Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Tricuspid atresia is a congenital cardiac malformation in which the right atrium, in the setting of ventricular D-loop, fails to open into a ventricle through an atrioventricular (AV) valve. Thus, there is univentricular AV connection consisting of a left-sided mitral valve between morphologically left atrium and left ventricle (LV). Atrial situs is almost invariably solitus (normal) in association with ventricular D-loop, and the right ventricle (RV) is hypoplastic. A ventricular septal defect (VSD) usually is present. Ventriculoarterial connection may be concordant or discordant (transposed great arteries). Rarely, tricuspid atresia occurs in situs inversus with ventricular L-loop (mirror-image pattern).
Patients with atrial situs solitus and ventricular L-loop in which the left-sided left atrium is separated from a left-sided hypoplastic morphologically RV by an atretic left-sided (tricuspid) AV valve are excluded from this discussion; they are considered in Chapter 56 .
Single-ventricle physiology is present when there is impossibility or inadvisability of surgically reconstructing a functional two-ventricle heart with separated in-series pulmonary and systemic circulations. The spectrum of surgical management of single-ventricle physiology is generally similar for tricuspid atresia, other types of univentricular AV connection (see Chapter 56 ), and other anomalies without two adequate ventricles (see “ Fontan versus Intracardiac Repair for Complex Morphology ” later in this section) and is based on the Fontan operation. All such anomalies have in common the limitation of single-ventricle physiology, regardless of their specific morphology. Therefore, the Fontan operation and all its modifications and applications, as well as the spectrum of pre-Fontan palliative operations, are discussed fully in this chapter.
In 1906, Kuhne apparently recognized the entity of congenital tricuspid atresia and described its two basic morphologic subsets: hearts with concordant ventriculoarterial connection and hearts with discordant connection. In 1949, Edwards and Burchell further emphasized these two subsets and added presence or absence of pulmonary stenosis as another categorizing feature. Tandon and Edwards added further descriptive features in 1974. The clinical features of tricuspid atresia were described by Bellet and colleagues in 1933 and by Taussig and by Brown in 1936. Controversy arose early and continues as to whether tricuspid atresia should be considered a subset of single ventricle (see Historical Note in Chapter 56 ). From a surgical point of view, it is best considered as such, but tradition and its prevalence support presenting it as a separate entity.
Systemic–pulmonary arterial shunts developed in 1945 by Blalock and Taussig and later by Potts and by Waterston (see Historical Note in Section I of Chapter 38 ) were soon applied to cyanotic patients with tricuspid atresia. In 1958, Glenn specifically applied the superior vena cava–to–right pulmonary artery anastomosis to patients with tricuspid atresia. The basis for the classic Glenn shunt was experimental studies reported by Carlon and colleagues in 1951, by Glenn and colleagues, and by Robicsek and colleagues, showing that systemic venous pressure was adequate to drive pulmonary blood flow. In Moscow, Bakuljev and Kolesnikov independently developed these same concepts. In 1966, Haller and colleagues demonstrated experimentally the possibility of performing a bidirectional superior cavopulmonary anastomosis. This was applied clinically by Azzolina and colleagues in 1974, and in 1985, Hopkins and colleagues further refined the procedure with an end-to-side anastomosis of superior vena cava (SVC) to undivided right pulmonary artery (RPA). Abrams and colleagues applied this idea in the form of a side-to-side anastomosis of SVC to undivided RPA. In 1984, Kawashima and colleagues added a further improvisation in patients with either one or two SVCs, and azygos or hemiazygos continuation of the inferior vena cava (IVC) into an SVC, with only splanchnic and coronary venous blood draining into the right atrium. They divided the SVC (both, if there were two), closed its cardiac end, and anastomosed the SVC end to side to the pulmonary artery after closing the pulmonary trunk. This total cavopulmonary shunt was an incompleted Fontan operation in which only splanchnic and coronary venous blood drained directly to the systemic arterial circulation.
Successful repair of tricuspid atresia with separation of right and left circulations was accomplished in 1968 by Fontan and colleagues and was reported in 1971. This was preceded by experimental studies in 1943 by Starr and colleagues, demonstrating that destroying a dog's RV did not result in systemic venous hypertension ; in 1949 by Rodbard and Wagner, demonstrating that the RV could be bypassed ; and in 1954 by Warden, DeWall, and Varco, demonstrating the feasibility of bypassing the RV with a right atrial–pulmonary artery anastomosis. Based on these experiments, Hurwitt and colleagues reported an unsuccessful attempt to correct tricuspid atresia by a right atrial–to–pulmonary artery anastomosis in 1955. Fontan's procedure involved constructing a cavopulmonary (Glenn) anastomosis with, in the first patient, a direct anastomosis between right atrial appendage and proximal end of the divided RPA. In the subsequent two patients, one of whom had discordant ventriculoarterial connection, an aortic allograft valved conduit was placed between right atrium and RPA. In all three patients, an allograft valve was inserted into the IVC ostium, foramen ovale closed, and pulmonary trunk ligated or divided. In 1973, Kreutzer and colleagues reported a modification of Fontan's operation in which the patient's pulmonary trunk with its intact pulmonary valve was excised from the RV and anastomosed to the right atrial appendage after closing the VSD and atrial septal defect (ASD). A Glenn procedure was not performed, and no IVC valve was used.
Other early reports of successful repairs were those of Ross and Somerville and of Stanford and colleagues. Fontan subsequently modified the operation that he and Baudet had originally performed ; many others have as well. Bjork described direct anastomosis between right atrial appendage and RV outflow tract in patients with a normal pulmonary valve, using a roof of pericardium to avoid a synthetic tube graft. Direct right atrial–pulmonary artery connection, used by Fontan in his first case, has been modified and widely used.
The extracardiac conduit Fontan modification, in which the IVC is disconnected from the right atrium and connected by a prosthetic tube to the RPA outside the heart, has become popular. A bidirectional superior cavopulmonary connection completes the procedure. This was first described by Humes for use only in special cases of complex venous anatomy. It was subsequently modified for use as the procedure of choice for all Fontan candidates by Marcelletti and colleagues and further modified as a closed heart, partial bypass, or off-bypass procedure by Petrossian and colleagues.
Choussat and Fontan and colleagues formalized a set of risk factors (“ten commandments”) for the Fontan operation in 1979. In 1988, Laks and colleagues introduced the concept of deliberately making the separation between caval and pulmonary venous pathways incomplete, and then adjusting or closing the communication early postoperatively by percutaneous manipulation of a snare. Bridges and colleagues modified this approach by closing the residual aperture using catheter techniques late postoperatively.
Although the Fontan operation was introduced as a treatment for tricuspid atresia, it was soon realized that it was applicable to many other forms of univentricular AV connection. In 1976, Yacoub reported its use for single ventricle, and by 1980, for many other anomalies with one severely hypoplastic ventricle. The Fontan operation has subsequently been used to treat a group of patients who have two adequate ventricles and AV valves but who are judged by some surgeons to have intracardiac morphology too complex for biventricular repair.
In tricuspid atresia, there is no direct connection between right atrium and RV, but the left atrium connects through the mitral valve to the LV. Atresia is usually muscular (75% of cases), meaning the AV connection is absent; it may be membranous with an imperforate AV connection. In the muscular type, presence of a tiny dimple in the right atrial floor may or may not represent the atretic valve. The dimple has been said to lie above the LV or ventricular septum in most cases and may then transilluminate from the LV. In such instances, it may represent a remnant of membranous AV septum.
The membranous type has three variants. In one, a fibrous diaphragm blocks the AV orifice, and remnants of the valvar apparatus are occasionally found beneath the membrane in the RV. This has been called tricuspid atresia with imperforate valve membrane . It is often associated with left-sided juxtaposition of atrial appendages and discordant ventriculoarterial connection. Overall, juxtaposed atrial appendages are found in 11% of patients with tricuspid atresia. In a second, there is classic Ebstein anomaly (see Chapter 42 ) that is imperforate because of completely fused leaflets that are also fused to the wall of a small RV ( Fig. 41-1 ). In a third and rarer variant, there is an AV septal defect in which the right-sided valve is imperforate and blocks the opening between right atrium and RV ( Fig. 41-2 ). An autopsy series, focused on left-sided structures in tricuspid atresia, identified a high prevalence of mitral valve and LV abnormalities, including cleft mitral valve, muscularized subvalvar apparatus, and abnormal muscle bundles.


There are two major morphologic subsets of tricuspid atresia:
Origins of great arteries are normal (concordant ventriculoarterial connection; 60% to 70% of cases).
Origins of great arteries are transposed (discordant ventriculoarterial connection; about 30% to 40% of cases).
Rarely, the ventriculoarterial connection is double outlet right ventricle (DORV) or double outlet left ventricle (DOLV) or single outlet with truncus arteriosus. Other rare variants exist, and it has been estimated that only about 80% of patients diagnosed as having tricuspid atresia actually have the typical two morphologies.
This form of tricuspid atresia was referred to as type 1 by Edwards and Burchell and by Vlad. Atria are usually in situs solitus. The right atrium and its appendage are enlarged and thick walled, and an interatrial communication is present, usually through a fossa ovalis ASD ( Fig. 41-3 ). The valve of the fossa ovalis may be redundant and bulge into the left atrium and contain multiple fenestrations. The ASD is generally large; by hemodynamic studies, Dick and colleagues found that only 4% of patients had a restrictive ASD. Uncommonly, the defect is an ostium primum ASD in association with a cleft left AV valve, and rarely there is a common atrium (see Morphology in Chapter 34 ).

The eustachian valve is often prominent, and in about 5% of cases it extends superiorly to form a veil or partition across the right atrium, so-called cor triatriatum dexter (see Morphology in Chapter 32 ). At operation, this may be confusing to the unprepared surgeon.
The left atrium is morphologically normal but enlarged from obligatory shunting of systemic venous blood across the ASD. The mitral valve is usually larger than normal, as is the LV, because both systemic and pulmonary venous return pass through them. The LV is also hypertrophied and its trabeculations typically fine, although anomalous muscle bands near the posterior papillary muscle are occasionally present.
The normally positioned RV is highly abnormal and typically similar to the small RV of double inlet left ventricle (DILV; see Morphology in Chapter 56 ). In most hearts it consists of a distal tubular smooth-walled portion with a thin free wall and a smaller proximal trabeculated portion into which a VSD usually opens ( Fig. 41-4 ). The VSD varies in size and position and is sometimes multiple. In general, the larger the VSD, the larger the RV. The VSD usually lies below the infundibular (conal) septum, and from the LV side is separated from the noncoronary aortic cusp by infundibular muscle. When the VSD is large, it may extend inferiorly to the membranous septum, or it may be entirely muscular, lower in the septum, and sometimes slit-like. The VSD and trabeculated portion of the RV into which it opens are frequently separated from the smooth-walled distal portion by a narrow opening that looks like an os infundibulum (see “Infundibulum” under Morphology in Section I of Chapter 38 ). Like other VSDs, it frequently narrows spontaneously and is therefore often small and may close completely. In some hearts, the RV is large and has a true sinus portion ( Fig. 41-5 ).


In 85% to 95% of patients, pulmonary blood flow is obstructed. Obstruction most commonly occurs at the os infundibulum, or it may occur at the VSD or throughout the entire infundibulum ( Fig. 41-6 ). The pulmonary valve is bicuspid in about 20% of cases, but usually it and the “anulus,” although a little smaller than normal, are not obstructive (diameter usually within 1 standard deviation of normal). The pulmonary trunk and branch pulmonary arteries are usually a little small, but only uncommonly (about 5% of patients) are they severely hypoplastic and restrictive to flow.

In about 10% of cases in this subset, the pulmonary valve is atretic. Under these circumstances, trunk and branch pulmonary arteries are usually small, and pulmonary blood flow ( ![]() ) is via a patent ductus arteriosus or aortopulmonary collateral artery. The RV is usually extremely small, represented only by a minuscule endothelium-lined slit that is often inapparent on gross examination. The same is usually true when a VSD is absent. However, an RV chamber may be found in tricuspid atresia without a VSD.
) is via a patent ductus arteriosus or aortopulmonary collateral artery. The RV is usually extremely small, represented only by a minuscule endothelium-lined slit that is often inapparent on gross examination. The same is usually true when a VSD is absent. However, an RV chamber may be found in tricuspid atresia without a VSD.
Absent pulmonary valve is rarely described with type 1 tricuspid atresia. In a report documenting three newly described cases, it was noted that only 24 previous cases had been described. A number of associated lesions that are atypical for the more usual forms of tricuspid atresia are commonly found when there is absent pulmonary valve, including absence of a VSD, RV myocardial dysplasia, and abnormalities or even absence of the right coronary artery.
Some 5% to 15% of cases have no infundibular or pulmonary stenosis and normal or increased ![]() . The VSD is larger than usual. Coronary arteries are normally distributed, and the system is usually right dominant. The well-formed anterior descending coronary artery is displaced rightward by the large LV. The conduction system is basically normal but is affected by abnormalities present. Thus, the AV node is in its usual position in the AV septum between coronary sinus and dimple of atretic tricuspid valve. It penetrates the abnormally formed central fibrous body to the left side of the ventricular septum and becomes the branching bundle in the lower confines of the pars membranacea. Here it gives off most of the posterior radiation of the left bundle branch. Bifurcation of the bundle and formation of the right bundle branch occur at the posteroinferior angle of the VSD on the LV side. The right bundle branch proceeds here on the LV side and then intramyocardially along the inferior (caudal) border of the VSD. Then it emerges on the RV side and proceeds along the hypoplastic trabecula septomarginalis (septal band) ( Fig. 41-7 ).
. The VSD is larger than usual. Coronary arteries are normally distributed, and the system is usually right dominant. The well-formed anterior descending coronary artery is displaced rightward by the large LV. The conduction system is basically normal but is affected by abnormalities present. Thus, the AV node is in its usual position in the AV septum between coronary sinus and dimple of atretic tricuspid valve. It penetrates the abnormally formed central fibrous body to the left side of the ventricular septum and becomes the branching bundle in the lower confines of the pars membranacea. Here it gives off most of the posterior radiation of the left bundle branch. Bifurcation of the bundle and formation of the right bundle branch occur at the posteroinferior angle of the VSD on the LV side. The right bundle branch proceeds here on the LV side and then intramyocardially along the inferior (caudal) border of the VSD. Then it emerges on the RV side and proceeds along the hypoplastic trabecula septomarginalis (septal band) ( Fig. 41-7 ).

Major associated anomalies in this subset of tricuspid atresia are uncommon, but a persistent left SVC entering the coronary sinus occurs in about 15% of cases. Partially unroofed coronary sinus with coronary sinus–left atrial communication (see Morphology in Chapter 33 ) occurs in 1% to 5% of patients. This is important for the atriopulmonary type of Fontan repair, when high right atrial pressure will produce an important right-to-left shunt through it, even though a shunt was not apparent preoperatively.
In this tricuspid atresia subset (called type 2 by Edwards and Burchell ), the aorta arises from the RV, and the pulmonary trunk from the LV ( Fig. 41-8 ). Generally, the aorta is anterior and to the right of the pulmonary trunk (D-malposition) in the position characteristic of transposition of the great arteries (see Morphology in Chapter 52 ), but uncommonly there is L-malposition.

Atrial anatomy is generally similar to that described in the preceding text. However, left juxtaposition of the atrial appendages occurs in about 10%, and in about half, the ASD is small.
The RV is larger and thicker walled than usual. It tends to be a single smooth-walled cavity without a proximal trabeculated portion and is, in actuality, a subaortic outlet chamber. The VSD is usually subaortic in position. Commonly it is small, or becomes small, and then represents important subaortic stenosis. In one series, aortic or subaortic stenosis was present in 40%. The VSD, however, may be large and unobstructive.
The LV is normal although enlarged. Pulmonary valve and anulus are usually normal or as large as the pulmonary trunk. Thus, ![]() is usually large. Subpulmonary stenosis in the LV occurs in about 20% to 30% of cases, and occasionally pulmonary atresia is present. These conditions result in low
is usually large. Subpulmonary stenosis in the LV occurs in about 20% to 30% of cases, and occasionally pulmonary atresia is present. These conditions result in low ![]() and hypoxia.
and hypoxia.
Coronary arteries usually arise from the posterior aortic sinuses of Valsalva, those facing the pulmonary trunk, as in transposition of the great arteries (see “Coronary Arteries” under Morphology in Chapter 52 ). Associated cardiac anomalies usually involve the aortic arch. Obstruction coexists in about 25% to 35% of cases, with coarctation occurring more frequently than interrupted aortic arch.
Wolff-Parkinson-White syndrome is associated with tricuspid atresia. The morphology that results in single-ventricle physiology has other systemic effects. For example, levels of natriuretic peptides (both atrial natriuretic peptide and B-type natriuretic peptide) are abnormal; furthermore, they are distinctively abnormal compared with other forms of heart disease, including congenital defects with biventricular physiology.
Patients in this subset of tricuspid atresia are usually cyanotic from birth because of limited ![]() from RV outflow obstruction. Dick and colleagues reported that in 50% of patients, congenital heart disease is recognized on the first day of life. Cyanosis is severe, progressive, and often accompanied by hypoxic spells characterized by increased cyanosis, dyspnea, and occasionally syncope. These spells may occur in the first 6 months and are a grave prognostic sign. In patients with increasing obstruction to pulmonary blood flow from progressive infundibular stenosis or VSD closure, cyanosis becomes rapidly more severe, and those who were previously acyanotic may become cyanotic in a matter of a few months. Clubbing of the fingers is common in children who survive beyond the first 2 years, but it may occasionally develop as early as 3 or 4 months. Squatting is uncommon, but dyspnea is often apparent with crying or feeding.
from RV outflow obstruction. Dick and colleagues reported that in 50% of patients, congenital heart disease is recognized on the first day of life. Cyanosis is severe, progressive, and often accompanied by hypoxic spells characterized by increased cyanosis, dyspnea, and occasionally syncope. These spells may occur in the first 6 months and are a grave prognostic sign. In patients with increasing obstruction to pulmonary blood flow from progressive infundibular stenosis or VSD closure, cyanosis becomes rapidly more severe, and those who were previously acyanotic may become cyanotic in a matter of a few months. Clubbing of the fingers is common in children who survive beyond the first 2 years, but it may occasionally develop as early as 3 or 4 months. Squatting is uncommon, but dyspnea is often apparent with crying or feeding.
Most patients have loud, harsh, ejection systolic murmurs that are loudest over the lower left sternal border; these may be associated with an apical mid-diastolic rumble from large mitral valve flow. In cases of progressive obstruction to pulmonary flow, murmurs decrease or disappear. A continuous ductus arteriosus murmur may also be heard in patients with pulmonary atresia and occasionally in infants with pulmonary stenosis.
A minority of patients have no obstruction to pulmonary blood flow and a nonrestrictive VSD. These patients may present in infancy with signs and symptoms of excessive pulmonary blood flow, or they may have more or less normal ![]() and only mild cyanosis. In the latter, physical findings, chest radiograph, and electrocardiogram (ECG) are similar to those of other patients with normal origin of the great arteries.
and only mild cyanosis. In the latter, physical findings, chest radiograph, and electrocardiogram (ECG) are similar to those of other patients with normal origin of the great arteries.
Patients in this subset of tricuspid atresia often present in early life with symptoms and signs of excessive pulmonary blood flow (see “Clinical Findings” under Clinical Features and Diagnostic Criteria in Section I of Chapter 35 ). Usually an apical mid-diastolic rumble is heard, and there is fixed splitting of the second heart sound at the base. However, moderate subvalvar pulmonary stenosis occasionally results in either mildly increased or normal ![]() . Such patients usually present after the neonatal period and sometimes after infancy, with mild cyanosis and few if any symptoms. Physical findings are similar to those in patients with tricuspid atresia and concordant ventriculoarterial connection. If aortic coarctation or interrupted aortic arch is present, the neonate presents with a duct-dependent systemic circulation and pulmonary overcirculation.
. Such patients usually present after the neonatal period and sometimes after infancy, with mild cyanosis and few if any symptoms. Physical findings are similar to those in patients with tricuspid atresia and concordant ventriculoarterial connection. If aortic coarctation or interrupted aortic arch is present, the neonate presents with a duct-dependent systemic circulation and pulmonary overcirculation.
Chest radiography is usually characteristic of reduced ![]() and RV hypoplasia in typical pulmonary undercirculated patients with tricuspid atresia and concordant ventriculoarterial connection . Pulmonary vascular markings are reduced and hilar shadows diminutive. The left apical heart border may be rounded, forming a high, arched contour. The vascular pedicle is narrow, and the left border in the area of the pulmonary trunk is usually concave. Radiographic appearance of the heart may resemble that of tetralogy of Fallot or occasionally appear normal.
and RV hypoplasia in typical pulmonary undercirculated patients with tricuspid atresia and concordant ventriculoarterial connection . Pulmonary vascular markings are reduced and hilar shadows diminutive. The left apical heart border may be rounded, forming a high, arched contour. The vascular pedicle is narrow, and the left border in the area of the pulmonary trunk is usually concave. Radiographic appearance of the heart may resemble that of tetralogy of Fallot or occasionally appear normal.
Chest radiography in patients with tricuspid atresia and discordant ventriculoarterial connection usually shows pulmonary plethora and cardiomegaly, and the narrow supracardiac waist and LV contour make it resemble simple transposition.
The ECG in the subset with concordant ventriculoarterial connection demonstrates left axis deviation (0° to −90°) in about 90% of patients, LV hypertrophy in virtually all, and abnormalities of the P wave, which is frequently tall (>2.5 mV) and notched.
The ECG may show left axis deviation in the subgroup with discordant ventriculoarterial connection, but a normal QRS axis between 0 and +90 degrees is present in more than half of patients.
Echocardiography with color flow Doppler interrogation confirms the clinical impression of tricuspid atresia and usually provides definitive diagnosis ( Fig. 41-9 ). Position of the great arteries and size and position of the diminutive RV and large LV can be determined ( Fig. 41-10 ). In discordant ventriculoarterial connection, size of VSD relative to aortic “anulus” must be determined because this importantly affects the surgical procedure chosen. The aortic arch is examined for obstruction. RV size is determined because in this setting, it is functionally a subaortic outlet chamber. LV contractility is assessed. Flow across the atrial septum is assessed, which is unobstructed in most but not all cases.


Cardiac catheterization and cineangiography are not routinely performed. Indications for catheterization include inadequate echocardiographic evaluation, suspicion of inadequate or abnormal pulmonary arteries, concerns about pulmonary vascular resistance (Rp), and need for catheter-based intervention (e.g., restrictive atrial septum).
Computed tomography (CT) and magnetic resonance imaging (MRI) are rarely indicated in the newborn period. They can, however, be of great value at subsequent stages to assess valve abnormalities, complex subaortic obstruction, arch obstruction, ventricular mass, ventricular function, peripheral and central vascular dynamics, and abnormal venous and arterial connections associated with chronic single-ventricle physiology.
Tricuspid atresia occurs more commonly than any other type of univentricular AV connection and accounts for 1% to 3% of congenital heart disease. The early natural history is determined primarily by presence and severity of obstruction to pulmonary blood flow and later by LV cardiomyopathy that develops in response to volume overload (see “ Cardiomyopathy ” later in this section).
Patients in this subset usually have important RV outflow obstruction and are cyanotic at birth. In most, the VSD narrows rapidly (in common with the general tendency of muscular VSDs to close spontaneously [see “Spontaneous Closure” under Natural History in Section I of Chapter 35 ]), ![]() diminishes still further, cyanosis worsens, and hypoxia increases, causing the death of 90% of surgically untreated patients by age 1 year ( Fig. 41-11 ).
diminishes still further, cyanosis worsens, and hypoxia increases, causing the death of 90% of surgically untreated patients by age 1 year ( Fig. 41-11 ).
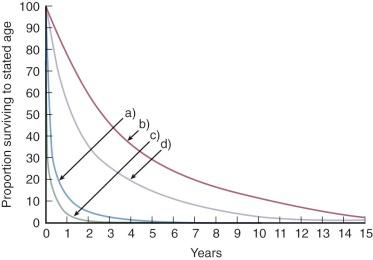
When these patients have a normal or increased ![]() , natural history is more favorable than in any other subset (see Fig. 41-11 ). Some die in early infancy of heart failure secondary to large
, natural history is more favorable than in any other subset (see Fig. 41-11 ). Some die in early infancy of heart failure secondary to large ![]() , but spontaneous VSD narrowing and progression of infundibular narrowing usually produce a more balanced flow and better hemodynamic state within a few months of birth. Mild cyanosis and mild to moderate exercise intolerance persist at a plateau level for several years. Spontaneous narrowing of most VSDs continues, however, and approximately 90% of patients are dead by age 10 years. A few survive into their second and third decades and even beyond, presumably because neither VSD nor RV outflow tract continues to narrow.
, but spontaneous VSD narrowing and progression of infundibular narrowing usually produce a more balanced flow and better hemodynamic state within a few months of birth. Mild cyanosis and mild to moderate exercise intolerance persist at a plateau level for several years. Spontaneous narrowing of most VSDs continues, however, and approximately 90% of patients are dead by age 10 years. A few survive into their second and third decades and even beyond, presumably because neither VSD nor RV outflow tract continues to narrow.
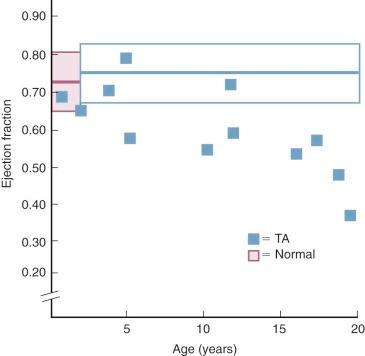
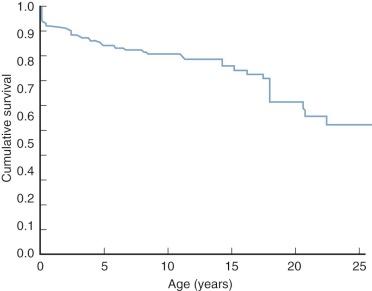
In patients who survive into the second decade and longer, chronic LV volume overload usually produces a secondary LV cardiomyopathy and reduced systolic function ( Fig. 41-12 ), and mitral regurgitation may develop. These factors produce a lower LV output and consequently increasing cyanosis and heart failure.
Surgically untreated patients in this subset usually have markedly increased ![]() , because the LV ejects directly and without restriction into the pulmonary trunk. Any tendency to VSD closure worsens the pulmonary plethora and, by producing subaortic stenosis, reduces systemic blood flow (
, because the LV ejects directly and without restriction into the pulmonary trunk. Any tendency to VSD closure worsens the pulmonary plethora and, by producing subaortic stenosis, reduces systemic blood flow ( ![]() ). This unfavorable situation results in death of most babies by age 1 year (see Fig. 41-11 ). If there is coexisting important aortic coarctation or interruption, natural history is heavily influenced by duct-dependent systemic perfusion. The majority of such infants suffer circulatory collapse and death in the first weeks of life within hours or days of ductal closure.
). This unfavorable situation results in death of most babies by age 1 year (see Fig. 41-11 ). If there is coexisting important aortic coarctation or interruption, natural history is heavily influenced by duct-dependent systemic perfusion. The majority of such infants suffer circulatory collapse and death in the first weeks of life within hours or days of ductal closure.
A few patients have mild or moderate LV (subpulmonary) outflow narrowing at birth and decreased ![]() . Progression of VSD narrowing (and RV outflow obstruction) is slower in this subset, so approximately 50% of patients survive to about age 2 years (see Fig. 41-11 ). Hypoxia worsens with time, however, and about 90% of surgically untreated subjects are dead by age 6 or 7 years.
. Progression of VSD narrowing (and RV outflow obstruction) is slower in this subset, so approximately 50% of patients survive to about age 2 years (see Fig. 41-11 ). Hypoxia worsens with time, however, and about 90% of surgically untreated subjects are dead by age 6 or 7 years.
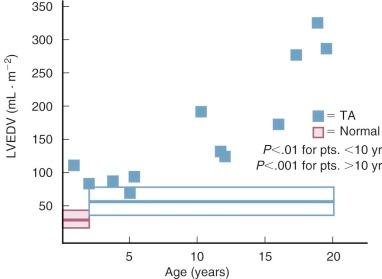
The volume-overloaded LV, receiving both pulmonary and systemic venous return in patients with tricuspid atresia, plays an important role in natural history. Surgically untreated infants with diminished ![]() have depressed LV systolic function (reduced ejection fraction) and end-diastolic volume larger than normal. Reduced ejection fraction at this young age may be related to hypoxia. In patients who live beyond about age 5 years, ejection fraction becomes progressively more depressed (see Fig. 41-12 ) and LV volume progressively larger ( Fig. 41-13 ). This is related to progression of LV cardiomyopathy secondary to chronic volume overload. In some patients, this leads to gradual development of mitral regurgitation in the second, third, and fourth decades. Recent evidence suggests the cardiomyopathy is due to a combination of volume overload and ischemia, with the ischemia partially due to an inadequately developed capillary network within the LV.
have depressed LV systolic function (reduced ejection fraction) and end-diastolic volume larger than normal. Reduced ejection fraction at this young age may be related to hypoxia. In patients who live beyond about age 5 years, ejection fraction becomes progressively more depressed (see Fig. 41-12 ) and LV volume progressively larger ( Fig. 41-13 ). This is related to progression of LV cardiomyopathy secondary to chronic volume overload. In some patients, this leads to gradual development of mitral regurgitation in the second, third, and fourth decades. Recent evidence suggests the cardiomyopathy is due to a combination of volume overload and ischemia, with the ischemia partially due to an inadequately developed capillary network within the LV.
The Fontan operation is considered the surgical end point for patients whose cardiac anomalies do not allow a two-ventricle circulation. The original Fontan operation was performed exclusively in patients with tricuspid atresia, but it is now applied to all forms of univentricular AV connection (see Chapter 40, Chapter 49, Chapter 56 ), as well as to a number of other conditions in which complete two-ventricle circulations cannot easily be achieved (see Chapter 58 ). Soon after the original operative description, Fontan himself modified the procedure; it therefore seems unnecessary to term each subsequent modification a “modified Fontan.” All forms of Fontan operation aim to divert systemic (with or without coronary) venous return to the pulmonary arterial circulation (either directly or by pathways through the heart), leaving one ventricle to provide essentially all energy driving blood flow in an in-series circulation. Each of the various techniques of achieving this separated pulmonary and systemic in-series circulation has advantages and disadvantages. Widely used techniques are described in sections that follow, and techniques that may be useful in specific cases are described under Special Situations and Controversies in Section IV .
Most patients with tricuspid atresia and those with other forms of univentricular AV connection (single-ventricle physiology) require preliminary surgical palliation before the Fontan operation. This usually involves:
First-stage palliation in the neonatal period (see Section II )
Commonly, second-stage palliation at some point between 3 months and 1 year to create a superior cavopulmonary connection in order to remove or reduce volume load on the single ventricle (see Section III )
Third-stage palliation, the Fontan procedure, is then performed, typically between ages 1 and 5 years, depending on a number of factors (see Section IV )
Physiology and presentation, technique of operation, special features of postoperative care, results, indications for operation, and special situations and controversies of each palliative stage are discussed in separate sections that follow.
Morphologic variations of tricuspid atresia encompass most of the physiologic circumstances encountered when managing all other forms of single-ventricle physiology; therefore, specific discussion of the surgical management of tricuspid atresia presents an excellent opportunity to outline most techniques for managing all forms of single-ventricle physiology, from birth to Fontan completion. Several unique exceptions exist and are discussed in Chapter 49, Chapter 58 .
Results of first-stage palliation are presented in Section II , those of second-stage palliation in Section III , and those of third-stage palliation (Fontan operation) in Section IV .
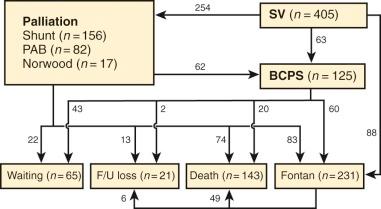
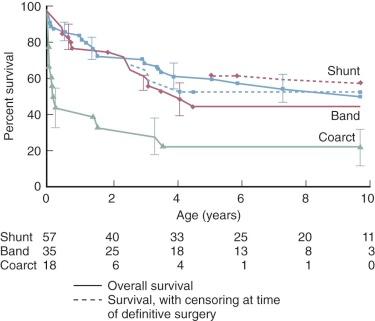
Overall outcome for single-ventricle patients followed from early infancy was assessed in 405 patients by Lee and colleagues. These patients had the entire spectrum of single-ventricle morphology, but included only 14 with hypoplastic left heart physiology; thus, this relatively high-risk lesion did not have a major influence on overall results. Tricuspid atresia, double inlet ventricle, DORV, and unbalanced AV septal defect accounted for 379 of the 405 cases. Patients were managed with a variety of surgical procedures ( Fig. 41-14 ), but the goal was to achieve a Fontan circulation. Survival was thus influenced by operative mortality at each operation, interstage mortality, and mortality following the Fontan operation. The study showed a 10-year survival of 60%. Lan and colleagues examined 140 cases of DILV or tricuspid atresia with discordant ventriculoarterial connection and reported survival of 80% at 10 years ( Fig. 41-15 ).
Patients in whom only one ventricle has an AV connection and is of sufficient size and power to provide energy for generating in-series pulmonary and systemic blood flows are considered for the Fontan operation. In most of these patients, the heart has a univentricular AV connection (see “Atrioventricular Connection” under Morphology in Chapter 56 ), one subset of which is tricuspid atresia. The Fontan operation may also be indicated for a few patients with concordant or discordant AV connections in whom one ventricle is too small or dysplastic, or both, to provide sufficient energy for generating adequate blood flow in a two-ventricle circulation (see Chapter 40, Chapter 49, Chapter 58 ). Also, there are some cases in which two adequately sized and functioning ventricles exist in association with adequate inlet valves, but they cannot be septated because of complex relationships among the ventricles, great arteries, and VSDs. Such patients may best be treated with a Fontan procedure (see Chapter 53 ).
Some data suggest that a hypoplastic RV of less than 30% normal size does not contribute to the circulation, indicating that the Fontan operation should be performed if this threshold value is met.
If the RV is hypoplastic but greater than 30% normal size, then it may be of benefit to incorporate it into the right-sided circulation with a functioning inlet and outlet valve and a superior cavopulmonary anastomosis. This procedure has been called the one-and-a-half ventricle repair .
Although definite proof of its efficacy is lacking, the one-and-a-half ventricle repair, used both to avoid the Fontan operation and to unload the normally developed but failing RV, has gained fairly wide acceptance as a useful procedure in both settings.
Under certain morphologic circumstances, biventricular repair, although theoretically possible, may not be advisable, and consideration should be given to performing the Fontan operation. These circumstances include but are not limited to:
Unbalanced AV septal defect
Moderate right heart hypoplasia
Moderate left heart hypoplasia
DORV with uncommitted VSD
Tricuspid atresia with VSD and moderately developed RV
Pulmonary atresia with intact ventricular septum with moderate RV hypoplasia or dysfunction
Ebstein anomaly with moderate RV hypoplasia or dysfunction
Marked straddling of one AV valve, with AV and ventriculoarterial discordant connections, in association with VSD and pulmonary atresia or stenosis
In these anomalies, there may be two reasons to question a standard biventricular repair:
Concern about ability of the hypoplastic ventricle or AV valve to function adequately
Overall complexity of the procedure required to achieve a standard biventricular repair
Occasionally, both ventricles and inlet valves are of normal size and morphology is not particularly complex, but one ventricle demonstrates marked dysfunction (e.g., tetralogy of Fallot with markedly reduced RV function). In these circumstances, biventricular repair, Fontan operation, superior cavopulmonary anastomosis with intracardiac repair (one-and-a-half ventricle repair) and transplantation are theoretically possible. No clear criteria exist in these complex clinical settings for deciding among the options. Furthermore, there are no compelling data to suggest that long-term functional status of patients with one type of reconstruction is superior to the alternatives (e.g., one-and-a-half ventricle repair vs. Fontan). One single-institution study suggests that based on short-term and midterm survival, the Fontan is superior to complex intracardiac reconstructions. Other centers, however, do not agree with the findings.
The classic Glenn operation (SVC-RPA anastomosis) is rarely performed in the current era. Most were performed as definitive operations prior to development, or at least wide acceptance, of the Fontan operation.
Early mortality for the procedure has been low, generally about 5%, when performed in infants older than about age 6 months and in children. In some respects it facilitates a later Fontan operation, because half the operation has been performed, similar to the situation following bidirectional superior cavopulmonary anastomosis. Interruption of the RPA, a necessary step in the classic Glenn operation, is in general disadvantageous from a technical standpoint.
Long-term results of the classic Glenn anastomosis in patients not suitable for complete repair are generally satisfactory. About 85% survive at least 10 years after creation of the shunt, which usually remains patent for at least that length of time. Kopf and colleagues report only 50% are still functional 20 years postoperatively, with 10- and 20-year survivals of 84% and 66%, respectively.
When symptoms recur, they may be caused by shunt closure, but also by rising hematocrit (with consequent decrease in right lung pulmonary blood flow), decreasing left lung pulmonary blood flow due to progressive narrowing of the VSD or pulmonary valve or subvalvar area, or by increasing flow through venovenous collaterals developing from the upper body to the lower body around the ligated SVC.
The operation is followed in many patients by redistribution of pulmonary blood flow in the right lung. Using ventilation/perfusion lung scans, Cloutier and colleagues demonstrated a decreased ratio of upper lobe–to–lower lobe pulmonary blood flow. Presumably as a more advanced manifestation of the same underlying process, right-sided pulmonary arteriovenous fistulae form late after the Glenn procedure, generally confined to the right lower lobe. In Kopf and colleagues’ 30-year follow-up study, pulmonary arteriovenous fistulae developed in the right lung of 33% of patients. They found that a longer interval between operation and observation increased the probability of these fistulae being present. Right-to-left shunting and cyanosis usually develop with sufficient severity to warrant therapeutic fistula embolization in about one third of those afflicted. If fistulae are multiple and diffuse, embolization may not be an option.
In most centers, the Fontan operation is considered the definitive operation for patients with single-ventricle physiology. Some patients will not meet physiologic criteria for the Fontan, most commonly because of elevated Rp or elevated ventricular end-diastolic pressure. For these patients, the definitive operation may be the superior cavopulmonary anastomosis, commonly with an additional source of pulmonary blood flow. In a minority of centers, the bidirectional superior cavopulmonary anastomosis with an additional source of pulmonary blood flow is considered the preferred definitive operation for patients with single-ventricle physiology. There are no data comparing long-term outcomes after this procedure with those after the Fontan.
The purpose of neonatal surgical intervention for tricuspid atresia and all forms of single-ventricle physiology is to balance systemic and pulmonary blood flow ( ![]() ), provide unobstructed mixing at the atrial level, and ensure unobstructed systemic cardiac output.
), provide unobstructed mixing at the atrial level, and ensure unobstructed systemic cardiac output.
Because in this subset, the patient most commonly presents with reduced ![]() , or with duct-dependent
, or with duct-dependent ![]() resulting from obstruction at or below the pulmonary valve, neonatal surgical palliation is required. This is achieved by some form of systemic–pulmonary arterial shunt designed to increase
resulting from obstruction at or below the pulmonary valve, neonatal surgical palliation is required. This is achieved by some form of systemic–pulmonary arterial shunt designed to increase ![]() .
.
Occasionally, there is little or no obstruction at or below the pulmonary valve, and the patient presents with excessive ![]() ; surgical palliation to reduce it is required. This is achieved with a pulmonary trunk band . On occasion, neonates present with either well-balanced or moderately increased
; surgical palliation to reduce it is required. This is achieved with a pulmonary trunk band . On occasion, neonates present with either well-balanced or moderately increased ![]() across the right ventricular (RV) outflow tract. If
across the right ventricular (RV) outflow tract. If ![]() is truly well balanced, neonatal surgical palliation may be avoided. Such patients need careful monitoring, because resistance across the VSD, hypoplastic RV, and pulmonary valve can change rapidly in the first few weeks of life. Some will require a systemic–pulmonary arterial shunt if blood flow to the lungs decreases over time; others will remain with relatively well-balanced
is truly well balanced, neonatal surgical palliation may be avoided. Such patients need careful monitoring, because resistance across the VSD, hypoplastic RV, and pulmonary valve can change rapidly in the first few weeks of life. Some will require a systemic–pulmonary arterial shunt if blood flow to the lungs decreases over time; others will remain with relatively well-balanced ![]() and may not require surgery until their superior cavopulmonary connection.
and may not require surgery until their superior cavopulmonary connection.
When there is little or no resistance across the VSD and RV outflow tract, however, markedly increased ![]() gradually develops. This usually is not a problem in the first week of life when resistance in the pulmonary microvascular bed remains somewhat elevated, but such patients eventually require a pulmonary trunk band to establish appropriate balance between
gradually develops. This usually is not a problem in the first week of life when resistance in the pulmonary microvascular bed remains somewhat elevated, but such patients eventually require a pulmonary trunk band to establish appropriate balance between ![]() and
and ![]() . Careful consideration should be given to timing of banding in such patients, even if diagnosis is made in the first few days of life. It is beneficial to delay placing the band until
. Careful consideration should be given to timing of banding in such patients, even if diagnosis is made in the first few days of life. It is beneficial to delay placing the band until ![]() increases somewhat, in concert with the normal postnatal decrease in pulmonary resistance (Rp) (see “ Timing of Pulmonary Trunk Banding ” under Indications for Operation later in this section).
increases somewhat, in concert with the normal postnatal decrease in pulmonary resistance (Rp) (see “ Timing of Pulmonary Trunk Banding ” under Indications for Operation later in this section).
Before undertaking any surgical procedure, overall cardiopulmonary stability should be ensured. These neonates are typically stable and come to the operating room breathing spontaneously and on little pharmacologic support other than, in some cases, prostaglandin E 1 (PGE 1 ) infusion to maintain ductal patency. However, if they present in an uncompensated state, either with overcirculation of the pulmonary circuit or with undercirculation and profound cyanosis, it is prudent to resuscitate them aggressively before operation. This may include use of PGE 1 , inotropic agents, diuretics, mechanical ventilation with appropriate manipulations, nutritional support, and treatment of sepsis. Following stabilization, a period of observation is usually beneficial to allow recovery of systemic end-organ damage before surgical intervention. However, circumstances may require urgent operation despite inadequate resuscitation. For example, a previously undiagnosed and stable infant may present at several weeks of life with a recently closed ductus, resulting in ongoing critical cyanosis.
After anesthesia induction, an indwelling arterial catheter is placed, preferably in the left radial artery. Reliable intravenous access is achieved, preferably via peripheral extremity vein; subclavian and internal jugular veins should be specifically avoided because they tend to develop deep venous thrombosis that can importantly complicate subsequent management at the time of superior cavopulmonary shunt (see Section III ). It is similarly important to avoid femoral vein cannulation, because most patients with univentricular AV connection require multiple cardiac catheterization evaluations, preferably via the femoral vein.
The preferred incision for performing a systemic–pulmonary arterial shunt is median sternotomy. This incision has multiple advantages over traditional lateral thoracotomy:
Both lungs can be completely ventilated throughout the procedure. This can be especially important in unstable infants.
The shunt can be placed more centrally on the left or right branch pulmonary artery, thereby reducing prevalence of right or left upper lobe pulmonary artery branch stenosis.
Maximal flexibility is achieved.
If the ductus arteriosus is to be ligated during the shunt procedure, it can be accomplished effectively.
If central pulmonary artery stenosis at the site of ductus insertion is present or suspected, pulmonary arterioplasty can be performed.
If the patient becomes unstable during the procedure and requires cardiopulmonary bypass (CPB), there is access for cannulation.
Occurrence of musculoskeletal deformities induced by a lateral incision, such as scoliosis, is eliminated.
The single disadvantage of median sternotomy is risk of hemorrhage from inadvertent cardiotomy on repeat sternotomy at subsequent procedures. This can be minimized by leaving intact the anterior aspect of the pericardial sack overlying the ventricular mass at the first procedure.
Median sternotomy is performed (see “Incision” in Section III of Chapter 2 ). The typically large thymus gland is mobilized or partially removed, and only the upper pericardial reflection over the great arteries is opened, leaving intact the portion overlying the ventricular mass. Sites on both systemic and pulmonary circuits are chosen for placing the shunt (see “ Systemic–Pulmonary Arterial Shunt ” under Special Situations and Controversies later in this section). Usually a modified Blalock-Taussig shunt is performed ( Fig. 41-16 ) using an expanded polytetrafluoroethylene (PTFE) vascular graft of specified internal diameter, placed between the brachiocephalic–right subclavian artery junction, and central portion of the RPA (see “ Systemic–Pulmonary Arterial Shunt ” under Special Situations and Controversies later in this section). Systemic and pulmonary arterial sites are prepared using sharp dissection. The patient is heparinized (3 mg · kg −1 intravenously). An appropriately sized partial occlusion vascular clamp is used to isolate the brachiocephalic–right subclavian arterial segment, which is incised over a length appropriate to create an orifice that matches the expanded PTFE graft.

Focus on detail is necessary to create a functional and reliable shunt. Attention is given to the angle of takeoff of the brachiocephalic artery origin and brachiocephalic-subclavian arterial junction. The expanded PTFE graft is tailored with a bevel to maximize laminar flow at the arterial graft anastomosis. Anastomosis is then performed with running 7-0 nonabsorbable monofilament suture.
After the anastomosis is completed, the partial occlusion clamp on the arterial segment is removed and replaced with a small clamp occluding the graft. This allows the arterial segment to assume its natural position, thereby permitting the surgeon to judge exactly the length of the graft in preparation for its anastomosis to the RPA. Attention to detail is necessary because a graft tailored to an inappropriate length may kink or distort the involved arteries. Typically, no bevel is necessary at the graft-RPA connection, and end-to-side anastomosis is performed at a 90-degree angle. After trimming the graft to an appropriate length, a partial occlusion clamp is placed on the central portion of the RPA that lies to the right of the ascending aorta and left of the SVC; it should not involve the right upper pulmonary artery. An incision of appropriate length is made in the sequestered segment of RPA, and anastomosis proceeds using a technique similar to that of the previous anastomosis. Before completing it, heparinized saline may be infused into the graft and pulmonary artery segments to flush remnants of blood that may have accumulated. The clamp is then removed from the RPA. Before removing the clamp on the shunt, the ductus arteriosus (if present) is exposed, and a heavy silk ligature with a snare is placed around it. The clamp is then removed from the shunt, and the snare on the ductus is gently tightened to occlude it.
A period of hemodynamic adjustment then ensues. The surgeon should pay careful attention to change in systemic arterial oxygen saturation (Sa o 2 ) as indicated by pulse oximetry and by change in hemodynamics as indicated by heart rate and systolic, diastolic, and mean blood pressures. New steady-state values for these variables are judged against baseline conditions, which may vary among infants. In general, Sa o 2 between 75% and 85% is considered acceptable. Sa o 2 below this range should raise concerns about a technical problem with the shunt, an inadequately sized shunt, or unsuspected distal pulmonary artery problems. Sa o 2 above this range should raise concern that the shunt is too large. This latter concern is heightened if systemic arterial diastolic blood pressure is less than 25 to 30 mmHg.
Once stability has been achieved, the snare is removed from the ductus, and it is permanently ligated. PGE 1 infusion is stopped. Mediastinal drainage and closing the median sternotomy are as usual (see “Completing Operation” in Section III of Chapter 2 ).
Pulmonary trunk banding is usually performed via median sternotomy, lateral thoracotomy, or anterior parasternal incision. Median sternotomy is preferred for the same reasons described in the preceding text for placing a systemic–pulmonary arterial shunt; patient preparation is also similar. If the surgeon prefers a lateral thoracotomy or anterior parasternal incision, it is performed on the left side. In other patients with univentricular AV connection with conotruncal abnormalities that result in position of the pulmonary trunk to the right of the aortic root, a right lateral incision is chosen.
Preferred median sternotomy is performed as described in Chapter 2 , and the thymus gland is mobilized or partially removed. The pericardium is opened only at its superior border over the great arteries, with care taken to leave it intact over the ventricular mass. The tissue plane between ascending aorta and pulmonary trunk is developed over a limited area halfway between the sinutubular junction of the pulmonary trunk and origin of the RPA ( Fig. 41-17, A ).

Aggressive dissection in this area is discouraged because it increases the chances of migration of the band over time. Once circumferential access to the pulmonary trunk is achieved, the band is placed around it. Choice of band material may vary; however, material that prevents important fibrosis and calcification and has a low risk of erosion into the pulmonary trunk should be chosen. Width of band material should be broad (at least 2.5 mm) to minimize erosion. Preferred choice of band is a 3-mm-wide strip fashioned from a relatively thick (0.3 to 0.4 mm) silicone rubber sheet. This material incites minimal reaction in surrounding tissues.
After placing the band around the pulmonary trunk ( Fig. 41-17, B ), its free ends are secured together to create a circumferential ring ( Fig. 41-17, C ). Formulas can be used to estimate the appropriate circumferential length, but individual physiologic variability usually dictates adjustments be made. Free ends of the band are initially secured together at a point that allows only minimal circumferential narrowing of the pulmonary trunk. Following this initial placement, two sutures are placed at points 180 degrees opposite each other on the circumference of the band, attaching the band to the adventitia of the proximal portion of the pulmonary trunk (see Fig. 41-17, C ). These sutures prevent pressure-driven distal band migration on the pulmonary trunk. Migration is common if the band is not secured.
Once the band is positioned, but before it has been adjusted to its final circumference, it is prudent to temporarily place a catheter into the distal pulmonary trunk to measure pressure distal to the band. Difference in systemic arterial and distal pulmonary artery pressure provides an accurate assessment of band gradient. The surgeon then gradually reduces band circumference, evaluating both band gradient and Sa o 2 as end points. Both vary depending on physiologic circumstances; however, a typical band gradient in a neonate will be in the range of 40 to 70 mmHg, and Sa o 2 should range between 75% and 85%. Band circumference is adjusted by placing metal clips in the vicinity where the two free ends of the band were initially secured together (see Fig. 41-17, C ). These clips are placed sequentially, with each subsequent clip placed just below the most recently placed one, gradually approaching the physiologic end points just described. Appropriately adjusting ![]() with a pulmonary trunk band can be somewhat difficult. This is because pulmonary blood flow occurs only in systole, and the band is a two-dimensional resistor with little length. As a result, small changes in band circumference result in marked resistance changes and, therefore, marked
with a pulmonary trunk band can be somewhat difficult. This is because pulmonary blood flow occurs only in systole, and the band is a two-dimensional resistor with little length. As a result, small changes in band circumference result in marked resistance changes and, therefore, marked ![]() changes. Because flow across the band occurs only in systole,
changes. Because flow across the band occurs only in systole, ![]() varies with changes in systemic arterial pressure. It is therefore critical that the anesthesiologist create circumstances during band adjustment such that systemic blood pressure approximates that expected in the awake infant. This can usually be achieved by appropriate choice of anesthetic and volume management.
varies with changes in systemic arterial pressure. It is therefore critical that the anesthesiologist create circumstances during band adjustment such that systemic blood pressure approximates that expected in the awake infant. This can usually be achieved by appropriate choice of anesthetic and volume management.
Once the band is appropriately adjusted, an indwelling right atrial catheter and atrial and ventricular pacing wires are placed as described earlier in this section for the systemic–pulmonary arterial shunt. Mediastinal drainage and median sternotomy closure are as described in “Completing Operation” in Section III of Chapter 2 .
After completing the procedure, it is not necessary to reverse the heparin with protamine; instead, the heparin is allowed to metabolize slowly. Beginning on the first postoperative night, aspirin is given rectally (1 mg · kg −1 · day −1 ) to prevent thrombus formation in the shunt.
Some degree of hemodynamic instability and modest metabolic acidosis are common in the first few postoperative hours. It is prudent to support the patient over the first postoperative day with mechanical ventilation and close observation. Occasionally, low-dose inotropic support is indicated.
As Rp gradually decreases after placement of a pulmonary trunk band, it is occasionally necessary to reoperate to tighten the band further. Need for readjusting it can be minimized by appropriately timing the initial banding (see “ Timing of Pulmonary Trunk Banding ” under Indications for Operation later in this section).
Early mortality for patients with tricuspid atresia and other types of univentricular communications and reduced ![]() is low ( Tables 41-1 and 41-2 ) and similar to that for shunts performed for palliation of tetralogy of Fallot (see “Interim Results after Classic Shunting Operations” in Section I of Chapter 38 ). As expected, early mortality from multi-institution reports is somewhat higher than in the single-institution reports cited in these tables. A three-institution report revealed 3 early deaths in 23 cases (13%; CL 6%-24%). Under most circumstances, pulmonary artery distortion by the shunt is uncommon.
is low ( Tables 41-1 and 41-2 ) and similar to that for shunts performed for palliation of tetralogy of Fallot (see “Interim Results after Classic Shunting Operations” in Section I of Chapter 38 ). As expected, early mortality from multi-institution reports is somewhat higher than in the single-institution reports cited in these tables. A three-institution report revealed 3 early deaths in 23 cases (13%; CL 6%-24%). Under most circumstances, pulmonary artery distortion by the shunt is uncommon.
| Hospital Deaths | |||||
|---|---|---|---|---|---|
| Operation | n | No. | % | CL (%) | |
| Systemic–pulmonary artery shunting | 69 | 5 | 7 | 4-12 | |
| Blalock-Taussig | 31 | 2 | 6 | 2-15 | } 3/44, 7%; CL 3%-13% |
| PTFE interposition | 13 | 1 | 8 | 1-24 | |
| Others | 25 | 2 | 8 | 3-18 | |
| Glenn operation | 11 | 2 | 18 | 6-38 | |
| Revisions of shunts | 8 | 2 | 25 | 9-50 | |
| Open palliative procedures | 7 | 1 | 14 | 2-41 | |
| Pulmonary trunk banding | 6 | 0 | 0 | 0-27 | |
| Coarctation repair and pulmonary trunk banding | 1 | 0 | 0 | 0-86 | |
| Miscellaneous other palliative procedures | 6 | 1 | 17 | 2-46 | |
| T otal | 108 | 11 | 10 | 7-14 | |
| Hospital Deaths | |||||
|---|---|---|---|---|---|
| Operation | n | No. | % | CL (%) | |
| Systemic–pulmonary artery shunting | 73 | 6 | 8 | 5-13 | |
| Blalock-Taussig | 41 | 1 | 2 | 0.3-8 | } 1/55, 2%; CL 0.2%-6% |
| PTFE interposition | 14 | 0 | 0 | 0-13 | |
| Other shunts | 18 | 5 | 28 | 16-43 | |
| Pulmonary trunk banding | 4 | 0 | 0 | 0-38 | |
| Atrial septectomy | 9 | 0 | 0 | 0-19 | |
| Repair only of associated cardiac anomaly | 7 | 2 | 29 | 10-55 | |
| Combined closed palliative procedures | 9 | 0 | 0 | 0-19 | |
| Others a | 9 | 2 | 22 | 8-45 | |
| T otal | 111 | 10 | 9 | 6-13 | |
a Seven exploratory cardiotomies, including or not including pulmonary valvotomy or a valved extracardiac conduit (seven cases, one hospital death), and two revisions of previous procedures (one hospital death).
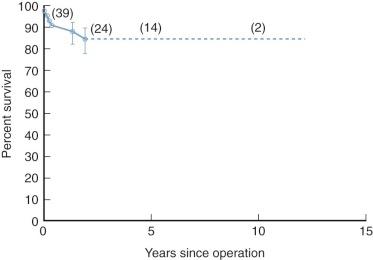
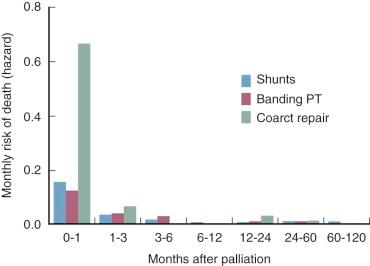
In patients who cannot subsequently have a Fontan operation, palliation has been good, and 5-year survival without definitive operation is about 90%. Intermediate time-related survival, including mortality of subsequent interventions, is about 85% at 10 years when the shunt is initially performed after the first few months of life ( Fig. 41-18 ). However, substantially worse survival was reported by Franklin and colleagues for a patient group in which many required operation early in life; this may be more representative ( Fig. 41-19 ). Risk of dying is highest in the first few months following the shunt procedure ( Fig. 41-20 ). Then, after 5 to 10 years, many patients begin to deteriorate. This is related to cyanosis, which is due to relative narrowing of the Blalock-Taussig shunt commensurate with patient growth, as well as to LV cardiomyopathy secondary to chronic volume overload, which worsens with time (see “ Cardiomyopathy ” under Natural History in Section I ).
Early mortality after pulmonary trunk banding has been reported to be substantial—25% to 35%. This high mortality likely reflects that this information spans many years and thus includes many patients receiving bands in the 1970s, when mortality in general was high for complex cases. It may also reflect difficulty of achieving physiologic balance of ![]() and
and ![]() compared with a systemic–pulmonary arterial shunt. In the current era, mortality of less than 5% should be expected (see Tables 41-1 and 41-2 ). Low mortality (no early deaths in 10 cases) (0%; CL 0%-17%) in the current era is confirmed even in multi-institutional reports.
compared with a systemic–pulmonary arterial shunt. In the current era, mortality of less than 5% should be expected (see Tables 41-1 and 41-2 ). Low mortality (no early deaths in 10 cases) (0%; CL 0%-17%) in the current era is confirmed even in multi-institutional reports.
Outcome following pulmonary trunk banding, like systemic–pulmonary arterial shunting, is somewhat influenced by intracardiac anatomy. For example, with tricuspid atresia or DILV and discordant ventriculoarterial connection, the tendency for subaortic stenosis to develop or progress is a frequent and unfavorable sequel to the banding procedure (see “Physiology and Presentation” under Discordant Ventriculoarterial Connection later in this section). Subaortic stenosis not only increases risk of death before definitive repair but also after the Fontan operation; this is due to the resulting increase in main ventricular chamber muscle mass and corresponding decrease in ventricular compliance.
Presence of severe cyanosis (Sa o 2 < 70%-75%) early in life or of duct dependency are indications for performing a systemic–pulmonary arterial shunt. Causes for cyanosis other than restrictive ![]() (e.g., reversible lung disease, anemia, obstructive pulmonary venous connection) must be ruled out.
(e.g., reversible lung disease, anemia, obstructive pulmonary venous connection) must be ruled out.
The shunt does not facilitate later decision making about a Fontan operation, and it somewhat complicates its later performance.
When ![]() is large enough to produce serious heart failure early in life, the pulmonary trunk should probably be banded. If increased
is large enough to produce serious heart failure early in life, the pulmonary trunk should probably be banded. If increased ![]() is insufficient to produce important heart failure in the early weeks of life, banding is not performed.
is insufficient to produce important heart failure in the early weeks of life, banding is not performed.
If a pulmonary trunk band is placed too early following birth when distal Rp is still high, the surgeon will be limited by the patient's cyanosis when attempting to tighten the band to an appropriate level. As Rp gradually decreases after placing such a band, it is commonly necessary to reoperate to tighten the band further. Need for readjusting the band can be minimized by appropriately timing the initial banding. The ideal time varies based on individual physiologic characteristics, but the procedure is usually best performed in the second, third, or fourth week of life. In the physiologic setting of low distal Rp and relatively high ![]() , the situation is optimal for placing the band with an appropriate tightness that ensures long-term balance between
, the situation is optimal for placing the band with an appropriate tightness that ensures long-term balance between ![]() and
and ![]() .
.
In this subset, neonates typically present a different set of physiologic considerations from those with concordant ventriculoarterial connection. Because the pulmonary valve is in fibrous continuity with the mitral valve and arises directly from the LV, obstruction to pulmonary blood flow is unusual and unrestrictive ![]() the rule. The aorta arises from the hypoplastic RV, and as a result, the LV must eject through the VSD (bulboventricular foramen) and underdeveloped RV into the aorta. If the outflow tract from LV to aorta and the aortic arch are well developed, the patient can be managed effectively in a fashion similar to that described for tricuspid atresia and concordant ventriculoarterial connection with excessive
the rule. The aorta arises from the hypoplastic RV, and as a result, the LV must eject through the VSD (bulboventricular foramen) and underdeveloped RV into the aorta. If the outflow tract from LV to aorta and the aortic arch are well developed, the patient can be managed effectively in a fashion similar to that described for tricuspid atresia and concordant ventriculoarterial connection with excessive ![]() using a pulmonary trunk band as described in the preceding text.
using a pulmonary trunk band as described in the preceding text.
Tricuspid atresia and discordant ventriculoarterial connection, however, commonly manifests with important obstruction in the systemic circulation. Obstruction typically occurs at two levels: subaortic and aortic arch. Subaortic obstruction is due to a combination of restrictive VSD and muscular obstruction in the underdeveloped incomplete RV. Arch obstruction may be due to discrete aortic coarctation alone, a diffusely hypoplastic arch in combination with discrete coarctation, or interrupted aortic arch. Many patients with DILV have physiology similar to that just described (see Clinical Features and Diagnostic Criteria in Chapter 56 ).
Patients in whom subaortic stenosis becomes evident shortly after birth typically have a small or moderate-sized VSD and often coexisting hypoplasia of the aortic arch with associated aortic coarctation or interrupted aortic arch. Any type of coexisting aortic arch obstruction increases by sevenfold the probability that severe subaortic stenosis will be present. Narrowing may be accelerated by maneuvers that reduce volume load on the heart, such as pulmonary trunk banding or takedown of a systemic–pulmonary arterial shunt at the time of a bidirectional superior cavopulmonary shunt or Fontan procedure. Some studies suggest that subaortic stenosis will ultimately develop in up to 80% of such patients who undergo pulmonary banding early in life.
Even when the VSD is large at the time of a Fontan operation, it may narrow thereafter and subaortic stenosis may appear. Narrowing may occur immediately at the time of the Fontan operation if important volume unloading occurs, either by removing a pulmonary artery band with pulmonary trunk occlusion or by removing a systemic–pulmonary arterial shunt. However, if the patient is undergoing three-stage palliation, it is more likely for the volume load to be dramatically reduced at the time of second-stage bidirectional superior cavopulmonary shunt, and subaortic stenosis is more likely to develop at that time.
In summary, subaortic stenosis is a potential problem in patients in whom the aorta arises above an incomplete ventricle (or outlet chamber). Probability of its appearance is increased by smallness of the VSD (bulboventricular foramen), coexisting aortic arch obstruction, and maneuvers that reduce ventricular volume load. Even in the absence of associated factors, it may still develop. Subaortic stenosis is least likely to occur when the aortic valve is large, the VSD is large, and no arch obstruction is present.
Neonates with this morphology have the potential to be acutely ill in a manner similar to those with hypoplastic left heart physiology; therefore, preoperative stabilization should be similar to that for patients with hypoplastic left heart physiology (see Box 49-1 under “Definition,” and “Preoperative Management” in Chapter 49 ). Even when the VSD is large at birth, it may spontaneously narrow, and subaortic obstruction then becomes apparent.
This technique is described for tricuspid atresia and discordant ventriculoarterial connection with aortic arch obstruction, but is applicable to any patient with univentricular AV connection, aortic arch obstruction, and excessive ![]() ( Fig. 41-21 ). The patient is positioned in right lateral decubitus position, and a standard left posterolateral thoracotomy is made through the fourth intercostal space (see “Alternative Primary Incisions” under Incisions in Section III of Chapter 2 ). Description of the arch repair is similar to that for isolated coarctation in the neonate (see Technique of Operation in Section I of Chapter 48 for details).
( Fig. 41-21 ). The patient is positioned in right lateral decubitus position, and a standard left posterolateral thoracotomy is made through the fourth intercostal space (see “Alternative Primary Incisions” under Incisions in Section III of Chapter 2 ). Description of the arch repair is similar to that for isolated coarctation in the neonate (see Technique of Operation in Section I of Chapter 48 for details).

Following arch reconstruction, a longitudinal incision in the pericardium is made 1 cm anterior to the left phrenic nerve. Depending on extent of the thymus gland, modest mobilization of its left lobe may be necessary. Once the pericardium is opened, the pulmonary trunk is identified in the transposed position, posterior and to the left of the ascending aorta. (In patients with DILV and L-transposition, the pulmonary trunk is posterior and to the right.) The plane between adjacent walls of ascending aorta and pulmonary trunk are carefully dissected, gaining circumferential access around the pulmonary trunk midway between its sinutubular junction and origin of the RPA. The RPA origin is particularly difficult to visualize through a left thoracotomy incision; however, it must be carefully located before positioning the band. Following this, details related to placing and adjusting the band are similar to those described previously (see “ Pulmonary Trunk Banding ” under Technique of Operation earlier in this section) for placing a pulmonary artery band through a median sternotomy (see Fig. 41-17 ).
This technique is described for tricuspid atresia and discordant ventriculoarterial connection with subaortic and arch obstruction, but is applicable to all forms of univentricular AV connection with subaortic and arch obstruction ( Fig. 41-22 ). After anesthesia induction, placing indwelling peripheral arterial and venous catheters, and supine positioning, a median sternotomy is performed (see “Preparation for Cardiopulmonary Bypass” in Section III of Chapter 2 ). The thymus gland is subtotally removed and the pericardium opened anteriorly over the heart. The plane between pulmonary trunk and ascending aorta is carefully dissected and the entire aortic arch mobilized, including the first 1 to 2 cm of each arch vessel. The central pulmonary artery, ductus arteriosus, and descending aorta to the level of the first pair of intercostal arteries are also mobilized. The patient is then prepared for CPB. If the aortic arch is hypoplastic but not preocclusive (>2-3 mm in diameter), adequate perfusion on CPB can be achieved by cannulating the aortic system alone using a 6F or 8F aortic cannula. If the ascending aorta is of adequate size, it can be cannulated directly (as shown in Fig. 41-22 ), or alternatively, if it is hypoplastic, the base of the brachiocephalic artery can be cannulated. The arterial cannula (or cannulae) is secured in place with standard purse-string sutures and snares. If the aortic arch is interrupted, or is in continuity but with preocclusive narrowing at the isthmus and coarctation, dual arterial cannulation of the proximal pulmonary trunk and the aortic system is performed. (This variation is not shown in Fig. 41-22 , but see Technique of Operation in Chapter 48 for a detailed description of cannulation technique and CPB management when the arch is interrupted.) Temporary occlusion of branch pulmonary arteries is achieved either with snares or vascular clamps if perfusion is performed through the pulmonary trunk and ductus arteriosus to the descending thoracic aorta.

Venous cannulation is through a purse string in the right atrial appendage. After cannulation, CPB is instituted and preferably carried out using continuous antegrade cerebral perfusion (see Fig. 41-22 ). Alternatively, some surgeons prefer to use circulatory arrest (see “Technique in Neonates, Infants, and Children” in Section IV of Chapter 2 ). These CPB management techniques are also discussed in detail in the description of the Norwood procedure for hypoplastic left heart physiology (see “Norwood Procedure Using Continuous Perfusion” under Technique of Operation in Chapter 49 ). In particular, the techniques described in Chapter 49 for performing continuous antegrade cerebral perfusion are widely applicable to all forms of neonatal arch obstruction with associated hypoplastic arch and ascending aorta. Once the target perfusion temperature is reached, antegrade cerebral perfusion established, and cardiac arrest induced with cardioplegia, the obstructed aortic arch is addressed, as described in Fig. 41-22 .
If the aortic arch is in continuity but hypoplastic, the aortic isthmus is ligated with a 5-0 polypropylene suture, and ductus and coarctation tissue distal to it are removed (see Fig. 41-22, A ). A small vascular clamp can be placed across the descending aorta at the level of the first set of intercostal vessels to stabilize the aorta and deliver it into the anterior mediastinum. A longitudinal incision is made in the posterior aspect of the upper ascending aorta and proximal aortic arch (see Fig. 41-22, A ), and the descending aorta is anastomosed to this incision with a running 7-0 monofilament absorbable suture, thereby repairing the arch obstruction ( Fig. 41-22, B ). In the case of true aortic arch interruption, the isthmus ligature is not necessary, and arch repair otherwise proceeds as described.
With the arch obstruction addressed, antegrade cerebral perfusion is terminated and full-body bypass is again established (see “Norwood Procedure Using Continuous Perfusion” under Technique of Operation in Chapter 49 ). Attention is turned to the proximal pulmonary trunk–to-aortic anastomosis. This can be accomplished in several ways, one of which is described in detail in Fig. 41-22, B . Although unusual in neonates with tricuspid atresia, if preoperative evaluation suggests that potential or real obstruction exists at the atrial septum, the right atrium is opened during continuous bypass, and the septum primum is removed to create an unobstructed atrial communication. During this maneuver, the single venous cannula in the right atrium must be temporarily clamped, and cardiotomy suction devices used within the two vena caval orifices to provide exposure for the atrial septal resection. The right atriotomy is then closed with a running 6-0 polypropylene monofilament suture. Venous drainage via the right atrial cannula is then reestablished and rewarming begun.
An appropriately sized expanded PTFE tube graft is then used to create a modified Blalock-Taussig shunt (see Fig. 41-22, B ). Details of the shunt procedure are similar to those described earlier in this section for isolated neonatal systemic–pulmonary arterial shunt.
Principles of rewarming and separation from bypass are those described in “Completing Cardiopulmonary Bypass” in Section III of Chapter 2 . Following separation from CPB, management considerations with regard to pharmacology, physiology, and sternal wound (immediate or delayed sternal closure) are the same as described in the management of hypoplastic left heart physiology following the Norwood procedure (see “Special Features of Postoperative Care” in Chapter 49 ).
The Norwood anastomosis used in this setting is a slight modification of the procedure used in first-stage repair of patients with classic hypoplastic left heart physiology. It combines the Damus-Kaye-Stansel anastomosis with extensive augmentation (enlargement) of the hypoplastic aortic arch and upper descending thoracic aorta (see Chapter 49 ). It is important to emphasize that the augmentation patch used is of a slightly different shape from that described for the typical patient with hypoplastic left heart physiology. This is necessary because with either tricuspid atresia and discordant ventriculoarterial connection, or with double inlet ventricle with L-loop and discordant ventriculoarterial connection, orientation of the great arteries is different from that in patients with normally related great arteries (i.e., found in aortic atresia and other classic forms of hypoplastic left heart physiology).
This technique is described for patients with tricuspid atresia and discordant ventriculoarterial connection with subaortic obstruction at the bulboventricular foramen (VSD) or incomplete RV, but is applicable to all patients with univentricular AV connection in whom the aorta arises from an incomplete ventricle. Other lesions that result in a similar problem include DILV with discordant ventriculoarterial connection, and mitral atresia with concordant ventriculoarterial connection (see Chapter 56 ).
Patient preparation, median sternotomy, and cardiac exposure are similar to that described in the preceding text. In the unusual case that there has been no previous surgery, upon opening the pericardium, it is immediately noticed that aorta and diminutive RV are anterior. More commonly, previous surgery (including previous median sternotomy) has been performed. Caution should be exercised upon repeat median sternotomy, because the anterior aorta may be in close proximity to the posterior sternal table. The patient is prepared for CPB by placing purse strings in the ascending aorta just below the brachiocephalic artery origin and in SVC and IVC. Aortic and bicaval cannulation is then performed in standard fashion, the patient is placed on CPB, and moderate hypothermia is achieved. The aorta is clamped, and cardioplegia is administered through the aortic root (see “Single-Dose Cold Cardioplegia in Neonates and Infants” in Chapter 3 ).
The VSD is best approached through an incision in the incomplete ventricle (outlet chamber) just below the aortic valve ( Fig. 41-23, A ). This incision is later closed by an enlarging patch. Alternatively, the VSD may be approached through the aorta; however, this exposure may be limited because the aortic valve and aorta are typically hypoplastic. Some have considered the risk of surgically induced heart block to be high in this procedure, but risk is substantially reduced using currently available knowledge about the location of the conduction tissue. When the VSD is observed through an incision in the outlet chamber (morphologic RV), the relationship of the conduction system to the VSD is the same as with any conoventricular VSD, with the conduction tissue at the posterior inferior rim of the defect. This is true whether the underlying morphology is tricuspid atresia and discordant ventriculoarterial connection, or double inlet single LV and discordant AV and ventriculoarterial connections. Confusion regarding this issue has arisen because the conduction system appears to be positioned on the “anterior” rim of the VSD when viewed from the perspective of the main ventricular chamber of either tricuspid atresia and discordant ventriculoarterial connection or DILV and discordant AV and ventriculoarterial connections. This confusion is the result of difficulty in conceptualizing the spatial arrangement of the two ventricular chambers and ventricular septum with this exposure, which involves an atrial incision, substantial rotation of the heart, and visualization of the VSD through an AV (typically mitral) valve.

The preferred approach is a vertical incision made in the outlet chamber free wall directly in line with the course of the ascending aorta (see Fig. 41-23, A ). This incision should be made only after all major coronary artery branches are identified, because the incision should not cut across any of these. Once the outlet chamber has been entered, the VSD is identified and a full-thickness wedge of septum is removed from the anterior and anterior apical aspects of the rim of the defect ( Fig. 41-23, B ).
Obstructing muscle bundles within the outlet chamber are excised. The outlet chamber is enlarged by closing the ventriculotomy with an enlarging patch, typically of polyester or glutaraldehyde-treated pericardium, using running monofilament suture ( Fig. 41-23, C ). The process of separation from bypass and chest closure is standard.
These are detailed under Special Features of Postoperative Care in Chapter 49 .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here