Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
blood oxygen level-dependent
cervical spondylosis
corticospinal tract
cervical spondylosis myelopathy
diffusion tensor imaging
fractional anisotropy
functional connectivity
gray matter
functional magnetic resonance imaging
mean diffusivity
magnetic resonance
magnetic resonance imaging
primary motor cortex
resting-state networks
resting-state functional MR imaging
spinal cord injury
sensorimotor cortex
supplementary motor area
primary sensory cortex
supramarginal gyri
white matter
Compression of the cervical spinal cord can be caused by a wide range of causes, but the most common is cervical spondylosis (CS), a chronic degenerative process that affects the vertebral bodies and inter-vertebral discs of the cervical spine ( Table 1 ). CS is frequently found in asymptomatic adults after the fifth decade of life, but may progress to disk herniation, bone spur formation, compression of the spinal cord, and cervical spondylotic myelopathy (CSM) ( ).
| Traumatic causes | Atraumatic causes |
|---|---|
| Vehicular accidents | Degenerative spondylosis |
| Falls | Intervertebral disk herniation |
| Violence | Metastatic disease |
| Sports | Primary spinal cord tumor |
| Recreation activities | Spinal epidural abscess |
| Spinal epidural hematoma | |
| Scoliosis | |
| Rheumatoid arthritis | |
| Bone diseases |
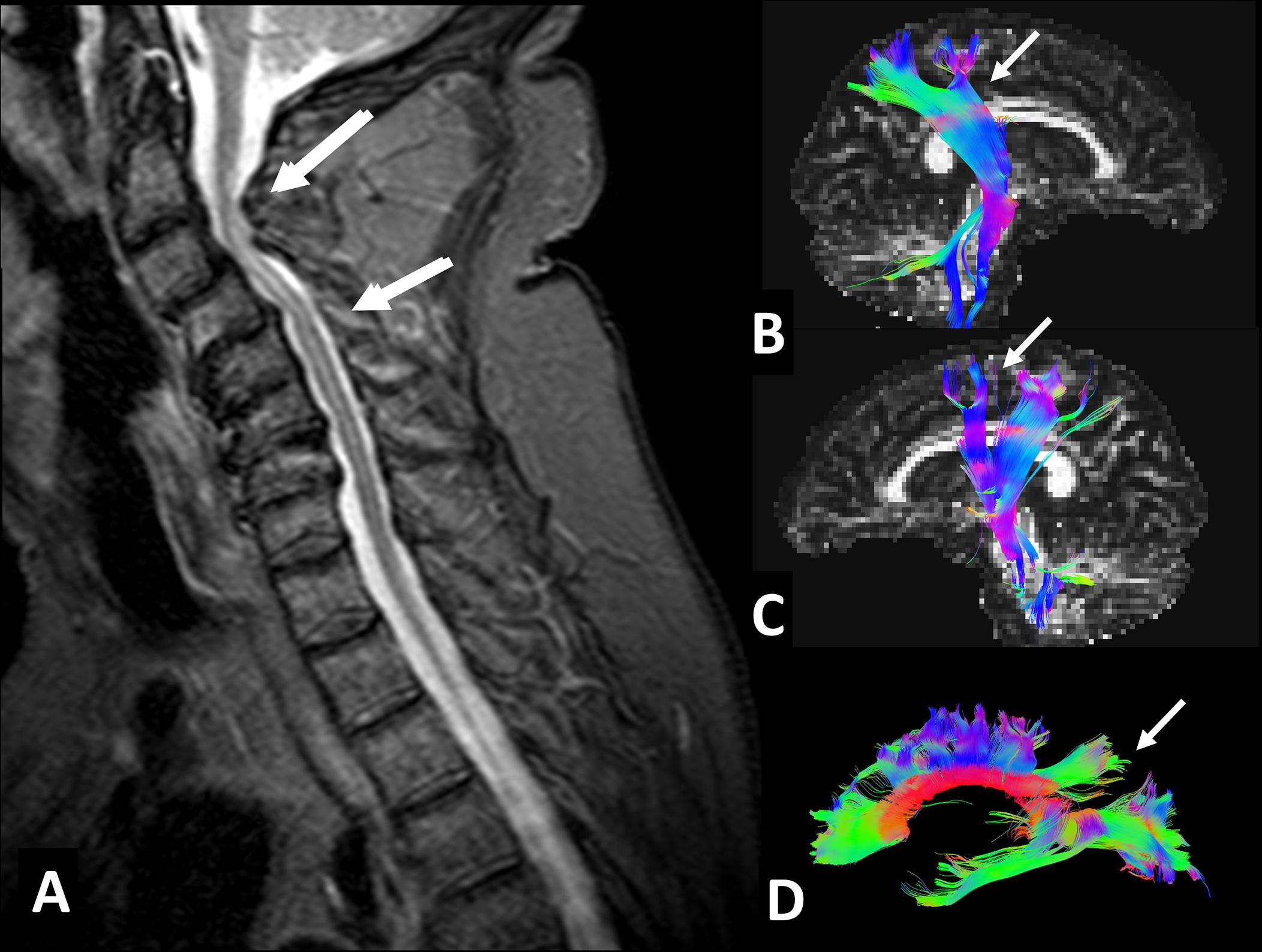
The compression of the spinal cord by CS is a slow and progressive process caused by the reduction of the spinal canal diameter by dynamic and static factors ( ). In CS, any flexion or extension of the cervical spine causes the intermittent compression of the spinal cord. This progressive compression leads to several changes such as apoptosis of the anterior horn neurons, ischemia, gradual spongy necrosis, demyelination of the white matter (WM), and Wallerian degeneration ( Fig. 1 ; ). Despite it may affect one single segment of the spine, multi-level involvement is very frequent, generally at the lower segments. Contrary to the lumbar spine, which encloses only a small part of the spinal cord, stenosis of the cervical spinal canal can induce a direct compromise of the whole spinal cord and severe neurological deficits.
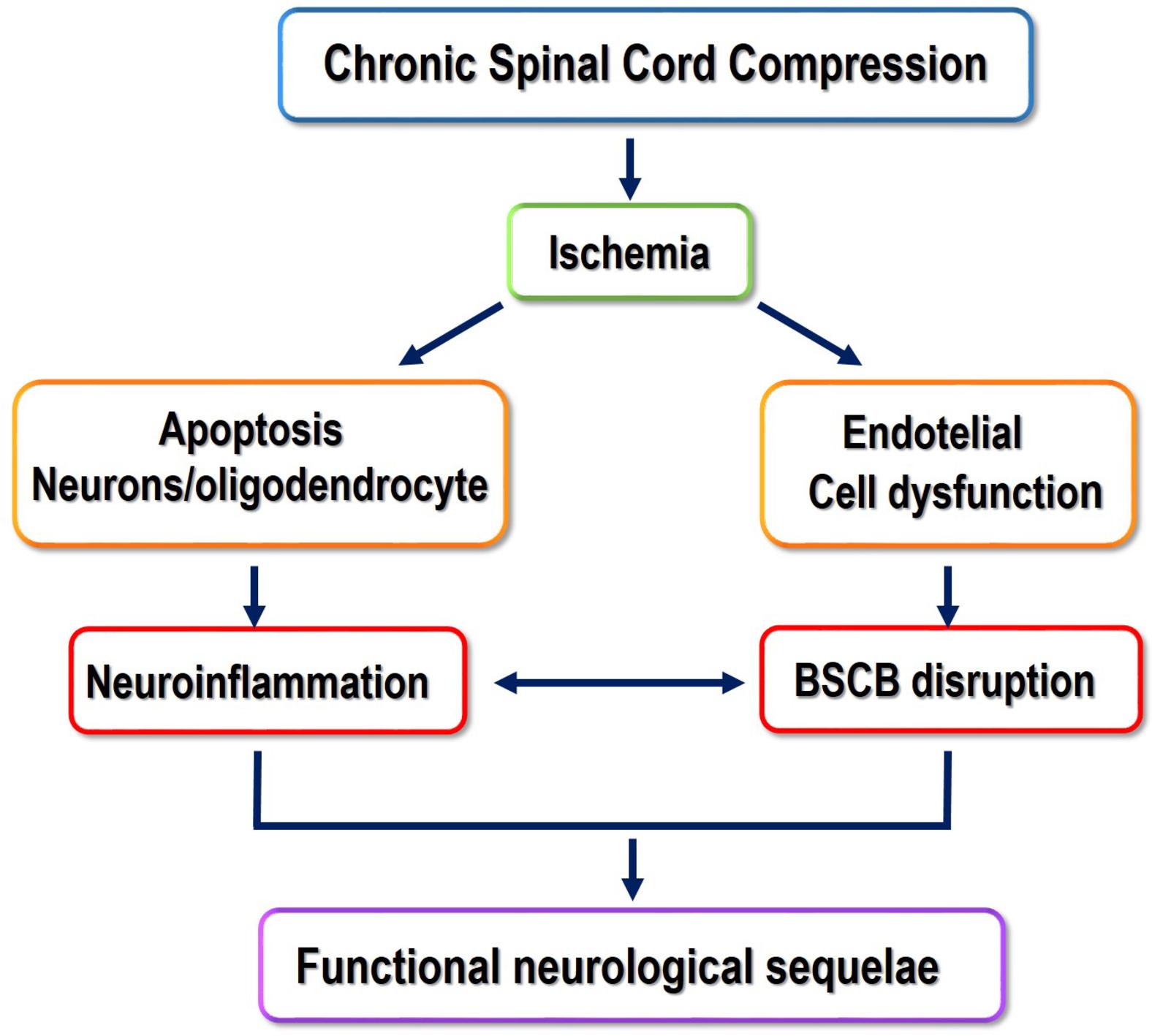
Characteristically, in CS, the severity of compression and deficits varies greatly across individuals. Some patients experience a benign form of the disease with stable periods lasting from months to years, while others develop CSM with neurological deficits and a considerable deterioration over time ( ; ). If CSM fully develops, the symptoms are progressive and in a stepwise manner ( ), with a wide range of clinical manifestations including weakening or sensory loss in one or more limbs, gait unsteadiness, fine motor deficits, and non-specific neck and shoulder pain with or without radiculopathy.
Diagnosis of CSM is based on physical examination and clinical imaging, usually with Magnetic Resonance Imaging (MRI). MRI allows to evaluate the vertebral discs, ligaments, subarachnoid space, spinal cord, and quantify the spinal stenosis. Still, it lacks sensitivity to provide information about the integrity of the spinal cord, presents a poor correlation with neurological and functional impairment, and does not provide reliable prognostic information to predict surgical outcomes ( ). Recent advances in MR techniques have started to overcome these limitations by providing invaluable information about CSM pathology, with several studies reporting that prolonged cervical spine compression affects not only local structures but also distally from the site of injury. For instance, numerous brain changes, such as cortical and subcortical atrophy, functional reorganization of the sensorimotor cortex (SMC), and connectivity impairment, have been reported ( ). These observations have had a great impact on the understanding of CSM, framing the disease as multi-factorial and complex, with its pathophysiology still being poorly understood. In this sense, a clear limitation to advancing in the field of CSM pathology has been the lack of reliable animal models.
Understanding the sequence of structural and functional changes of CSM at the brain level, and defining their effects on the clinical outcome is of crucial importance to better understand the disease, to prevent its fatal consequences, and to develop the best evidence-based rehabilitation therapies. In the next sections, we will summarize the current knowledge on the effects of prolonged spinal cord compression on the brain ( Figs. 2–5 ).
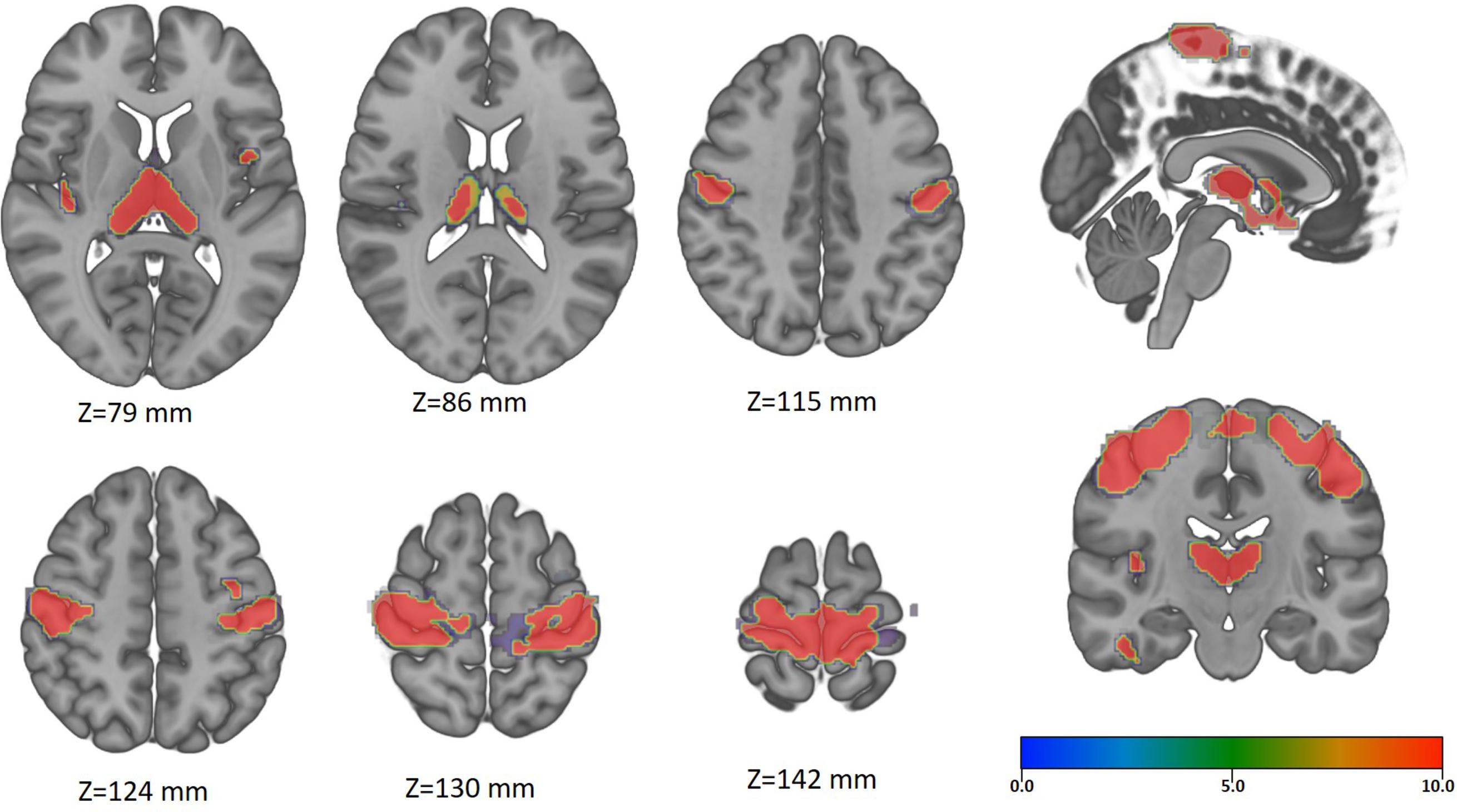
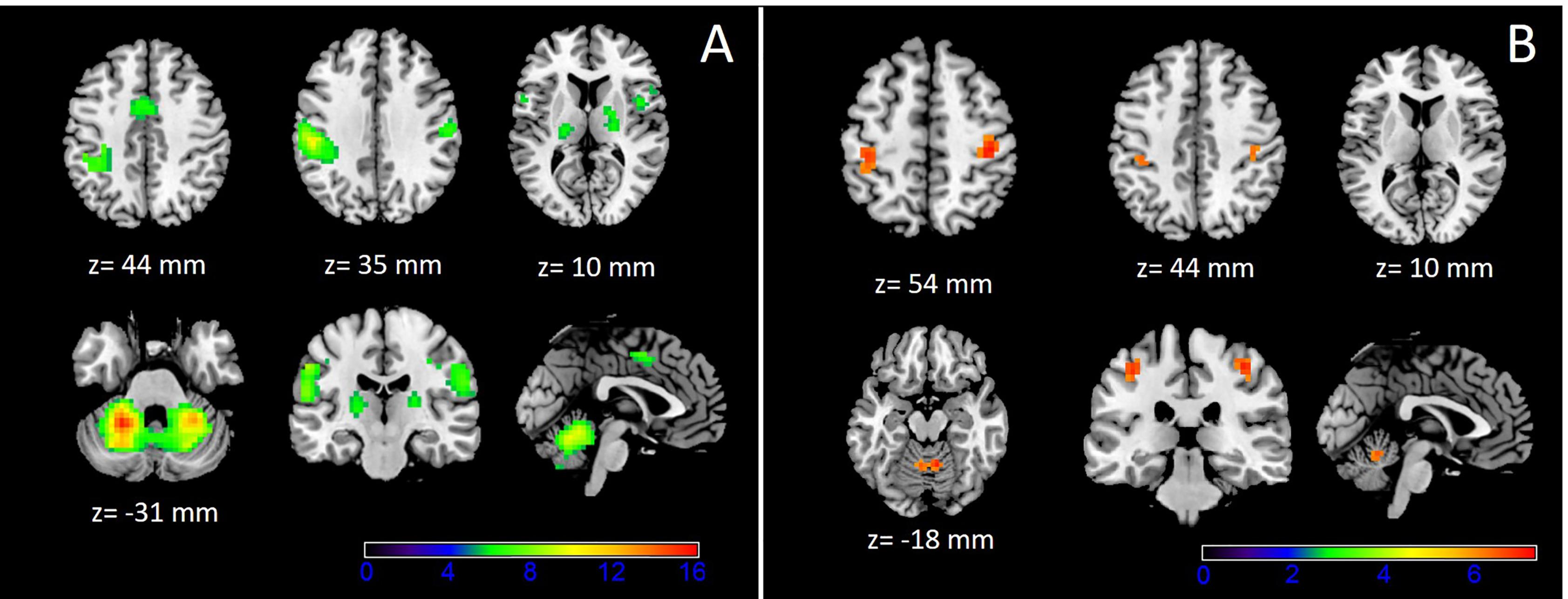
There is increasing evidence supporting that spinal cord injury (SCI) may cause destructive cortical changes ranging from atrophy of projecting neurons to cell death ( ; ). In animal models, after SCI, the disruption of the motor efferents and sensory afferents results in atrophic changes of the neural sensorimotor systems ( ). Indeed, several studies have demonstrated that SCI can induce cortical atrophy on the supraspinal network, whose degree correlates with the spinal cord atrophy ( ) and the functional status ( ).
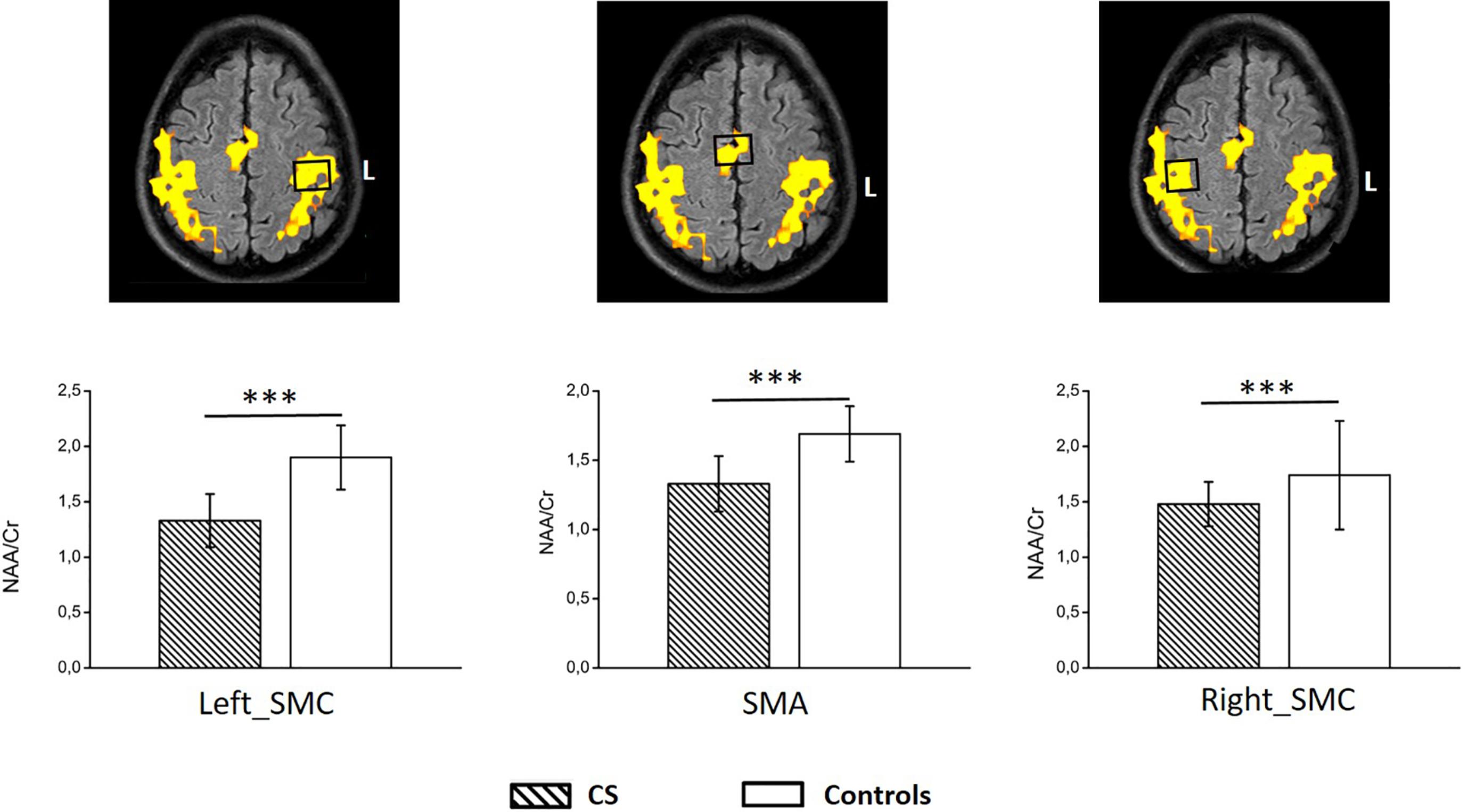
There are still a few works exploring the effect of prolonged spinal cord compression by CS in the brain. However, the results are consistent reporting cortical atrophy in the primary SMC, with additional volume changes at the thalami, specifically at the pulvinar nuclei ( ; ). Furthermore, supplementary brain areas related to the execution of complex movements (such as gait, grasp, and fine hand-motor coordination) are also atrophied ( ; ). Metabolic studies using MR-spectroscopy on CSM patients have also reported neuronal damage and impaired metabolism on the SMC that is not recovered or may worsen after surgery in spite of clinical improvement ( ; ).
In a recent study regarding chronic CS, a consistent pattern of brain changes associated with neurological function and neck pain was observed ( ). Specifically, cortical thinning in the superior frontal regions, anterior cingulate, insula, and precuneus were present. Furthermore, atrophy in the putamen, BA3a, and primary motor regions when worsening neurological symptoms was reported. Taking into account the role of BA3a as an afferent receiver of proprioception ( ), the observed atrophy in this study may explain the deficits in proprioception (gait and hand function) reported in CSM patients ( ).
CS patients may also present brain changes to chronic pain in the same fashion as other chronic pain conditions. Specifically, a cortical thinning within the insula and anterior cingulate cortex has been reported ( ). Finally, a faster rate of cortical thinning and atrophy compared to age-matched healthy subjects in CS patients has been observed ( ).
The significance of these findings is that despite CS has been traditionally considered as a benign disease typically present in the old-age population, it may, if not properly monitored, harm the brain inducing progressive atrophy. In this sense, the CS-related brain atrophy may become a potential obstacle to clinical recovery after surgery, even if significant repair is achieved at the site of compression.
One of the most fascinating characteristics of the central nervous system is its ability to self-repair, reorganize after injury, and compensate for functional loss. This mechanism, known as neural plasticity (or functional reorganization), is present in numerous brain conditions ( ) and has also been observed in SCI. The exact mechanism is not fully understood but may occur through synaptic modifications of pre-existing connections and/or by the development of new circuitries ( ).
Several studies have shown that SCI, although it does not directly involve cortical neurons, may alter the functional activation pattern of the SMC. These observations have been achieved with different physiological and imaging techniques such as functional MRI (fMRI). fMRI is a non-invasive imaging modality that allows to detect changes in brain activation over a period of time while a specific task is being performed. It can be used to localize areas of brain activation secondary to several cognitive and motor tasks, allowing to map the cortical representation of neural activation ( ).
In CSM patients, fMRI studies have shown the existence of significant changes in the cortical activation pattern during sensorimotor tasks. These changes consist of the expansion of the cortical representation for the affected limb by the inclusion of adjacent motor territories ( ). Recruitment of supplementary areas outside the SMC including globus pallidus , left thalamus, caudate nucleus, and both cerebellum hemispheres have been reported. Additionally, decreased activation in SMA and both SMC, as well as increased activation of both cerebellum hemispheres, has been observed ( ). Interestingly, the displacement and activation of the sensorimotor areas in CSM appeared in the vicinity of the areas with gray matter (GM) atrophy, suggesting that the displacement and recruitment of new cortical areas may be mediated by adaptive changes to GM atrophy ( ). This functional reorganization is closely associated with the severity of the SCI ( ), helping to maintain a residual motor function, and contributing to its restoration if the disease progression is halted ( ). Indeed, these adaptive changes may also explain why some CSM patients with significant SCI are able to perform motor activities with apparently minor neurological deficits.
Comparison of pre-operative and post-operative studies has shown that surgical decompression of the cervical spine leads to cortical reorganization, probably by the recovery of conduction in the preserved axons at the spinal cord ( ). After surgical decompression, the altered SMC recruitment pattern gradually disappears ( ), and everything progresses toward an activation pattern similar to the observed in healthy controls ( ). There is an increased activation in the contralateral primary motor cortex (M1) concomitant with the improvement of the motor function and the extent of spinal cord expansion ( ; ). Also, a gradual disappearance of the ipsilateral primary SMC activation in addition to a progressive increase in the contralateral SMC and SMA is observed ( ). This normalization continues over time and can be enhanced by activity-dependent mechanisms related to task practice and learning.
In summary, according to recent studies, CSM patients present compensatory mechanisms that consist of an increase and expansion of the cortical representation of the motor areas, especially those devoted to the hands. After the surgery, there is a tendency to normalization of the sensorimotor representation. However, the reported results are not completely consistent. This is probably because CSM patients form a heterogeneous group with different levels of impairment, different degrees of SCI, and different natural histories. In addition, there is no uniformity in the timing of imaging after the surgery. Still, the reported observations provide direct evidence of neuronal plasticity in the SMC of CSM patients that may play a main role in the post-operative motor functional recovery.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here