Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The salivary gland system comprises three pairs of major glands (parotid, submandibular, and sublingual) and about 500 to 1000 lobules of minor glands dispersed in the submucosa of the oral cavity. The seromucinous glands of the nasal cavity, larynx, and bronchi, although not producing saliva by definition, are histologically similar to the minor salivary glands and share a similar repertoire of neoplasms.
The normal adult parotid gland weighs 15 to 30 g. The superficial and deep lobes are separated by the facial nerve. Most salivary gland tumors arise from the superficial lobe and present as facial swellings. Tumors occurring in the deep lobe often expand through the parapharyngeal space, manifesting as pharyngeal swelling. The accessory lobe of the parotid gland, situated adjacent to the Stenson duct and separate from the main body of the parotid gland, is found in 21% of normal subjects. Tumors arising in this lobe often present as midcheek masses. About 20 lymph nodes and randomly distributed lymphoid aggregates are normally present in the parotid gland, with the latter component representing the mucosa-associated lymphoid tissue (MALT). Conventional types of nodal lymphoma occurring in intraparotid lymph nodes may present clinically as tumor of the parotid gland. Conversely, salivary gland tissues can be found in intraparotid, paraparotid, and cervical lymph nodes; and are believed to give rise to Warthin tumor and other salivary gland tumors in lymph nodes mimicking metastatic disease.
The submandibular and sublingual glands weigh approximately 7 to 15 g and 2 to 4 g, respectively. Unlike the parotid gland, there is no lymph node or large nerve coursing through the parenchyma, although lymph nodes are normally present adjacent to the submandibular gland. Minor salivary glands can be found in the lateral margins of the tongue, lips, buccal mucosa, palate, and glossopharyngeal area. Among them, the palate is the predilection site for salivary gland neoplasms.
Heterotopic salivary gland tissue can occur in the soft tissues of the neck. It typically presents as a draining sinus and/or asymptomatic nodule of the neck along the lower anterior sternocleidomastoid muscle, often with cutaneous involvement.
The major salivary glands are enveloped by a thin fibrous capsule, except the sublingual gland, where the capsule is incomplete. The salivary lobules consist of variable proportions of serous and mucous cells. The parotid gland is exclusively serous, the submandibular gland mixed seromucinous, and the sublingual gland predominantly mucous; the minor glands are seromucinous or predominantly mucous depending on location.
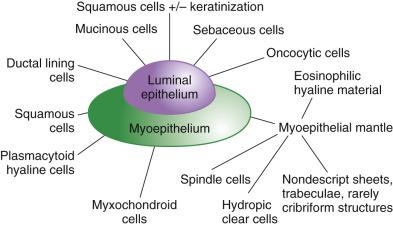
Salivary glands are tubuloacinar exocrine glands. The acini are the secretory units. The secretion reaches the oral cavity via the conducting unit, consisting of intercalated, striated, interlobular, excretory, and salivary ducts ( Fig. 7.1 ). Preservation of the lobular architecture is an important feature favoring a diagnosis of nonneoplastic process over a tumor. The entire glandular structure is a two-tiered organization which comprises the inner luminal cells (acinar and ductal) and the outer abluminal cells (myoepithelial and basal). The secretory acini and the intercalated ducts are wrapped by myoepithelial cells ( Fig. 7.2 ). The striated ducts and the subsequent conducting units are lined by simple or pseudostratified columnar epithelium which gradually transforms into stratified squamous epithelium in the salivary duct, and they are surrounded by basal cells.
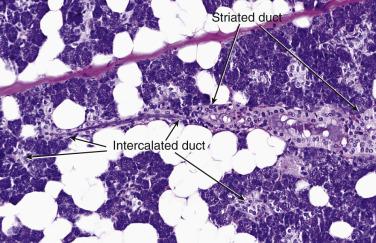
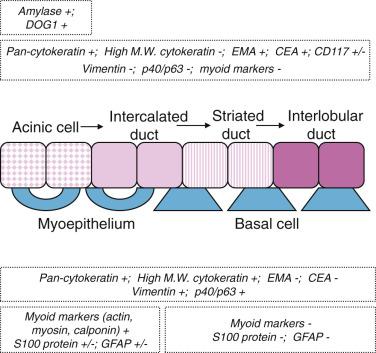
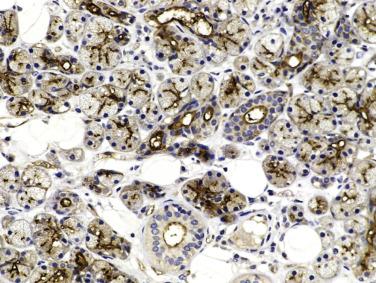
Serous cells are pyramidal, with basal nuclei and abundant basophilic cytoplasm rich in zymogen granules that are periodic acid–Schiff (PAS) positive (diastase resistant) and mucicarmine negative. The mucous cells are cuboidal to columnar, and have pale, finely vacuolated cytoplasm containing sialomucins. They are PAS positive (diastase resistant) and mucicarmine positive. The luminal cells of the intercalated ducts are cuboidal, with lightly eosinophilic cytoplasm and centrally located nuclei. The striated ducts are lined by columnar cells with eosinophilic granular cytoplasm (mitochondria rich), with subnuclear vertical striations produced by prominent basal folds in the plasma membrane (see Fig. 7.1 ). The luminal cells are readily highlighted by immunostaining for cytokeratin, carcinoembryonic antigen (CEA), and epithelial membrane antigen (EMA) ( Fig. 7.3A ; also see Fig. 7.2 ). DOG1 shows luminal staining in serous acini, and weaker staining in mucous acini and luminal cells of the intercalated ducts, whereas luminal cells of the striated ducts and excretory ducts are negative (see Fig. 7.3B ). CD117/c-kit is negative or weakly positive in the normal ductal-acinar cells but is often strongly positive in the luminal cells of various types of salivary gland tumors.
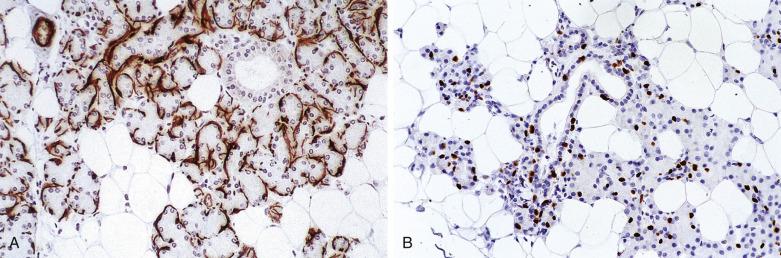
Myoepithelial cells are modified epithelial cells situated between the luminal cells and the basement membrane. They are stellate shaped with cytoplasmic processes embracing the acini or spindle shaped surrounding the intercalated ducts, and cannot be reliably distinguished from basal cells on light microscopy. They possess a dual epithelial and smooth muscle phenotype characterized ultrastructurally by the presence of desmosomes, intermediate filaments, pinocytotic vesicles, and myofilaments. Myoepithelial cells produce basement membrane materials and myxoid substances, which account for the diverse morphology of salivary gland tumors. Myoepithelial cells are best highlighted by immunostaining for p40/p63, high-molecular-weight cytokeratin (including CK14), calponin, or actin. They are variably positive for S100 protein, glial fibrillary acidic protein (GFAP), CD10, and maspin ( Fig. 7.4A and B ; also see Fig. 7.2 ).
The basal cells located around the striated ducts, excretory ducts, and salivary ducts differ from myoepithelial cells in the absence of myofilaments and the lack of myoid markers (calponin, actin) while retaining expression of p40/p63 and high-molecular-weight cytokeratin. They maintain the capacity of multidirectional differentiation and play an important role in the processes of regeneration and metaplastic changes.
Oncocytic cells, characterized by abundant eosinophilic granular cytoplasm due to accumulation of mitochondria, are uncommon below the age of 50 but increase thereafter until being almost universal above the age of 70. They show variable replacement of the normal cells in the ductoacinar units. Oncocytic metaplasia has been implicated in the development of oncocytic hyperplasia, nodular oncocytosis, and even oncocytoma.
Adipose tissue is normally a conspicuous component of the parotid gland, which increases in proportion with age. Groups of sebaceous glands may occur in the parotid gland and represent the normal counterpart of the salivary sebaceous neoplasms ( Fig. 7.5 ).
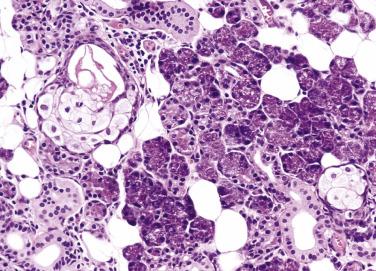
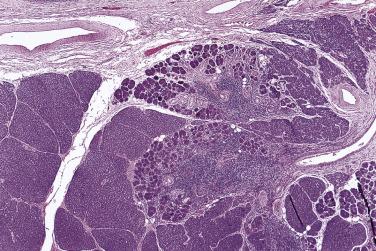
Salivary gland neoplasms are uncommon. The worldwide annual incidence ranges from 0.4 to 13.5 cases per 100,000 people. In general, women are more commonly affected than men, except for Warthin tumor and high-grade carcinomas. Epithelial tumors constitute 80% to 90% of all salivary gland tumors, with the majority being benign (75%) and pleomorphic adenoma being the most common (about 65% of all tumors) ( Table 7.1 ). The distribution of tumors in the parotid gland, submandibular gland, and minor salivary glands roughly follows the 10 : 1 : 1 proportion rule, but some tumor types show a predilection for either the major or minor glands, and thus knowledge of the site of tumor can aid in diagnosis ( Box 7.1 ).
| Benign Epithelial Tumors | Malignant Epithelial Tumors |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warthin tumor
Acinic cell carcinoma
Mammary analogue secretory carcinoma
Basal cell adenoma/adenocarcinoma
Oncocytoma/oncocytic carcinoma
Epithelial-myoepithelial carcinoma
Salivary duct carcinoma
Lymphoepithelial carcinoma
Canalicular adenoma (lip, buccal mucosa)
Polymorphous low-grade adenocarcinoma (palate)
Cystadenoma/cystadenocarcinoma (lip, buccal mucosa)
Inverted papilloma (lip, buccal mucosa)
Intraductal papilloma (lip, buccal mucosa)
Sialadenoma papilliferum (palate)
Cribriform adenocarcinoma of minor salivary glands (tongue, oral mucosa)
Signet ring cell adenocarcinoma (oral mucosa)
Primary carcinomas of the salivary glands account for less than 0.3% of all cancers. The sites of occurrence with respect to the number of cases in descending order are parotid gland, submandibular gland, palate, cheek, and tongue. Tumors have the highest chance of being malignant if they arise from the sublingual gland (95% malignant) or minor salivary glands (80% malignant), while only 20% of all parotid tumors are malignant. Among salivary gland carcinomas, the most common histologic types are mucoepidermoid carcinoma, adenoid cystic carcinoma, and acinic cell carcinoma.
The age at presentation of malignant tumors is similar to or slightly older compared with benign tumors. Mucoepidermoid carcinoma, adenoid cystic carcinoma, and acinic cell carcinoma tend to have an earlier age of onset compared with adenocarcinoma not otherwise specified (NOS), squamous cell carcinoma, and carcinoma ex pleomorphic adenoma. In practice, most malignant salivary gland tumors are not clinically distinguishable from the benign ones, except when they show rapid increase in size, pain, fixation to adjacent structures, ulceration, or cervical lymph node metastasis. Facial nerve paralysis is a more consistent sign of malignancy, more often seen in high-grade tumors such as squamous cell carcinoma and undifferentiated carcinoma, although it can also rarely occur in Warthin tumor and pleomorphic adenoma.
Salivary gland tumors are rare in children. Previous studies reported a higher proportion of epithelial tumors in children to be malignant compared with adults, but recent series have shown a similar malignant to benign tumor ratio in both populations. Low-grade mucoepidermoid carcinoma is the most common malignant epithelial tumor in children. In infants, mesenchymal tumors (hemangioma and lymphangioma) are the most common. Some unusual tumors such as sialoblastoma and salivary gland anlage tumor occur almost exclusively in this age group.
Some salivary gland carcinomas are clinically low grade (e.g., acinic cell carcinoma, polymorphous low-grade adenocarcinoma, basal cell adenocarcinoma, epithelial-myoepithelial carcinoma, clear cell carcinoma, cystadenocarcinoma), some are high grade (e.g., salivary duct carcinoma, most cases of carcinoma ex pleomorphic adenoma, undifferentiated carcinoma, oncocytic carcinoma), while some span a range of behavior according to the histologic grade (e.g, mucoepidermoid carcinoma, adenoid cystic carcinoma). The prognosis of an individual tumor type is further influenced by the clinical stage and margin status. The TNM (tumor, nodes, metastasis) staging is listed in Table 7.2 .
| DEFINITION OF PRIMARY TUMOR (T) | |
| T Category | T Criteria |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor 2 cm or smaller in greatest dimension without extraparenchymal extension* |
| T2 | Tumor larger than 2 cm but not larger than 4 cm in greatest dimension, without extraparenchymal extension* |
| T3 | Tumor larger than 4 cm and/or tumor having extraparenchymal extension* |
| T4 | Moderately advanced or very advanced disease |
| T4a | Moderately advanced disease Tumor invades skin, mandible, ear canal, and/or facial nerve |
| T4b | Very advanced disease Tumor invades base of skull and/or pterygoid plates, and/or encases carotid artery |
| *Extraparenchymal extension is clinical or macroscopic evidence of invasion of soft tissues or nerves, except those listed under T4a and T4b. Microscopic evidence alone does not constitute extraparenchymal extension for classification purposes. | |
| DEFINITION OF REGIONAL LYMPH NODE (N) | |
| Clinical N (cN) | |
| N Category † | N Criteria |
| NX | Regional lymph node cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in a single ipsilateral node, 3 cm or smaller in greatest dimension and ENE(−) |
| N2 | N2a: Metastasis in a single ipsilateral node larger than 3 cm but not larger than 6 cm in greatest dimension and ENE(−) |
| N2b: Metastasis in multiple ipsilateral lymph nodes, none larger 6 cm in greatest dimension and ENE(−) | |
| N2c: Metastasis in bilateral or contralateral lymph nodes, none larger than 6 cm in greatest dimension and ENE(−) | |
| N3 | N3a: Metastasis in a lymph node larger than 6 cm in greatest dimension and ENE(−) |
| N3b: Metastasis in any node(s) with clinically overt ENE(+) | |
| † Note: A designation of “U” or “L” may be used for any N category to indicate metastasis above the lower border of the cricoid (U) or below the lower border of the cricoid (L). Similarly, clinical and pathologic ENE should be recorded as ENE(−) or ENE(+). | |
| PATHOLOGIC N (pN) | |
| N Category † | N Criteria |
| NX | Regional lymph node cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in a single ipsilateral node, 3 cm or smaller in greatest dimension and ENE(−) |
| N2 | N2a: Metastasis in a single ipsilateral ot contralateral node, 3 cm or smaller in greatest dimension and ENE(+); or a single ipsilateral node larger than 3 cm but not larger than 6 cm in greatest dimension and ENE(−) |
| N2b: Metastases in multiple ipsilateral nodes, none larger than 6 cm in greatest dimension and ENE(−) | |
| N2c: Metastasis in bilateral or contralateral lymph nodes, none larger than 6 cm in greatest dimension and ENE(−) | |
| N3 | N3a: Metastasis in a lymph node larger than 6 cm in greatest dimension and ENE(−) |
| N3b: Metastasis in a single ipsilateral node larger than 3 cm and ENE(+) or multiple ipsilateral, contralateral or bilateral nodes, any with ENE(+) |
|
| † Note: A designation of “U” or “L” may be used for any N category to indicate metastasis above the lower border of the cricoid (U) or below the lower border of the cricoid (L). Similarly, clinical and pathologic ENE should be recorded as ENE(−) or ENE(+). | |
| DEFINITION OF DISTANT METASTASIS (M) | |
| M Category | M Criteria |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
| When T Is… | And N Is… | And M Is… | Then the Stage Group Is… |
|---|---|---|---|
| Tis | N0 | M0 | 0 |
| T1 | N0 | M0 | I |
| Any N | N0 | M0 | II |
| T3 | N0 | M0 | III |
| T0, T1, T2, T3 | N1 | M0 | III |
| T4a | N0, N1 | M0 | IVA |
| T0, T1, T2, T3, T4a | N2 | M0 | IVA |
| Any T | N3 | M0 | IVB |
| T4b | Any N | M0 | IVB |
| Any T | Any N | M1 | IVC |
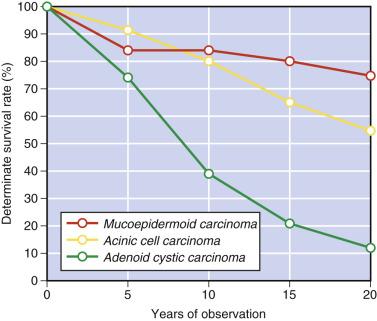
Some studies have shown high proliferative fraction (Ki67 index), DNA aneuploidy, and expression of p53 protein to be associated with a worse prognosis. Since some salivary gland carcinomas are notorious for pursuing a protracted clinical course with late recurrence or metastasis, long-term follow-up is required. The survival curves of the three major types of salivary gland carcinoma ( Fig. 7.6 ) are most instructive. The plateau in the curve after an initial drop indicates that a significant proportion of patients with mucoepidermoid carcinoma can be cured. Acinic cell carcinoma is indolent, but adverse events may manifest after 10 years. Although the short-term survival for adenoid cystic carcinoma is good, the long-term outcome is bleak—that is, a high proportion of patients eventually succumb to the tumor.
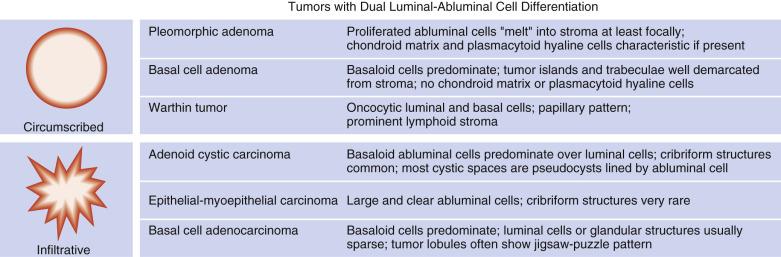
Salivary gland tumors often pose problems in diagnosis due to their rarity, broad morphologic spectrum, and morphologic overlap between the different tumor types. It is important therefore to understand the basic cytoarchitectural features of each tumor type, in particular whether the tumor shows dual cell type (luminal and abluminal) differentiation ( Fig. 7.7 ), so that a diagnosis can be made logically through analysis of the cellular components, cell arrangement, and extracellular components (see “ Analytic Approach to Diagnosis of Epithelial Tumors of the Salivary Gland ”).
Histochemical studies have only a limited role in the diagnosis of salivary gland tumors. Staining for mucosubstances and basement membrane materials offers little help in diagnosis, because any salivary gland tumors with luminal cell differentiation can have epithelial-type mucin (usually PAS-positive diastase resistant) in the lumens, and tumors with abluminal cell differentiation can be associated with production of basement membrane–like material (PAS-positive diastase resistant) or acidic stromal mucin (Alcian blue positive, but PAS negative). The limited applications of histochemistry include:
Aid in diagnosis of high-grade mucoepidermoid carcinoma by demonstrating intracytoplasmic mucin. Mucin stains are not required for the diagnosis of low- to intermediate-grade mucoepidermoid carcinomas because mucin is often obvious in routine histologic sections.
PAS-diastase can aid in detection of focal luminal cell differentiation in a predominantly basal cell or myoepithelial cell neoplasm, such as the solid type of adenoid cystic carcinoma and clear cell–rich epithelial-myoepithelial carcinoma. Immunostaining for epithelial membrane antigen (EMA), carcinoembryonic antigen (CEA), or c-kit can similarly highlight the small and abortive glandular lumens.
Phosphotungstic acid hematoxylin (PTAH) stain may help in the diagnosis of the clear cell variant of oncocytoma, by highlighting the mitochondria. This role can alternatively be achieved by immunostaining for mitochondria.
Immunohistochemical staining may aid in diagnosis in selected circumstances, as follows:
To delineate whether there is dual-cell differentiation in tumors with complex architecture; luminal cells are best highlighted by c-kit/CD117 and abluminal cells by p40 and/or calponin.
To confirm the diagnosis of specific salivary gland tumor types, such as myoepithelial tumors (expression of myoepithelial markers), mammary analogue secretory carcinoma (extensive positive staining for S100 and mammaglobin), acinic cell carcinoma (luminal DOG1 staining), polymorphous low-grade adenocarcinoma (positive for p63 and negative for p40), and basal cell adenoma (nuclear staining for β-catenin and LEF-1).
To confirm the in situ nature of intraductal carcinoma.
Ki67 proliferative index may be useful in distinguishing an adenoma from a carcinoma (Ki67 index usually <5% versus >10%).
There have been significant advances in knowledge of the molecular genetics of salivary gland neoplasms in recent years ( Table 7.3 ). These findings are particularly helpful in the diagnosis of difficult cases (including small biopsies), unusual variants (such as oncocytic and Warthin-like variants of mucoepidermoid carcinoma), specific entities (such as mammary analogue secretory carcinoma), and cases occurring outside the salivary glands (often with a differential diagnosis of adenosquamous carcinoma or squamous cell carcinoma).
| Tumor Type | Genetic Alterations and Implicated Genes | Prevalence |
|---|---|---|
| Pleomorphic adenoma | t(3;8) or t(5;8) involving PLAG1 (8q21) t(3;12) involving HMGA2 (12q14) |
Common Uncommon |
| Basal cell adenoma, nonmembranous type | Activating point mutation in CTNNB1 (3p22): I35T | Common |
| Basal cell adenoma, membranous type | Inactivating mutation in CYLD1 (16q12) | Common |
| Mucoepidermoid carcinoma | t(11;19) and t(11;15) involving MAML2 (11q21) and CRTC1 (19p13), or less frequently, CRTC3 (15q26) t(6;22) involving EWSR1 (22q12) and POU5F1 (6p21) |
Common Rare |
| Adenoid cystic carcinoma | t(6;9) involving MYB (6q23) and NFIB (9p22) t(8;9) involving MYBL1 (8q13) and NFIB |
Common Uncommon |
| Mammary analogue secretory carcinoma | t(12;15) involving ETV6 (12p13) and NTRK3 (15q25) t(11;12) involving ETV6 and RET (11q11) [rare] |
Defining feature |
| Clear cell carcinoma | t(12;22) involving EWSR1 (22q12) and ATF1 (12q13) | Defining feature |
| Myoepithelial carcinoma, clear cell type | Translocation of EWSR1 (22q12) with unknown partners | A proportion of cases |
| Polymorphous low-grade adenocarcinoma | Activating point mutation in PRKD1 (14q12): E710D Translocation of PRKD2 (19q13) with unknown partners |
Common Rare |
| Cribriform adenocarcinoma of minor salivary gland origin | t(1;14) and t(X;14) involving PRKD1 (14q12) and ARID1A (1p36), or DDX3X (Xp11) Translocation of PRKD2 or PRKD3 with unknown partners |
Common |
| Salivary duct carcinoma | Amplification of HER2 (17q21) Activating mutation in PIK3CA |
Common |
| Intraductal carcinoma, intercalated duct type | NCOA4-RET gene fusion | Common |
Little is known about the precursor lesions of the salivary gland neoplasms. In rare cases of basal cell adenoma, basal cell adenocarcinoma, pleomorphic adenoma, epithelial-myoepithelial carcinoma, or some other tumor types, proliferative lesions of intercalated ducts can be identified in the vicinity of the tumor or in the background salivary gland tissue, suggesting that they might represent precursor lesions of some salivary gland tumors. Further evidence however is needed to ascertain their precursor role, if any.
Intercalated duct lesions, which are rare, are usually incidentally discovered small and nodular lesions in the salivary gland, most commonly the parotid. They can be unifocal or multifocal, and have a mean size of 3.1 mm. There is a morphologic spectrum, including:
Intercalated duct hyperplasia—a nonencapsulated proliferation of closely packed intercalated ducts with little intervening tissue, blending imperceptibly with some acinar units in the peripheral portion ( Fig. 7.8 ). The intercalated ducts are lined by cuboidal cells, which have uniform round nuclei. Although myoepithelial cells may not be evident on routine histologic sections, they are consistently present around the individual ducts, as can be demonstrated by actin or p40 immunostaining.
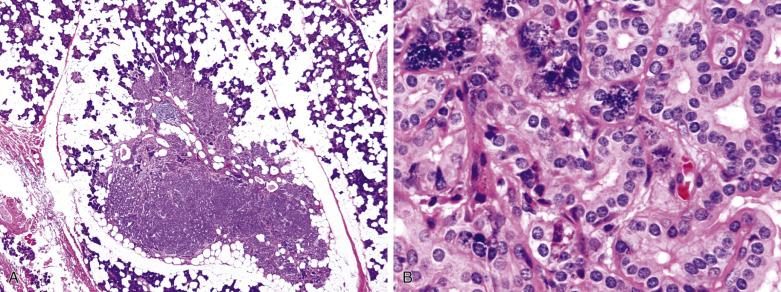
Intercalated duct adenoma—a discrete, rounded, partially to completely encapsulated nodule with well-defined contours. It usually comprises pure intercalated ducts, but some acinic cells can be present. There is minimal intervening stroma.
Hybrid intercalated duct lesion—a lesion with mixed features of hyperplasia and adenoma.
The majority of salivary gland tumors are benign or low-grade malignant. It is thus not surprising to encounter biologic progression in longstanding neoplasms as a result of acquisition of additional genetic alterations (such as TP53 mutation, HER2 amplification). Tumor progression can manifest as malignant transformation, stromal invasion, high-grade progression, overgrowth, or dedifferentiation (high-grade transformation) ( Table 7.4 ).
| Phenomenon | Relationship of Components | Examples |
|---|---|---|
| Malignant transformation | Benign tumor → Malignant tumor |
|
| Stromal invasion | In situ carcinoma → Invasive carcinoma |
|
| Overgrowth | Carcinoma with dual cell differentiation → monomorphic carcinoma with one-line differentiation |
|
| High-grade progression | Same tumor type, from low grade → high grade |
|
| Dedifferentiation (high-grade transformation) | Carcinoma → High-grade malignant neoplasm with loss of original line of differentiation |
|
Hybrid tumor, which is very rare, comprises two or more histologic distinct components at a single site. The most common components of hybrid carcinomas are salivary duct carcinoma, epithelial-myoepithelial carcinoma, and adenoid cystic carcinoma. Others include mucoepidermoid carcinoma, acinic cell carcinoma, squamous cell carcinoma, basal cell adenocarcinoma, and polymorphous low-grade adenocarcinoma. Treatment would probably be best based on the histologic component with higher grade malignancy. Rare hybrid adenomas include combinations of basal cell adenoma with canalicular adenoma, Warthin tumor with sebaceous adenoma, and Warthin tumor with oncocytoma. Hybrid tumors are postulated to arise through divergent differentiation from the same tumor clone, or collision of two separate tumors. Many cases of hybrid carcinoma however can be interpreted as a dedifferentiation process (such as combination of a lower grade carcinoma with salivary duct carcinoma).
Pleomorphic adenoma is a benign neoplasm characterized by dual cell type differentiation and melting of the outer basal/myoepithelial cells into a stroma which commonly shows chondromyxoid features. The coexistence of epithelial and mesenchymal elements gives rise to the synonym mixed tumor, but pleomorphic adenoma is now widely accepted as a monoclonal epithelial tumor with divergent differentiation.
Pleomorphic adenoma occurs more frequently in women than men, and is most prevalent from the fourth to sixth decades, with a mean age of 45 years. Bimodal age distribution peaks at 38 and 64 years are also found in women. The tumor usually presents as a slow-growing and painless swelling. When it occurs in the minor glands, ulceration of the overlying mucosa or apparent fixation to the surrounding tissue can be seen rarely. Pleomorphic adenoma can occur in various mucosal sites such as nasal cavity, bronchus, skin (also known as chondroid syringoma), breast, and soft tissues.
The treatment of choice is complete surgical excision. The overall 20-year recurrence rate is 6.7% and the median time to first recurrence is 7 years. Enucleation alone, rupture or spillage of tumor during removal, presence of protuberances beyond the main tumor, abundance of chondromyxoid stroma, and young age are associated with a higher recurrence rate. Malignant transformation occurs in 0.15% of all cases or 3.2% of recurrent cases. Factors associated with an increased risk of malignant transformation are older patient age, longstanding tumor, submandibular location, large tumor size, prominent zones of hyalinization, and at least moderate mitotic activity.
The tumor size ranges from a few millimeters to several centimeters. The tumor is typically thinly encapsulated and solitary. Intraoral examples, especially those arising from the palate, may lack a well-defined capsule. The cut surface is rubbery, fleshy, mucoid, or glistening, depending on the amount of stroma in the tumor. In areas where the capsule is deficient, tumor buds may lie in direct contact with the adjacent salivary tissue. Recurrent tumor typically appears as multiple nodules scattered over the field of the previous operation ( Fig. 7.9 ).
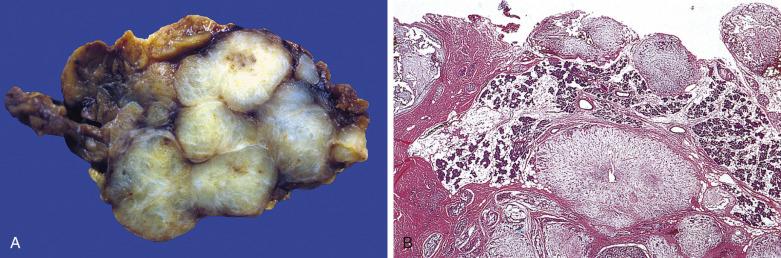
Pleomorphic adenoma is thinly encapsulated ( Fig. 7.10A ). A few small, smooth-contoured buds (protuberances) may protrude through the fibrous capsule. Occasionally, tumor islands may appear outside the capsule at a short distance from the main tumor mass (see Fig. 7.10B ), but serial sectioning usually demonstrates that such satellites are in fact outgrowths continuous with the main tumor mass and should not be regarded as invasion. Pleomorphic adenoma can grow entirely within a dilated duct. Vascular invasion can be found in 1% to 3% of cases, but this is not associated with adverse outcome. Perineural invasion can also rarely occur, but its significance is currently unclear.
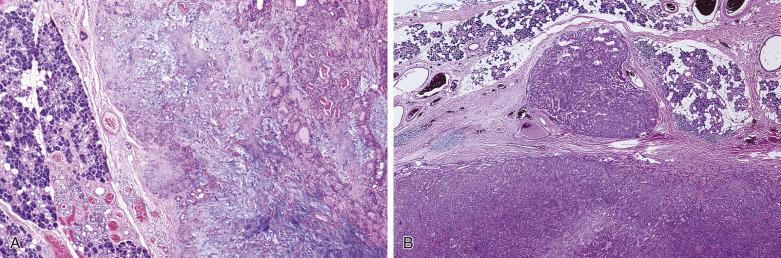
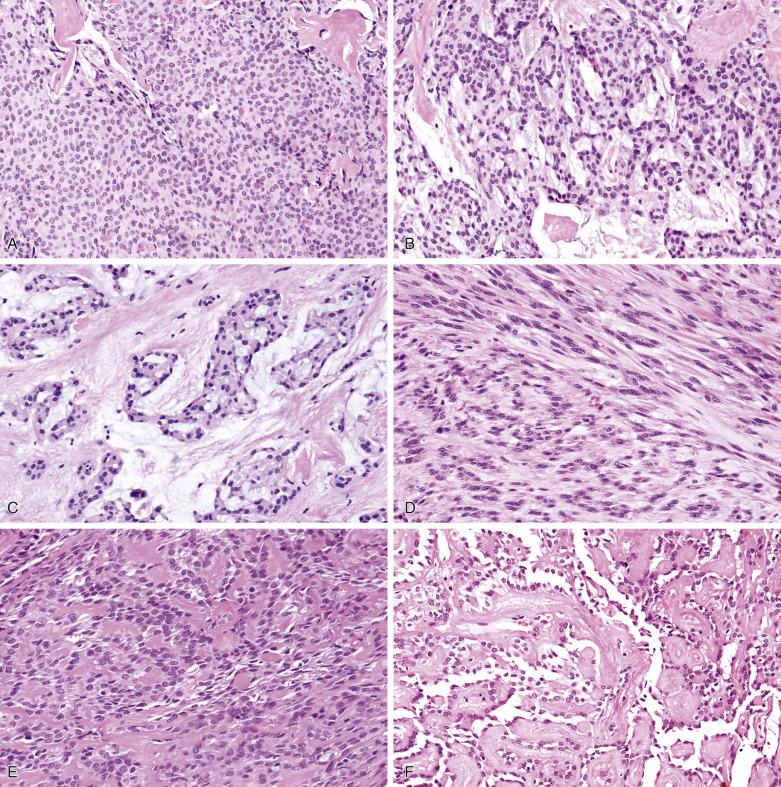
Pleomorphic adenoma is characterized by highly variable growth patterns in different areas of the same tumor ( Fig. 7.11 ).
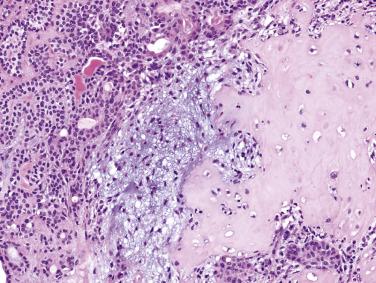
The prototypic histologic appearance consists of tubular structures enveloped by myoepithelial mantles submerging in a chondromyxoid stroma. The interface between the tumor islands and the stroma is usually poorly demarcated. The myoepithelial mantle radiates centrifugally, forming sheets, clusters, lattices, and isolated cells, where they appear to melt into the sea of stroma they produce ( Fig. 7.12 ). While the melting phenomenon is characteristic, it can be focal, and some areas of the tumor can be composed of tubules or trabeculae well delineated from the stroma ( Fig. 7.13 ). There may even be foci architecturally resembling mucoepidermoid carcinoma or adenoid cystic carcinoma ( Fig. 7.14 ), but carcinoma ex pleomorphic adenoma should not be diagnosed unless a discrete expansile lesion is formed.
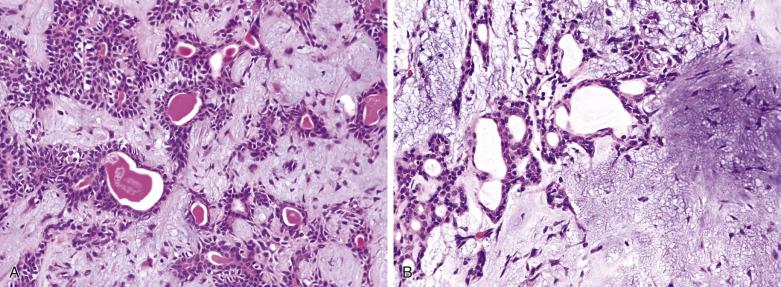
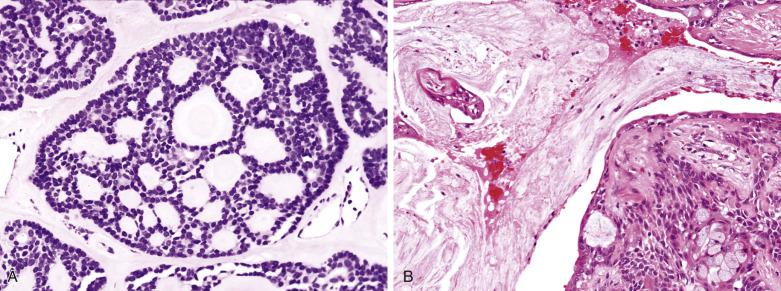
The luminal cell component takes the form of anastomosing tubules, cysts, ribbons, and solid sheets ( Fig. 7.15 ). The cells are columnar, cuboidal, or flat. The duct lumens may be empty or contain eosinophilic colloid-like material, which is PAS-positive diastase resistant and variably mucicarmine positive (see Figs. 7.12 and 7.15 ). Rarely, metaplastic change to squamous, sebaceous, oncocytic, or clear cells can occur ( Figs. 7.16, 7.17, and 7.18 ). Very occasionally, the epithelium may form goblet or mucous cells, which in association with squamous epithelium can lead to an erroneous interpretation of mucoepidermoid carcinoma (see Fig. 7.14B ).
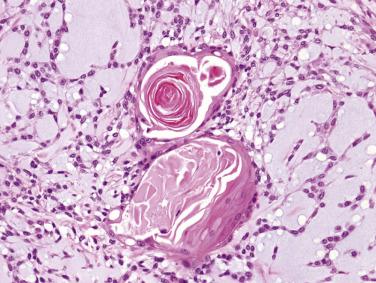
Myoepithelial or modified myoepithelial cells appear as cuboidal, spindle, stellate, plasmacytoid hyaline, nondescript epithelioid, or hydropic clear cells (see Fig. 7.18 ). The spindle or cuboidal cells surround the ducts in a single layer, thick mantle, or radiating corona ( Fig. 7.19 ; also see Figs. 7.12 and 7.17 ). They can form nondescript sheets, trabeculae, and even cribriform structures.
Plasmacytoid hyaline cells represent the most distinctive form of modified myoepithelial cells; they are oval shaped, with homogeneous eosinophilic hyaline cytoplasm ( Fig. 7.20A ). The nucleus is round and eccentrically located, with a tendency for peripheral margination of the dense chromatin. Plasmacytoid hyaline cells are so named because of their superficial resemblance to plasma cells, but they are larger, show less coarse clumping of the chromatin, lack a perinuclear Golgi zone, and possess eosinophilic rather than amphophilic cytoplasm. They are usually arranged in aggregates or sheets, often with focal areas of noncohesive growth (see Fig. 7.20 ). Since their occurrence is restricted to pleomorphic adenoma and myoepithelioma, their identification is of great diagnostic value, especially in small biopsies. There can be cells with morphologic features intermediate between plasmacytoid hyaline cells and other types of myoepithelial cells.
Stellate or spindled myoepithelial cells occur singly or form anastomosing strands, suspended in an abundant myxoid matrix ( Fig. 7.21 ). Uncommonly, myoepithelial cells may merge into squamous nests or cystic squamous-lined structures filled with keratin, suggesting an ability to differentiate directly toward the squamous lineage (see Fig. 7.17 ). In occasional cases, myoepithelial cells dominate the tumor. Rarely, skeletal muscle differentiation and scattered melanocytes can occur, the latter also imparting a pigmented macroscopic appearance to the tumor.
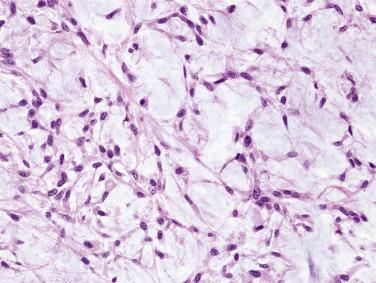
Extracellular stroma is one of the defining components of pleomorphic adenoma, although its quantity can range from scanty to abundant. It is composed mostly of acidic mucosubstances produced by the modified myoepithelial cells, and is positive for Alcian blue but variably positive for PAS. The stroma takes the form of a mixture of chondroid (hyaline cartilage), myxoid, chondromyxoid, hyaline, and very rarely, osseous and adipose tissues (see Figs. 7.11, 7.12, and 7.21 ). Of interest, adipocytic cell differentiation is uncommon except in cutaneous sites. Isolated or groups of stellate, oval or polygonal cells are suspended in the matrix. The presence of chondromyxoid stroma in a salivary gland tumor is practically pathognomonic of pleomorphic adenoma (see Fig. 7.11 ). In tumors in which chondromyxoid matrix predominates, the sparse ductal structures are often confined to the subcapsular zone. Tumors with very scanty or no extracellular stroma are often called cellular pleomorphic adenomas ( Fig. 7.22 ; also see Figs. 7.13 and 7.15 ); they can be recognized by the focal melting of the myoepithelial mantles. It has been suggested that recurrence is more frequent for stroma-rich tumors, which have a higher chance of spillage of mucoid stroma during operation. Highly cellular tumors, on the other hand, may be more prone to malignant change.
Homogenous, fibrillary, or radiating hyaline material can be interspersed among the epithelial or myoepithelial cells (see Fig. 7.19A ). Crystalloids composed of collagenous substance, tyrosine, and oxalate are sometimes found between the cellular or stromal components. Tyrosine crystals often appear as daisy-heads in the myxoid stroma. Elastic fibers are present in variable amounts in most pleomorphic adenomas, and they are particularly abundant in longstanding lesions. They show up as globular masses or irregular bands with fluffy outlines on histochemical staining ( Fig. 7.23 ). These thick elastic fibers are diagnostically helpful because they are very uncommon in other salivary gland tumors.
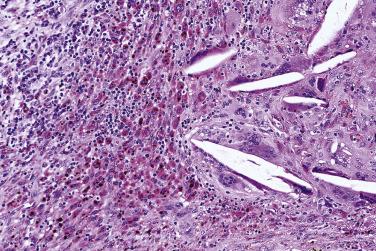
Fine-needle aspiration commonly results in hemorrhagic tracts and micronecrosis, accompanied by variable reparative changes ( Fig. 7.24 ). Complete or incomplete infarction can also occur ( Fig. 7.25 ). There can be florid reactive proliferation of the myoepithelium, which can protrude into the fibrous capsule or show nodular bulging beneath the endothelium of veins. Atypical squamous metaplasia is also common (see Fig. 7.25B ).
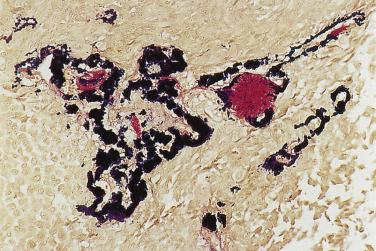
The main application of immunohistochemistry is to demonstrate the dual cell composition when this is not obvious. The glandular component, which may be inconspicuous, can be highlighted by EMA, CEA, or c-kit ( Fig. 7.26 ). The myoepithelial and modified myoepithelial cells are positive for cytokeratin, but not EMA and CEA. Although CK14, p40/p63, and various myoid markers (such as actin, myosin, and calponin) highlight the normal myoepithelium consistently, the pattern of staining in pleomorphic adenoma is anarchic: the staining of the neoplastic myoepithelium can be patchy or totally negative (see Fig. 7.26D ). The myoepithelial component is commonly positive for S100 protein and GFAP, although S100 immunoreactivity can also be variably observed in the luminal cells (see Fig. 7.26 ). Currently the more reliable markers for the neoplastic myoepithelial component are p40/p63 and calponin, although the staining can be focal ( Fig. 7.27 ).
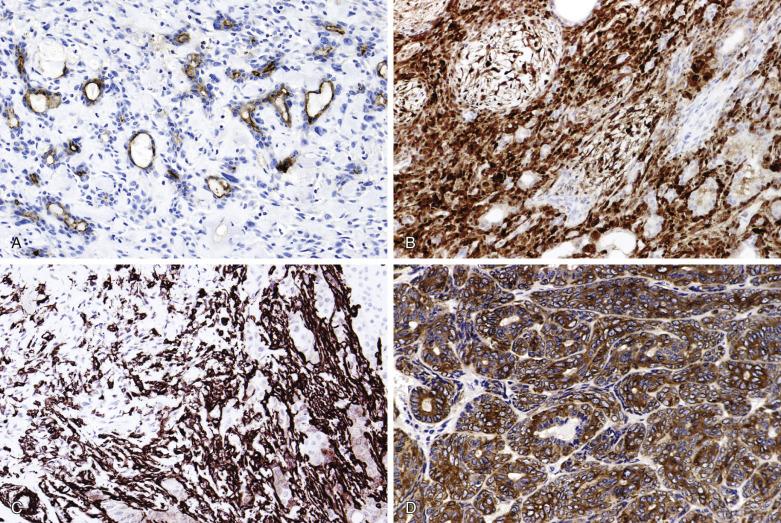
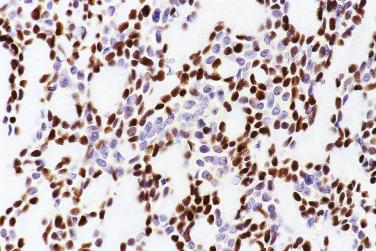
Pleomorphic adenoma shows a low Ki-67 proliferative index (mean 1.6%), rare immunoreactivity for P53 protein (mean 1.2% positive cells), and weak BCL2 staining. These parameters can help to distinguish this tumor from adenoid cystic carcinoma, because the latter tumor shows a mean Ki67 index of 20.5% to 54% depending on grade, mean P53 protein positive cells of 4.3% to 24%, and intense staining for BCL2.
Cytogenetic studies have demonstrated recurrent translocation involving 8q ( PLAG1 ) and 12q ( HMGA2 , also known as HMGIC ), which encode transcription factors (see Table 7.3 ). The resulting overexpression of these genes has been postulated to play an important role in the pathogenesis. Since PLAG1 or HMGA2 gene translocations have not been identified in other salivary gland tumor types, their detection can potentially aid in the diagnosis of pleomorphic adenoma. PLAG1 immunostaining, however, is not specific for pleomorphic adenoma. This protein is expressed in the myoepithelial cells of pleomorphic adenoma as well as other salivary gland tumors with myoepithelial cell differentiation. Strong immunostaining for HMGA2 has been claimed to be a specific (96%) but not sensitive (30%) marker for pleomorphic adenoma.
Monomorphic adenoma (e.g., basal cell adenoma, myoepithelioma)
Adenoid cystic carcinoma
Polymorphous (low-grade) adenocarcinoma
Epithelial-myoepithelial carcinoma
Mucoepidermoid carcinoma
Various mesenchymal tumors (e.g., solitary fibrous tumor, nerve sheath tumor, smooth muscle tumor)
Metastasizing pleomorphic adenoma is a rare complication of pleomorphic adenoma. Generally, metastases occur after a relatively long interval (ranging from 1.5 to 52 years, mean 15 years), and may develop synchronously with or following local recurrence. Rarely, the metastatic tumor may represent the initial manifestation of an occult pleomorphic adenoma in the salivary gland.
The metastatic tumor characteristically retains the benign histologic features of pleomorphic adenoma. Retrospective analysis and flow cytometry both fail to identify any features that can predict the occurrence of metastasis. The most common metastatic sites are bone (50%), lungs (30%), and lymph nodes (30%). Scalp, abdominal wall, and liver metastases have also been reported. Despite the bland histology of the metastatic lesion, up to 37% of patients die of the disease. The disease apparently pursues a more rapidly aggressive course in immunocompromised hosts. Malignant transformation of metastasizing pleomorphic adenoma has also been reported.
With rare exceptions, there is a history of one or multiple operations for the salivary gland pleomorphic adenoma. Vascular permeation secondary to mechanical implantation has been postulated as a probable mechanism for the development of metastasis. The treatment of choice is local excision of both the primary and metastatic tumors.
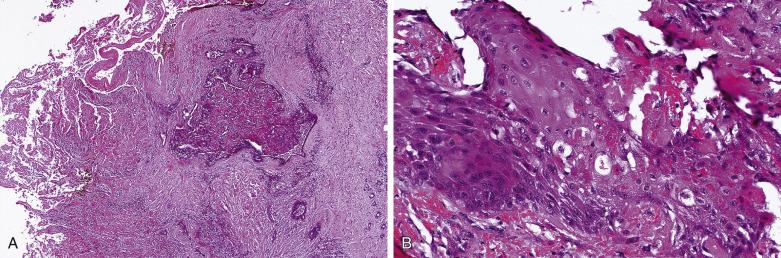
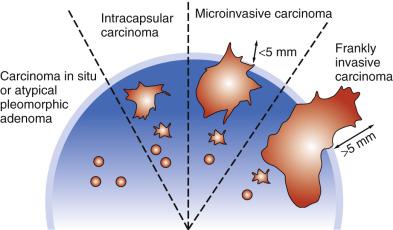
Carcinoma ex pleomorphic adenoma represents malignant transformation of a preexisting pleomorphic adenoma, usually in a longstanding pleomorphic adenoma or in tumor with multiple recurrences. It occurs in 0.15% of pleomorphic adenomas, and constitutes around 10% of all malignant salivary gland tumors. Malignant transformation may follow a stepwise sequence manifested as follows ( Figs. 7.28, 7.29, 7.30, and 7.31 ):
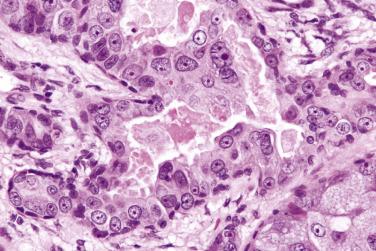
Carcinoma in situ (intraductal carcinoma) : In the earliest phase, carcinoma cells replace the luminal cells while retaining an intact, nonatypical neoplastic myoepithelial layer (see Fig. 7.29 ). The transformed luminal cells may exhibit a clinging (without expansion of involved duct) or solid (with expansion of involved duct) growth pattern.
Intracapsular carcinoma : Stromal invasion develops upon further progression of the carcinoma, but without violation of the fibrous capsule of the parent pleomorphic adenoma ( Fig. 7.32A ).

Invasive carcinoma : Extracapsular invasion subsequently follows (see Figs. 7.30 and 7.31 ).
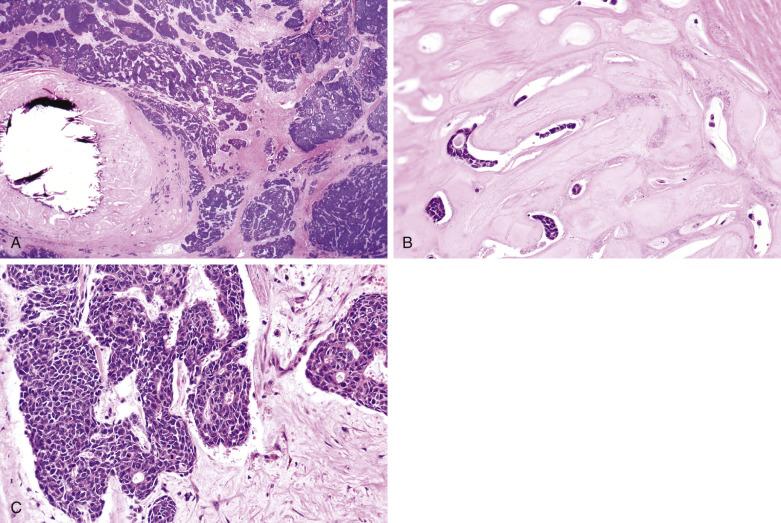
Malignant transformation is heralded by rapid growth after a long period of minimally perceptible increase in size. Signs of malignancy also include fixation to surrounding tissues, ulceration, facial nerve palsy, and regional lymphadenopathy. The mean age at presentation is 61 years, about one decade older than that of pleomorphic adenoma. The majority (65%) of patients present with stage III/IV disease. Recurrence and metastases are common, and the overall 5-year survival is only 30%.
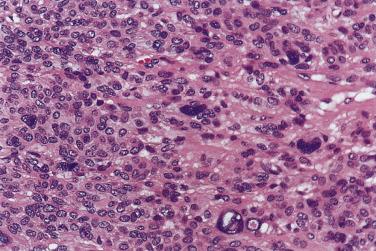
The tumor is usually larger than its benign counterpart. Most cases are frankly infiltrative; areas of necrosis or hemorrhage are common. Histologically, the malignant component is characterized by an expansile or infiltrative nodule within the parent adenoma, widespread significant cellular pleomorphism, high mitotic count, atypical mitotic figures, and coagulative necrosis (see Figs. 7.30 and 7.31 ).
In most cases, the malignant component dominates the tumor and is most frequently a high-grade carcinoma (85% of cases) such as adenocarcinoma NOS or salivary duct carcinoma (see Fig. 7.30C ), but sometimes adenosquamous carcinoma, undifferentiated carcinoma, small cell carcinoma, or sarcomatoid carcinoma. Low-grade carcinoma such as polymorphous (low-grade) adenocarcinoma, adenoid cystic carcinoma, mucoepidermoid carcinoma, epithelial-myoepithelial carcinoma, and myoepithelial carcinoma can also occur. Transitional zones with morphologic features intermediate between the malignant and benign components may be present. Exceptionally, there is a component of melanoma in the carcinoma ex pleomorphic adenoma.
Often the residual pleomorphic adenoma is difficult to find and appears hypocellular or markedly hyalinized. The clues to its existence are as follows:
Hyalinized or calcified nodule within or directly adjacent to the carcinoma (see Fig. 7.30 )
S100 protein, actin, or p40/p63-positive spindle cells in the hyalinized nodule
Thick, fluffy elastic fibers (best highlighted by elastic stain)
Clinical history of recurrent pleomorphic adenoma or longstanding mass
Hence when a salivary gland carcinoma is not easily classifiable into the recognized entities, the possibility of carcinoma ex pleomorphic adenoma should be seriously considered.
In most cases (75%), it is the luminal epithelial cells that undergo malignant change. In the other cases, the supervening carcinoma shows dual epithelial-myoepithelial differentiation (19% of cases) or pure myoepithelial differentiation (6% of cases). The in situ phase has not been well characterized for cases with pure myoepithelial differentiation.
The most important prognostic factor is the extent of invasion. Carcinoma in situ and intracapsular carcinoma have no metastatic potential, with the exception of a single reported case with cervical lymph node metastasis. In several large series, excellent prognosis has been found in tumors with extracapsular invasion less than 8 mm, 5 mm, or 1.5 mm beyond the capsule, respectively. That is, the optimal cutoff point to define a category of invasive carcinoma ex pleomorphic adenoma with no or minimal metastatic potential is currently unsettled, but the different findings in the different series may reflect difficulties in making reproducible measurements. Based on the available data, adoption of 5 mm as the cutoff appears reasonable. To complicate matters, one study suggests that the concept of minimal invasion is not applicable if salivary duct carcinoma is the supervening carcinoma, since aggressive behavior is observed even for tumor with 2 mm or less capsular invasion.
Other features associated with a worse outcome are (1) high histologic grade of the malignant component (5-year survival 30% versus 96% for low histologic grade), (2) high pathologic stage, (3) proportion of carcinoma more than 50% of the tumor, and (4) HER2 gene amplification. Occasionally, carcinoma in situ can extend beyond the capsule for up to 6 mm, and the prognosis of these cases appears to be favorable.
Rearrangements of chromosomes 8q21 and 12q13-15 are frequently found in carcinoma ex pleomorphic adenoma, reflecting its molecular origin. In addition, amplification and overexpression of genes in chromosome 12q13-15, including CDK4, HMGIC, and MDM2 , may represent important genetic events in the malignant transformation.
HER2 overexpression or HER2 gene amplification occurs in 21% to 82% of cases. HER2 amplification is more common in cases with extracapsular growth (50%) than those with intracapsular or in situ growth (23%). It has been suggested that HER2 immunostaining may aid in distinguishing carcinoma ex pleomorphic adenoma from atypical pleomorphic adenoma (see Fig. 7.32B ), and S100P overexpression may serve a similar purpose. Androgen receptor is expressed in a proportion of cases.
Alterations of the TP53 gene are found in 29% to 67% and P53 protein overexpression in 41% to 75% of cases, suggesting that the gene may play a role in the transformation. The Ki67 proliferative index is increased (mean 35%) compared with the parent pleomorphic adenoma.
Capsular involvement and vascular tumor plugs are acceptable findings in benign pleomorphic adenoma. Isolated enlarged or pleomorphic nuclei in a background of bland-looking cells can also be disregarded because they do not signify a worse outcome ( Fig. 7.33 ). However, rare tumors may exhibit atypical features such as diffuse mild nuclear atypia and occasional mitotic figures but without coagulative tumor necrosis or formation of an expansile mass ( Fig. 7.34 ). Under such circumstance, the designation atypical pleomorphic adenoma may be appropriate. A conservative designation is justified because of the excellent prognosis even if there were already carcinomatous changes, as long as there is no invasion beyond the capsule.
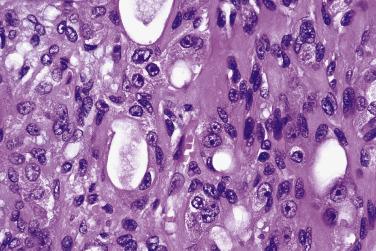
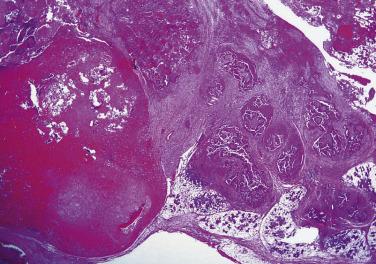
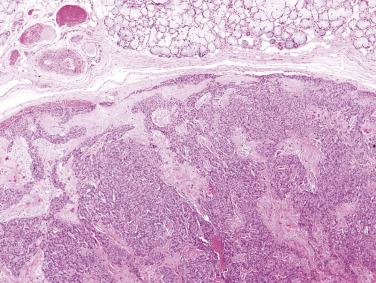
Carcinosarcoma, or true malignant mixed tumor, is very rare. The mean age at presentation is 62 years with no sex predilection. The tumor most frequently affects the major salivary glands. In one-third of cases, there is clinical or histologic evidence of coexisting pleomorphic adenoma. Histologically the neoplasm comprises frankly malignant epithelial and mesenchymal elements. The epithelial component is most often a squamous cell carcinoma or adenocarcinoma, and the most common malignant mesenchymal component is chondrosarcoma, followed by fibrosarcoma, leiomyosarcoma, osteosarcoma, liposarcoma, and rhabdomyosarcoma ( Fig. 7.35 ). It is an aggressive tumor with a mean survival of only 29.3 months.
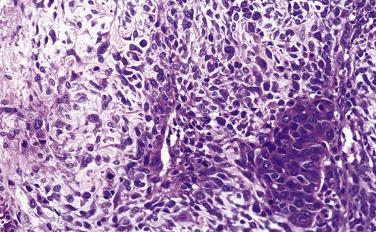
Basal cell adenoma is a benign tumor composed predominantly of basaloid cells forming solid, trabecular, and tubular structures that are sharply demarcated from the stroma. Peripheral nuclear palisading is frequently seen, but chondromyxoid stroma, by definition, is absent.
Basal cell adenoma typically presents as a solitary, slow-growing asymptomatic mass in the parotid gland (80%) or, far less frequently, upper lip, submandibular gland, and minor salivary glands. Age at presentation peaks in the sixth to seventh decades with a female predilection (2 : 1).
Surgical excision is the treatment of choice. Recurrence is rare except for the membranous type, which is associated with a recurrence rate of 25% because of its multifocal nature. Basal cell adenoma may rarely undergo malignant transformation (4%) to basal cell adenocarcinoma, adenoid cystic carcinoma, salivary duct carcinoma, or adenocarcinoma NOS. The transformation rate is much higher in the membranous type, being up to 28%.
The gross appearance is that of a well-circumscribed solid tumor with homogeneous, light tan to brown cut surfaces. The cartilage-like or mucoid quality characteristic of pleomorphic adenoma is lacking. Membranous basal cell adenoma is usually multifocal (50% of cases) and multinodular.
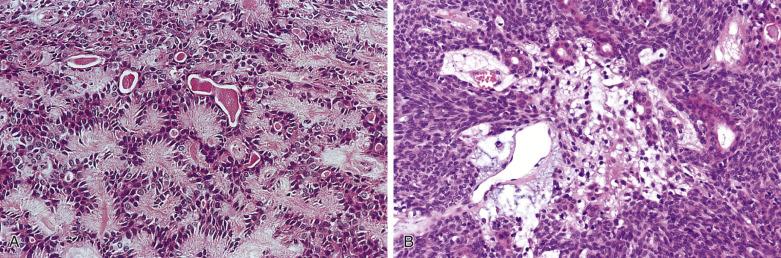
Histologically the tumor is often surrounded by a fibrous capsule ( Fig. 7.36A ). It typically comprises anastomosing jigsaw puzzle–like islands and trabeculae, imparting a plexiform appearance ( Fig. 7.37 ; also see Fig. 7.36 ). Discrete or anastomosing tubules can be present focally or extensively, and their lumens often contain eosinophilic secretion. Rarely, there are focal or extensive cribriform structures (cribriform variant), mimicking adenoid cystic carcinoma ( Fig. 7.38 ). The tumor islands and trabeculae are sharply demarcated from the hyalinized, often highly vascularized stroma by basement membrane–like material, differing from the centrifugal or melting growth of pleomorphic adenoma (see Fig. 7.37 ).
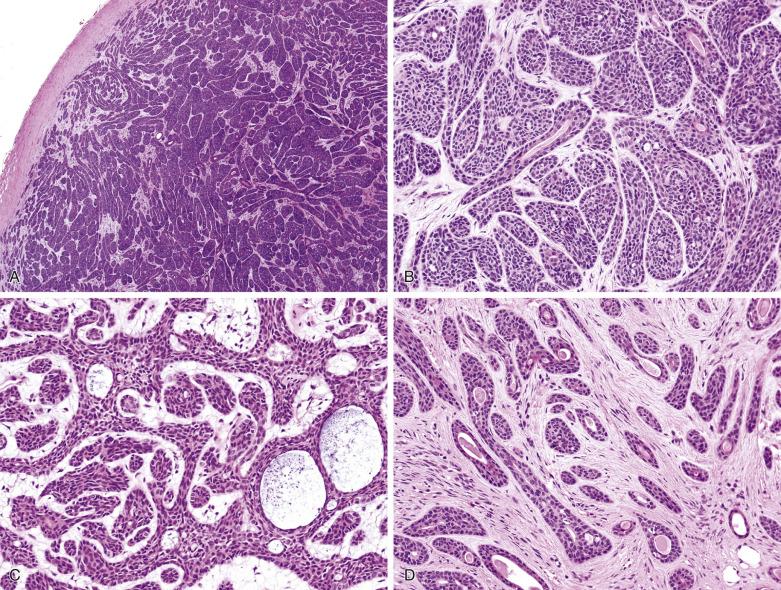
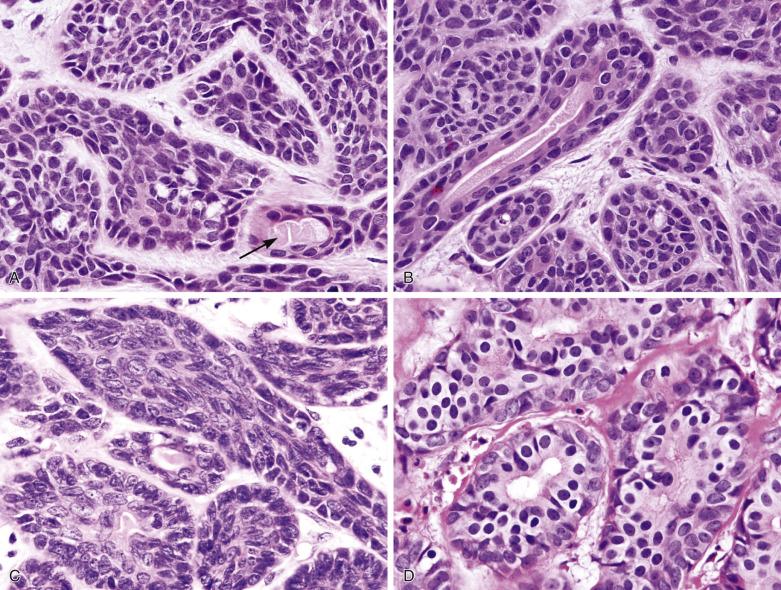
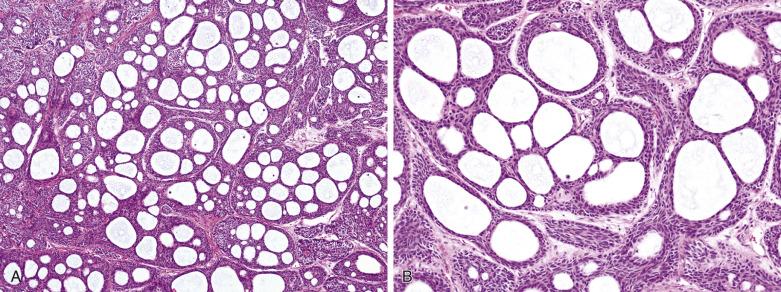
Basal cell adenoma comprises dual cell types: abluminal basaloid cells with a basal cell or myoepithelial phenotype and luminal cells. Basaloid cells predominate except in the tubular component. They possess scanty cytoplasm; and uniform, round or oval, sometimes grooved nuclei with finely granular chromatin. There is often prominent peripheral palisading (see Fig. 7.37 ). Mitotic figures are rare. Sometimes the basaloid cells appear plump and spindled, streaming in the longitudinal direction of the tumor islands (see Fig. 7.37C ). Small ductal structures lined by luminal cells with eosinophilic cytoplasm, smaller nuclei, and more dense chromatin are commonly interspersed within the basaloid islands, but can be difficult to appreciate (see Figs. 7.36 and 7.37 ). The luminal cells can be enlarged due to oncocytic change (see Fig. 7.37D ). Squamous and sebaceous components may be present. When cribriform structures are present, most of the rounded spaces are surrounded by abluminal rather than luminal cells (see Fig. 7.38 ).
Basal cell adenoma with myoepithelium-derived stroma is a variant with plump spindled cells in the stroma ( Fig. 7.39 ). The spindled cells are intensely positive for S100 protein and show ultrastructural features of myoepithelium, but are negative for actin and p40/p63 (see Fig. 7.39B ). This tumor is distinguished from pleomorphic adenoma by the sharp demarcation of the basaloid islands from the stroma, without transition to the spindle cells. In our experience, focal myoepithelium-derived stroma (S100+) is a common and characteristic finding in basal cell adenoma, and its presence may aid in the distinction from adenoid cystic carcinoma.

The membranous variant is characterized by the presence of thick, eosinophilic and PAS-positive hyaline basal lamina material around the basaloid islands in more than 50% to 75% of the tumor. Droplets of hyaline material are also found within the islands. Interspersed small glandular lumens lined by cells with eosinophilic cytoplasm are present ( Figs. 7.40 and 7.41 ). Since membranous basal cell adenoma commonly grows in a multinodular fashion with entrapment of normal salivary gland tissue, it can be misinterpreted as a malignant tumor.
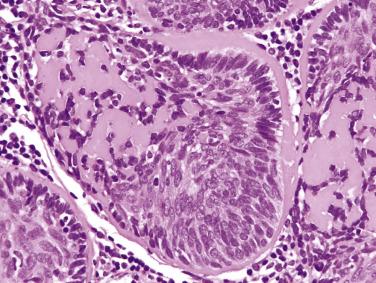
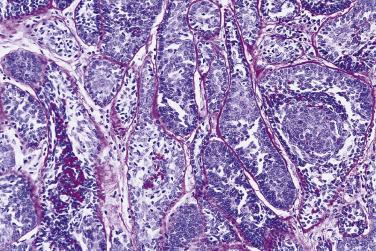
Basal cell adenomas exhibit dual-cell differentiation. Epithelial markers (low-molecular-weight cytokeratin, CEA, EMA) can be demonstrated in the luminal cells. Myoepithelial markers (p40/p63, calponin, actin, GFAP, S100) can variably be demonstrated in the peripherally located basaloid cells.
Activating somatic mutation in exon 3 of CTNNB1 (ß-catenin) gene I35T is demonstrated in 30% to 80% of basal cell adenomas, suggesting that Wnt pathway activation is important in the tumorigenesis. Nuclear expression of ß-catenin can be demonstrated in 50% to 82% of basal cell adenomas, but not in the membranous variant, pleomorphic adenoma, and adenoid cystic carcinoma. LEF-1, a downstream protein of the Wnt pathway, is overexpressed in 67% of basal cell adenomas. In contrast to ß-catenin, LEF-1 is also expressed in some (40%) membranous basal cell adenomas.
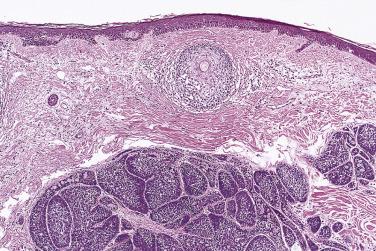
Membranous basal cell adenoma, also known as dermal analogue tumor, does not have CTNNB1 mutation but frequently harbors somatic mutations of cylindromatosis gene ( CYLD ), a tumor suppressor gene located at chromosome 16q12-q13. Germline mutation of CYLD has been implicated in familial cases of membranous basal cell adenoma that, when accompanied by multiple cylindromas, trichoepithelioma, eccrine spiradenoma, and milia, constitutes the autosomal Brooke-Spriegler syndrome (familial cylindromatosis or Turban tumor syndrome) ( Fig. 7.42 ).
Basal cell adenocarcinoma (see next section)
Canalicular adenoma
Adenoid cystic carcinoma—The cribriform variant of basal cell adenoma is often mistaken for adenoid cystic carcinoma. Features favoring the former diagnosis include (a) tumor encapsulation, (b) jigsaw puzzle–like basaloid islands in at least some areas, (c) cribriform structures that appear to be formed by expanded jigsaw puzzle–like islands punctuated by multiple holes, and (d) focal presence of S100+ spindle cells in stroma.
Pleomorphic adenoma
Basal cell adenocarcinoma is a low-grade malignant neoplasm with architectural and cytologic resemblance to basal cell adenoma but shows invasion of the surrounding salivary lobules, nerves, or blood vessels.
Basal cell adenocarcinoma is a rare carcinoma accounting for 1.4% of major salivary gland cancers. The average age is 64 to 67 years with no gender predilection. Most tumors arise in the parotid gland, and others arise in the submandibular gland, oral cavity, or upper respiratory tract. The tumors may rarely arise from pleomorphic adenoma or preexisting basal cell adenoma, particularly the membranous variant.
Basal cell adenocarcinomas are low-grade carcinomas. Regional (11.9%) and distant (1.8%) metastasis are uncommon. The overall 5- and 10-year survival rates are 79% and 62%, respectively. Age over 65 years and high tumor stage are independent poor prognosis factors on multivariant analysis. For high stage disease, surgery with radiation results in significantly better survival than surgery alone.
Basal cell adenocarcinoma is morphologically similar to basal cell adenoma. It usually invades in multinodular broad fronts ( Figs. 7.43 and 7.44 ). It shows a predominantly solid growth characterized by jigsaw puzzle–like islands of basaloid cells with peripheral palisading, and areas with a trabecular or membranous arrangement. Well-defined tubular structures with two-cell type lining are sometimes present focally. Focal squamous differentiation is seen in 25% of cases. The tumor cells may assume a spindled appearance ( Fig. 7.45 ). Within the basaloid cell islands, there are small numbers of interspersed glandular spaces lined by cuboidal cells.
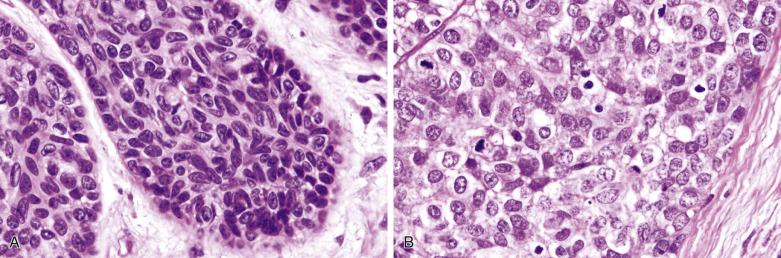
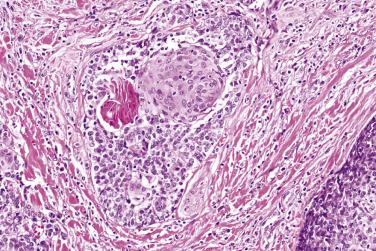
The mean mitotic count and Ki67 index are higher than those in the basal cell adenoma (9 versus 1 per 10 high-power fields [hpf] and 16% versus 3%, respectively). Cases showing nuclear atypia and readily identified mitotic figures are easy to recognize as being malignant (see Fig. 7.44B ). Most cases however have a relatively bland cytologic appearance, and the diagnosis of malignancy depends on the identification of infiltrative growth ( Fig. 7.46 ; also Fig. 7.44 ). Perineural infiltration or intravascular invasion are present in 25% to 35% of cases. The immunohistologic profile is similar to basal cell adenoma.
Activating mutations of PIK3CA , biallelic inactivation of NFKBIA, and loss of CYLD but not CTNNB1 mutation are found in basal cell adenocarcinomas. These molecular findings suggest the pathogenetic pathway of basal cell adenocarcinoma differs from that of basal cell adenoma.
Basal cell adenoma
Adenoid cystic carcinoma, solid variant
Undifferentiated carcinoma
Basaloid squamous cell carcinoma
Myoepithelial carcinoma
Myoepithelioma is a benign tumor composed exclusively of neoplastic cells exhibiting myoepithelial differentiation.
Pleomorphic adenoma, basal cell adenoma, and myoepithelioma can be envisaged to lie on a continuum: Myoepithelioma may represent an extreme form of basal cell adenoma without a ductal component, whereas basal cell adenoma is a “pleomorphic adenoma minus the characteristic stroma,” and pleomorphic adenoma lies in the middle of this continuum (see Fig. 7.22 ). However, given the benign nature of all these tumor types, problems in nomenclature of cases exhibiting overlapping features is a matter of semantics.
Myoepithelioma most frequently affects the parotid gland and palate. Less commonly, it can occur in the skin, breast, or soft tissue. Clinically it presents as a painless mass. The peak age is from the third to fifth decade with no sex predilection. The treatment of choice is surgical excision.
The prognosis is excellent and recurrence is not expected after complete excision. Nonetheless, the biologic behavior of myoepithelial tumor is not always predictable on histologic grounds, in that metastasis may develop unexpectedly in an apparently benign-looking tumor (see Fig. 7.46 ).
Myoepithelioma is often thinly encapsulated and has a solid, tan or yellow cut surface ( Fig. 7.47 ). The neoplastic myoepithelial cells can be spindled, plasmacytoid hyaline, epithelioid, clear, or oncocytic, with the first two cell types being the most common ( Figs. 7.48 and 7.49 ). Either a single cell type predominates in a tumor or there can be a mixture of cell types. Myoepitheliomas of the minor glands tend to be composed of plasmacytoid hyaline cells, and those of the parotid gland, spindly or epithelioid cells. The stroma ranges from being scanty to myxoid, hyaline, or sclerotic. Radially arranged collagenous fibers and oxalate crystals can be present ( Fig. 7.50 ), but cartilaginous matrix characteristic of pleomorphic adenoma is not allowable.
The spindle cells, which have central vesicular nuclei and eosinophilic cytoplasm, form interlacing fascicles (see Fig. 7.48A ). Myoepithelioma consisting predominantly of spindle cells tends to be more cellular, with little fibrous stroma; however, some cases can be collagen rich, mimicking solitary fibrous tumor.
Plasmacytoid hyaline cells in myoepithelioma are identical to those seen in pleomorphic adenoma (see Fig. 7.49B and C ); they are frequently accompanied by a loose myxoid stroma. They form nondescript islands and sheets, or are suspended in myxoid matrix in the form of isolated cells, cords, or aggregates, and in the absence of true chondroid matrix (see Figs. 7.48C and 7.49B ). Plasmacytoid hyaline cells show variable staining for calponin, actin, and p40/p63. Epithelioid cells are large polygonal cells with eosinophilic cytoplasm and centrally located bland nuclei. They commonly show a reticular, trabecular, or solid growth pattern (see Figs. 7.48 and 7.49 ).
Clear cells are rich in glycogen. They are usually present only focally, but can occasionally be so prominent as to pose difficulties in distinction from other clear cell tumors. A mucinous variant has recently been recognized, characterized by cells with abundant cytoplasm that contains mucin ; some tumors reported as signet ring cell carcinoma of the salivary gland may represent this variant.
Infiltrative growth, cytologic pleomorphism, easily identified mitotic figures, and coagulative necrosis should not be present. However, the presence of rare enlarged hyperchromatic nuclei in a background of benign-appearing cells is acceptable.
Pan-cytokeratin as well as myoepithelial-related markers (calponin, actin, p40/p63, S100, GFAP, CK14) are generally positive, but the frequency of positivity and percentage positive cells with the individual markers are highly variable. S100 has been reported to be the most useful marker, but it lacks specificity. Many cases also express EMA, but only rarely CEA. Ultrastructural studies can help confirm myoepithelial differentiation by identifying both epithelial (hemidesmosomes) and myoid features (myofilaments with focal densities; pinocytotic vesicles).
Myoepithelioma of the soft tissues commonly exhibits EWSR1-POU5F1 or EWSR1-PBX1 gene fusion, but these molecular alterations have not been found in salivary gland myoepitheliomas.
Pleomorphic adenoma or basal cell adenoma
Myoepithelial carcinoma
Various mesenchymal lesions (e.g., nerve sheath tumor, nodular fasciitis, solitary fibrous tumor)
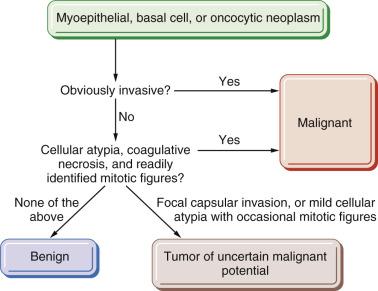
Myoepithelial carcinoma is a malignant myoepithelial tumor which demonstrates infiltrative growth and variable cytologic atypia and mitotic activity. A designation myoepithelial neoplasm of uncertain malignant potential may be appropriate for a tumor that exhibits some worrisome features but falls short of frank infiltrative growth (see Fig. 7.46 ).
The peak age is in the sixth decade, about 10 years older than the benign counterpart. Approximately half of all cases arise from a preexisting pleomorphic adenoma or myoepithelioma, particularly in recurrences. It most commonly involves the parotid gland, but other major or minor glands and the breast can be affected. Myoepithelial carcinoma is an intermediate- to high-grade carcinoma. Approximately one-third of patients die, another one-third have recurrences (mostly multiple), and the remaining one-third are disease free. The most common site of distant metastasis is the lung, followed by the liver, brain, bone, and soft tissue. The diverse clinical outcomes reported in different series probably reflect different leniency in rendering a diagnosis of malignancy in a myoepithelial tumor.
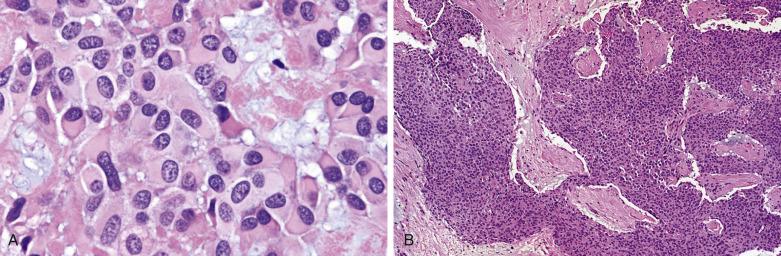
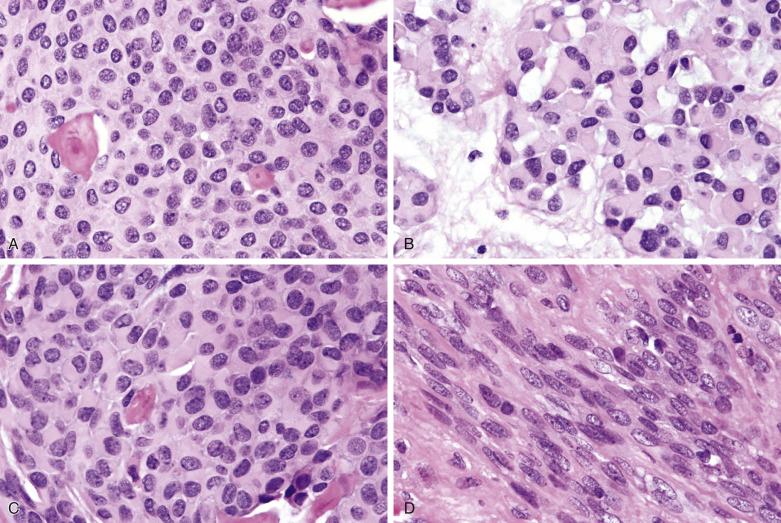
Myoepithelial carcinoma is usually unencapsulated and may exhibit areas of necrosis and cystic degeneration, although rare ones may be predominantly encapsulated. The median size is 3 cm. Most tumors show a pushing type of infiltration ( Fig. 7.51 ). Solid, fascicular, trabecular, festooning, and lacelike growth patterns are common, but glandular structures are not found ( Fig. 7.52 ). A characteristic pattern, observed in half of all cases, is a zonal arrangement with a hypercellular peripheral rim exhibiting solid/trabecular growth pattern, surrounding a hypocellular center that is usually myxoid and/or necrotic (see Fig. 7.52B ). There are often variable amounts of hyaline, myxoid, or collagenous stroma (see Fig. 7.52 ). The tumor may comprise one or more of the following cell types: spindly, epithelioid, plasmacytoid hyaline, clear, stellate, and basaloid cells ( Fig. 7.53 ). A rare secretory variant characterized by signet ring cells containing intracytoplasmic mucin is also recognized. Nuclear atypia ranges from mild to marked (see Fig. 7.53 ). There can be metaplastic changes to squamous (often with keratinization) or sebaceous cells.
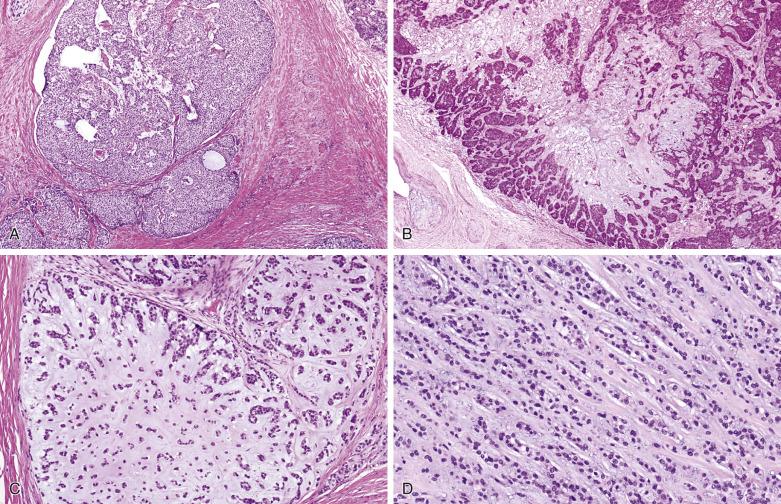
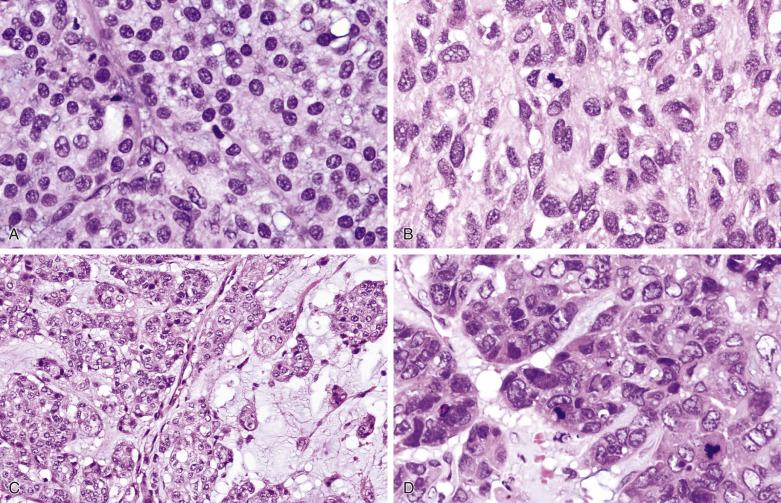
According to Nagao et al, myoepithelial carcinoma can be distinguished from benign myoepithelioma by a mitotic count greater than 7 per 10 hpf or Ki67 index greater than 10%. On the other hand, rare cases of myoepithelial carcinomas can be bland looking and mitotically inactive (see Fig. 7.52A ); demonstration of definite invasive growth is essential to establish their malignant nature.
Cytologic grading apparently has no prognostic value in myoepithelial carcinoma. There are conflicting reports on the prognostic implication of myoepithelial carcinoma arising from pleomorphic adenoma versus de novo myoepithelial carcinoma. Dedifferentiated myoepithelial carcinoma has rarely been described.
To render a diagnosis of myoepithelial carcinoma, myoepithelial differentiation has to be substantiated by immunohistochemistry. The immunohistochemical profile is similar to that of the benign counterpart. Tumor cells commonly express cytokeratin and S100 protein, and variably express p40/p63, calponin, actin, CK14, EMA, CD10, and GFAP, but not CEA and HMB45 ( Fig.7.54 ).
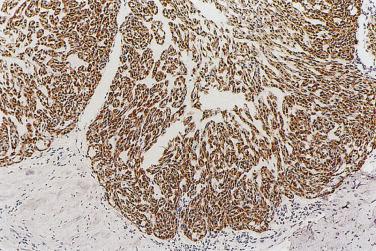
EWSR1 rearrangement has been found in 39% of clear cell myoepithelial carcinomas, but the partner genes are not certain. The morphologic features commonly seen in EWSR1 -rearranged tumors include necrosis, nested growth pattern, hyalinized septa, hyaline droplets, and squamous metaplasia.
Differential diagnoses include myoepithelioma (see Fig. 7.46 ), various sarcomas (leiomyosarcoma, synovial sarcoma, and malignant peripheral nerve sheath tumor), interdigitating dendritic cell sarcoma, and melanoma. The latter two tumors also express S100 protein but not cytokeratin and myoid markers.
Clear cell myoepithelial carcinoma also raises the differential diagnosis of various clear cell tumors in the salivary glands ( Table 7.5 ).
| Clear Cell Oncocytoma | Clear Cell Carcinoma | Mucoepidermoid Carcinoma | Epithelial-Myoepithelial Carcinoma | Clear Cell Myoepithelioma and Myoepithelial Carcinoma | Acinic Cell Carcinoma | Metastatic Renal Cell Carcinoma | |
|---|---|---|---|---|---|---|---|
| Nature of clear cells | Oncocytes | Ductal cells (with squamous features) | Intermediate cells, mucinous cells | Myoepithelial cells | Myoepithelial cells | Acinic cells | Neoplastic renal epithelial cells |
| Cause of cytoplasmic clearing | Glycogen | Glycogen | Glycogen and mucin, respectively | Glycogen | Glycogen | Tissue processing artifact | Glycogen and lipid |
| Growth patterns | Encapsulated or circumscribed; trabeculae or packets | Infiltrative; solid or trabecular; sclerotic or hyalinized stroma |
Infiltrative; inflamed fibrous stroma; islands of epidermoid, intermediate, and mucinous cells; some cystic spaces | Infiltrative; ductal structures lined by inner cuboidal cells and outer clear myoepithelial cells | Lobules, nests, trabeculae, and fascicles; may have collagenous spherules | Infiltration in broad fronts; microcystic pattern | Prominent sinusoids; hemorrhage and hemosiderin deposition; some glandular structures |
| Cytologic features of clear cells | Centrally located, round nuclei; peripheral rim of cytoplasm may retain pink granularity | Polygonal cells with water-clear cytoplasm; nuclei central or eccentric | Intermediate clear cells are large cells with water-clear cytoplasm; mucinous cells have flocculent cytoplasm | Polygonal cells, with basally located or central nuclei; water-clear cytoplasm | Cells polygonal or spindly; variable degrees of nuclear atypia; often admixed with a population of cells with eosinophilic cytoplasm | Peripherally located nuclei; sparse basophilic granules in some cells | Water-clear cytoplasm; variable nuclear atypia |
| Staining properties of clear cells | PTAH+, mitochondrial antibody+ | CK+, EMA+, p40/p63+, myoepithelial markers (actin, calponin)− | PAS+, PASD-, mucin− for intermediate clear cells; PAS+, PASD+, mucin+ for mucinous cells | S100+, actin+, calponin+, p40/p63+ | S100+, actin+, calponin+, p40/p63+ | PASD+ granules, amylase+ | Lipid+, PAS+, PASD-, CK+, EMA+, myoepithelial markers-, PAX8+, CD10+ |
Oncocytoma is a discrete, encapsulated tumor consisting exclusively of oncocytes and lacking features of other defined tumor types. Oncocytoma, when accompanied by lymphoid stroma, can simulate a Warthin tumor. Occasionally, oncocytoma and Warthin tumor may coexist.
Oncocytosis is a diffuse oncocytic metaplastic process in the salivary gland, often associated with atrophy of the surrounding parenchyma ( Fig. 7.55 ). The lobular architecture is preserved.
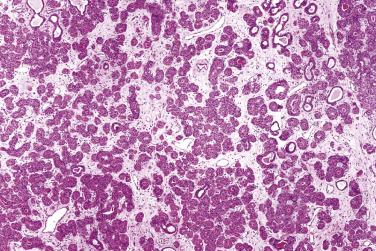
Multifocal nodular oncocytic hyperplasia is characterized by multiple nodular proliferations of closely packed oncocytes ( Fig. 7.56 ). Features favoring this diagnosis over oncocytoma include (1) multiple rather than single nodule, (2) lack of capsule, (3) nodules that usually show a lobular configuration (often triangular in shape) and gradual merging into the normal ducts and acini, and (4) frequent clear cell change.
Since oncocytosis can be induced pharmacologically in rats and these lesions may subsequently transform into oncocytoma, the various oncocytic lesions described above could represent a spectrum of a single entity. Although distinguishing features have been described for these three conditions, given the similarly excellent prognosis and treatment modality, it is not worthwhile to make great efforts to achieve definite distinction in problematic cases.
Oncocytoma most commonly occurs in the parotid gland of older adults (mean age 58–77 years) without gender predilection, but the submandibular gland can also be affected. Radiation exposure to the head and neck region has been implicated in the genesis in about 20% of cases; this risk factor is also associated with a presentation 20 years younger than those without such a history. Surgical excision is the treatment of choice and recurrence is uncommon (0%–10%).
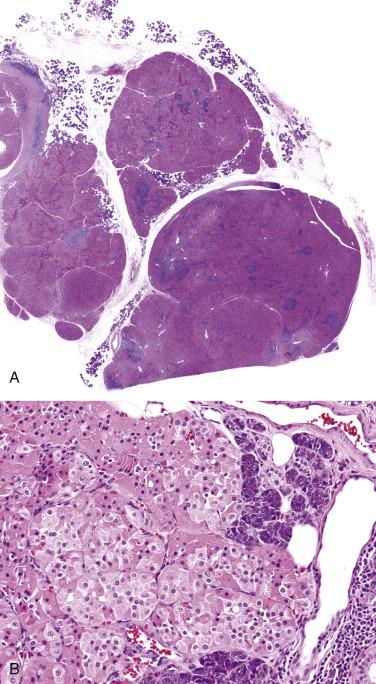
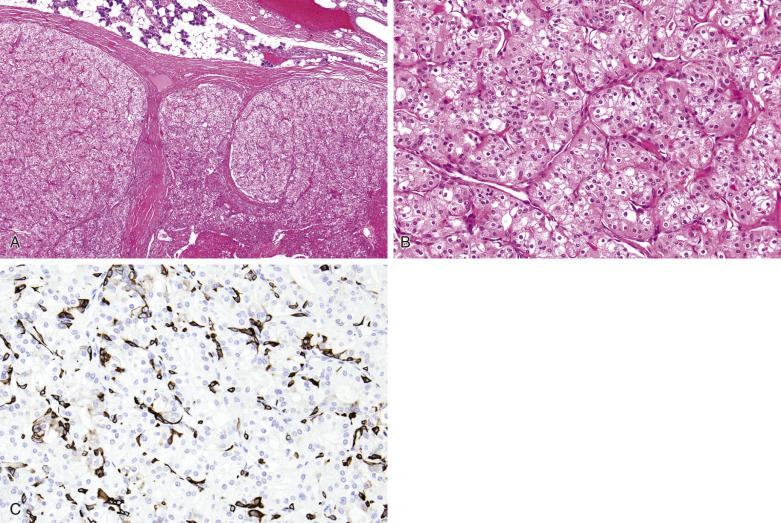
Macroscopically, oncocytoma is a thinly encapsulated, mahogany brown, solid lesion ( Fig. 7.57A ). Histologically the tumor cells form trabeculae, packets, diffuse sheets, and rarely glands, separated by scanty loose vascularized stroma (see Fig. 7.57 ). The oncocytes are polygonal or cuboidal, with abundant eosinophilic granular cytoplasm, central round nuclei, and often distinct nucleoli (see Fig. 7.57 ). The cytoplasm is packed with mitochondria, which can be highlighted by PTAH stain or immunostaining with antimitochondrial antibody. Focally, sebaceous, goblet, or squamous cells and psammoma bodies may be present. Although inconspicuous, attenuated basal cells can be demonstrated around the trabeculae and packets of oncocytic cells by immunostaining for p40/p63 or CK14 (see Fig. 7.57C ).
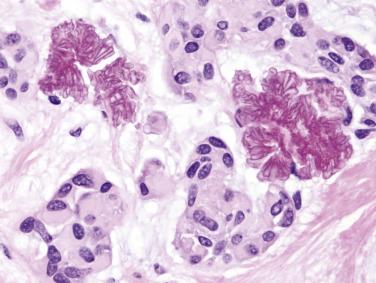
Tyrosine-rich crystals can be found in the tumor and adjacent striated ducts of some oncocytomas. These eosinophilic crystals are needle shaped or platelike. They are seen extracellularly as well as within the oncocytic cells. Similar crystals can also be found in Warthin tumor and oncocytic cystadenoma.
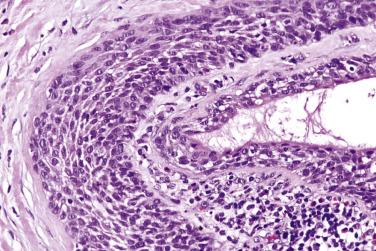
Oncocytoma is prone to infarction either spontaneously or following fine-needle aspiration. The necrotic cells manifest as ghost shadows or eosinophilic granular material. The residual viable tumor or adjacent salivary epithelium commonly undergoes squamous metaplasia with atypical (reparative) nuclei, mimicking squamous cell carcinoma ( Fig. 7.58 ) (see “ Analytic Approach to Diagnosis of Epithelial Tumors of the Salivary Gland ”).
Clear cells are present in 11% of oncocytomas as a dominant or partial component. The architecture is otherwise the same as that of conventional oncocytoma ( Fig. 7.59 ). The cytoplasmic clearing is due to glycogen accumulation, but sparse granularity is still evident. Transition of clear cells to typical oncocytes can often be identified. Clear cell oncocytoma appears to show a higher frequency of bilateral tumors and recurrence in comparison to conventional oncocytoma.
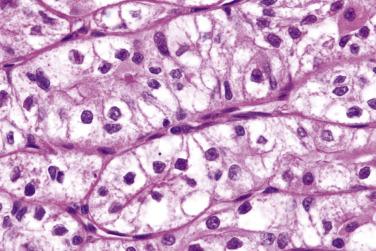
Typical oncocytoma poses no difficulty in diagnosis. The major diagnostic challenge is to distinguish oncocytoma from salivary gland tumors showing prominent oncocytic change, most notably Warthin tumor, pleomorphic adenoma, basal cell adenoma, and oncocytic variant of mucoepidermoid carcinoma (see “ Analytic Approach to Diagnosis of Epithelial Tumors of the Salivary Gland ”). Oncocytoma may be mistaken for acinic cell carcinoma by virtue of the similar cell arrangement (cellular groups or cords) and cytoplasmic granularity, especially at intraoperative frozen section. However, the nuclei of acinic cell carcinomas are peripherally located, in contrast to the central round nuclei in oncocytoma. Clear cell oncocytoma may also be mistaken for other clear cell salivary gland tumors (see Table 7.5 ).
Oncocytic carcinoma is an oncocytic tumor that demonstrates malignant histologic features and lacks the diagnostic criteria of other specific tumor types.
Oncocytic carcinoma is very rare. Most cases occur in the parotid gland, with a median age of 67 years and a male predilection of 1.6 : 1. Some cases may arise from a preexisting oncocytoma. In contrast to oncocytoma, oncocytic carcinoma is not associated with prior radiation exposure. It is a high-grade neoplasm with frequent tumor recurrence (56%) and metastasis (80%), most commonly to the lung, kidney, liver, thyroid, mediastinum, and bone. The overall survival at 5 and 10 years are 64% and 39%, respectively.
Oncocytic carcinoma is an unencapsulated, single or multinodular tumor ( Fig. 7.60 ). The oncocytic cells, in contrast to their benign counterparts, show variation in size and shape and nuclear pleomorphism, although nuclear atypia may be minimal in some cases. They form trabeculae, sheets, nests, or ducts that infiltrate the salivary gland parenchyma and surrounding connective tissue (see Fig. 7.60 ). Frequent mitotic figures, atypical mitoses, perineural invasion, and vascular invasion can be seen (see Fig. 7.60C ). Coagulative tumor necrosis appears to be specific for oncocytic carcinoma versus oncocytoma, and may confer an ominous prognosis; it must not be confused with tumor infarction, which can also be seen in oncocytoma.
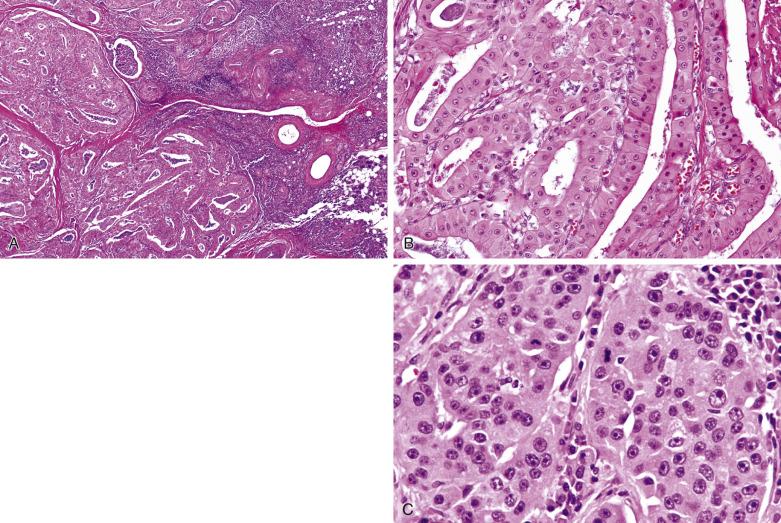
The malignant nature of most oncocytic carcinomas is easily recognized by the nuclear pleomorphism and infiltrative growth. Some investigators however suggest that there may not be a sharp histologic distinction between oncocytoma and oncocytic carcinoma, and an otherwise bland-looking tumor may rarely develop metastasis unexpectedly. Sugimoto reported a case of encapsulated oncocytoma with minimal nuclear atypia and low mitotic count that had lymph node metastasis at presentation; multiple distant metastases occurred at 7 months and the patient succumbed at 18 months. These tumors may represent metastasizing oncocytoma, akin to metastasizing pleomorphic adenoma, both of which portend a poor clinical outcome.
For tumors showing borderline atypical features, such as cellular atypia alone, occasional mitotic figures, or limited local invasion, use of the designation oncocytic neoplasm of uncertain malignant potential may be appropriate to indicate uncertainties about their behavior (see Fig. 7.46 ).
Canalicular adenoma occurs most commonly in the elderly, with a mean age of 65 to 70 years and female predilection. It is primarily an oral lesion. The upper lip is the site of predilection, which accounts for 74% of the cases, followed by the buccal mucosa (12%), palate, and rarely major salivary glands. The patients present with a nonulcerated, painless mass that grows slowly. Infrequently, multifocal nodules, ulceration, necrosis, and bone destruction may be seen. Recurrence does not occur after complete excision.
Canalicular adenoma is usually small (<3 cm) and well circumscribed, with or without a capsule. Multifocal lesions are not uncommon. It is composed of bilayered strands of cells which abut and separate haphazardly, giving rise to single files, beads, canaliculi, and pseudopapillae ( Fig. 7.61 ). The tumor shows pure luminal cell differentiation without a myoepithelial component. The epithelial cells that form the strands are cuboidal to columnar, with a moderate amount of amphophilic cytoplasm and regular oval nuclei ( Fig. 7.62 ). Cellular pleomorphism and mitoses are not seen. The stroma is characteristically edematous with many capillaries and sinusoids; it can be so loose that tumor strands may appear to be floating in the air. Intraluminal squamous morules or balls are found in 60% of cases, being positive for CK5/6, p63, and p16 but not p40. Foci of basaloid cells may be present, mimicking basal cell adenoma, but the presence of parallel rows of tumor cells, the edematous rather than sclerohyaline quality of the stroma, and absence of a myoepithelial/basal cell component on immunostaining allow for the diagnosis to be made.
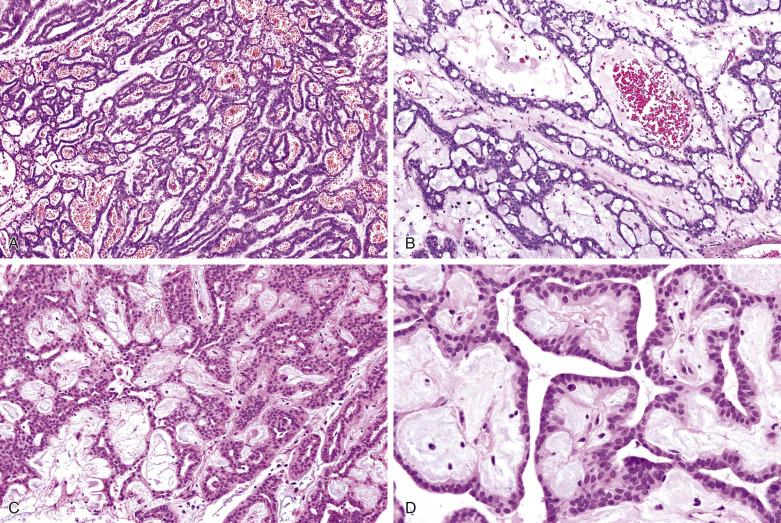
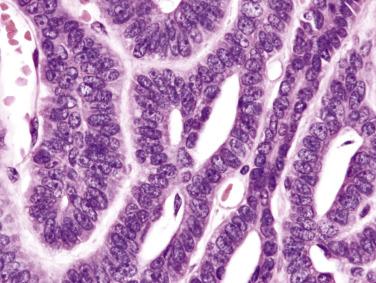
Immunohistochemistry and electron microscopy have shown exclusive luminal cell differentiation. The tumor cells are positive for cytokeratin, vimentin, S100 protein, and EMA. There is a distinctive pattern of linear GFAP immunoreactivity at the interface between tumor cells and connective tissue.
Warthin tumor, also known as adenolymphoma or papillary cystadenoma lymphomatosum, is composed of bilayered oncocytic and basaloid epithelium forming cystic structures, papillae, and glands accompanied by a dense lymphoid stroma.
Warthin tumor is the second most common salivary gland tumor, accounting for one-fourth of all primary parotid tumors. There is almost restricted occurrence to the superficial lobe of the parotid glands and the periparotid lymph nodes. It commonly presents in the sixth to seventh decade and is rare below the age of 40 years, with a definite male predominance (5–26 : 1). Interestingly, a decline of the incidence in men and concurrent increased incidence in women has been observed in recent years. This change is probably due to a decline in the smoking habit, an established risk factor for this tumor, in men and a reverse trend in women. Studies conducted among atomic bomb survivors suggest that radiation may also be implicated in the tumorigenesis. This tumor typically presents as a doughy to cystic mass in the inferior pole of the parotid gland. In contrast to other adenomas, Warthin tumor can manifest a variety of symptoms. The patients can be asymptomatic or have pain, facial weakness, or ipsilateral ear symptoms such as earache, tinnitus, and deafness. Sudden painful increase in size associated with acute pain (known as papillary cystadenoma lymphomatosum syndrome) has been postulated to be caused by leakage of fluid into the surrounding tissue and retrograde infection from the oral cavity via the Stensen duct. Rarely, facial nerve palsy is seen in tumors complicated by inflammation and fibrosis, which may be mistaken clinically or intraoperatively for carcinoma. Warthin tumor is multicentric in 12% to 20% of patients (either synchronous or metachronous), and bilateral in 5% to 14%. In addition, serial sectioning may reveal additional subclinical lesions in 50% of cases. This tumor is sometimes seen in association with other benign salivary gland tumors, especially pleomorphic adenoma.
Superficial parotidectomy or enucleation of tumor is curative. The rare recurrences (<2%) are believed to represent a second primary or an expression of multifocal tumor. In older patients or those with poor surgical risk, observation without surgery may be an option.
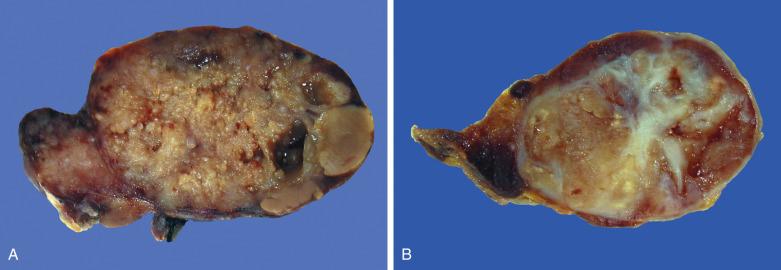
Warthin tumor is a well-circumscribed spherical to ovoid lesion. The cut surfaces often reveal solid tumor interspersed with cystic spaces containing clear, mucoid, brown or caseous semisolid debris; and the latter may sometimes give a false impression of tuberculous lymphadenitis on gross examination. There are usually fine papillary projections protruding into the cystic spaces ( Fig. 7.63A ). Prior fine-needle aspiration commonly results in areas of hemorrhage, necrosis, or fibrosis (see Fig. 7.63B ).
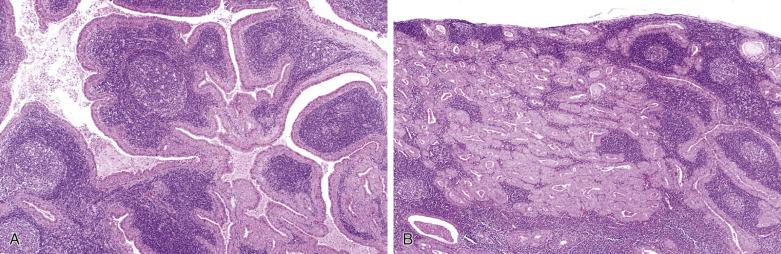
Warthin tumor comprises irregular cystic structures with the lining epithelium thrown into papillary folds ( Fig. 7.64A ). The epithelium can also show downward extension to form loosely arranged or closely packed tubular glands (see Fig. 7.64B ). The epithelium consists of two layers—a luminal layer of oncocytic columnar cells supported by a discontinuous layer of oncocytic basal cells ( Fig. 7.65 ). The nuclei of the luminal cells appear uniform and display palisading toward the free surface. Their brightly eosinophilic granular cytoplasm is due to accumulation of mitochondria. The basal cells possess round to oval nuclei and small but conspicuous nucleoli. The lumens of the cysts contain thick proteinaceous secretions, cellular debris, cholesterol crystals, and sometimes laminated bodies that resemble corpora amylacea.
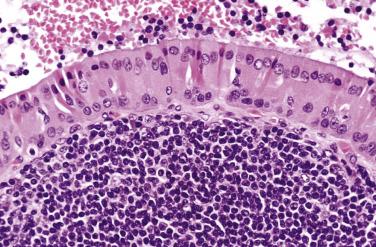
A distinct layer of basement membrane separates the cystic lining from the lymphoid stroma, which consists of small lymphocytes mixed with some plasma cells, histiocytes, and mast cells. Germinal centers and sinusoids can be seen in some cases. Sometimes there may be a granulomatous reaction with Langhans-type giant cells. The intimate relationship between glandular structures and lymphoid stroma earned the designation of adenolymphoma for this tumor. The origin of the lymphoid cells is still controversial: residual normal nodal lymphoid tissue versus tumor-associated reactive lymphoid proliferation. Tumors developing in extraparotid (such as cervical) lymph node can potentially be misinterpreted as metastatic Warthin tumor.
The epithelial component can undergo metaplastic change to squamous, mucous cells or even ciliated cells, especially in response to inflammation or infarction ( Fig. 7.66 ); tumors with florid metaplastic changes are often called metaplastic Warthin tumor . Sometimes the tumor undergoes infarction, either spontaneous or following fine-needle aspiration, resulting in tumor necrosis, granulation tissue formation, inflammatory reaction, and fibrosis. Worse still, cellular atypia and a pseudoinfiltrative appearance of the metaplastic squamous epithelium in the residual tumor may invite an erroneous diagnosis of squamous cell or mucoepidermoid carcinoma. Lack of true infiltrative growth into the surrounding parenchyma and merging of the atypical squamous islands with oncocytic epithelium should point to the correct diagnosis ( Fig. 7.67 ).
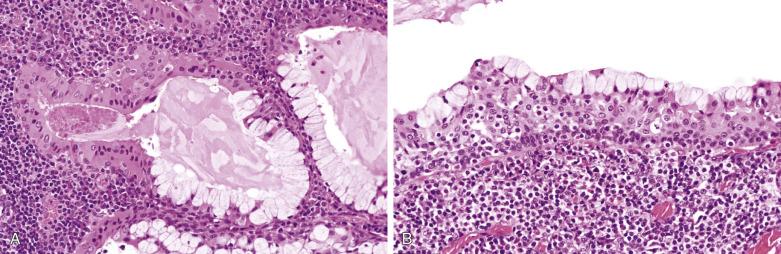
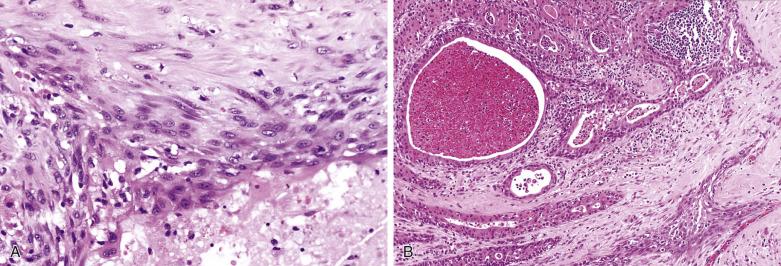
The relative proportions of epithelial and lymphoid components in Warthin tumors vary. Cases with scanty lymphoid component may overlap morphologically with oncocytoma, such that some authors suggest that Warthin tumor and oncocytoma constitute the two extremes of a spectrum of lesions.
Rarely, the epithelial or lymphoid component of Warthin tumor can undergo malignant transformation, with an estimated incidence of less than 0.1%. In order of frequency, the commonest carcinomas are squamous cell carcinoma, oncocytic carcinoma, adenocarcinoma, undifferentiated carcinoma, mucoepidermoid carcinoma, and Merkel cell carcinoma. One-third of patients have regional lymph node metastasis, and some may also develop distant metastasis.
Lymphoma arising from the lymphoid stroma is characterized by a relatively monomorphic infiltrate with distortion of the epithelial and lymphoid architecture. Various types of non-Hodgkin lymphoma and Hodgkin lymphoma have been reported.
Typical Warthin tumor has a highly distinctive morphology and poses no problem in diagnosis. It differs from oncocytoma in the presence of a prominent lymphoid component, predominance of papillae and glands rather than trabeculae and packets, and conspicuous presence of abluminal cells on light microscopy. The squamous metaplastic Warthin tumor, particularly if infarcted, can be mistaken for squamous or mucoepidermoid carcinoma. Squamous metaplasia of Warthin tumor usually lacks keratinization, a feature seen in most squamous cell carcinomas. In contrast to low-grade mucoepidermoid carcinoma, there is no definite infiltrative growth and the tumor cells appear too frankly squamous.
Sebaceous cells can occur normally in the parotid gland, submandibular gland, and oral minor salivary glands. Sebaceous tumors are very rare neoplasms which are believed to arise from these sebaceous-differentiated cells. It should also be noted that different types of salivary gland tumors can exhibit focal sebaceous differentiation, such as pleomorphic adenoma, Warthin tumor, mucoepidermoid carcinoma, and epithelial-myoepithelial carcinoma.
Affected patients are generally in their sixth or seventh decade of life with a slight male predilection. These tumors usually present as an asymptomatic, slow-growing mass. The parotid gland is the most common site, in keeping with the natural occurrence of sebaceous glands in this anatomic site. Complete excision is curative.
Sebaceous adenoma is an encapsulated tumor comprising multiple incompletely differentiated sebaceous lobules accompanied by a fibrous stroma. Each lobule consists of groups of mature sebaceous cells surrounded by basaloid cells. Some cells can show transitional features between the two cell types. The sebaceous cells contain multiple small honeycombed vacuoles of lipid that can be highlighted by Oil Red O stain on frozen section or immunostaining for adipophilin on paraffin section. Focal squamous, mucous, or oncocytic metaplasia is common. Disintegration of mature sebaceous cells can result in cystic spaces in the lobule. There can also be cystic structures lined by squamous, columnar, or cuboidal cells, with or without sebaceous cells. The fibrous stroma can be infiltrated by copious inflammatory cells, including lipogranuloma formation, probably in response to extravasated sebum.
Sebaceous lymphadenoma bears a strong resemblance to Warthin tumor. Islands of sebaceous lobules, solid nests, trabeculae, ductlike structures, or cysts are intimately mixed with a dense lymphoid stroma ( Fig. 7.68 ). The cells at the periphery of the cell nests and tubuloglandular structures have a basaloid appearance. There can be foci of squamous differentiation with keratinization. The lymphoid stroma often contains many reactive lymphoid follicles. Foreign body granulomas associated with extruded sebum are frequently present. It has been postulated that sebaceous lymphadenoma, similar to Warthin tumor, originates from ectopic salivary gland tissue in parotid lymph node. Nonetheless, in parallel to the controversy over Warthin tumor, the lymphoid stroma has alternatively been interpreted as a tumor-associated reaction. Rarely, the epithelial component can undergo malignant transformation to carcinoma, such as sebaceous carcinoma or basal cell adenocarcinoma.
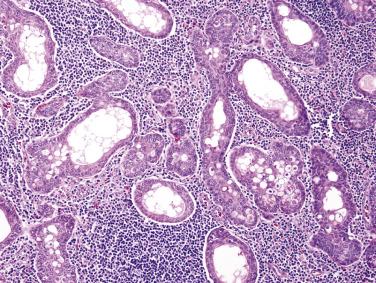
Sebaceous carcinoma is a rare, intermediate-grade malignancy. There is a bimodal age distribution, with peaks in the third and seventh to eighth decades. The gender distribution is equal. Patients present with a mass, pain, or facial paralysis. The treatment of choice is wide surgical excision for low-stage carcinomas. Adjunctive radiation therapy is recommended for higher stage and grade tumors. The overall 5-year survival rate is 62%.
These tumors are partially circumscribed but show infiltrative margins at least focally. There are variable-sized islands, sheets, and infiltrative cords of basaloid, squamous, and sebaceous cells. Ductlike and cystic spaces are common. Many cells are undifferentiated, but distinct sebaceous cells with foamy cytoplasm are present in the center of most or some tumor islands. All cases exhibit cellular pleomorphism, nuclear atypia, and frequent mitoses. Tumor necrosis is common and perineural invasion is noted in 20% of cases.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here