic Rim/Nuclear Membrane (Feature 3) | Chromatin Quality (Feature 4) |
Mandatory (Major) Features | Minor Features |
|---|---|---|---|---|---|---|
| Atypical urothelial cells a | > 0.5 | Similar to umbrella cells or dark/very dark a | Fine and even or uneven shape and thickness a | Finely granular or coarsely clumped a | 1 | 2-4 (one of the features) 2-4 noted with footnote “a” must be second features identified in the cells of interest in addition to 1 |
| Suspicious b and high-grade urothelial carcinoma b | > 0.7 | Very dark | Uneven shape and thickness | Coarsely clumped | 1, 2 | 3, 4 (at least one of the above must be a third feature identified) |
a Only one minor feature required.
b Only difference is the cellular quantity: suspicious, very few cells; high-grade carcinoma, 5-10 cells or more.
N/C ratio is a critical component of the Paris System, but just how reliable is it? Hang et al. confirmed the importance of the ≥ 0.5 cut point for the diagnosis of atypical urothelial cells using digital image analysis; receiver operating characteristic analysis demonstrated that the maximum N/C ratio alone was highly predictive of high-grade carcinoma on follow-up (area under the curve [AUC], 79%), with a sensitivity of 73% and a specificity of 85%. However, visual quantitation of N/C ratio showed only a fair correlation with actual N/C ratio, with correlation decreasing with increasing N/C ratio. In the critical range, N/C ratio of 0.5 to 0.7, interobserver correlation (75%), and correlation with true N/C ratio (53%) may be insufficiently accurate for precise category assignment in the Paris System.
Compared with previous classification systems, the Paris System 2013 resulted in a great increase in the rate of “atypical” cases while improving sensitivity but lowering specificity. Granados et al. found that the incidence of “atypical” increased from 3% to 24% in benign cases, from 2.5% to 25% in low-grade carcinoma, and from 6.6% to 16% in high-grade carcinoma. The false-positive rate (abnormal cytology in negative or low-grade carcinoma cases) increased from 11% to 34%. Sensitivity was higher (63% versus 49%) at the expense of lower specificity (73% versus 91%). The agreement between prior classification and Paris System 2013 was moderate for negative and high-grade carcinoma cases (κ = 0.42 and 0.56, respectively) and weak for low-grade tumors (κ = 0.35). Conversely, Hassan et al. found fewer cases were diagnosed as “atypical” with the Paris System compared with their original diagnoses (26% versus 39%), whereas the correlation of “atypical” with subsequent high-grade cancer increased from 33% to 53%. The new system also resulted in a higher number of low-grade carcinomas diagnosed as “negative” (40%) rather than “atypical” (22%). In another study, 70% of cases of “atypical” cases were reclassified by Paris System 2013 as “negative”; however, 18% of these were found to have high-grade cancer. The sensitivity and specificity of fluorescence in situ hybridization (FISH) with Paris System 2013 were 86% and 33%, respectively, in the “atypical” group and 63% and 100%, respectively, in the “negative” group.
The category of “atypical urothelial cells” no longer includes cellular changes attributed to the BK polyomavirus cytopathic effect, according to the Paris System 2013. Reclassification of such cases as “negative” decreased the rate of “atypical” from 25% to 21%, although the high rate of subsequent “high-grade cancer” among nonsurveillance patients suggested that the reclassification may be “inappropriate.”
In Paris System 2013, nonatypical urothelial cell groups are classified as “negative for high-grade carcinoma” except in cases that display fibrovascular cores that are now diagnosed as “low-grade urothelial neoplasm.” However, because of the correlation of nonatypical urothelial cells with high-grade carcinoma (high specificity and negative predictive value [87.1% and 94%, respectively]) despite low sensitivity (30.4%), Granados et al. concluded that the presence of nonatypical urothelial cell clusters in voided urine (even without fibrovascular cores) should not be diagnosed as “negative.”
The predictive values of “suspicious” and “high-grade carcinoma” diagnoses were unchanged (94% each) after reclassification with Paris System 2013 despite the new exclusion criterion of cellular degeneration for “suspicious.” Joudi et al. found that “high-grade carcinoma” with the Paris System 2013 yielded a higher predictive value for carcinoma than the cytologic diagnosis of “suspicious” (79% versus 55%, respectively), similar to results with the Bostwick Laboratories Classification (74% versus 54%, respectively).
Addition of anisonucleosis and India ink nuclei (but not tumor diathesis, ragged edge of urothelial cells, apoptotic bodies, or pleomorphism) significantly improved the predictive accuracy of the Paris System 2013 according to Suh et al. With their modification the reporting rate of “atypical” decreased from 25% in their original system to 15% in Paris System 2013 and 11% in Suh’s proposed modification; likewise, sensitivity increased from 59% to 71% and 90.0%, respectively.
Interobserver agreement with the Paris System 2013 was adequate for the category of “negative for high-grade carcinoma,” but not for the other categories, with mean absolute agreement of 65% and a mean expected agreement of 44%; the mean chance-corrected agreement (κ) was only 0.32. Approximately 15% of disagreements were classified as high clinical impact. The authors concluded that this low level of diagnostic precision may negatively impact the applicability of Paris System 2013 for widespread clinical application.
The most common cellular elements are benign superficial urothelial cells, followed by intermediate and basal urothelial cells that are more commonly observed in instrumented specimens. Superficial squamous cells from the female genital tract often outnumber urothelial cells. Benign glandular cells (from cystitis glandularis), squamous cells originating in squamous metaplasia of urothelium or external genital tract skin, and, rarely, benign seminal vesical cells also fall into this category. Clusters or fragments of urothelial cells that may be seen in both instrumented and noninstrumented urine specimens should be classified as “negative” unless the cytomorphology of the cells forming the group fulfills the criteria for “atypical.” Similarly, changes associated with urolithiasis, treatment-related changes, and polyomavirus cytopathic changes should all be classified as “negative,” according to Paris System 2013.
Urothelial cells are the most variably sized cells in the urinary sediment, ranging from 20 μm in diameter for intermediate and basal cells up to 100 μm for typical “umbrella” or superficial cells. Urothelial cells typically have single round to oval nuclei with abundant, homogenous, predominately basophilic cytoplasm. Cells from the basal urothelium are smaller, round, and display well-defined thickened cytoplasmic membranes. Chromocenters and multiple eosinophilic micronucleoli may be prominent, especially in cases with accompanying inflammation.
Fragments of urothelial cells are commonly found in catheterized specimens, as well as bladder washes; however, it is abnormal to see urothelial fragments in spontaneously voided urine, and their presence may be associated with papilloma or low-grade urothelial cancer. Occasionally large urothelial fragments may display cytoplasmic vacuoles containing neutrophils. Multinucleation, nuclear enlargement, and hyperchromasia can be found in inflammatory processes within the lower urinary tract.
Regardless of the type of sample and collection technique used, superficial urothelial cells are a common component of the urine sediment. These cells have one or more nuclei that are large, measuring up to 3 μm in diameter, comparable with superficial squamous cells ( Fig. 7.1 A ). Binucleate and multinucleate cells are common. Such cells are often larger than the mononucleate superficial cells, and their nuclei are somewhat smaller. Large multinucleate superficial cells are by far the most striking component of the urinary sediment, particularly in washings or brushings of the bladder or ureter. Multinucleate superficial cells are particularly large and may be mistaken for giant cells. A potential error in diagnosis is misinterpretation of large superficial cells as macrophages or tumor cells. The DNA content of superficial cells may be polypoid.
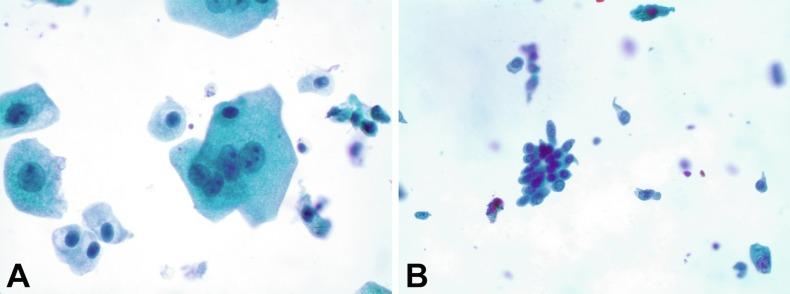
The chromatinic rim of the nucleus is thick and sharply demarcated. The chromatin is finely granular, often with a “salt and pepper” appearance, and may contain one or more prominent chromocenters. The structure of the nucleus is better preserved in bladder washings than in voided urine. In women there may be a sex chromatin body attached to the nuclear membrane. The cytoplasm of these cells is usually basophilic, often finely granular, and sometimes vacuolated. The cell border is convex (luminal) and concave (deep).
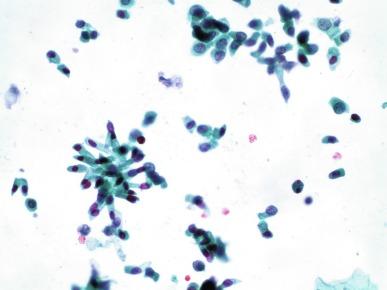
All other urothelial cells are smaller than the superficial cells, and often exfoliate in clusters, particularly in instrumented specimens. Single small urothelial cells are observed in voided urine. Clusters of urothelial cells may be tightly packed and assume spherical “pseudopapillary” configurations with sharp borders. Such clusters are often misinterpreted as low-grade papillary carcinoma. When deep (basal) cells are removed by instrument, they often appear in loose clusters. These cells are polygonal or elongate, sometimes columnar, and almost always display cytoplasmic extensions in contact with other cells. The amount of basophilic cytoplasm in such cells depends on the layer of origin and is more abundant in cells derived from upper layers. Single cells resemble parabasal squamous cells in size and configuration. These cells are often spherical or round, particularly in voided urine, but may also show cytoplasmic extensions. The nuclei of the smaller urothelial cells are approximately the same size, measuring about 2 to 5 μm in diameter ( Fig. 7.1 B). They are usually finely granular and benign appearing, containing one or rarely two small chromocenters. In voided urine the nuclei may be pale or opaque and occasionally somewhat darker.
Columnar urothelial cells are common, particularly in specimens obtained by instrumentation. Columnar cells often derive from cystitis cystica or the urethra. They can be single or in small groups, often with a tail by which they are attached to the basement membrane ( Fig. 7.2 ).
Occasionally urine specimens contain mucus-secreting columnar epithelial cells with peripheral nuclei and distended clear cytoplasm. These cells may be ciliated. Such cells often derive from cystitis cystica or cystitis glandularis but may represent cells from urachal remnant, nephrogenic metaplasia, or Müllerian rest (endometriosis or endocervicosis).
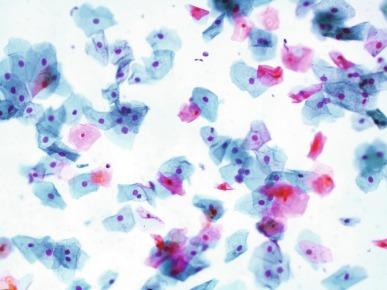
Squamous cells of varying size and degrees of maturation are common in urine sediment, particularly in voided specimens ( Fig. 7.3 ). Such cells are more abundant in female than male patients. In women these cells originate in the urethral squamous epithelium and in the trigone of the urinary bladder, and are often glycogenated. Voided urine sediment may also contain squamous cells derived from the vulva, vagina, or uterine cervix. In men the origin of the squamous cells is the terminal portion of the urethra or, in rare cases, vaginal type of squamous metaplasia with bladder origin. Among the benign squamous cells, there may be superficial cells, intermediate cells, and small parabasal cells. Navicular cells are intermediate squamous cells with abundant cytoplasmic glycogen content and peripheral nuclei; these cells stain yellow with Papanicolaou stain. Such cells may be observed during pregnancy, early menopause, and sometimes in women or men receiving hormonal therapy (androgen deprivation therapy for prostate cancer). Squamous cells may also be anucleate and fully keratinized. In such cases these should be reported, because the presence of such “ghost” cells may be of considerable significance, representing leukoplakia or squamous cell carcinoma of the bladder.
Cells derived from renal tubules sometimes appear in the urine sediment. These cells are small and usually poorly preserved, with pyknotic, hyperchromatic, condensed, spherical nuclei, and granular eosinophilic cytoplasm. Occasionally the tubular cells form small clusters or casts. The significance of tubular cells in urine sediment remains uncertain. In patients after kidney transplant the presence of renal tubular cells may indicate rejection of the allograft.
Cells from the convoluted tubular epithelium are the largest cells in the nephron, present at the entrance to the Bowman capsule and extending to the beginning of the loop of Henle. These cells are rarely seen in healthy individuals but are shed in large numbers in cases of renal toxicity and renal ischemia caused by a wide variety of drugs, heavy metals, immunosuppressant, and other toxins.
Proximal tubular cells in urine are easily identified by their large size (20 to 60 μm in diameter); irregular, elongate, or cigar-like appearance; and coarsely granular basophilic cytoplasm ( Fig. 7.4 A ). Cytoplasmic borders are indistinct and may be ragged or torn. The granular cytoplasm contains large numbers of mitochondria by ultrastructure. Nuclei are slightly larger than erythrocytes and may occasionally be multinucleate. Interestingly, proximal and distal tubular cells appear singly, never in fragments or clusters. These cells are often mistaken for granular casts in unstained bright-field microscopy. Proximal and distal renal tubular cells slough from their basement membranes and can be found in urine as intact preserved cells or as “ghost” or necrotic forms that retain their size and cytoplasmic characteristics ( Fig. 7.4 B).
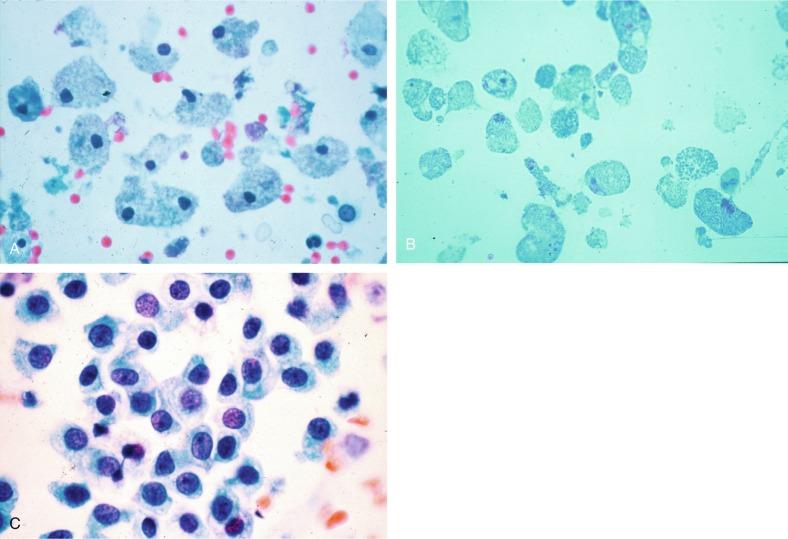
Renal tubular cells lining the proximal and distal collecting ducts are small (12 to 18 μm in diameter), and each contains a single slightly eccentric nucleus with coarse and evenly distributed chromatin. There may be an occasional prominent nucleolus, because these cells may be reactive, but they are never multinucleate. The cytoplasm is polygonal to columnar, finely granular, and uniform basophilic, with distinct borders ( Fig. 7.4 C). Vacuolization may occasionally be seen, especially in reactive states. The cells may phagocytize castlike material, crystals, and pigments.
Collecting duct cells in urine may be present in very low numbers in normal individuals, but are significant when found with renal casts or as fragments. An abnormal number (greater than one per hpf) may be found in a wide variety of clinical conditions, including shock, trauma, burn, and exposure to toxins; also, an increased number of cells in renal transplant patients heralds clinical rejection up to 48 hours early.
Renal epithelial cell fragments in urine indicate a severe form of renal tubular injury (“ischemic necrosis”) and are exclusively from the collecting duct. This reflects loss of blood flow (ischemic injury) to the renal tubules and subsequent sloughing of entire segments or portions of the renal tubules with regeneration of lost epithelium, a process similar to repair in cervical smears. There are five types of fragments, and these are classified according to morphology: (1) spindle fragments; (2) fragments attached to or surrounding cast material; (3) pavement or “en face” fragments; (4) fragments with reactive cellular or noncellular inclusions (castlike, crystal, or pigmented [bile] inclusions); and (5) cylindrical, tubelike fragments.
Occasionally cells of prostatic and seminal vesicle ( Fig. 7.5 ) origin may be present in the urinary sediment. Such cells accompany spermatozoa and are common after prostatic massage. Erythrocytes are a frequent component of the urinary sediment, particularly in patients with clinical evidence of hematuria (see later).
Macrophages are often observed in inflammatory reactions of the urinary tract. The cells may be mononucleate or multinucleate and contain fine cytoplasmic vacuoles, sometimes with phagocytic debris. Normal urine sediment contains very few lymphocytes or neutrophils. The presence of large numbers of such cells may precede clinical evidence of inflammation. For example, when there were more than 12.5 white blood cells/μL by image analysis, sensitivity and specificity for predicting Chlamydia infection were 87% and 89%, respectively, in first voided urines in men at high risk.
In addition to viral inclusions, a variety of intracellular and extracellular findings may be diagnostically valuable in the urine sediment.
Numerous normal and pathologic processes result in extracellular pigmented material in the urine and pigmented cells ( Table 7.4 ).
| Finding | Description |
|---|---|
| Lipofuscin pigment | Granular yellow-brown pigment scattered around nuclei, often obscured by degenerative changes |
| Hemosiderin | Coarse golden-brown, brown, or black pigment usually in the cytoplasm of macrophages or rarely in urothelium; often observed in the setting of injury, blood transfusions, calculi, or foreign bodies; may be mistaken for melanin |
| Melanin | Dusty brown pigment found in melanosis, nevi, or melanoma; the greatest difficulty is when melanin pigment is associated with atypical urothelial cells; distinguishing urothelial cells from melanoma cells may require biopsy; with uncertainty, biopsy is indicated |
Nonspecific cytoplasmic inclusions may appear as products of degenerating cells in multiple body fluids and can be seen with careful examination in 43% of urine samples. There is no relationship with any disease. The round, opaque bodies are 12 to 15 μm in diameter, and may be single or multiple, with eosinophilia standing in contrast with the pale-staining urothelial cytoplasm. Nuclei are usually degenerate, with hyperchromasia, karyorrhexis, or pyknosis, but may also be intact.
Nonspecific cytoplasmic eosinophilic inclusions should be distinguished from acid-fast–positive nuclear inclusions in renal tubular cells associated with lead poisoning, as well as nonspecific acid-fast–negative red nuclear inclusions of uncertain significance in older women.
Polygonal transparent crystalline precipitates of urates are common in voided urine. Their presence results from changes in the acidity of urine after collection but has no diagnostic significance. Crystals derived from true uric acid are rare, and other crystals are rarely of diagnostic value. Voided urine and occasional specimens obtained by instrumentation may contain contaminants and renal casts. For a complete review, refer to other texts.
Renal casts are observed in urine sediment in patients with glomerular and renal parenchymal diseases. Casts are composed of Tamm-Horsfall protein and originate in the distal tubules and collecting ducts. In healthy individuals hyaline and rare granular casts may occasionally appear because of dehydration, fever, exercise, and other factors; these casts are considered physiologic. Conversely, nonphysiologic casts made of abnormal urine protein and those that contain cells of various types are easily identified. The type of cells contained within the cast matrix, the width of the casts, and the number of casts is indicative of the severity of the underlying disease. The presence of abnormal amounts of protein, blood, leukocytes, nitrites, and bilirubin all correlate with the type of cast.
“Round cells” are recently described cells in patients with end-stage renal failure that appear to be predictive of early hemodialysis. They are distinct from known cells in sediment and are similar to proximal convoluted tubule-derived cells based on morphology and molecular marker expression (GGT1, but not podocalyxin). These cells also express PAX2, Wilms tumor 1 (WT1), OSR1, and SIX2. The number of round cells correlates with the severity of chronic kidney disease.
The severity of lupus nephritis correlates strongly with voided urine cytology findings, including erythrocytes (isomorphic and dysmorphic), acanthocytes, and leukocytes (0.65 for each); classification tree has an accuracy rate of 84.3%.
Dysmorphic red blood cells may be indicative of urologic or glomerular diseases (see later).
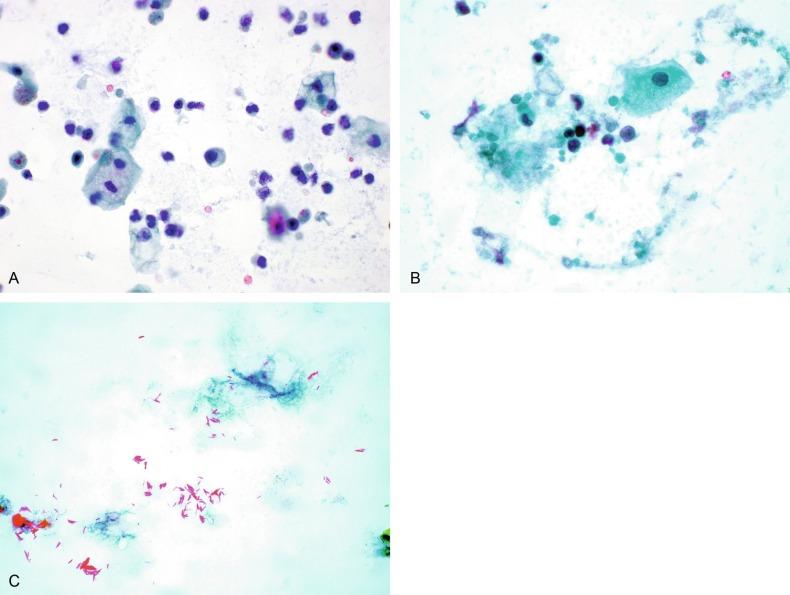
A wide variety of bacteria may affect the epithelium of the urinary tract. Most are coliforms and other gram-negative rods. Cystitis may be acute or chronic. Acute cystitis is usually associated with symptoms that rarely require confirmatory tissue biopsy or cytologic examination. The sediment may contain numerous exfoliated urothelial cells, necrotic material, and inflammatory cells, with a predominance of neutrophils ( Fig. 7.6 A to C ). Marked necrosis and inflammation may also occur in the presence of necrotic tumors, particularly high-grade urothelial carcinoma and squamous cell carcinoma.
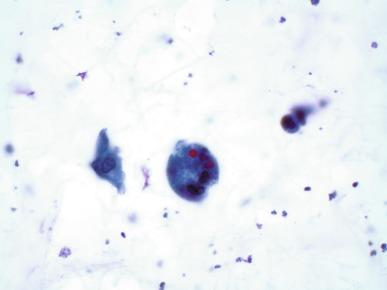
The sediment in chronic cystitis usually contains a background of chronic inflammation with macrophages and erythrocytes. Urothelial cells may be abundant and poorly preserved, occasionally forming small clusters. The cytoplasm in these cells tends to be granular and vacuolated; when the cells are degenerate, the cytoplasm contains spherical eosinophilic inclusions (Melamed-Wolinska bodies) ( Fig. 7.7 ). There may be slight nuclear enlargement and hyperchromasia, but the contours of the nuclei are usually regular and the chromatin texture is finely granular without the coarse granularity of cancer cells. There may be necrosis of urothelial cells, with nuclear pyknosis and marked cytoplasmic vacuolization. In ulcerative cystitis, large sheets of urothelial cells may exfoliate.
Interstitial cystitis, a form of chronic cystitis associated with chronic inflammation, displays nonspecific cytologic changes. Eosinophilic cystitis has a predominance of eosinophils, a pattern that may be seen in patients with allergic disorders, previous biopsies, or after mitomycin C treatment.
Tuberculous cystitis may be observed in patients with AIDS and those receiving treatment for urothelial carcinoma with bacillus Calmette–Guérin (BCG). In such patients the urine has inflammatory cells, necrosis ( Fig. 7.6B ), and rarely contains fragments of tubercles consisting of clusters of elongate carrot-shaped epithelioid cells, sometimes accompanied by multinucleated Langhans-type giant cells, and reactive atypia of urothelial cells. Ziehl-Neelsen staining may reveal acid-fast bacilli ( Fig. 7.6C ). The sediment occasionally contains “decoy” cells with glassy hyperchromatic nuclei. Similar findings may occur in patients with tuberculosis of the bladder.
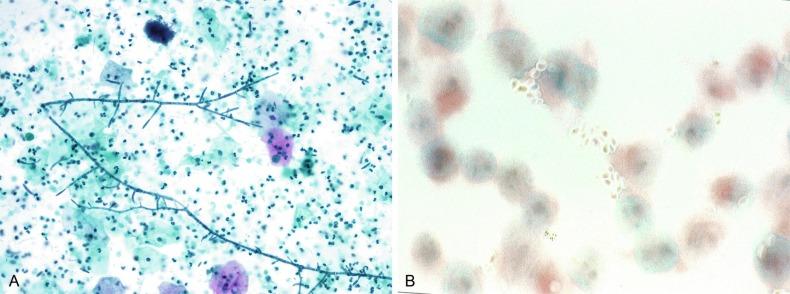
Fungi occasionally affect the lower urinary tract, particularly the urinary bladder, and Candida albicans is the most common, usually seen in pregnant women, diabetics, and those with impaired immunity such as patients with AIDS, those undergoing chemotherapy for cancer, and bone marrow transplant recipients. In the sediment the fungi may appear as yeast forms, with small oval bodies, or pseudohyphae, with oblong branching nonencapsulated filaments ( Fig. 7.8A and B ). Other fungi are uncommon, including Blastomyces dermatitidis , Aspergillus , and mucormycosis. A fungus of the species Alternaria is a common laboratory contaminant.
Several important viruses cause significant morphologic changes in the urothelial cells, many of which may be confused with malignancy. The dominant feature of viral infection is the formation of nuclear and cytoplasmic inclusions ( Table 7.5 ).
|
You're Reading a PreviewBecome a Clinical Tree membership for Full access and enjoy Unlimited articles If you are a member. Log in here |