Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() Additional content is available online at Elsevier eBooks for Practicing Clinicians
Additional content is available online at Elsevier eBooks for Practicing Clinicians
Peripheral vascular disease is a general term that includes pathologic processes affecting arteries, veins, and lymphatics (see Chapter 43 ). This chapter focuses on catheter-based endovascular treatment of large- and medium-sized arteries predominantly affected by atherosclerosis, as well as large-vein obstruction secondary to chronic disease. Although peripheral artery disease (PAD) refers to lower limb arterial disease, sometimes the term is used to describe disease in the large and medium arteries of the upper limbs, neck, and aortomesenteric arteries. The incidence and prevalence of PAD increase with age and with other risk factors for atherosclerosis. Thus, these two demographic forces will likely lead to a global increase in PAD (see Chapter 2 ).
Increasing awareness of PAD, the impact that PAD has on cardiovascular (CV) risk and quality of life, and the rapid development of percutaneous techniques for revascularization continue to accelerate the number of endovascular procedures for PAD. Appropriate use of this expensive technology requires a clear understanding of the goals of medical and revascularization therapies.
Chronic PAD may be asymptomatic or may manifest as claudication, critical limb ischemia (CLI), or embolic infarction of a distal organ (e.g., stroke). Asymptomatic disease is common. In the lower extremities, asymptomatic disease occurs in at least half and in as many as 80% of patients with abnormal functional test results indicative of obstructive arterial disease (e.g., abnormal ankle-brachial index [ABI]). Even asymptomatic disease indicates elevated CV risk. These considerations warrant intensive modification of atherosclerosis risk factors as a prime goal of therapy to reduce the risk for myocardial infarction (MI) and stroke, the most common causes of death in patients with PAD. , , ,
Claudication classically refers to leg discomfort, weakness, or pain related to exercise and relieved by rest, but it also describes discomfort in the upper limbs caused by effort-related ischemia. Claudication affects function (the ability to walk or use a limb) and quality of life. Therefore, treatment of claudication aims to improve function and reduce discomfort at the maximum level of activity desired by a patient. Stopping cigarette smoking and starting a regular walking regimen are the two most important lifestyle interventions for claudication. Supervised exercise training consisting of 1-hour sessions two to three times a week for 12 weeks is particularly useful at improving walking, with or without endovascular intervention (see Chapter 43 ). , , , Together, these interventions reduce the mechanisms responsible for the progression of disease and favorably change arterial biologic state, including vasodilator function, muscle metabolism, and angiogenesis. , , , , It is important to tell patients that the pain or discomfort associated with claudication is not harmful, and that once this discomfort abates with rest, they should continue to push their activity again to improve endurance. Revascularization strategies aim to improve arterial blood flow in obstructed large- and medium-sized arteries when noninvasive therapies fail. Catheter-based interventions, when indicated, should be deployed together with lifestyle and medical treatment. , ,
Critical limb ischemia , also referred to as critical limb-threatening ischemia, refers to PAD with ischemic pain at rest or tissue loss (e.g., ulcer or gangrene). , , This scenario has clinical urgency because of near-term risk for limb jeopardy requiring major amputation. Major amputation in the lower limbs refers to amputation at or above the level of the ankle and requires a prosthesis for the patient to walk. Amputation is disfiguring and at higher levels (above versus below knee amputation) has greater impact on functional independence of the patient. In contrast, minor amputations (e.g., toe or transmetatarsal) usually have little impact on the patient’s ability to walk. Catheter-based therapies for CLI are used to improve blood flow and heal ischemic tissue, to salvage the limb (prevent major amputation), or to enable a lower level of amputation that might have less impact on the patient’s ability to walk.
Acute limb ischemia (ALI) is a sudden loss of limb perfusion (defined as within 14 days) typically caused by embolus or in situ thrombus. Thrombolysis may be indicated for acute thrombosis with a threatened but viable extremity, but an immediately threatened limb (e.g., with sensory or early motor deficits) is more often treated by surgical revascularization, , , which offers more rapid reperfusion, the ability to débride devitalized tissue, and the opportunity to relieve compartment syndromes (see Chapter 43 ).
Cervical carotid, vertebral, and subclavian disease, although often asymptomatic, can lead to artery-to-artery embolism with transient ischemic attack (TIA) and stroke. The risk for major stroke is high shortly after a symptomatic event but declines to the level of asymptomatic disease after approximately 3 months. Mesenteric and renal artery disease affects organ function. Chronic ischemia of the gut causes postprandial abdominal discomfort and food avoidance leading to weight loss, but it may progress to frank mesenteric infarction with a high mortality rate. Renal artery stenosis can precipitate hypertensive crises associated with pulmonary edema, hypertension resistant to treatment, and rapidly worsening renal dysfunction.
Symptomatic disease that threatens a distal organ (e.g., CLI, TIA, mesenteric angina) justifies a more aggressive approach because these manifestations entail the highest risk for functional loss and death without treatment. PAD associated with less-threatening clinical scenarios (e.g., claudication) may allow a less aggressive approach, with more time to assess the response to lifestyle and medical therapies ( Fig. 44.1 ). There is rarely justification for catheter-based or surgical revascularization of asymptomatic lower or upper limb PAD, mesenteric disease, or subclavian or vertebral artery disease. Revascularizing asymptomatic extracranial carotid disease beyond medical therapy has uncertain value, although guidelines support such interventions for patients at higher risk for stroke and low risk for periprocedural adverse events. ,
Increasingly, well-controlled randomized-controlled studies are used to evaluate endovascular treatment of PAD and venous disease. However, many studies are single arm, and most focus on patency (lack of restenosis) and repeated revascularization over a relatively short period. Although these endpoints provide information on the mechanisms likely to lead to improved control of symptoms, function, quality of life, and tissue preservation, they do not provide direct guidance on symptoms and function in patients with claudication or CLI. Interventionalists should recognize the limitations in many studies and encourage future studies to address patient-oriented endpoints and adjudicated CV outcomes.
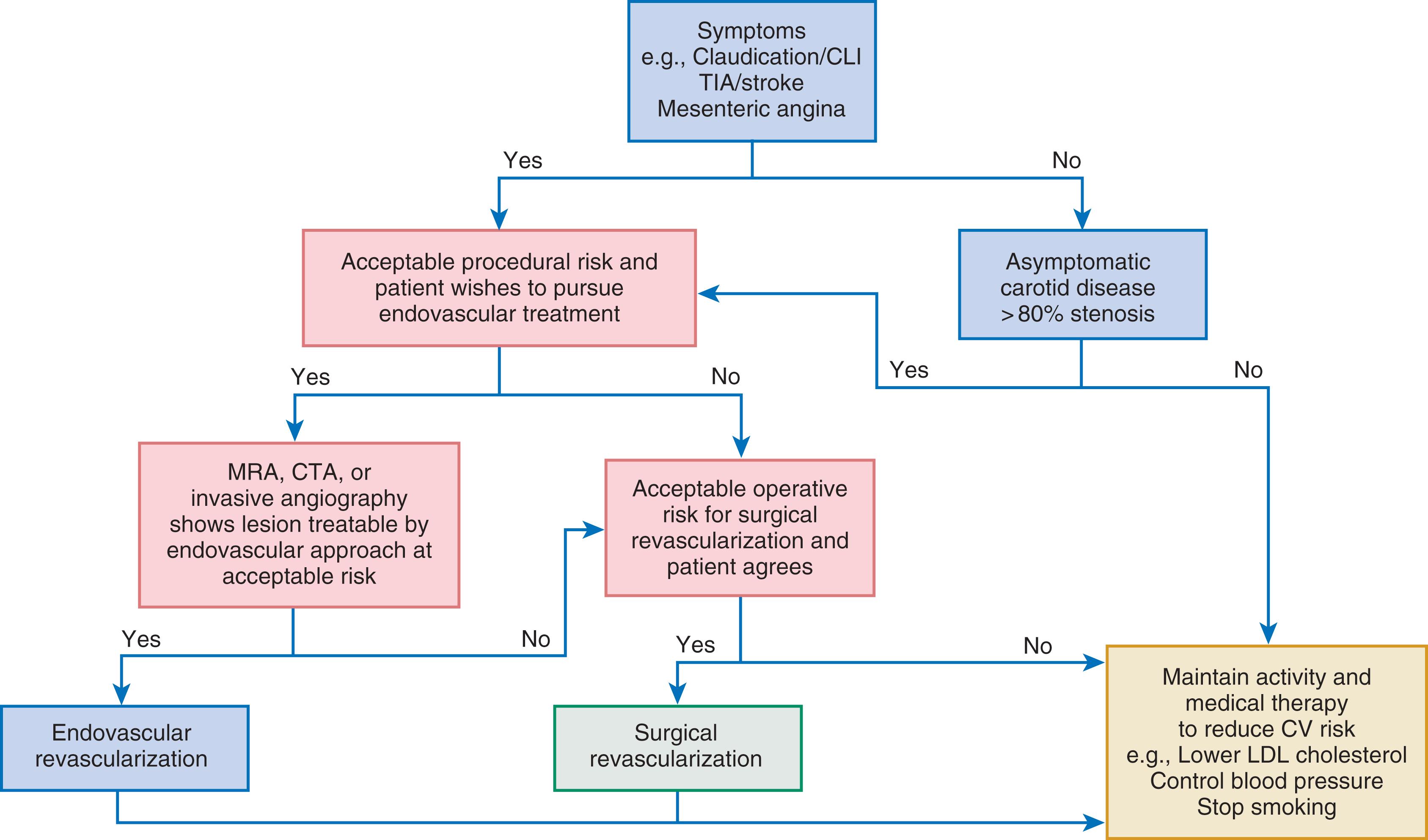
Balloon angioplasty remains the mainstay of endovascular intervention for PAD and venous disease ( Fig. 44.2 ). Angioplasty remodels the artery by expansion and accommodates the atherosclerotic plaque to expand the vessel lumen. This procedure usually causes dissection of the plaque that may or may not impair blood flow. Angioplasty is limited in the short term by acute recoil of the artery and flow-limiting dissections, which may cause abrupt closure of the artery. In the intermediate time frame, overexuberant neointimal hyperplasia and negative remodeling of the artery may lead to symptomatic restenosis. Despite these limitations, balloon angioplasty can achieve durable results, particularly with shorter lesions, and is less likely than stenting to obstruct side branches associated with the lesion. Most operators use prolonged inflations (at least 1 minute or more). Both rapid-exchange and over-the-wire platforms are available, as well as short and long shaft lengths for lesions close to or farther from the access site.
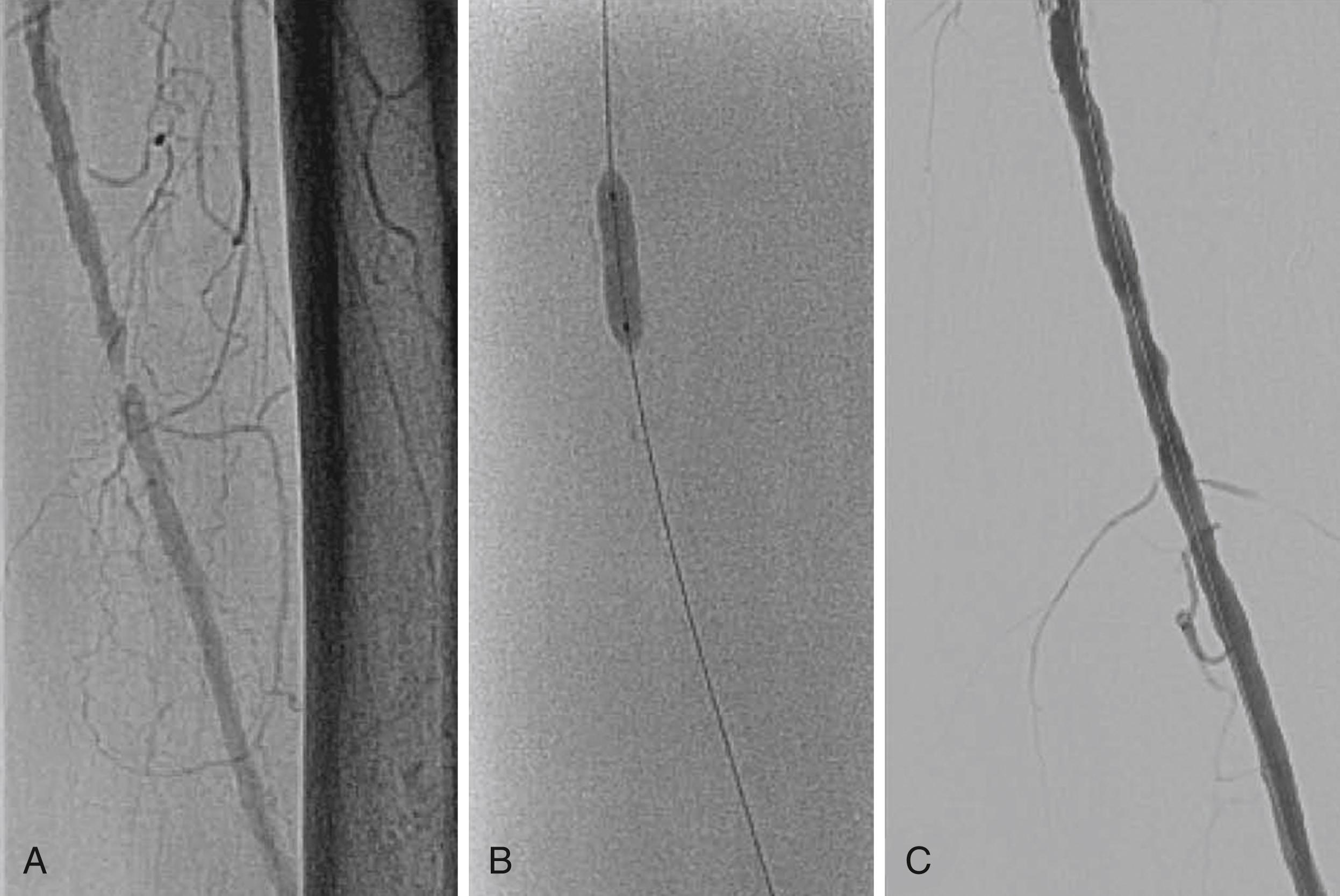
Bare-metal stents (BMSs) come in two types: balloon-expandable stents ( Fig. 44.3 ) and self-expanding stents ( eFig. 44.1 ). Stent implantation requires aspirin therapy and an adenosine receptor antagonist (i.e., clopidogrel), although the evidence for dual-antiplatelet therapy (DAPT) is largely derived by extrapolation from the coronary stent literature.
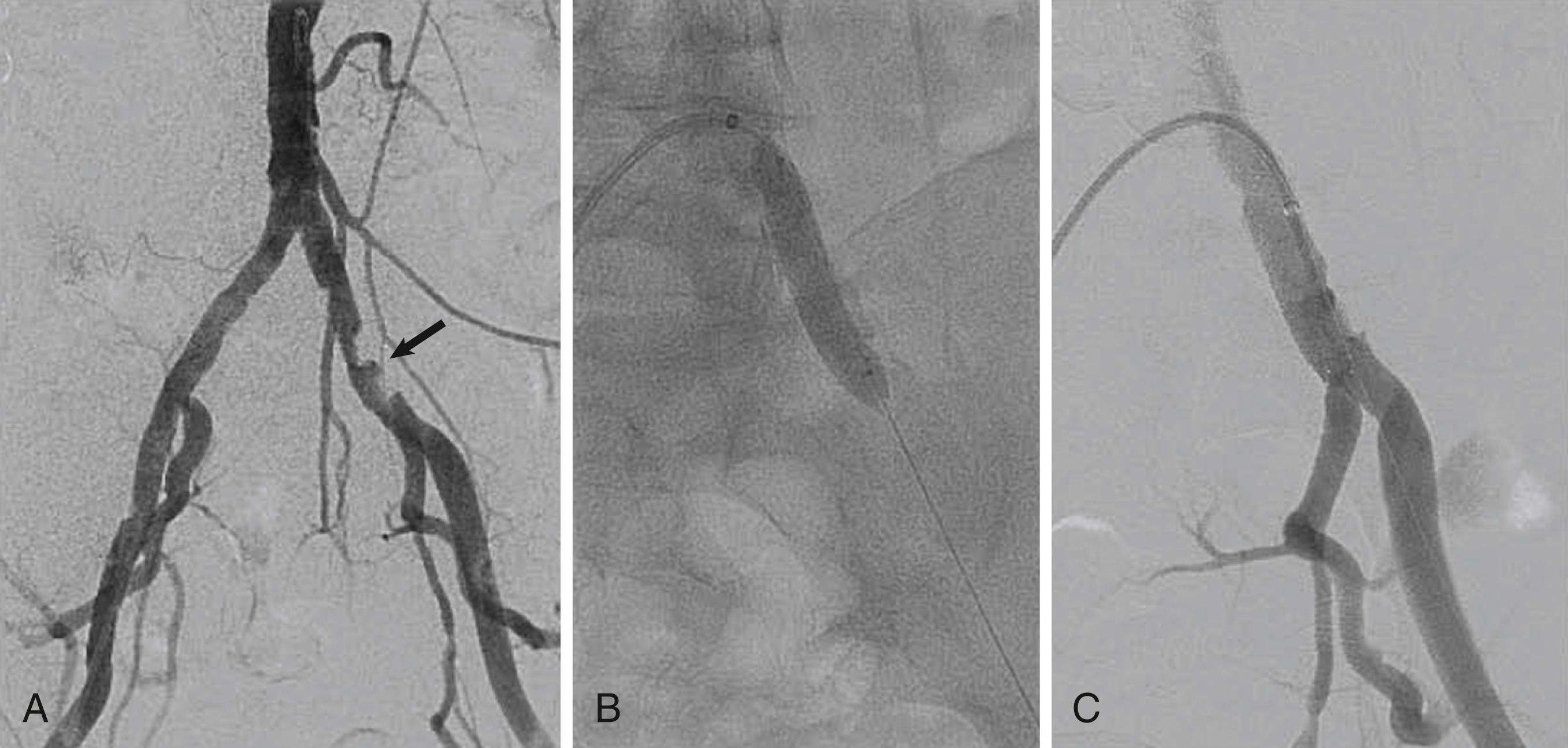
Balloon-expandable stents have greater radial strength and are less likely to move on deployment, which is important for ostial placement. Such stents can be crushed by external compression and are therefore avoided outside the torso. They are sometimes used to treat tibial disease, but only for CLI, for which long-term patency may be less of an issue once tissue healing has occurred.
Self-expanding stents were originally made of stainless steel but are now usually made of nitinol. Nitinol stents reexpand on compression and are therefore used outside the torso, where external compression is more likely to occur. They may also be used in tortuous arteries, where they probably conform better than balloon-expandable stents. Their lower radial strength, however, increases the risk for recoil. Contemporary self-expanding stent designs are more durable and less likely to fracture than older designs. Nitinol stents cannot be overdilated if the stent is undersized for the artery, which may lead to stent malapposition or even embolization.
Drug coated self-expanding stents using a polymer or polymer-free coating of paclitaxel offer lower rates of restenosis than bare-metal self-expanding stents. The 5-year follow-up of the Zilver PTX study demonstrated a sustained benefit from self-expanding drug-eluting stents (DESs) over balloon angioplasty and BMSs on freedom from clinical symptoms of ischemia (80% versus 59%) and repeat revascularization (66% versus 43%) in femoral-popliteal arteries. In the IMPERIAL study, a polymer-coated paclitaxel stent had similar 12 months of efficacy and safety outcomes to a polymer-free paclitaxel stent, and a single-arm cohort study suggested no “catch-up” target vessel revascularization over 3 years with this device. In one trial, drug-coated self-expanding stents had similar patency and target lesion revascularization at 12 months with lower complication rates and shorter lengths of hospital stay than surgical bypass with prosthetic graft. The duration of DAPT required for these stents is uncertain, but recent randomized trials have generally used 2 to 6 months of treatment with an adenosine receptor antagonist. ,
Balloons coated with antirestenosis agents (drug-eluting balloons) are designed to decrease restenosis without the use of stents. This technology uses a non–stent-related method to deliver drugs such as paclitaxel into the arterial wall after angioplasty or atherectomy. Compared with plain balloon angioplasty, drug-coated balloons have less restenosis and repeat revascularization in the femoral-popliteal arteries, and similar patency to drug-coated self-expanding stents. , Drug-coated balloons also offer a lower risk of restenosis at 1 year compared with plain balloon angioplasty for treating in-stent restenosis lesions. Late follow-up of two randomized trials at 2 and 5 years shows a sustained benefit on patency and repeat revascularization with drug-coated versus plain balloon angioplasty in femoral-popliteal arteries, without concerns of aneurysm or late stenoses. , The duration of DAPT with drug-coated balloons in the femoral-popliteal arteries is uncertain but varies between 1 and 6 months in most randomized trials.
The effect of drug-coated balloons in below-knee angioplasty, primarily for CLI, is less certain. A meta-analysis of eight randomized controlled trials (RCTs) compared drug-coated balloon angioplasty to plain balloon angioplasty in tibial arteries. Overall, there was a significant decrease in target vessel repeat revascularization with drug-coated balloons, but an increased risk of death or amputation, although with significant heterogeneity in some of the outcomes in this meta-analysis. Currently the paclitaxel drug-coated technologies are under increased scrutiny due to some meta-analyses suggesting higher rates of all-cause mortality 3 to 5 years after treatment (see later).
Lesion length is a key determinant of restenosis, , and a meta-analysis suggests that long lesions have less restenosis and need for target revascularization with paclitaxel drug-coated balloons and stents. However, in 2018 a group-level meta-analysis of randomized trials suggested that patients treated with paclitaxel drug-coated balloons or stents for femoral-popliteal disease had higher rates of mortality 2 to 5 years after their procedure compared with non-paclitaxel balloons and stents. In a subsequent meta-analysis, drug-coated balloons for infrapopliteal disease were associated with increased all-cause mortality two or more years after treatment. Meta-regression analyses in both studies suggested greater risks from devices with higher doses of paclitaxel coating. In response to these reports, investigators of the clinical trials reported patient-level long-term outcomes data and meta-analyses refuting the higher late mortality risk 3 to 5 years after treatment with DESs or balloons in the femoral-popliteal artery. Analyses of large administrative databases from Medicare/Medicaid in the United States and Germany suggested lower mortality and amputation at 5 years after drug-coated balloons or stents for CLI or intermittent claudication. Furthermore, late mortality risks were not observed with drug-coated balloons used to treat coronary artery disease.
The group-level meta-analyses were limited by the relatively small number of deaths in studies that were not powered to assess mortality, and the lack of trials with long follow-up beyond 1 to 2 years. Until more data are available, the Federal Drug Agency recommends discussing the potential long-term risks with patients, using alternative technologies if possible, and reserving paclitaxel technologies for patients at very high risk of restenosis. Currently, drug-coated devices that deliver the limus class of drugs are under investigation and may replace paclitaxel devices if they have improved efficacy and safety.
Stents covered with or sandwiching a polymer such as polytetrafluoroethylene (PTFE) are very useful for treating perforations related to endovascular treatment or excluding aneurysms ( eFigs. 44.2 and 44.3 , and ![]() ). Results from randomized trials and meta-analyses of covered stents compared with BMSs are inconsistent for the outcome of restenosis at 1 year and found no difference in limb salvage or survival. , , In one series, covered stents that cross the knee joint were associated with higher rates of occlusion and major amputation than those deployed above the knee (34% versus 10%). Potential disadvantages of covered stents include unintentional occlusion of important branch vessels, concerns about the risk for late stent thrombosis, and whether restenosis was merely delayed rather than prevented.
). Results from randomized trials and meta-analyses of covered stents compared with BMSs are inconsistent for the outcome of restenosis at 1 year and found no difference in limb salvage or survival. , , In one series, covered stents that cross the knee joint were associated with higher rates of occlusion and major amputation than those deployed above the knee (34% versus 10%). Potential disadvantages of covered stents include unintentional occlusion of important branch vessels, concerns about the risk for late stent thrombosis, and whether restenosis was merely delayed rather than prevented.
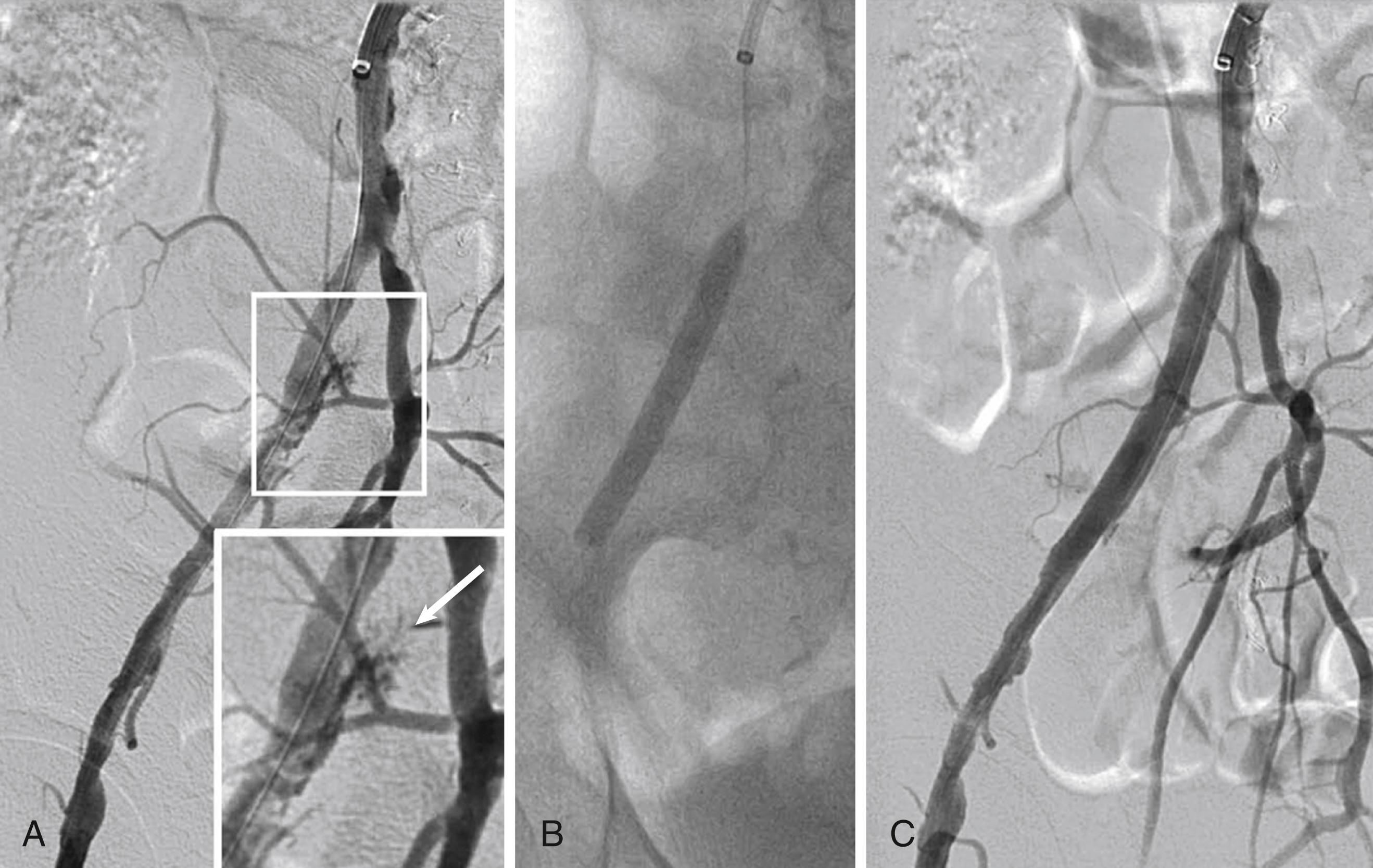
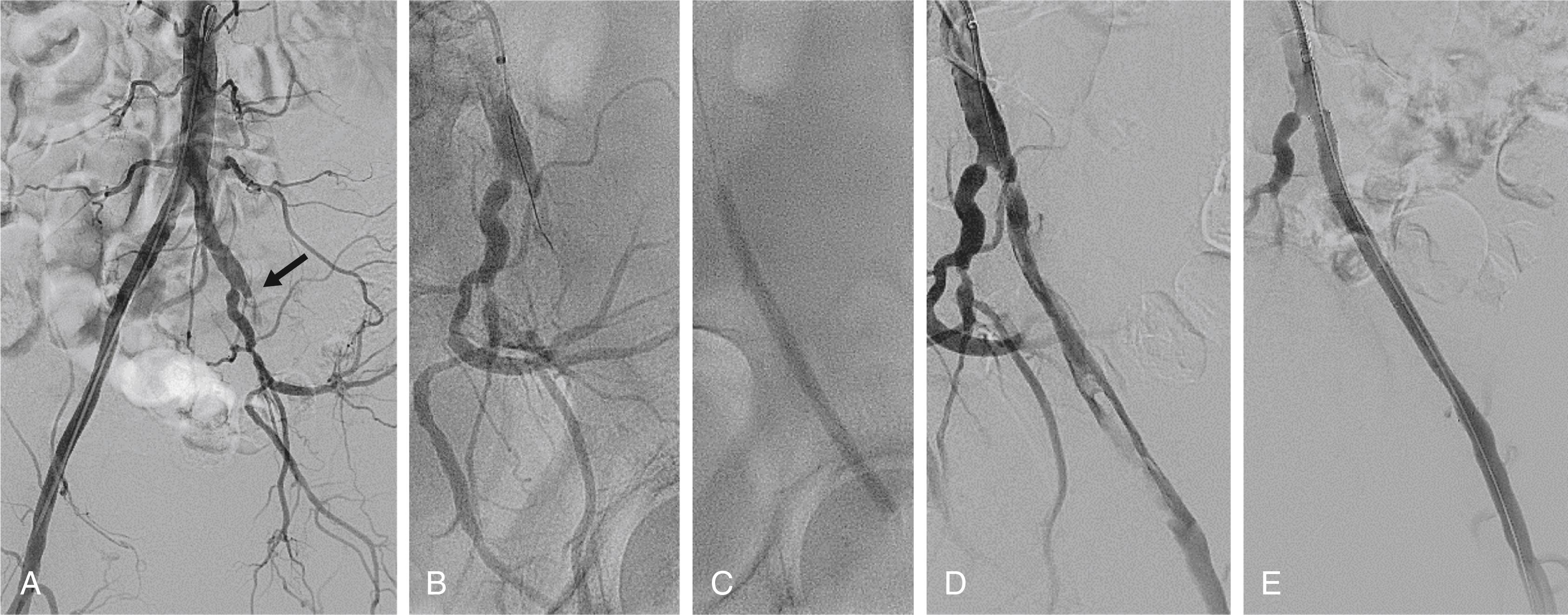
Catheter-directed thrombolysis is an important adjunctive therapy for arterial thrombosis, stent thrombosis, and occlusive thrombotic venous disease. Thrombolysis may be indicated for acute thrombosis with a threatened but viable extremity, but an immediately threatened limb (e.g., with sensory or early motor deficits) is more often treated by surgical revascularization. , , Much of the experience with catheter-based thrombolysis comes from its use for ALI, venous thrombosis, or pulmonary embolism. It serves as an adjunctive treatment of semiacute manifestations such as peripheral stent thrombosis. Long-term results tend to be better when thrombolysis reveals an anatomic stenosis that probably precipitated the thrombosis and is treatable, for example, by repeated angioplasty.
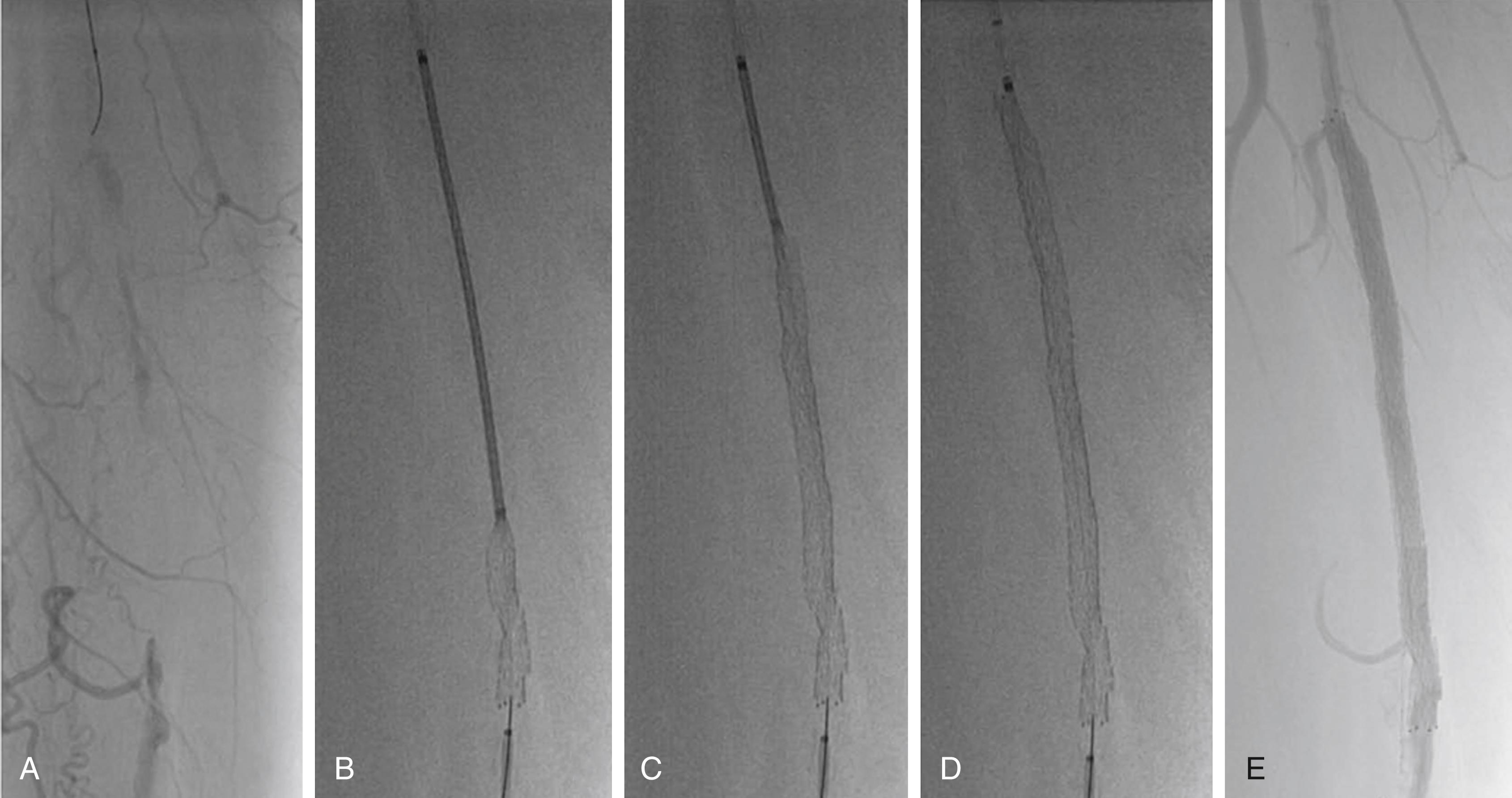
Catheter-directed thrombolysis is more effective than intravenous thrombolysis only if an infusion catheter (with multiple infusion holes) is inserted into the thrombosed vessel ( Fig. 44.4 and ![]() ). It is also less effective if given more than 14 days after thrombosis. , , Typically, the infusion continues for 12 to 24 hours, because treatment over 48 hours is associated with depletion of circulating fibrinogen and a higher risk for major bleeding. A meta-analysis comparing catheter and mechanically assisted thrombolysis versus surgical thrombectomy showed no difference in limb salvage but higher bleeding rates with thrombolysis. Lower dose thrombolysis regimens may offer a lower risk of bleeding complications, but may require a longer treatment duration. Catheter-based thrombolysis with or without angioplasty or stenting also reduces the incidence of post-thrombotic syndrome in patients with proximal (iliac) deep venous thrombosis (DVT), and it is used as adjunctive therapy for massive pulmonary emboli (see Chapter 87 ).
). It is also less effective if given more than 14 days after thrombosis. , , Typically, the infusion continues for 12 to 24 hours, because treatment over 48 hours is associated with depletion of circulating fibrinogen and a higher risk for major bleeding. A meta-analysis comparing catheter and mechanically assisted thrombolysis versus surgical thrombectomy showed no difference in limb salvage but higher bleeding rates with thrombolysis. Lower dose thrombolysis regimens may offer a lower risk of bleeding complications, but may require a longer treatment duration. Catheter-based thrombolysis with or without angioplasty or stenting also reduces the incidence of post-thrombotic syndrome in patients with proximal (iliac) deep venous thrombosis (DVT), and it is used as adjunctive therapy for massive pulmonary emboli (see Chapter 87 ).
Any thrombolysis regimen increases risk for fatal or major bleeding. Absolute contraindications to thrombolysis include (1) a cerebrovascular event less than 2 months previously, (2) active bleeding, (3) gastrointestinal bleeding less than 10 days previously, and (4) neurosurgery (intracranial or spinal surgery) or trauma less than 3 months previously. Relative contraindications include (1) cardiopulmonary resuscitation less than 10 days previously, (2) nonvascular surgery or trauma less than 10 days previously, (3) uncontrolled hypertension (sustained systolic blood pressure [BP] >180 mm Hg or diastolic BP >110 mm Hg), (4) puncture of a noncompressible vessel, (5) intracranial tumor, and (6) recent eye surgery.
Mechanical and aspiration thrombectomy are used with and without thrombolytic agents. At this stage, the quality of studies comparing these methods to catheter-based thrombolytic infusion does not allow any firm conclusions to assess their incremental benefit. , Catheter aspiration thrombectomy designed initially for aspirating thrombi in coronary arteries uses 5 to 7 French (F) catheters with a rapid-exchange port to direct the catheter to the thrombus and an aspiration port to aspirate the catheter with a large syringe (e.g., Export Advance catheter, Medtronic, Minneapolis; Pronto catheter, Vascular Solutions, Minneapolis). These catheters can aspirate smaller thrombi but are generally inadequate for a large burden of thrombus (e.g., long femoral stent thrombosis). The Inari flow retriever system (Inari Medical, Irvine, CA) uses a much larger catheter (up to 24F) and is designed mainly for venous thromboembolism, but case reports show large thrombi extracted with this system.
The Penumbra Indigo (Penumbra, Almeda, CA) aspiration system uses a mechanical aspirator and a range of 3 to 8F catheters to generate a greater suction than manual aspiration catheters. This was initially developed for aspirating intracranial arterial emboli, but has peripheral vascular catheters designed to aspirate peripheral arterial and venous thrombi.
Other mechanical thrombectomy devices break up a clot before aspiration and may use adjunctive locally delivered thrombolytic agents before extraction. These include the AngioJet device (Boston Scientific, Marlborough, MA), which uses a Venturi effect to break up and aspirate a thrombus. The Jetstream (Boston Scientific, Marlborough, MA) is primarily a rotational atherectomy device but also aspirates debris and is used for atherectomy and aspiration of a thrombus. Mechanical thrombectomy is a more rapid treatment than catheter-directed thrombolysis; embolization can occlude the distal arterial bed and lead to infarction and tissue loss, although combination with an embolic protection device might theoretically reduce this risk.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here