Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Acknowledgments: The authors wish to acknowledge the contributions of Professors Rod Hentz and Kazuteru Doi, who wrote an earlier rendition of this chapter on brachial plexus injury in adults, and those of Marie-Noëlle Hébert-Blouin, who assisted with a previous version of the chapter.
In the early 19th century, well before microsurgical equipment and techniques were developed, surgeons published encouraging results of brachial plexus reconstruction. Subsequently, others reported poor results and high complication rates. Little else was published on reconstruction of the brachial plexus until the 1970s and 1980s, when surgeons began to apply the fascicular nerve grafting techniques described by Millesi to birth and traumatic injuries of the brachial plexus. These pioneers established indications and techniques for plexus reconstruction and reported better recovery in operated patients than in those observed without surgical reconstruction.
In the late 1980s and early 1990s, exciting diagnostic and microsurgical advances further advanced the field. New surgical procedures and experimental nonoperative treatments were introduced internationally. In the late 1990s, aggressive reconstructions using extraplexal sources for reinnervation of vascularized muscle transfers were reported. In the past 20 years, the role of nerve transfers has expanded. The introduction of novel distal nerve transfers has changed the way in which brachial plexus surgery is being performed. Although full recovery of function after brachial plexus reconstruction still remains unachievable, the exciting developments of the past two decades have considerably improved surgical outcomes, and future directions for research are promising.
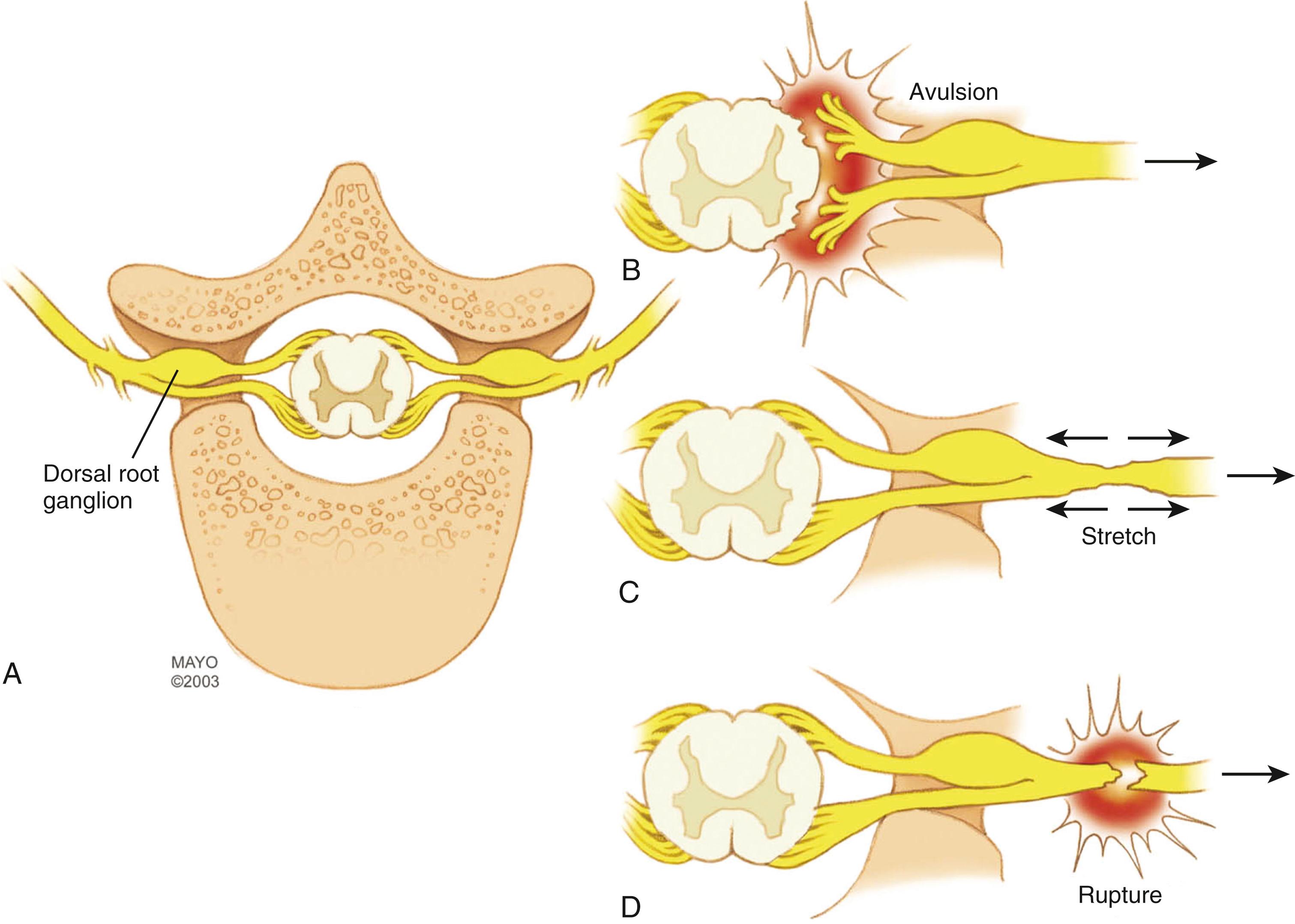
The brachial plexus is formed from five cervical spinal nerves, typically C5, C6, C7, C8, and T1 ( Fig. 34.1 ), and is generally divided into five anatomic sections: nerves, trunks, divisions, cords, and terminal branches. The spinal nerves are formed by coalescence of the ventral and dorsal nerve rootlets as they pass through the spinal foramen ( Fig. 34.2 ). The dorsal root ganglion holds the cell bodies of the sensory nerves and lies within the confines of the spinal canal and foramen. Each spinal nerve sends a small dorsal ramus to the paraspinal musculature. The remaining large ventral rami of the lower four cervical (C5-C8) and T1 spinal nerves emerge from the foramina as the first section of the brachial plexus.
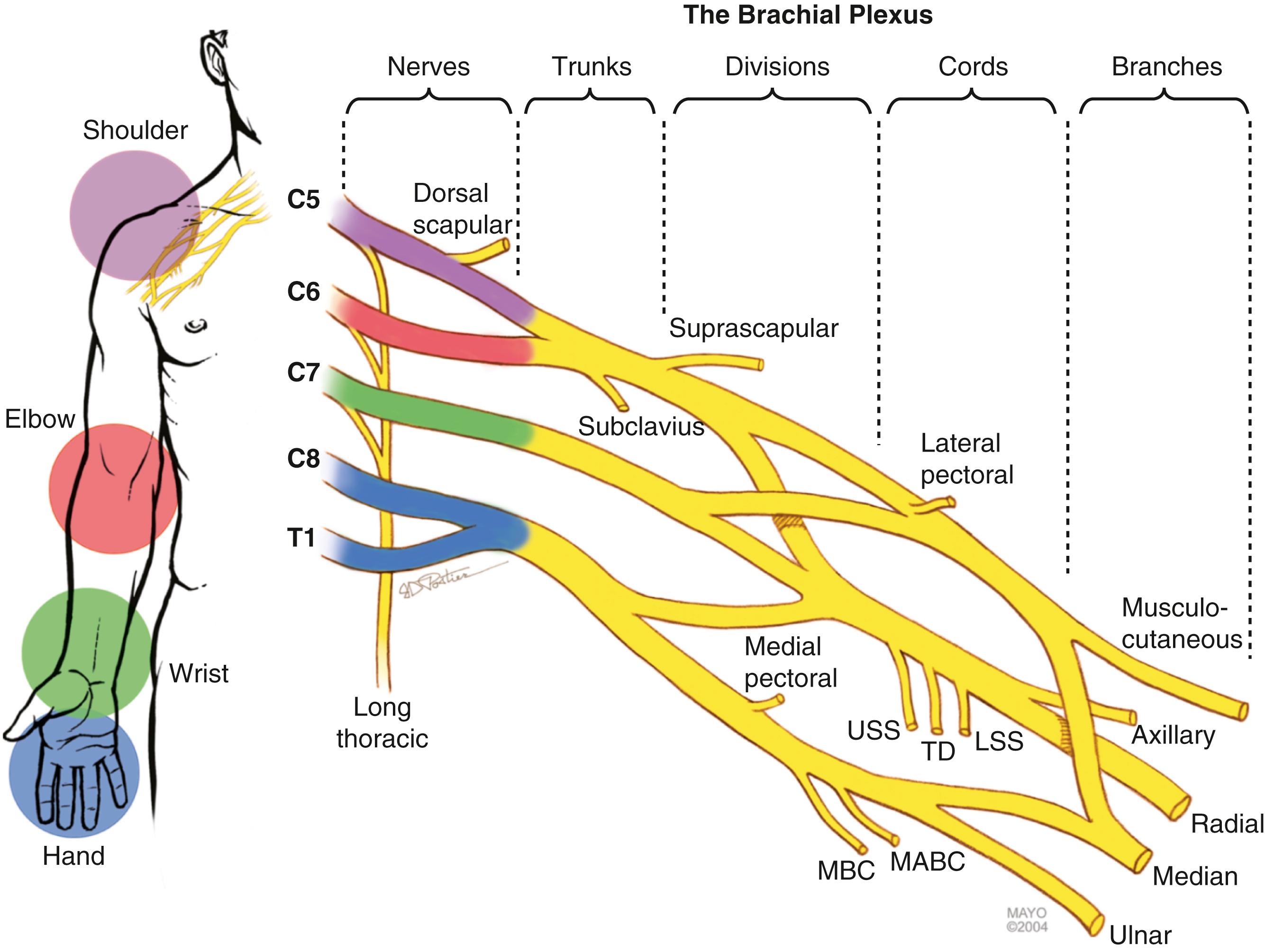
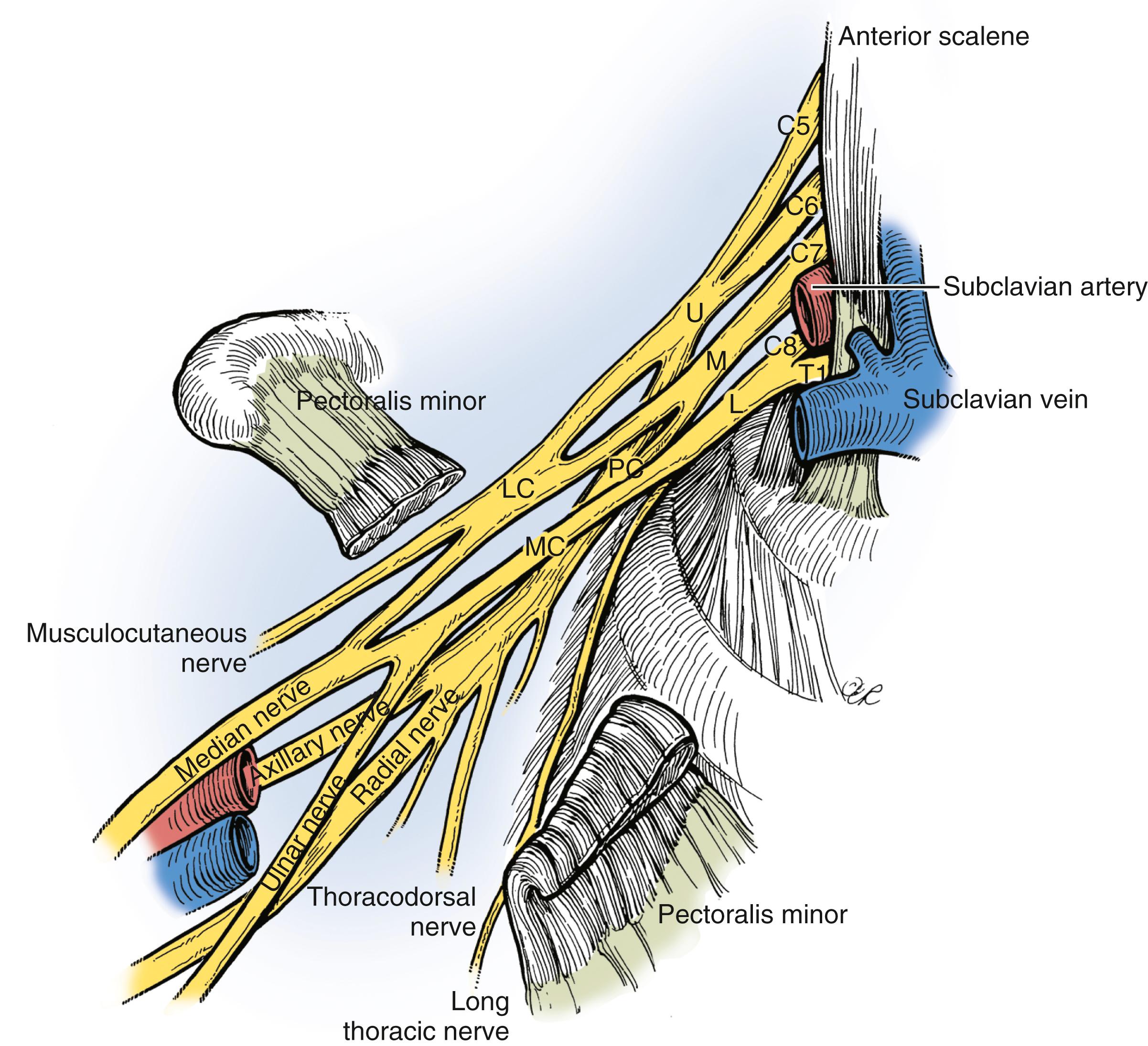
The C5 and C6 and the C8 and T1 nerves merge and form the upper and lower trunks, respectively. C7 continues distally as the middle trunk, with no clear demarcation point for this change in nomenclature. The point of convergence of the C5 and C6 nerves, known as Erb’s point, marks the spot at which the suprascapular nerve emerges. Nerves and trunks are located above the clavicle and collectively form the supraclavicular plexus. Each trunk then divides beneath (dorsal to) the clavicle into anterior and posterior divisions (the retroclavicular plexus ). The posterior divisions from all trunks merge to become the posterior cord, and the anterior divisions of the upper and middle trunks merge to form the lateral cord. The anterior division from the lower trunk continues distal to the clavicle as the medial cord. The lateral cord splits into two terminal branches, the musculocutaneous nerve and the lateral cord contribution to the median nerve. The posterior cord forms the axillary nerve and the radial nerve, and the medial cord gives off the medial cord contribution to the median nerve, as well as the ulnar nerve. The cords and terminal branches arising from them collectively make up the infraclavicular plexus.
Additional branches of clinical importance at the proximal nerve level include a C5 contribution to the phrenic nerve, the dorsal scapular nerve (C5), and the long thoracic nerve, with contributions from C5, C6, and C7. The only trunk branches are the suprascapular nerve and the nerve to the subclavius muscle, both from the upper trunk. The lateral pectoral nerve arises at the division level in the retroclavicular plexus. In the infraclavicular region, the posterior cord gives off branches (proximal to distal) that include the upper subscapular nerve, the thoracodorsal nerve, and the lower subscapular nerve; the medial cord gives off the medial pectoral nerve, the medial antebrachial cutaneous nerve, and the medial brachial cutaneous nerve.
Nerve injuries are generally classified according to the Seddon and Sunderland classifications. Both classifications overlap; Seddon classified nerve injuries into three different categories: neurapraxia, axonotmesis, and neurotmesis; Sunderland expanded Seddon’s axonotmetic category, for a total of five degrees of nerve injury: first-degree (neurapraxia), second- to fourth-degree (axonotmesis), and fifth-degree (neurotmesis) injuries. Basic knowledge of the different degrees of nerve injury is necessary to understand the need for observation in some patients and for surgical intervention in others. (See also Chapter 30 .)
In neurapraxia (first-degree injury), there is a focal conduction block at the site of injury. Wallerian degeneration does not occur, and as the conduction block resolves, neurologic function returns. These injuries do not require surgical intervention, and recovery occurs within hours to weeks. In axonotmesis (second- to fourth-degree injury), the axons are disrupted and Wallerian degeneration occurs distal to the injury. However, the nerve remains in gross continuity. Stretch injury is the most common mechanism. Depending on the severity of the injury, regeneration may occur (at a rate of 1 mm/day or 1 inch/month) or not. To better describe axonotmetic lesions, Sunderland further classified them into second-degree injury (axon disrupted, but intact endoneurium, perineurium, and epineurium), third-degree injury (axon and endoneurium disrupted, but intact perineurium and epineurium), and fourth-degree injury (axon, endoneurium, and perineurium disrupted, but intact epineurium). In second-degree injury, regeneration and neurologic recovery are expected but will require several months or even years. In very proximal injuries with distal targets (such as the proximal ulnar nerve or lower trunk lesions), muscle atrophy or motor endplate degeneration may occur before regeneration is completed and prevent functional recovery. In third- and fourth-degree injury, regeneration may occur to a variable degree or may not occur at all, and these lesions may require surgical intervention. Finally, in neurotmesis (fifth-degree injury), the nerve is completely disrupted and there is no continuity. These lesions cannot spontaneously recover and surgical intervention is necessary. In brachial plexus injuries, neurotmetic lesions include postganglionic ruptures and preganglionic avulsions.
Most traumatic injuries of the brachial plexus are due to traction or stretch injury. , These brachial plexus injuries are caused by varying amounts of energy imparted to the plexus that may result in different injuries ranging from mild stretch to rupture or avulsion (see Fig. 34.2 ). Low-energy traction trauma, not severe enough to cause rupture or avulsion, typically causes injuries with a potential for spontaneous regeneration, such as neurapraxia (Sunderland type I) or lower degrees of axonotmesis (Sunderland types II and III). Experimental evidence also suggests that low-energy injuries may interfere with brachial plexus microcirculation and cause ischemic injury. High-energy injuries are associated with more significant damage to the plexus that may include rupture of plexal elements (Sunderland type V) or avulsion of nerve roots from the spinal cord. When a combination of incomplete rupture and avulsion occurs, some spontaneous recovery may take place, but early microneurosurgical reconstruction generally improves the functional outcome. If the shoulder-neck angle is forcibly widened by downward traction on the arm, damage is imparted first to the upper spinal nerves (C5 and C6)/upper trunk and/or C7/middle trunk ( Fig. 34.3 ); if the scapulohumeral angle is forcibly widened, damage is imparted first to the lower nerves (C8 and T1) and the lower trunk. If the impact is extreme, all levels can sustain damage. Anatomic studies have determined that the supporting tissue anchoring the upper nerves (C5 and C6) to the vertebral foramina is significantly stronger than the supporting tissue of the lower nerves (C8 and T1). This anatomic arrangement predicts that the more caudal structures of the brachial plexus (i.e., the C8-T1 nerves) are more likely to be avulsed from the spinal cord, whereas the more cranial structures of the brachial plexus are more likely to stretch or rupture after exiting the neural foramina (i.e., the C5-C6 nerves). In approximately two-thirds of traction injuries involving the brachial plexus, the injury is located in the supraclavicular region; in the other third, the injury is retroclavicular or infraclavicular and involves the divisions, cords, or terminal nerves. In some patients, a double-level (supraclavicular and infraclavicular) injury can occur and should be considered. The principal factors determining the extent of injury are the energy imparted by the blow and, to a lesser degree, the direction and the relationship of the arm to the neck during the injury.
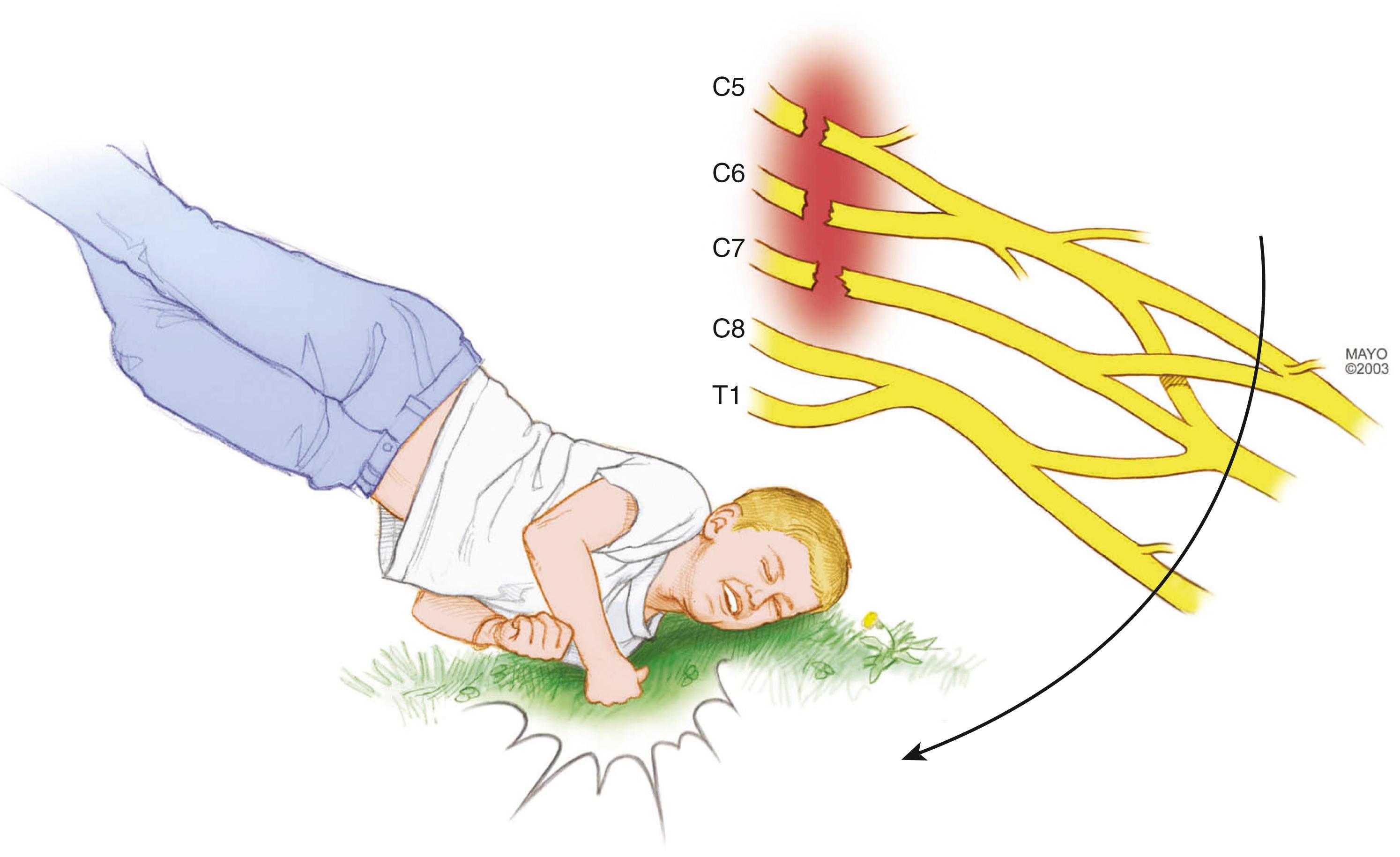
An individual patient may have different degrees of injury at different nerve levels; for example, the C8 and T1 nerves may be avulsed, the C7 nerve or middle trunk may be ruptured (Sunderland type V), and the upper trunk may remain intact but with varying degrees of internal damage (Sunderland types I to IV). Within an individual brachial plexus element, individual axons and fascicles may also suffer various degree of injury. This variability in the degree of injury affecting each plexal element explains why spontaneous recovery may occur in some plexal elements but not in others. Extensive longitudinal injury is also common and at times results in segmental injuries to the retroclavicular and infraclavicular plexus (divisions and cords or terminal branches, respectively), as well as the roots, nerves, and trunks cephalad to the clavicle.
The same high-energy traction injury that damages the brachial plexus may also cause other injuries. Approximately 75% of traumatic brachial plexus injuries are associated with head injury, thoracic trauma, and fractures or dislocations (or both) of the cervical spine or ipsilateral upper extremity; 20% of patients have associated vascular trauma. These associated injuries, such as fractures, dislocations, and vascular injuries (from either an associated hematoma, pseudoaneurysm, or arteriovenous fistula), can further damage the brachial plexus.
Most commonly, the plexal elements are in continuity following a gunshot injury, but in a small proportion of patients, some elements may be transected. The injury will depend on the caliber, velocity, and angle of incidence of the missile. Low-velocity missiles usually damage nerve elements by direct impact; high-velocity missiles can injure nerves by direct impact (which transects the nerves) or, more commonly, by shock wave and cavitation effects (which contuse or stretch the nerves). Because the injury is often in continuity, some patients will have spontaneous recovery. Others do not recover, however, and will require surgical exploration; high-velocity injuries are more likely to cause extensive damage and to fail to recover spontaneously. Gunshot wounds may cause associated vascular injuries that may require emergency or delayed repair; the latter may occur over time as a result of the formation of a pseudoaneurysm, which can compress the plexus, produce progressive loss of function, and require an intervention on the vessel(s) or nerves, or both.
The brachial plexus is occasionally injured by sharp (caused by knives or glass) or blunt (caused by fan and motor blades, chainsaws, or animal bites) penetrating trauma. Iatrogenic injuries have also been reported from various surgical procedures. As expected, most patients have brachial plexus elements in discontinuity (i.e., transected); however, despite the mechanism of injury, some patients have a lesion with a degree of continuity. Because of the high suspicion of transection of one or more nerves, these patients require nerve exploration. A third of these patients require vascular surgery, which is often performed concomitantly. In these patients, as in patients with gunshot wounds or traction injuries, an associated hematoma, pseudoaneurysm, or arteriovenous fistula may compress the plexus and require intervention because of progressive neurologic deterioration.
In brachial plexus injuries, any combination of stretch, rupture, and avulsion can occur. However, certain patterns are more frequent, such as supraclavicular injuries (including the roots, nerves, and trunks, and often described as C5-C6, C5-C7, C8-T1, or pan-plexus), retroclavicular injuries (divisions), and infraclavicular injuries (cords and terminal branches).
Approximately 15% of patients have injured the C5 and C6 nerves, often extending to the junction where they form the upper trunk (Erb’s point). These patients have deficits in shoulder stability, abduction, and external and internal rotation (supraspinatus and infraspinatus, deltoid, subscapularis), as well as in elbow flexion (biceps, brachialis, and brachioradialis) and forearm supination (biceps, supinator). Sensory deficit will be present in the C5 and C6 distributions. Elbow extension is normal, as is wrist and hand function. This pattern of injury is commonly referred to eponymously as Erb or Erb-Duchenne palsy in recognition of the early work of Wilhelm Heinrich Erb and Guillaume Duchenne on peripheral nerve injury.
In approximately 20% to 35% of patients, weakness of muscles innervated by C5 and C6, as described earlier, will be accompanied by a partial or complete C7 or middle trunk injury. In these patients, there will be variable weakness of the elbow, wrist, and sometimes finger extensors. The C7 contribution to wrist and finger extension and even to the flexor digitorum profundus muscles varies between patients and leads to different degrees of weakness. Sensory disturbances in the proximal part of the arm, as well in the thumb and index and middle fingers, may be present. This at times is referred to as an Erb-plus pattern. Some cases of C5-7 injury may mimic C5-6 injury.
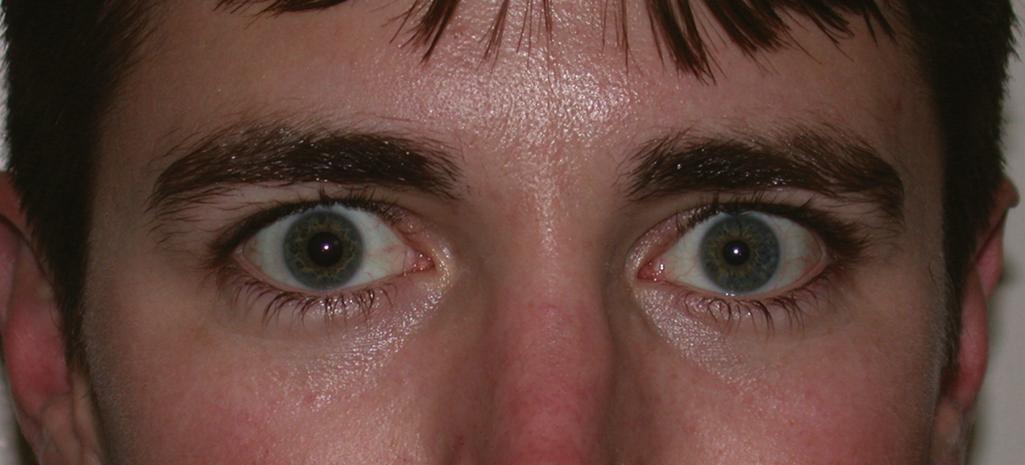
Approximately 10% of patients (even less in our experience) have supraclavicular lesions isolated to the C8 and T1 nerves at presentation (typically the upper elements have recovered). These patients will have weakness of the hand intrinsics, as well as variable weakness of the hand extrinsics and finger extensors, depending on the C7 contribution to these territories. Sensory loss in the ulnar digits, medial aspect of the forearm, and distal part of the arm may be present. Involvement of the lower roots can result in Horner syndrome, which is noted on examination by meiosis (constricted pupil), ptosis of the upper eyelid, anhidrosis, and enophthalmos (see the section Physical Examination). This rare pattern of injury is often referred to as Klumpke or Dejerine-Klumpke palsy.
Traumatic injury to the entire brachial plexus (C5-T1) occurs in 50% to 75% of patients with supraclavicular brachial plexus injuries. These patients most commonly have a completely flail arm and insensate hand. Occasionally, certain elements may be partially injured. Even with complete pan-plexus injuries, postganglionic injury (particularly of C5) is often present, with preganglionic lesions affecting other nerves.
Retroclavicular, infraclavicular, or axillary region brachial plexus injuries may involve injuries at the division, cord, or terminal branch level. Clavicular and/or scapular fracture is often present in these cases. A few patterns are more commonly seen, including injuries to the posterior cord (radial and axillary nerve distributions), as well as isolated axillary or suprascapular nerve injuries.
A comprehensive preoperative evaluation that includes a detailed history, physical examination, imaging, and electrodiagnostic evaluation is essential to determine the location and severity of the brachial plexus injury and appropriate management. The goal is first to determine whether there is potential for spontaneous and functionally significant recovery, which warrants further observation. If no recovery is seen within the first 2 to 3 months in patients with stretch injury, surgery is indicated. In cases in which surgery is indicated, determining whether the injury is preganglionic or postganglionic will also help in planning the surgical approach and possible reconstructive options. In preganglionic injuries, the nerve root is nonfunctional (avulsed from the spinal cord) and, for practical purposes, cannot be repaired or grafted. The presence of a preganglionic injury at one or more levels indicates a more severe injury with a lower probability of spontaneous recovery; thus earlier surgical intervention may be warranted. No single finding or evaluation can tell the surgeon whether the injured plexus requires a surgical intervention. All the information must be analyzed and each patient requires an individualized approach.
Patients at our institution are evaluated in a multidisciplinary clinic, first by a neurologist and then by a team of surgeons skilled in primary and secondary reconstruction. A physiatrist (with expertise in pain management) and a physical/occupational therapist are also involved in the care of each patient. They teach and emphasize the importance and timing of passive and active range-of-motion exercises before and after any operation. Some groups have also incorporated social workers and psychological evaluation. Decisions regarding management are discussed and planned collectively. We believe that this type of team approach best addresses the short- and long-term needs of these patients with complex injuries.
A good history of the injury mechanism is helpful in determining its severity. High-energy traction injuries, such as motor vehicle or motorcycle injuries, will probably be more severe, with less potential for spontaneous recovery than low-energy traction injuries, such as falls. Still, some falls, especially those associated with musculoskeletal injuries, may result in severe nerve lesions that do not recover spontaneously. Nerve deficits from lacerations, either sharp or blunt, will not recover spontaneously (except in the rare case in which the nerve elements were not transected) and should be explored acutely or subacutely (see the section Timing of Surgery). On the contrary, the majority of gunshot wounds associated with brachial plexus injury should be observed because most lesions are in continuity and have the potential for spontaneous recovery.
When a patient with brachial plexus injury is evaluated acutely in the emergency room, the airway, breathing, and circulation should be evaluated and stabilized, and the standard trauma evaluation guidelines should be followed. If the patient sustained high-energy trauma, a thorough search for associated life- and limb-threatening injuries should be conducted. In patients with head or spinal cord injuries, brachial plexus injury is often underdiagnosed; the opposite may also occur if the surgeon focuses on the upper extremity and fails to diagnose an associated head or incomplete spinal cord injury. Every patient should therefore be asked about loss of consciousness and paresthesias or weakness affecting the other extremities.
The presence of associated injuries (especially vascular injury, ipsilateral upper extremity musculoskeletal injury, scapulothoracic dissociation, rib fractures, pneumothorax and hemothorax, lung contusion, spine fracture and spinal cord injury, and traumatic brain injury) should be considered and documented. Associated injuries may indicate a more severe injury with less potential for spontaneous recovery and may influence the available reconstructive options. Severe pain in an anesthetic extremity may be due to deafferentation, as seen in patients with root avulsion injuries. When evaluated weeks to months after the injury, the patient should be questioned about any improvement in function or pain since the injury.
The examination of patients with brachial plexus injury must be carried out in a standardized and detailed manner. The objective is to establish the location of the nerve injury (preganglionic [i.e., root] or postganglionic [i.e., nerve, trunk, division, cord, or branches]) and the severity of the lesion (i.e., partial or complete for each element of the brachial plexus) (see Figs. 34.1 and 34.2 ). Careful systematic motor and sensory examination of the entire limb should be performed and recorded ( Fig. 34.4 ). Serial examinations over the first few months after injury may help determine the presence or absence of ongoing nerve regeneration and the prognosis for functional recovery.
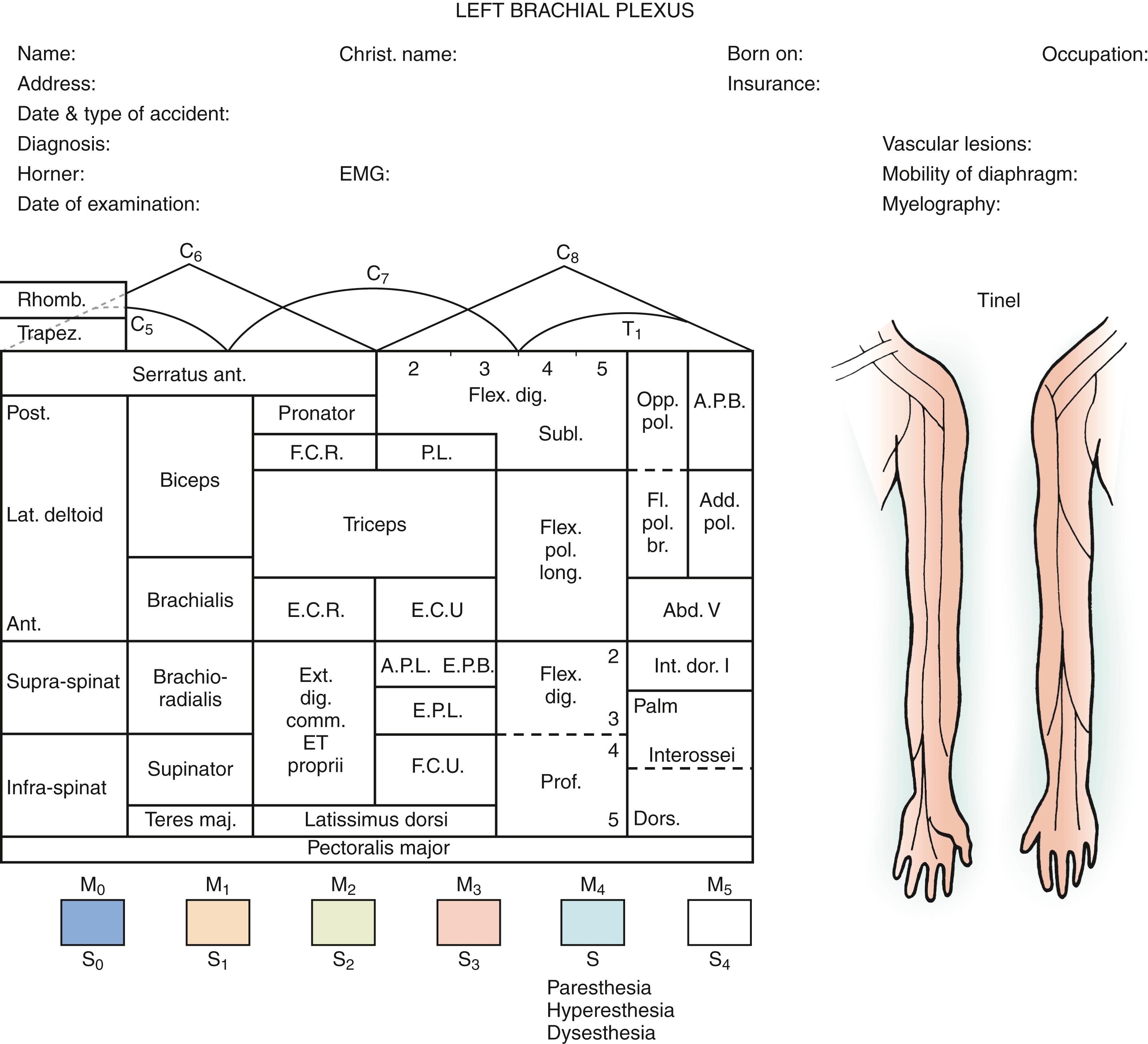
The motor and sensory innervation of the upper extremity is well known and assists the surgeon in predicting the location of nerve lesions. Terminal branches can be assessed by examination of the fingers and wrist (median, ulnar, and radial nerves), elbow (musculocutaneous and high radial nerves), and shoulder (suprascapular or axillary nerves). Involvement of the latissimus dorsi (thoracodorsal nerve), sternal head of the pectoralis major (medial pectoral nerve), and clavicular head of the pectoralis major (lateral pectoral nerve) implies a lesion at least at the posterior, medial, and lateral cord levels, respectively. Involvement of the suprascapular nerve (supraspinatus and infraspinatus for shoulder abduction and external rotation, respectively) indicates a lesion at this terminal branch or at the upper trunk level. Finally, loss of function of the rhomboid (dorsal scapular nerve derived from C5) and serratus anterior (long thoracic nerve from C5 to C7) is an important indicator of a proximal lesion or preganglionic injury.
There is variability in plexus anatomy and nerve level contribution to individual muscle innervation. For example, a prefixed plexus receives significant innervation from the C4 nerve and a postfixed plexus receives innervation from the T2 nerve; these variations are reported commonly by anatomists. The dorsal scapular nerve may on occasion receive innervation from C4, such that rhomboid activity may be identified by electrical studies in the presence of a C5 avulsion injury. Unusual motor and sensory impairment that is not distributed in the proper territory of the cervical and peripheral nerves may indicate incomplete nerve injury and some recovery.
Certain findings on physical examination suggest preganglionic injury of the brachial plexus; if present, these findings imply a lower probability of spontaneous recovery and a higher probability of finding an avulsed root at exploration. As stated earlier, injury to the rhomboid and serratus anterior indicates a proximal nerve injury and suggests a preganglionic injury of the upper neural elements, whereas the presence of Horner syndrome ( Fig. 34.5 ) on the affected side strongly correlates with avulsion of the lower elements (i.e., the C8 or T1 roots, or both). Because the T1 sympathetic ganglion is close to T1, an avulsion injury may interrupt sympathetic outflow and be manifested as Horner syndrome. Absence of a Tinel sign or of tenderness to percussion in the neck also suggests a preganglionic injury. Finally, muscular atrophy of the paraspinal muscles, which is associated with root avulsion, may be associated with shifting of the head away from the injured side.
Physical examination findings suggesting a postganglionic or more distal injury include the presence of tenderness to percussion in the supraclavicular or infraclavicular region, absence of sweating in the distribution of the injured nerve (sympathetic interruption), and minimal preservation of movement (partial injury). An advancing Tinel sign is suggestive of a recovering lesion.
The examination should also include testing of the distal spinal accessory nerve (cranial nerve XI), which supplies the trapezius muscle. It is frequently used for reconstruction options as a nerve transfer but can occasionally be injured concomitantly at the time of traumatic brachial plexus injury. In all patients, stability and active and passive range of motion of the joints should be assessed preoperatively. To rule out a concomitant spinal cord injury, a lower extremity neurologic examination (that includes reflexes) should be performed. The vascular system of the affected upper extremity should be assessed by palpation or Doppler examination of distal vascular flow, as well as for thrills or bruits in the infraclavicular and supraclavicular fossae.
Radiographs of the cervical spine, chest, shoulder girdle, and humerus should be obtained in patients with high-energy traumatic brachial plexus palsy to rule out associated upper limb fractures and dislocations. Chest radiographs should include anteroposterior inspiration and expiration views and should be examined for the presence of rib fractures and for activity of the diaphragm. A paralyzed (i.e., elevated) hemidiaphragm indicates a proximal (probably preganglionic) brachial plexus injury because it is supplied by the phrenic nerve, which originates from the proximal C3-C5 nerves. The presence of cervical transverse process fractures is also associated with brachial plexus avulsion injuries. First or second rib fractures are also associated with preganglionic brachial plexus injuries. Rib fractures may be important if the intercostal nerves are to be considered for nerve transfers (≈10% of the intercostal nerves will be damaged by rib fractures in our experience.
Myelography and computed tomographic (CT) myelography, , although invasive, have been considered the most reliable methods for detecting root avulsions ( Fig. 34.6 ). Myelographic findings have been classified by Nagano and colleagues’ modified scheme into six categories, from normal roots to ventral or dorsal root avulsions (or both), to the presence of traumatic pseudomeningoceles. Myelography or CT myelography is typically performed at least 3 to 4 weeks after the injury; such a delay will allow a potential blood clot in the area of the root avulsion to dissolve and the pseudomeningocele to form and be visualized on imaging.

More recently, magnetic resonance imaging (MRI) has been used increasingly , to evaluate patients with traumatic brachial plexus injury; in some centers it has supplanted CT myelography. MRI can reveal traumatic pseudomeningoceles, large neuromas, rootlet abnormalities, associated inflammation and edema, and mass lesions. Newer techniques have improved the detection of nerve root avulsions by MRI. ,
Finally, the vasculature to the injured upper extremity should be assessed, especially when a functioning free muscle transfer (FFMT) is being considered. In the authors’ experience, a normal peripheral pulse on examination does not preclude a vascular injury affecting the major vessels. We generally evaluate the vasculature with magnetic resonance angiography; special attention is given to our preferred source of arterial supply for FFMT, the thoracoacromial trunk. Other adequate options to formally evaluate the vasculature include CT angiography and conventional angiography.
Patients with a history of chest wall trauma (e.g., rib fractures, pneumothorax, hemothorax) and those found to have phrenic nerve dysfunction should have pulmonary function tests performed to better characterize their pulmonary function. These evaluations, with assessment by a pulmonologist if necessary, will help determine whether the patient will tolerate intercostal nerve transfers. Unless the preoperative pulmonary function test results are very low (i.e., <40% of expected, but no definitive criteria exist), a patient can generally tolerate up to four or five intercostal nerve transfers even if concomitant phrenic nerve dysfunction is present.
Electrodiagnostic studies are an integral component of the evaluation of traumatic brachial plexus injury. These studies can confirm the diagnosis, localize and characterize (i.e., partial versus complete) the nerve lesion, and assess for subclinical evidence of recovery. A baseline examination should be done 3 to 4 weeks after the traumatic injury, when Wallerian degeneration has occurred, to ensure that the electrodiagnostic study reflects the degree of nerve injury. Serial electrodiagnostic studies complement the physical examination when assessing for recovery and reinnervation. The electrodiagnostic evaluation should include electromyography (EMG) and nerve conduction studies (NCSs). EMG evaluates and records the electrical activity of muscles both at rest and with activity. Changes reflective of a denervation injury include the presence of fibrillation potentials at rest and absent (complete injury) or reduced (partial injury) motor unit potentials with voluntary effort. Over time, nascent motor unit potentials (low in amplitude, polyphasic in configuration, and of variable duration) may appear and suggest reinnervation. Several muscles, difficult to test clinically, can be evaluated by EMG to help localize the lesion. For example, the rhomboids, serratus anterior, and cervical paraspinal muscles, if affected and abnormal on EMG, may suggest a very proximal or preganglionic injury. The trapezius muscle should be evaluated, especially if the spinal accessory nerve is being considered as a potential donor for nerve transfer. NCSs, especially sensory nerve action potentials (SNAPs), are also helpful to evaluate the level of the nerve injury. With preganglionic injuries, the dorsal root ganglion, where the sensory neuron cell body is located, is intact. The distal axons do not undergo Wallerian degeneration because they remain connected to the cell body; thus the SNAP is preserved. However, the patient is insensate because the sensory neurons are not connected to the central nervous system (i.e., spinal cord and brain). The finding of intact SNAPs in the presence of dermatomal anesthesia is pathognomonic of a root avulsion injury. In postganglionic injuries, the sensory axons will degenerate and the SNAP will be lost. On occasion, a segmental or longitudinal injury will cause both preganglionic and postganglionic lesions, and the SNAP will be absent in the presence of a root avulsion injury. On the contrary, motor conduction will be absent with both preganglionic and postganglionic injuries because the motor axons will have undergone Wallerian degeneration (i.e., because their cell body is located in the anterior horn of the spinal cord).
Whenever possible, a follow-up electrodiagnostic study is performed 6 to 12 weeks after the initial study to assess for recovery. If surgery is being considered, electrodiagnostic interrogation of those muscles innervated by potential nerve transfer donors can be performed to ensure normal or near-normal motor unit recruitment. Although an advancing Tinel sign and the presence of nascent units are encouraging findings, they need to be taken into consideration along with improvement in clinical function; these findings do not necessarily eliminate the need for surgery.
Multiple studies on the exploration and reconstruction of brachial plexus traction injuries suggest the value of surgical intervention in many of these patients. Surgery is indicated in patients with functional deficits and no hope for spontaneous recovery or for further recovery. This principle applies to patients with traumatic brachial plexus injuries from any mechanism. All patients with laceration injuries in proximity to the brachial plexus should undergo exploration because in the vast majority of these injuries (sharp or blunt), there is no possibility of spontaneous improvement and surgery is clearly indicated. In patients with gunshot injuries, an initial potential for spontaneous recovery exists because the nerves are usually in continuity. The same applies to patients with traction injuries. After a period of observation (see Timing of Surgery), surgery is indicated if there is no clinical or electrophysiologic evidence of recovery. Signs of electrophysiologic recovery that do not translate to clinical recovery within a short period of observation should not be viewed as meaningful recovery, and such patients should be considered for a surgical intervention. If it were possible to clearly identify patients with complete nerve root avulsion or nerve rupture, a surgical intervention could be performed without the observation period because these injuries cannot recover spontaneously. However, at present, no definitive study or sign is sufficiently reliable; the decision should be made after analyzing the different evaluations (history, physical examination, imaging studies, electrodiagnostic studies) in the context of time.
Absolute contraindications to surgery for the reconstruction of brachial plexus injury are few. Stiffness and contractures, age, medical comorbidity, associated traumatic brain injury, and associated spinal cord injury should be considered in the decision process and may need to be addressed, but they are not generally contraindications. The main contraindication is unrealistic goals or unwillingness of the patient to undergo the procedure. Before a decision to operate is reached, it is therefore crucial to inform the patient about the possible surgical options, risk-benefit ratio, postoperative rehabilitation program, success rate, long recovery time, and possibility of additional surgery. This discussion should be individualized to each patient according to that patient’s specific injury, needs and expectations, and goals and priorities (set by the treating team and patient). Surgery is also not usually indicated for patients with spontaneous ongoing recovery in all involved elements because outcomes will generally be better without surgery in such patients. We believe that the rare patient with a C8-T1 lesion has a relative contraindication to primary nerve repair or grafting because of the limited ability for successful regeneration to reach the hand. Distal nerve transfers or secondary reconstruction with tendon transfer may yield more predictable results. Finally, if more than a year has passed since the injury, primary plexus reconstruction is generally contraindicated, except perhaps in a young patient. In select cases, distal nerve transfers may be considered up to 18 months after the injury.
Timing of brachial plexus reconstructive surgery is based on three principles: (1) better functional outcomes occur in patients with spontaneous recovery who do not require a surgical intervention; (2) surgical intervention is indicated for patients with no hope for spontaneous recovery or for further recovery; and (3) most series demonstrate that the surgical outcome is inversely proportional to the time interval from injury to surgery (i.e., outcomes are better if surgery is performed earlier). Based on these principles, primary nerve reconstruction may ideally be initiated immediately (within days) after injury or in a delayed fashion (within months). Timing of secondary reconstructive procedures is discussed separately.
In patients with plexus deficits and a sharp open injury, immediate exploration plus repair is indicated (best within several days). Immediate surgery allows direct repair of the lacerated elements. In patients with blunt laceration injury (e.g., fan or motor blades, auto metal fragments, chain saws, etc.) to the plexus, surgical intervention is also clearly necessary, but the timing of the surgery is controversial and less definite. A subacute (within 3 to 4 weeks) surgical intervention may be preferable; time will allow better definition of the zone of nerve injury. Occasionally, a reconstructive brachial plexus surgeon gets the opportunity to assess the degree of damage to the plexus at the time of injury when emergency vascular reconstruction of an injured subclavian or axillary artery is performed by the vascular surgeons. This may occur with sharp or blunt laceration injuries and with traction injuries. The supraclavicular and infraclavicular regions may be explored, the damage to the plexus mapped, and the plexus reconstruction planned. If the nerves are sharply divided, they can be repaired immediately; if they are bluntly transected or ruptured, they may be tagged with radiopaque vascular clips (to allow identification) and loosely coapted to prevent retraction. The injury can then be reexplored 3 to 4 weeks later to allow the zone of damage to be better demarcated. If the nerves are avulsed, reconstruction can be performed immediately or at a second setting, depending on the situation and condition of the patient; finally, if the nerves are stretched but in continuity, a period of observation will be necessary. ,
Surgery is delayed in patients who have a potential for spontaneous recovery or for injuries that require time to become better defined. In patients with closed traction injuries or gunshot injuries (because most are in continuity), surgery is performed after a period of observation if there is no evidence of spontaneous clinical or electrophysiologic recovery. Even though outcomes are better if surgery is performed earlier, a balance must be reached such that patients demonstrating early spontaneous recovery (i.e., who will probably fare well) do not undergo surgery. The timing of surgery needs to be individualized based on multiple factors, including the mechanism of injury, findings on physical examination and imaging, and the surgeon’s preference. Patients with a low probability of spontaneous recovery may undergo surgery earlier, and patients with a high probability of spontaneous recovery may be observed longer. However, there are no universal guidelines and different opinions exist in the literature. Our preference is to explore and perform primary nerve reconstruction early in patients in whom there is a high suspicion of root avulsion based on the history/mechanism, physical examination, electrophysiologic studies, or imaging studies. At our institution we are not performing surgery ultra-early, as is advocated by some. We operate on patients with pan-plexal lesions 2 to 3 months after injury if they have no evidence of recovery but have imaging evidence of avulsions. In patients without this high suspicion of root avulsion and no adequate signs of recovery, we perform exploration 3 to 6 months after the injury. We may wait longer (5 to 6 months) for patients with a higher chance of spontaneous recovery, such as those with incomplete brachial plexus palsy or complete brachial plexus palsy without evidence of root avulsion. An early intervention may not allow sufficient time for spontaneous recovery; however, we believe that routine use of intraoperative monitoring (such as nerve action potentials [NAPs]) will help determine whether there is early subclinical evidence of recovery at the time of exploration. We seldom wait for more than 6 months because long delays diminish the outcomes of the reconstructive procedure. Still, many patients present to us for initial evaluation more than 6 months after injury.
For adult patients initially seen late (i.e., >12 months after the injury), primary nerve reconstructive procedures are associated with significantly poorer results. In select cases, distal nerve transfers can still be considered even later (i.e., <18 months). However, secondary reconstructive procedures, such as tendon transfer, FFMT (gracilis muscle), and bony or soft tissue procedures, may be indicated and can be done at any time.
The possible surgical options, risk-benefit ratio, site of incisions, hospitalization time, postoperative precautions and rehabilitation program, expected outcome with or without surgery, and long reinnervation period should be discussed in detail with the patient and his or her family. The patient should have realistic expectations and a clear understanding of the goals and priorities of the planned surgery. The surgical approach should be individualized according to the patient’s comfort level (acceptable risks and set of goals).
The surgical procedure, in particular its indeterminate duration and the need to perform intraoperative stimulation and recordings, should be discussed with the anesthesiologist. Long-acting paralytic agents, muscle relaxants, and agents depressing cortical responses should be avoided. Adequate vascular access should be obtained and an indwelling urinary catheter should be inserted. After induction of anesthesia and intubation, the patient is positioned in the supine position. The head is turned to the contralateral side, the upper part of the body is elevated, and a small pillow is placed beneath the ipsilateral scapula to bring the shoulder forward. Proper padding and positioning to protect bony prominences are necessary because brachial plexus reconstructive procedures are lengthy. Surgical preparation and draping should include the ipsilateral neck, mandible, hemithorax, axilla, entire upper extremity, and both lower extremities (for possible nerve grafts). Some surgeons use tourniquets placed on the upper part of the thighs that can be inflated just before sural nerve graft harvesting. Given the long duration of plexus surgeries, it is recommended that the tourniquet’s pneumatic hose not be connected until just prior to inflation and disconnected again after use. The arm is prepared and draped freely; this will permit the response to nerve stimulation to be observed and appropriate changes in arm positioning to be made during the procedure if needed. Tight circumferential elastic drapes are avoided on the extremities because of the long duration of surgery and possible swelling. If planning is being done to use the contralateral C7 nerve for transfer, the contralateral neck and hemithorax should be included in the surgical field. The surgeon should be prepared to expose both the supraclavicular and infraclavicular portions of the brachial plexus, harvest nerve grafts (sural nerve, superficial radial nerve, or vascularized ulnar nerve), or perform nerve transfers if required.
Electrodes required for intraoperative electrophysiologic monitoring (such as motor evoked potentials [MEPs] and somatosensory evoked potentials [SSEPs]) are placed. A bipolar microcoagulator, nerve stimulator, magnifying loupes, and operative microscope should be available for the surgical procedure.
The elements of the brachial plexus are exposed through a supraclavicular approach (i.e., nerves, trunks, suprascapular nerve) or an infraclavicular approach (i.e., cords, terminal branches). Depending on the pattern of injury, a combined approach may be necessary. The divisions, which are retroclavicular, can be accessed through one or both approaches. With introduction of the newer nerve transfers, some surgeons do not routinely explore the brachial plexus in all patients, even if a proximal nerve would be available for grafting, believing that nerve transfers provide a more reliable option. We recommend routinely exploring the proximal brachial plexus, except in specific patients who are initially seen late and have a more reliable, distal nerve transfer option. When a pan-plexus injury is present, less than 20% of patients are reported to have avulsions of all five roots of the plexus ; we therefore believe that exploration is warranted because a proximal nerve can often be found for grafting.
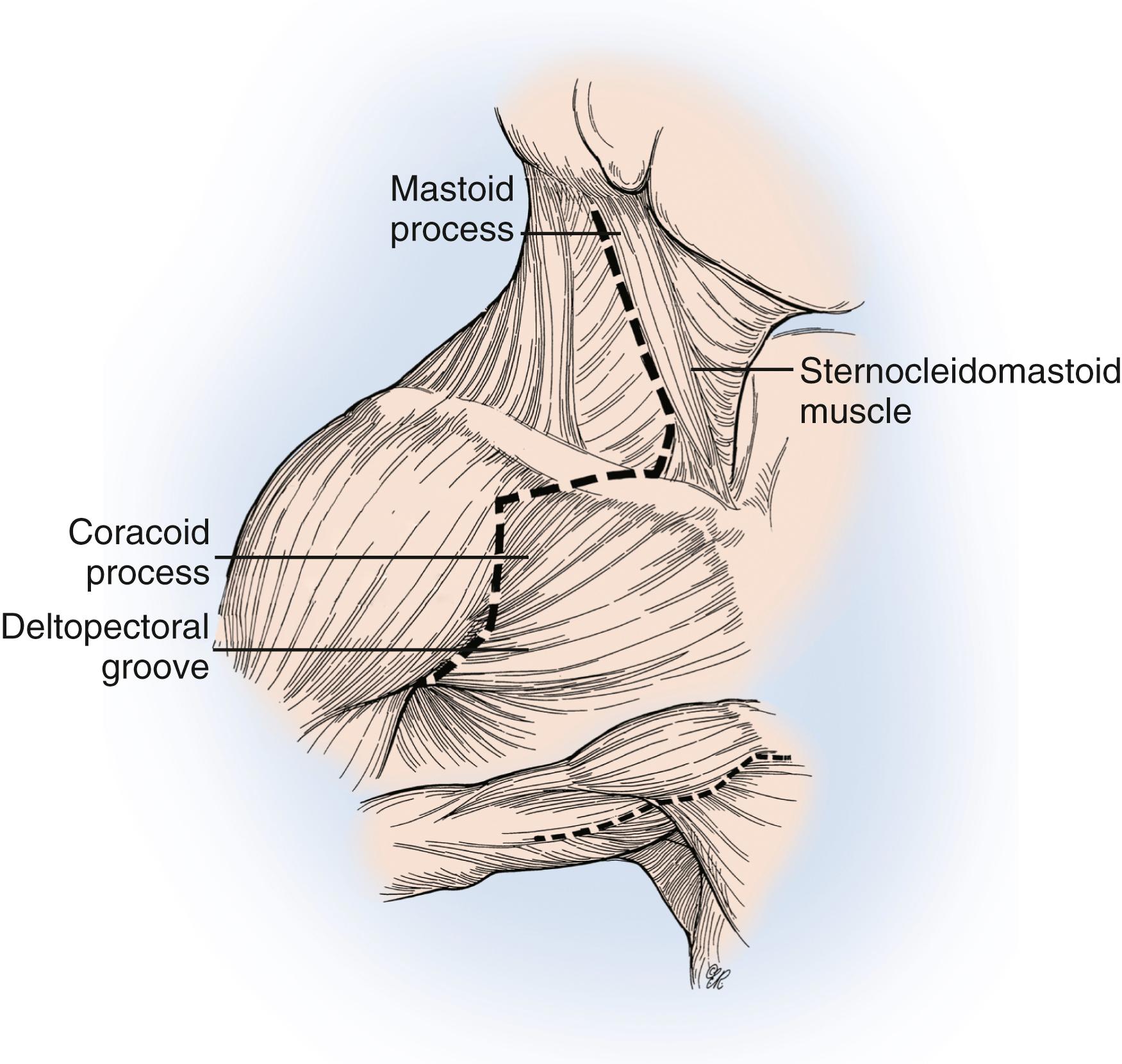
The variability in surgical approaches to the brachial plexus is mainly in the incisions used to expose the supraclavicular or infraclavicular brachial plexus and in the reconstructive approach used. Traditionally, a zigzag skin incision ( Fig. 34.7 ) was used: The supraclavicular incision begins at the angle of the jaw and follows the posterior border of the sternocleidomastoid to the midclavicular area; the infraclavicular incision begins at the clavicular insertion of the sternocleidomastoid (connecting with the supraclavicular incision), courses laterally toward the coracoid process, and extends laterally to the deltopectoral groove and into the arm. These incisions are connected to achieve a wide exposure. However, connecting these incisions increases the risk for scar hypertrophy. An alternative is to expose the supraclavicular plexus with a transverse incision, parallel to the clavicle, and the infraclavicular plexus with an incision over the deltopectoral groove. These two incisions can be connected if required. Some surgeons use a transverse cervical skin incision between the posterior tubercle of the C5 transverse process and the anterior tubercle of the C6 transverse process to expose the C5 and C6 nerves and a second incision that runs transversely over the clavicle to expose the C7, C8, and T1 nerves. Our preference, for exposure of the supraclavicular plexus, is to use a single transverse 5- to 6-cm cervical incision, off midline, parallel to and approximately two to three fingerbreadths above the clavicle ( Fig. 34.8 ). With this approach we can expose the C5 through T1 nerves (and the spinal accessory nerve laterally, as necessary). We expose the infraclavicular plexus with a second incision overlying the deltopectoral interval. The skin incision may be infiltrated with a vasopressor agent to decrease bleeding.
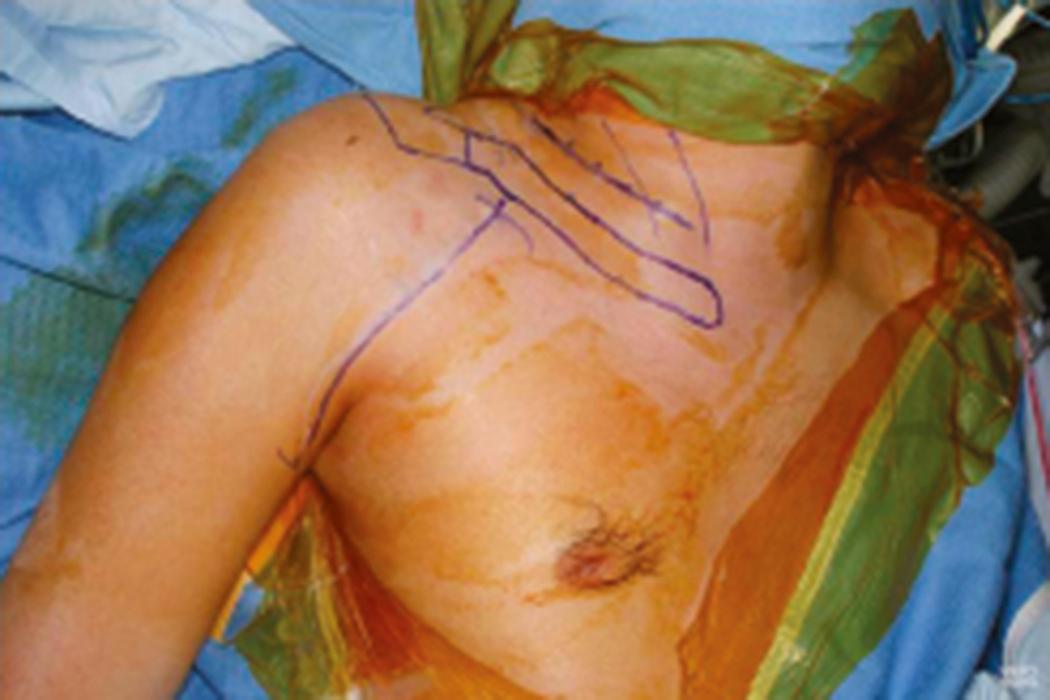
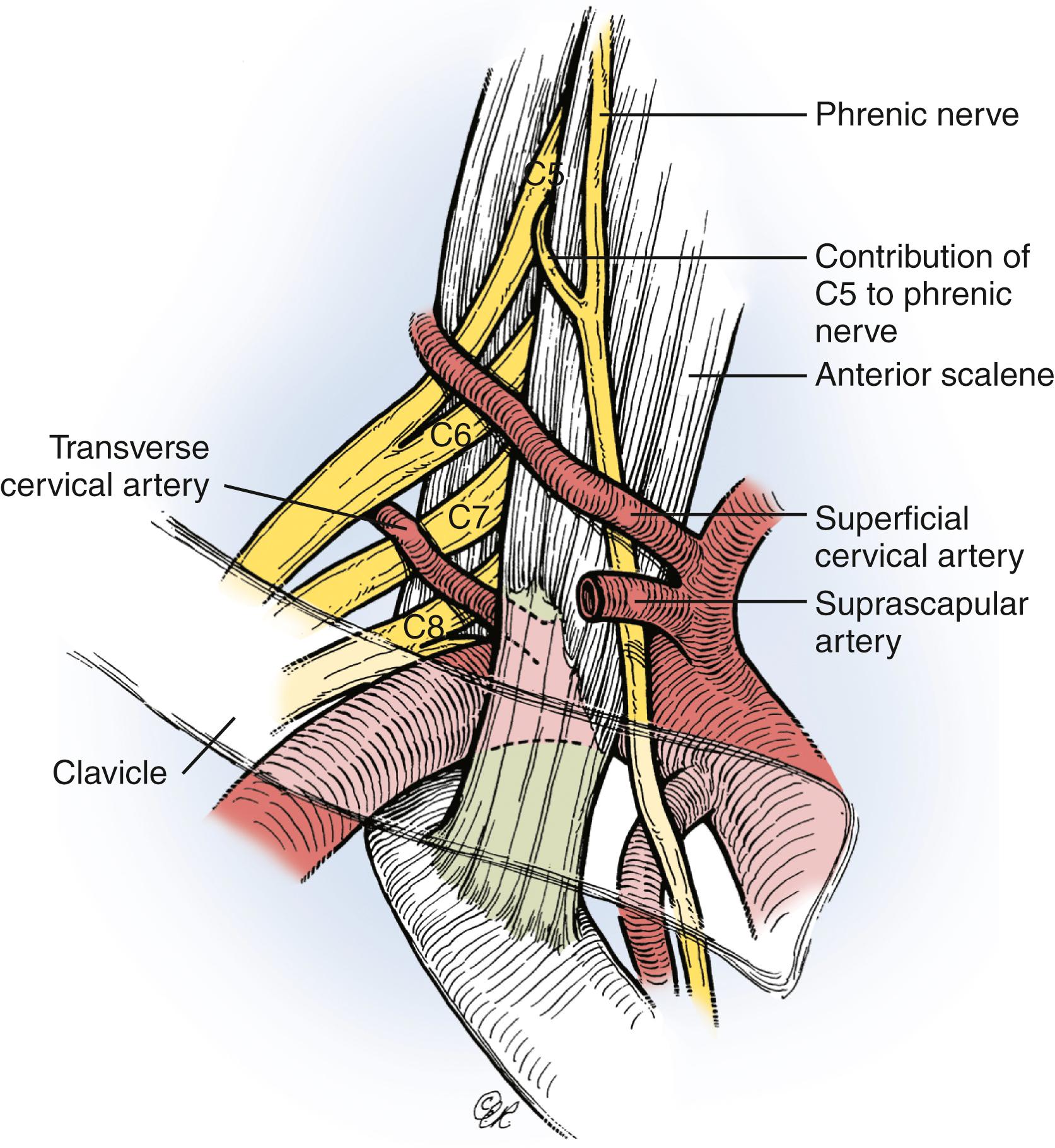
After the skin incision, the platysma is incised and the cutaneous branches of the cervical plexus are identified and protected. Skin flaps that include the platysma are carefully raised both superiorly and inferiorly. We generally do not encounter the spinal accessory nerve in the proximal portion of this exposure where it exits beneath the sternocleidomastoid muscle; however, we can easily identify it more distally near the medial border of the trapezius muscle if necessary (see the section Nerve Transfer). The external jugular vein on the surface of the sternocleidomastoid muscle is dissected and retracted. Dissection proceeds along the posterolateral margin of the sternocleidomastoid muscle, which is retracted medially; the cleidal head of the sternocleidomastoid can also be released. The superficial layer of the deep cervical fascia and cervical fat pad are opened and retracted. The dissection is extended to the superior border of the clavicle. The omohyoid muscle and transverse cervical vessels are identified, divided, and retracted medially and laterally. The first brachial plexus element to be visualized is usually the upper trunk (lateral to the anterior scalene). Medially, the phrenic nerve is found on the anterior surface of the anterior scalene muscle ( Figs. 34.9 and 34.10 ). Contraction of the diaphragm after electrical nerve stimulation can confirm identification of this nerve except in patients with phrenic nerve dysfunction (e.g., very proximal or preganglionic C5 nerve injury). The phrenic nerve should be mobilized with care. The anterior scalene muscle can then be resected safely to expose the proximal cervical nerves. When traced proximally, the C5 phrenic contribution will be visualized, thereby confirming identification of the C5 nerve. Careful dissection medial to the phrenic nerve may be necessary in patients with proximal C5 injury; iatrogenic injury to vital neck structures should be avoided. The upper trunk is exposed proximally and its C5 and C6 nerve origin identified. The C5 nerve is usually smaller, superior, lateral, and more vertical than the C6 nerve. The upper trunk is dissected distally to its anterior and posterior divisions, as well as to the origin of the suprascapular nerve. Long thoracic nerve may be identified lateral to the middle scalene (deep to the suprascapular nerve) and traced proximally to identify C6. The C7 nerve and middle trunk can be isolated between the anterior and middle scalene muscles, more medial, posterior, and horizontal than the C6 nerve. Even more inferior and posterior are the C8 and T1 nerves (forming the lower trunk), which are closely associated with the subclavian artery. Mobilization of the clavicle may help improve exposure. Osteotomy of the clavicle in our experience is only seldom performed in treating posttraumatic brachial plexopathies. In most cases, however, the C8 and T1 nerves are not specifically exposed, because their repair is rarely, if ever, indicated in our opinion, given the poor outcome after reconstruction of these lower nerve injuries in adults.
If the upper roots are avulsed, the rootlets and swollen dorsal root ganglia may be found twisted and lying either behind the clavicle or slightly above it in the region of the C8 nerve. If the upper plexus nerves or the upper trunk or middle trunk (or both) is ruptured, the distal ends typically lie behind the clavicle. The avulsed (often) or ruptured (infrequently) C8 and T1 structures are usually found much closer to their respective foramina than is the case with C5 or C6.
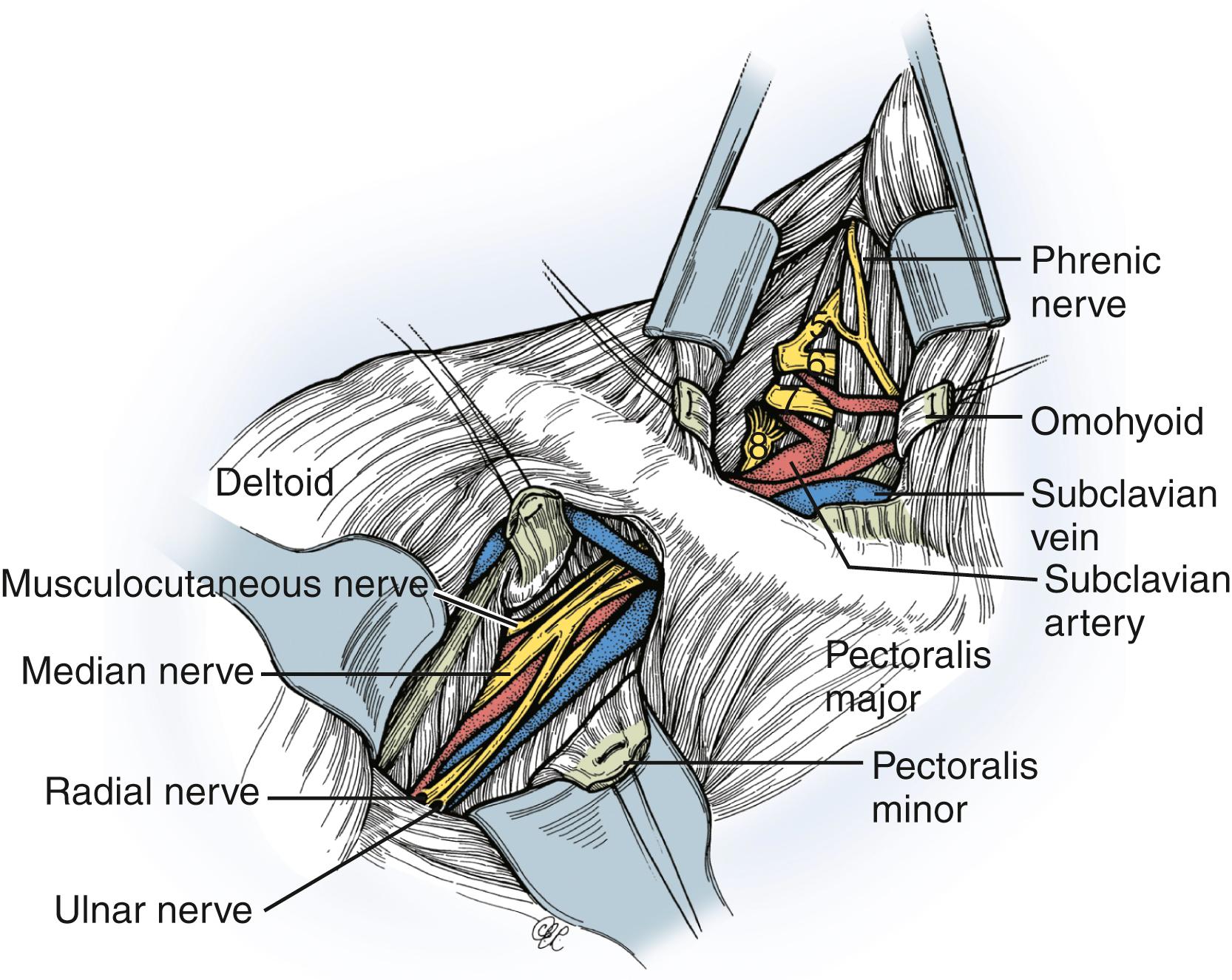
When indicated, the infraclavicular approach to the brachial plexus will expose the cords and terminal branches ( Fig. 34.11 ). After the skin incision, the deltopectoral interval is developed to expose the cephalic vein, which is preserved and retracted laterally. Some or all of the pectoralis major may be taken down from its humeral insertion and retracted as needed for exposure. A portion of its tendon is left on the humerus for reattachment, and the tendinous footprint is tagged with sutures for ease of closure. The infraclavicular plexus is explored by detaching the tendinous origin of the pectoralis minor from the coracoid process and tagging and retracting it. The clavipectoral fascia, which envelops the pectoralis minor, is incised to expose the lateral cord, which is superficial and lateral to the axillary artery. The thoracoacromial artery and its accompanying veins are identified and tagged if a FFMT or vascularized nerve graft is planned. The posterior cord is slightly lateral and deep to the artery, and the medial cord is medial and deep to the axillary artery. Care should be taken to preserve the medial and lateral pectoral nerves coursing in this area. Electric stimulation can be helpful for identifying these nerves, which often consist of multiple branches. The lateral cord is identified by following the median nerve from distal to proximal. At this point the musculocutaneous nerve can be seen branching laterally and piercing the coracobrachialis muscle. The median nerve can also be followed proximally to identify the medial cord, which is then followed distally to the ulnar, medial brachial cutaneous, and medial antebrachial cutaneous nerves. Medial and deep to the axillary artery and vein and coursing laterally is the radial nerve. It is followed proximally to the posterior cord; the axillary nerve can be seen branching from the posterior cord. Once the supraclavicular and infraclavicular brachial plexuses are exposed, the retroclavicular area can be addressed. A tunnel is made under the clavicle from the neck to the deltopectoral area with a gloved finger. The most lateral part of the clavicular attachment of the pectoralis major muscle is detached; the subclavius muscle is divided. Crossing vessels, including the suprascapular vessels, can be ligated as needed. By elevating the clavicle with a retractor or heavy tape, the divisions of the brachial plexus are exposed. If and when clavicular osteotomy is needed, predrilling of the screws for plate fixation should be done before the clavicle is divided. Patients with a history of clavicular fracture or hypertrophic callus require special attention; in these cases, blunt dissection underneath the clavicle can be difficult. The risk for major vascular and pleural damage must be considered, especially if the lower brachial plexus elements are exposed medially.
If extensive scarring is present, especially fibrosis related to previous vascular injury or reconstruction, identification of major neurovascular structures in “normal” tissue planes is the rule before proceeding toward the zone of pathology. Release of the insertion of the pectoralis major and in some cases the conjoined tendon may improve the exposure and facilitate safe dissection. Use of a Doppler device allows mapping of an arterial bypass graft.
Operative inspection and palpation of the brachial plexus in our experience has not proven to be a reliable means of predicting preganglionic versus postganglionic injury or of assessing (postganglionic) neuromas-in-continuity. For example, in some cases of preganglionic injury, there is little scarring of the brachial plexus, which might suggest a minor injury but in fact reflects a more proximal injury (i.e., at the root–spinal cord interface). In other cases, a large neuroma-in-continuity may be recovering but may not have yet reached the distal musculature.
For these reasons, intraoperative electrodiagnostic assessment can be performed; we recommend and routinely use it. Surgical strategies are tailored to the intraoperative findings. Our intraoperative assessment includes the use of SSEPs, MEPs, and NAPs. Spinal evoked potentials can replace SSEPs. Histologic evaluation by determining choline acetyltransferase (ChAT) activity , is also used by some groups. If a spinal nerve or peripheral nerve is divided surgically in preparation for nerve grafting, frozen-section histologic analysis of the transected portion can be performed quickly for assessment of relative fascicular preservation and intraneural scarring. ,
SSEPs and MEPs help determine whether the spinal nerves are in continuity with the spinal cord; they evaluate the integrity of the intraforaminal and intraspinal sensory and motor pathways. SSEPs are based on the principle that it is possible to record very low amplitude potentials on the scalp (over the parietal cortex) after stimulating a contralateral peripheral nerve in the upper or lower limb. MEPs are motor compound action potentials recorded from surgically exposed spinal nerves after central stimulation. Both SSEPs and MEPs have been used to identify root avulsion during brachial plexus surgery. ,
For SSEPs and MEPs, an electrode (which can act either as a stimulator or as a recorder) is placed on the available proximal cervical nerves and stimulations are performed. We rely partly on this examination to confirm the integrity of a proximal nerve that is being considered for grafting.
An important limitation is the fact that the sensory (posterior) roots and motor (anterior) roots may be differentially injured. When an SSEP is present, it does not signify that the motor roots are intact, and vice versa. Another limitation is that SSEPs are easily affected by the depth of anesthesia and technical difficulties; a useful normal control may be established by testing the SSEPs in the unaffected upper limb simultaneously. MEP recording and interpretation are also sometimes challenging and require a team experienced with the technique.
NAPs evaluate the presence of functioning axons, both sensory and motor, over a segment of nerve. Although they can be used to assess preganglionic injury, we routinely use SSEPs and MEPs instead for this purpose. We do use NAPs to assess all postganglionic neuromas-in-continuity. Stimulating and recording electrodes are placed directly on the nerve, proximal and distal to a neuroma-in-continuity ( Fig. 34.12 ). At least 4 cm of separation is required to record reliable NAPs. If a good NAP is present across a neuroma-in-continuity, the likelihood of significant functional recovery over time is high and the lesion should undergo neurolysis but not resection. We do not routinely use compound muscle action potential recordings in assessing incomplete lesions. Based on a large basic science and clinical experience, Kline and colleagues have shown that when no NAP is obtained across a neuroma-in-continuity, this lesion has minimal potential for spontaneous recovery and should be excised and grafted. , ,
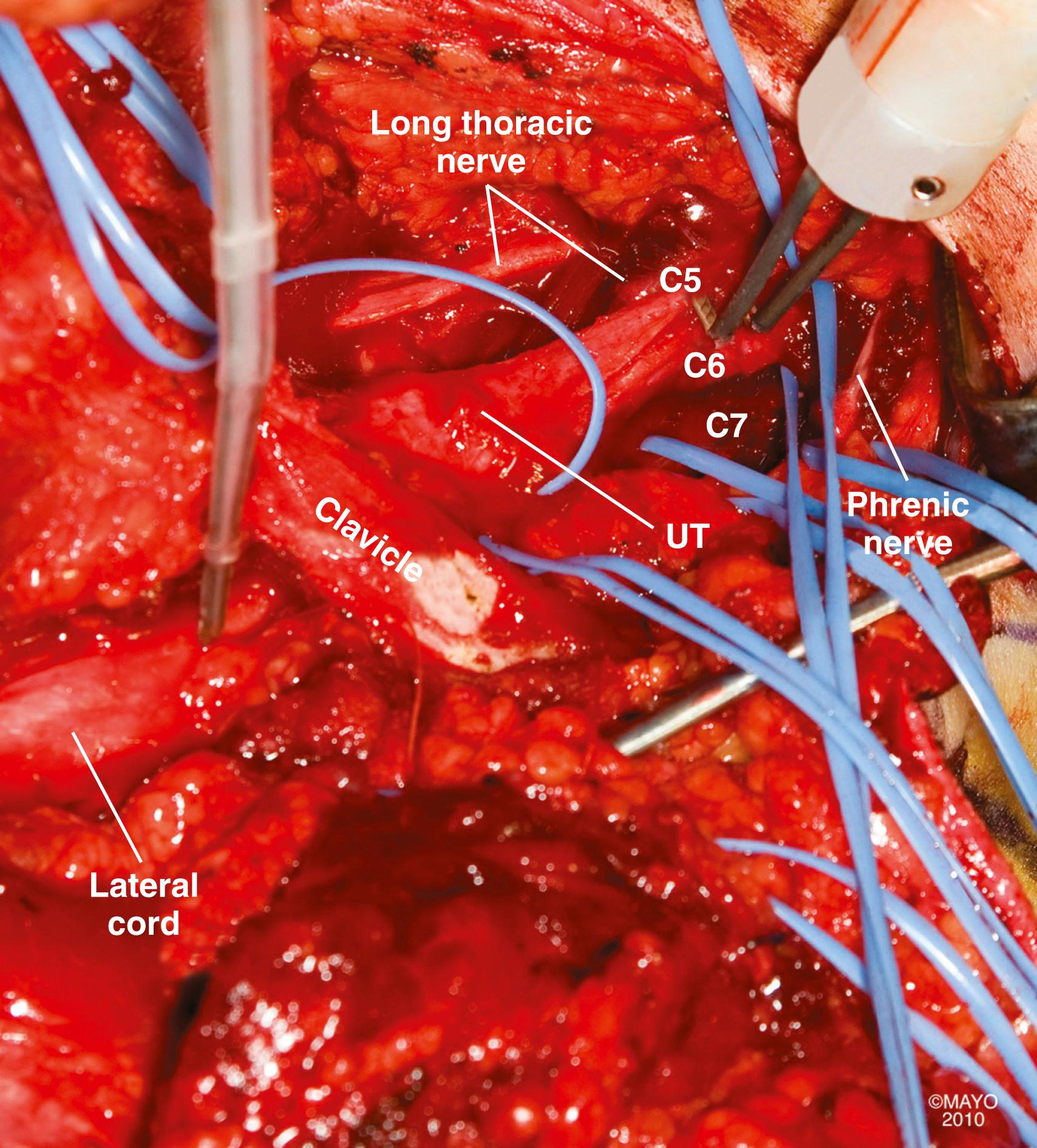
While performing intraoperative electrodiagnostic testing, we also observe the effects of direct nerve stimulation on muscle contraction. The phrenic or other functioning proximal nerves can serve as a normal positive control.
ChAT activity has been used clinically to differentiate between motor and sensory fascicles intraoperatively during nerve grafting procedures , and also to determine the availability of the proximal nerve stump as a donor motor nerve during brachial plexus surgery. ChAT is an enzyme of acetylcholine synthesized in the nerve cell body and transported by axonal flow to the nerve terminals. Its activity is higher in motor fascicles than in sensory fascicles, and this differential expression can be used to identify motor fascicles of good quality (ChAT activity >2000 cpm). , Some investigations have demonstrated that ChAT may not be as reliable as previously reported. This fact, in addition to the prolonged time of testing, makes the use of this test difficult in the clinical situation.
Primary brachial plexus nerve reconstruction is based on functional priorities, depends on intraoperative assessment of the nerve damage and available motor axon donors, and is individualized intraoperatively. When only some elements of the brachial plexus are injured or the nerve gaps are short, we prefer to reestablish continuity to all injured parts by using every available donor nerve. When all brachial plexus elements are injured or the nerve gaps are long, this approach becomes impossible; autologous donor nerve tissue is not available to restore continuity to all parts of the brachial plexus, and priorities for reconstruction should be set. The priorities for reconstruction are based on three factors: (1) the functional importance, (2) the likelihood of regaining function after nerve reconstruction (proximal muscles are reinnervated more successfully than distal muscles), and (3) the degree of difficulty in restoring function by secondary reconstructive surgery.
For a patient with an injury limited to the upper plexus nerves (C5 and C6), the priorities are regaining elbow flexion and shoulder stability, abduction, and external rotation. Elbow flexion is generally achieved by reinnervation of the biceps and brachialis muscles (via the anterior division of the upper trunk, the musculocutaneous nerve proper, or the motor branches to the biceps and brachialis). Shoulder stability, abduction, and external rotation are usually obtained with reinnervation of the supraspinatus and infraspinatus muscles (via the suprascapular nerve) and the deltoid (via the posterior division of the upper trunk, the axillary nerve proper, or the anterior division of the axillary nerve).
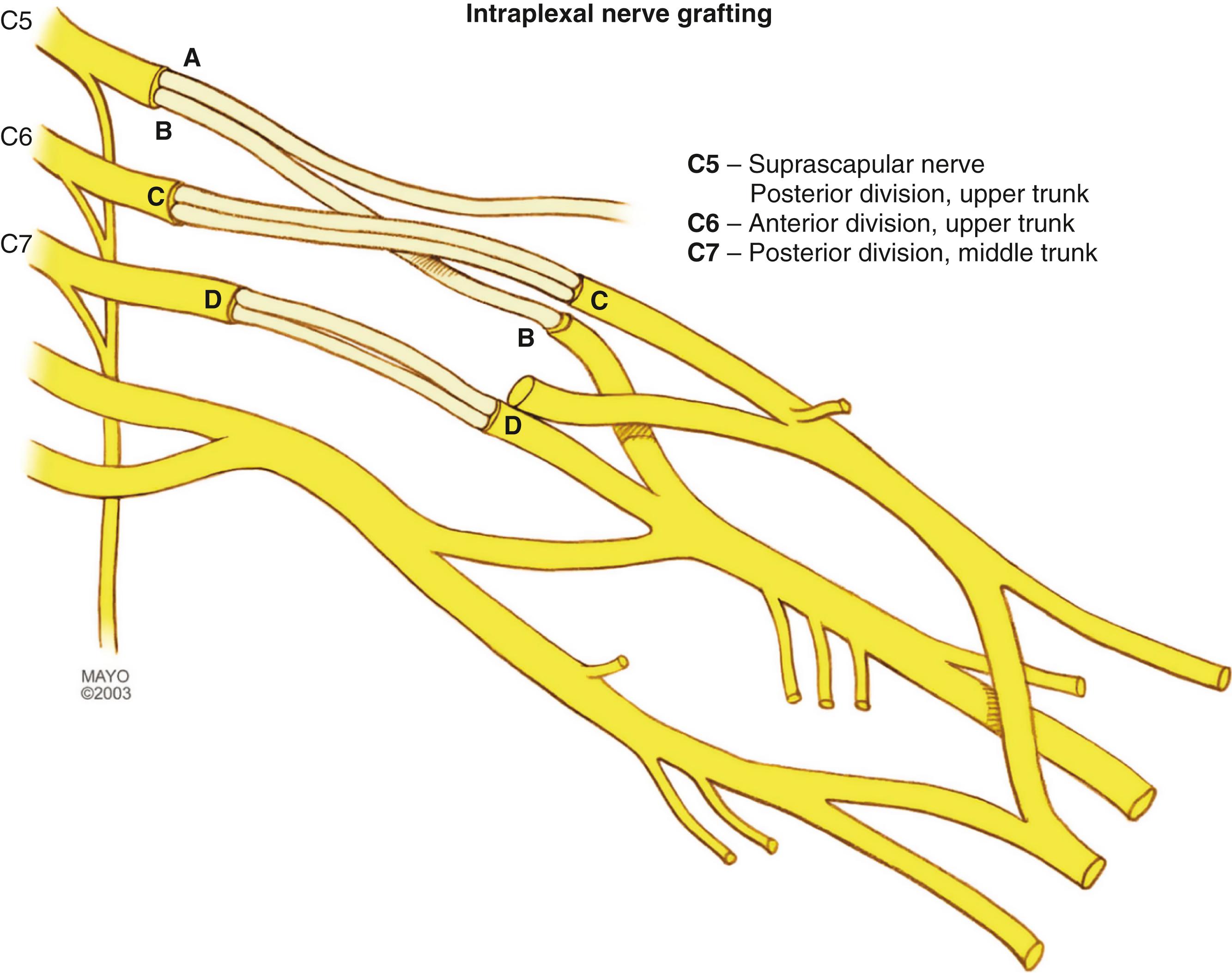
In the majority of patients with C5-C6 loss of function, we explore the brachial plexus and perform intraoperative recordings. In a patient with a nonrecovering postganglionic injury (i.e., a postganglionic neuroma-in-continuity with a negative NAP or a postganglionic nerve rupture), we typically reinnervate the shoulder by using interposition grafts between the C5 nerve stump and the suprascapular nerve and the posterior division of the upper trunk. Another option that we may use for shoulder reinnervation is an interposition graft between the C5 nerve stump and the posterior division of the upper trunk combined with transfer of the spinal accessory nerve to the suprascapular nerve. In patients in whom a functional C5 nerve stump is not identified or in those explored late (>6 to 9 months after injury), we perform double-nerve transfers (to suprascapular and axillary nerves) for shoulder reinnervation. Our preferred technique includes a spinal accessory nerve transfer to the suprascapular nerve and a triceps motor branch transfer to the anterior division of the axillary nerve. Consistent with recent literature authored by others, for elbow reinnervation, we typically perform fascicular nerve transfer(s) preferentially over C6 nerve grafting. Our preferred combination is transfer of an ulnar nerve fascicle to the biceps motor branch, sometimes combined with a second nerve transfer of a median nerve fascicle to the brachialis motor branch. Several reports have demonstrated that the addition of the second transfer has not statistically improved outcomes. ,
Patients with C5-C7 injuries have deficits in shoulder motion and elbow flexion (as do patients with C5-C6 injury), as well as variable deficits in elbow extension (triceps) and wrist extension. The priorities for primary nerve reconstruction are similar to those in patients with C5-C6 injury: regaining elbow flexion and shoulder stability, abduction, and external rotation. In addition, if elbow extension (uncommon) or wrist extension is severely affected, these problems should be addressed. The approach for nerve reconstruction differs because of the variable degree of weakness and the available donor motor axons. Generally, a combination of nerve grafts (if proximal nerves are available) and nerve transfers (spinal accessory, intercostals, pectoral, thoracodorsal, ulnar and median nerve fascicles) is performed.
Patients with this pattern of injury have weakness in the extrinsic and intrinsic muscles of the hand and more variable wrist weakness. The indications, timing, and approach to reconstruction are controversial. As stated previously, nerve grafting is not appropriate for these rare injuries because of the long reinnervation distances. Some surgeons are performing distal nerve transfers in patients with C8-T1 lesions when seen early. Because patient numbers are small, the results of such procedures have yet to be firmly determined. One can certainly perform early or delayed tendon transfers for an isolated C8-T1 injury. An individualized approach is required, with careful clinical and electrodiagnostic assessment of the strength of potential available donors. Reconstruction of thumb flexion, finger flexion, opposition, and intrinsic-minus claw deformity, as well as finger and thumb extension, will be necessary. If C7 is also affected and tendon transfer options are limited, the use of FFMT may also be appropriate.
In patients with complete pan-plexal injuries, our priorities of repair, in order of importance, include the following:
Elbow flexion by reinnervation of the biceps/brachialis muscle
Shoulder stabilization, abduction, and external rotation by reinnervation of the suprascapular, axillary nerves, and long thoracic nerve if possible
Hand sensation by reinnervation of the lateral cord (C6-C7 distribution)
Wrist and finger flexion (When hand reconstruction is performed, the triceps muscle should also be considered for reinnervation because it is a useful antagonist of elbow flexion; as such, it potentiates the strength of a FFMT that crosses the elbow joint.)
Wrist and finger extension
Intrinsic hand muscle function (not typically attainable in a pan-plexus injury)
In patients with complete root avulsions of the brachial plexus, no functioning spinal nerves are available proximally to restore function to the extremity. In these situations, there are no local musculotendinous transfers available for restoration of extremity function. Intact extraplexal nerves (spinal accessory, intercostal, C3 and C4 nerves) can be transferred and coapted to the distal peripheral nerve of the brachial plexus as a method for reinnervation of critical sensory or motor nerves.
Conventional goals in patients with pan-plexus injury include a stable shoulder and elbow flexion with or without some hand sensation. Advances in microsurgical techniques, as well as novel application of FFMTs in the acute setting (<6 months after injury), have enabled us to achieve rudimentary prehension in select patients. In all patients, we explore the supraclavicular brachial plexus in the hope of finding some viable and usable nerves from the proximal stumps; even in cases with evidence of multiple avulsions based on preoperative testing, exploration may occasionally reveal a viable nerve stump. In our experience, only rarely do we find more than one proximal nerve stump that can be used for nerve grafting in severe brachial plexus injuries. If available, we use this stump to reinnervate shoulder targets (suprascapular and axillary nerves). Our highest priority for nerve reconstruction in patients with complete injury is elbow flexion. For this reason, we have chosen to doubly reinnervate elbow flexion when possible, often using intercostal nerve transfers to the biceps motor branch in addition to a gracilis FFMT innervated by the intercostals as well. Shoulder abduction, stability, and especially external rotation are provided either by using spinal accessory nerve transfer to the suprascapular nerve (when no available nerves are present) or by leaving the spinal accessory nerve intact and performing a delayed lower trapezius tendon transfer for external rotation. Other options remain for later shoulder reconstruction if these measures prove unsuccessful. When additional nerve resources are available (i.e., more than one viable nerve), we will also target the serratus anterior (with a single intercostal nerve) or pectoralis major.
If hand reinnervation is attempted, a single-stage gracilis FFMT is used for both elbow flexion and finger flexion. The shoulder is stabilized with nerve grafts to either the suprascapular nerve or axillary nerve, or both, from an available cervical nerve stump or with a planned secondary trapezius tendon transfer. The spinal accessory nerve is transferred to a triceps branch with an intervening nerve graft for reinnervation of elbow extension. Sensation in the median nerve distribution of the hand is provided by the transfer of sensory branches of the intercostal nerves to the lateral cord contribution of the median nerve. Secondary surgeries to provide stability to the hand and assist in rudimentary grasp are performed between 4 and 6 months after the index brachial plexus reconstruction. Secondary surgeries include selected fusions and at times, soft tissue balancing. The fusions include wrist arthrodesis, thumb carpometacarpal arthrodesis (to place the thumb in palmar abduction and pronation), and thumb interphalangeal joint arthrodesis. The soft tissue balancing is primarily achieved with passive flexor digitorum superficialis lasso procedures to create a metacarpophalangeal joint contracture for prevention of a claw deformity. Other options for hand reanimation have also been proposed, including a two-stage FFMT and contralateral C7 (entire or half [hemi]) transfer to the median nerve, as well as extended phrenic nerve transfers to the median nerve.
Variable findings are due to different injury patterns in isolation or combination. Intraoperative NAP recordings are used in each case, and nerve grafting is commonly performed when NAPs are absent, when the zone of injury is relatively short, and provided good proximal and distal nerve architecture is maintained. Concomitant or staged tendon transfers may be considered for denervated muscles with long reinnervation distances. Nerve transfers, if available donors exist, may be preferable in patients initially seen 6 to 12 months after injury. For example, in cases of posterior cord injury, we would typically excise the neuroma and perform nerve grafting to the axillary nerve and to triceps nerve branches, and standard tendon transfers would be used for wrist, finger, and thumb extension.
The indication for neurolysis after traumatic brachial plexus injury is the intraoperative finding of a postganglionic neuroma-in-continuity and a positive (present) NAP. In most of these cases, the integrity of the proximal nerves is confirmed with intraoperative SSEPs and MEPs; however, in patients with a clearly isolated infraclavicular injury, testing of evoked potentials may not be necessary.
Microsurgical neurolysis consists of liberating the nerve from adhesions to neighboring tissue at the epineurial level (external neurolysis). Occasionally, internal neurolysis is indicated and performed under the microscope. This consists of longitudinal incisions made in the fibrotic layers of the epineurium and the epifascicular epineurium; all fascicles are completely separated to remove the surrounding scar tissue.
Direct nerve repair is preferable to nerve grafts if it can be performed without excessive tension. However, neurorrhaphy is rarely possible after traumatic brachial plexus injury, except in cases of sharp transection that are explored early. Mobilization of the nerve stumps may make direct repair possible in some situations. In rare cases, direct repair of individual nerves or nerve branches with focal neuromas that are resected may be possible, provided that the nerve ends can be mobilized sufficiently and sutured without tension.
Nerve grafting is indicated in patients with postganglionic nerve ruptures or with postganglionic neuromas that do not conduct a NAP (i.e., after resection of which, a gap exists that cannot be sutured directly without tension). In these cases, the nerve is in continuity with the spinal cord and has maintained viable motor axons that can be grafted to specific targets. When nerve transfer options exist, some surgeons prefer them to nerve grafting. Our preference, unless the surgery is performed late, the muscle targets are very distal, or the graft length is long (>10 cm), is to use nerve grafting when proximal nerves are available and in good condition.
Nerve grafting consists of the coaptation of interposition grafts (e.g., cable grafts of the sural or other cutaneous nerves) between the desired nerve stumps and targets. Generally, the C5 nerve is used to achieve shoulder stability, abduction, and external rotation (suprascapular nerve, axillary nerve, or posterior division of the upper trunk), the C6 nerve to target elbow flexion (anterior division of the upper trunk or musculocutaneous nerve), and the C7 nerve to target elbow extension (posterior division of the middle trunk or radial nerve) ( Fig. 34.13 ).
The most commonly used donor nerve for brachial plexus injury is the sural nerve, a purely sensory nerve that innervates a small area on the dorsolateral region of the foot ( Fig. 34.14 ). If harvested from the popliteal fossa to the lateral aspect of the ankle, up to 35 to 40 cm of donor nerve can be obtained from each leg. The sural nerve can be harvested in the supine position by placing a pad beneath the donor-side buttock to internally rotate the leg because this nerve is located posteriorly in the calf. The sural nerve can be dissected and mobilized with the leg in extension or with the knee flexed. Alternatively, an assistant can hold the leg elevated during harvest. The use of a tourniquet at the upper thigh level is optional; however, if used, surgeons must make certain that the limb tourniquets are deflated and disconnected or removed after harvesting the nerve grafts. Different techniques can be used, including multiple transverse or vertical incisions, a single incision along the course of the nerve (which is our preference), and minimally invasive techniques, using a tendon passer or an endoscope. The nerve is first identified between the lateral malleolus and Achilles tendon in the subcutaneous tissues adjacent to the lesser saphenous vein. Gentle traction is applied, and the remaining portion is harvested up to the popliteal fossa; dissection of the nerve should be as atraumatic as possible. The nerve is transected as far distally and proximally as possible. Communicating branches should be harvested. The skin wound is closed in layers, and the legs are wrapped in elastic bandages.
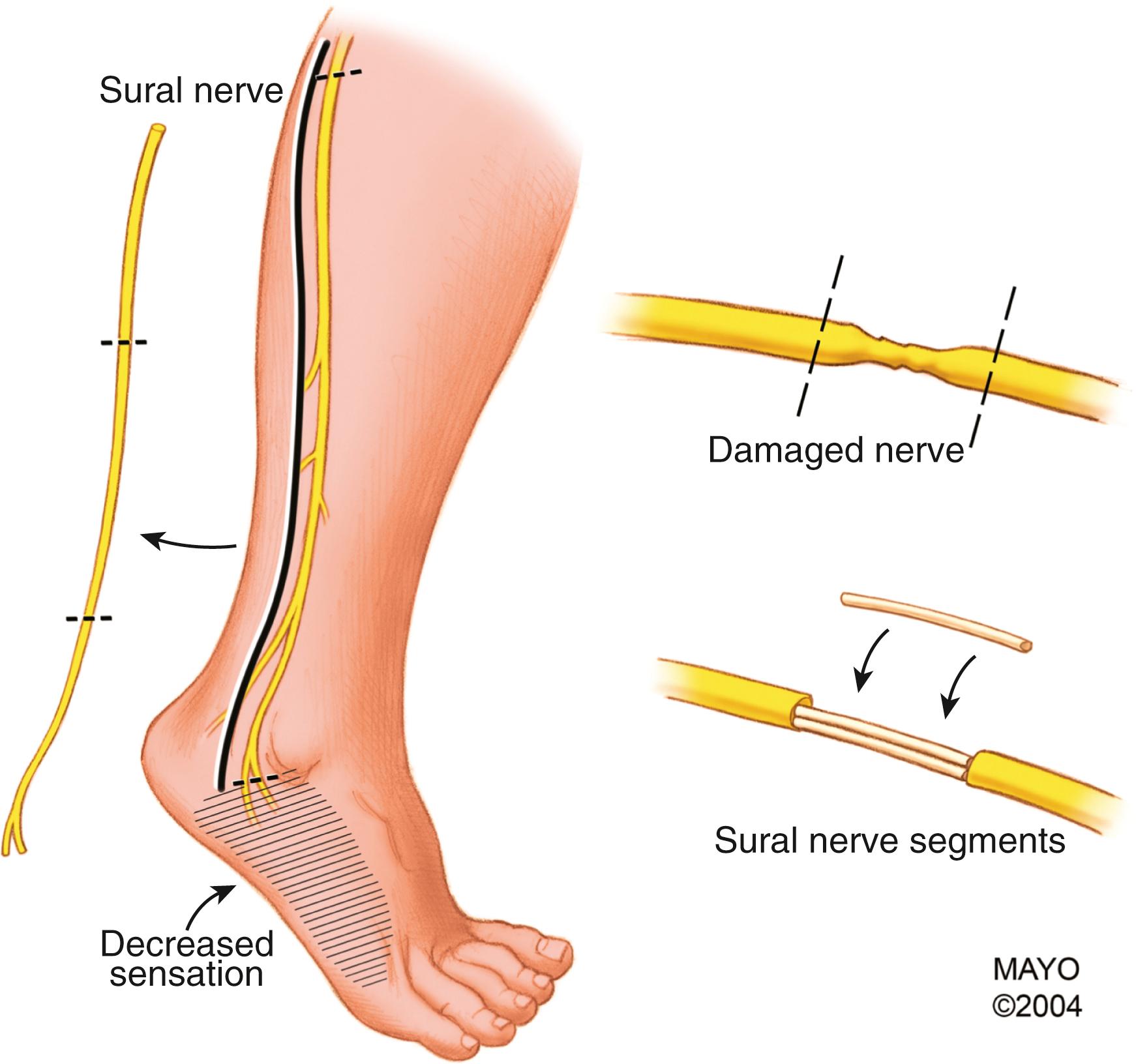
If needed, other cutaneous nerves may be used as interposition grafts, including the superficial radial, medial brachial or antebrachial cutaneous, lateral antebrachial cutaneous, and dorsal cutaneous branch (of the ulnar) nerves. For long defects or when a large-diameter graft is required, some recommend vascularized nerve grafts (pedicled or free). The most common vascularized nerve graft is the ulnar nerve (when it is nonfunctional), as described later with contralateral C7 transfer, but the superficial radial nerve and even the sural nerve may also be used as vascularized nerve grafts.
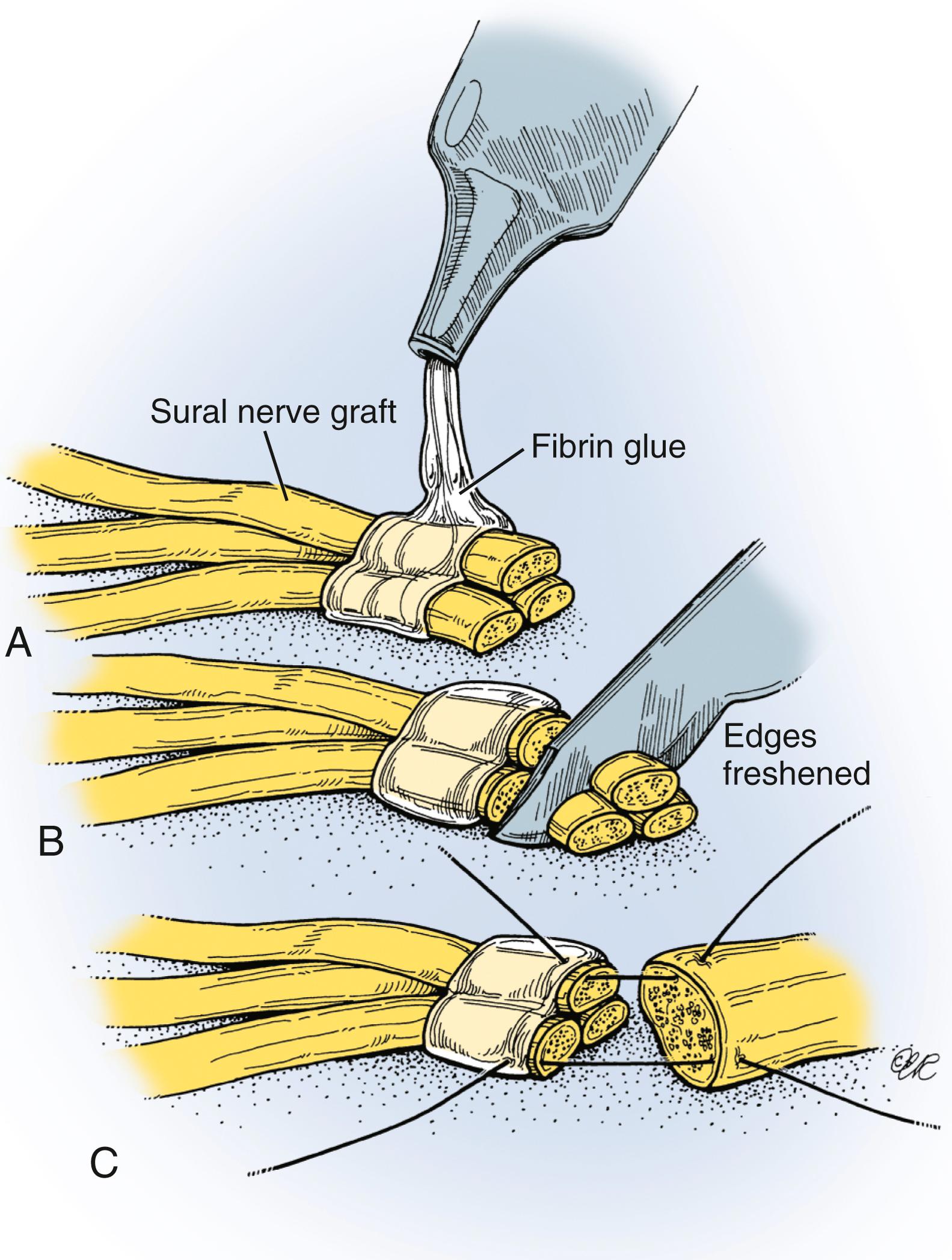
Coaptations should be performed without tension, and the grafts should ideally fill the cross-sectional area of the stumps. Usually, two or three segments of graft are cut and used to fill the area ( Fig. 34.15 ). When possible, different fascicular patterns are matched individually to lead the regenerating axons to the distal nerve, typically prioritizing motor fascicles. An understanding of the anatomic topography of the nerves at different levels can be helpful to align fascicles. Several 8-0 to 10-0 nylon epineurial sutures are commonly placed between each nerve graft and the proximal and distal nerves under microscope or loupe magnification. Tissue adhesive can be used to approximate multiple strands of nerve graft and simplify the coaptation with proximal and distal nerves ( Fig. 34.16 ). We generally reinforce the nerve coaptation sites with the use of a bivalved nerve conduit and tissue adhesive.
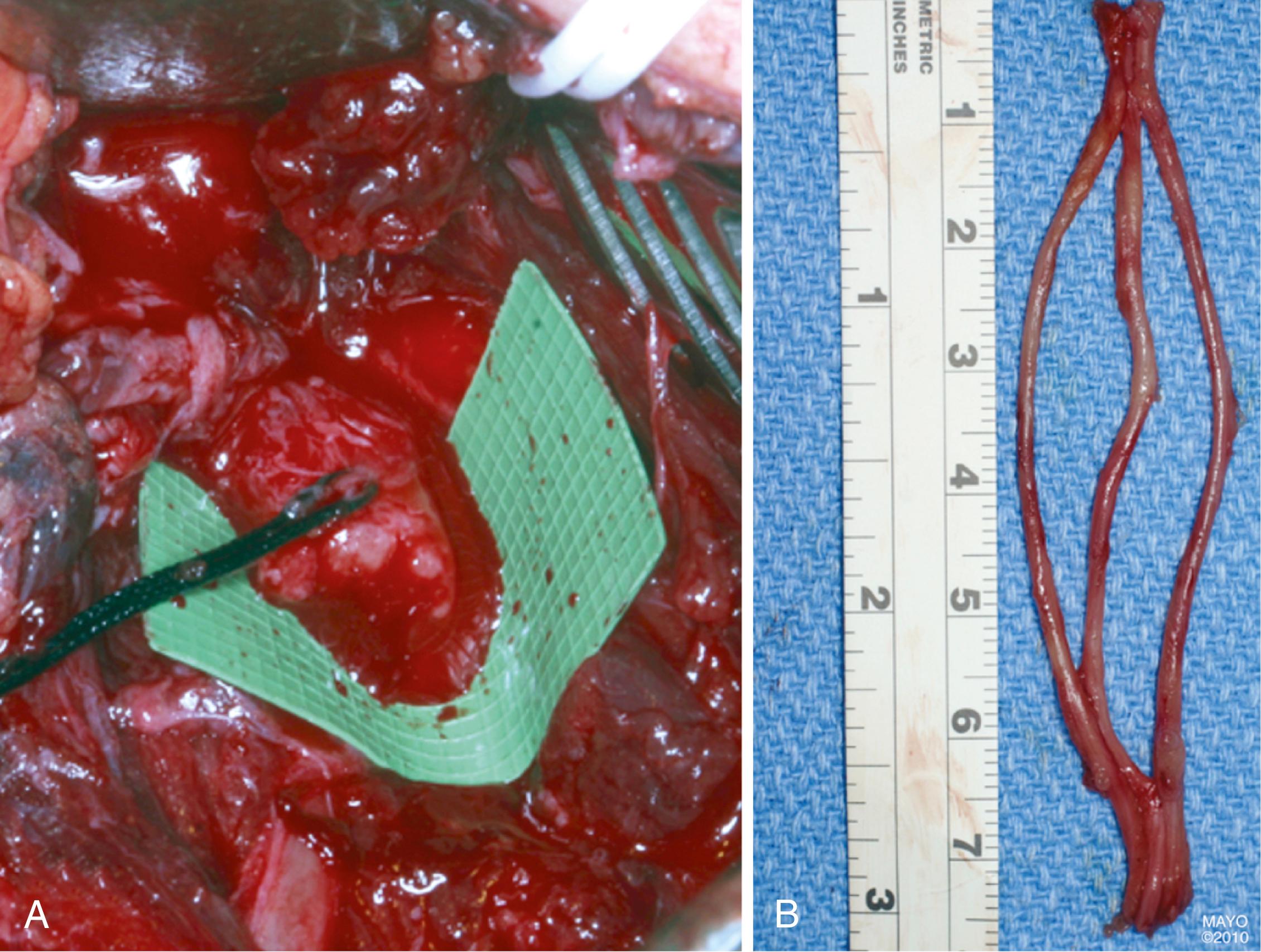
Nerve conduits are increasingly being used in nerve surgery for small-caliber (more commonly sensory) nerves, for short nerve gaps, or as an adjunct to nerve repair or transfer; however, in proximal brachial plexus injuries, this technique is not recommended by our group in adults at this time as a substitute for nerve grafts.
The use of nerve allografts that have been decellularized by a variety of methods has gained popularity in short-gap digital sensory nerve defect reconstruction as well as larger mixed nerve defect reconstruction. Use of these allografts in proximal brachial plexus reconstruction or for long nerve or motor graft reconstruction is not recommended at this time unless nerve autograft is unavailable.
The terms nerve transfer, neurotization, and nerve crossing have been used interchangeably in the literature, and it is recommended that the term neurotization be used exclusively for transfer of a nerve directly into muscle for purposes of reinnervation. Nerve transfer is used most frequently in the current literature and describes the transfer of a normal or nearly normal fascicle or nerve branch to an important sensory or motor nerve that has sustained irreparable proximal damage.
The indications for nerve transfer are irreparable preganglionic injury, selected postganglionic injury, and reinnervation of FFMTs. Important attributes of nerve transfers are as follows: They are closer to the end organ (which means that there is increased likelihood of more rapid recovery or more reliable recovery, or both). There is delivery of a large number of “pure” axons. Repair takes place outside the injured and scarred zone. The use of an intercalated nerve graft introduces a second repair site and should be avoided when possible in nerve transfer. Rehabilitation is more easily accomplished, especially with synergistic transfers. Common intraplexal and extraplexal nerve transfers are described in the following sections.
Spinal Accessory Nerve Transfer
The spinal accessory nerve (cranial nerve XI) innervates the sternocleidomastoid and trapezius muscles. It originates in the posterior cranial fossa from spinal and cranial nerve roots, passes through the jugular foramen, and divides into an internal branch (containing fibers originating from the “cranial part”), which joins the vagus nerve (cranial nerve X), and an external branch (consisting of fibers from the “spinal part”), which innervates the sternocleidomastoid and trapezius muscles. After supplying the sternocleidomastoid muscle, the nerve descends obliquely in the posterior triangle of the neck between the superficial and deep layers of the deep cervical fascia ( Fig. 34.17 ). In this area the nerve is embedded in loose connective tissue and is in contact with the cervical lymph node chain. This is the most common location for iatrogenic injuries to the spinal accessory nerve during lymph node biopsies. The spinal accessory nerve provides two or three branches to the upper part of the trapezius muscle before passing under its anterior edge. Intramuscularly, the nerve follows an oblique caudal course toward the middle and lower parts of the trapezius and gives off branches to the muscle throughout its course. There is great variability in connections between the spinal accessory nerve and the roots of the cervical plexus.
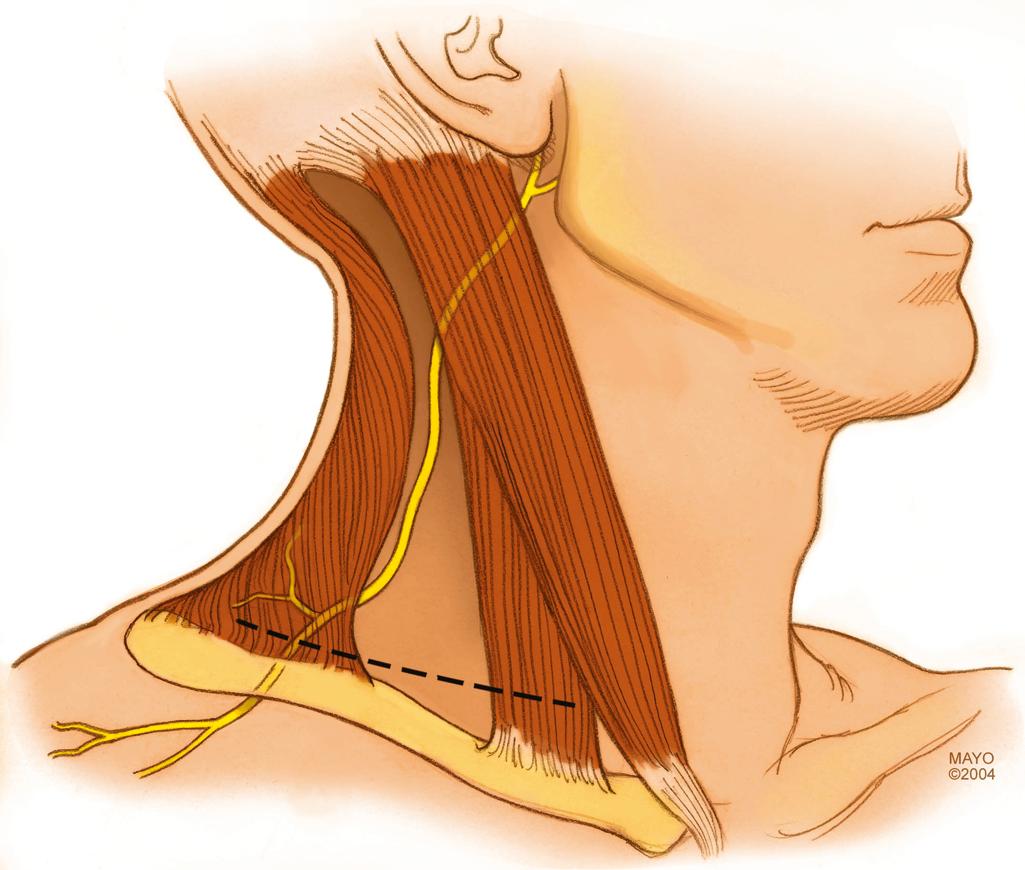
As with exploration of the supraclavicular plexus through the described skin incisions, turning the neck to the contralateral side and elevating the shoulder and neck off the surgical table facilitate access to the spinal accessory nerve. Muscle relaxation is avoided to allow the use of electrical stimulation to confirm identification of the spinal accessory nerve.
The spinal accessory nerve is often identified in an incision that parallels the posterior border of the sternomastoid. The nerve may be traced from the posterior border of the sternocleidomastoid muscle and traced as far as possible along the undersurface of the trapezius to its decussation, where it is transected. The nerve can be transferred to the supraclavicular area for coaptation to any of several denervated targets, most frequently the suprascapular nerve. Although this is the most popular approach to the spinal accessory nerve, there are some disadvantages. First, it creates a large longitudinal scar on the neck. Second, this extensive approach can inadvertently denervate proximal branches to the upper part of the trapezius, which should be preserved.
Our preferred technique uses the lateral portion of the transverse supraclavicular cervical incision described above; this approach makes a less conspicuous scar over the clavicle. The spinal accessory nerve can be identified several centimeters above the clavicle on the anterior surface of the trapezius. This nerve should not be confused with more superficial cervical plexus elements that may run in similar directions. These branches from the cervical plexus can be distinguished from the spinal accessory nerve by their size, which is comparatively small, and by the response of the nerves to electrical stimulation. The spinal accessory nerve should produce vigorous contraction of the trapezius.
The spinal accessory nerve should be dissected as far distally as possible. A proximal branch to the upper portion of the trapezius should be preserved. The nerve can then be divided and transferred. Some authors transect the spinal accessory nerve more proximally, just distal to the sternocleidomastoid muscle; we do not recommend this approach because an intervening nerve graft will be required to reach the distal target, which may reduce the success of the procedure.
Patients with spinal accessory nerve dysfunction preoperatively are not candidates for this procedure. Because trapezius function is difficult to assess on physical examination in patients with paralyzed shoulder muscles, preoperative EMG can help identify an abnormality. Intraoperative stimulation is the definitive assessment of spinal accessory nerve function.
There are generally no serious complications from this procedure as long as the proximal branch of the spinal accessory nerve to the upper fibers of the trapezius muscle is preserved. If the proximal branch to the trapezius is injured, shoulder depression and dysfunction may occur.
The suprascapular nerve, which innervates the supraspinatus and infraspinatus muscles, originates from the upper trunk 2 to 3 cm above the clavicle. In some patients, its origin can be higher, whereas in those with ruptures or avulsion injuries of the upper trunk, the nerve can retract to the retroclavicular or infraclavicular area. The suprascapular nerve courses laterally and posteriorly, deep to the omohyoid muscle, and enters the supraspinatus fossa through the suprascapular notch, deep to the superior transverse scapular ligament. The nerve then traverses the supraspinatus fossa deep to the supraspinatus muscle and winds around the lateral border of the spine of the scapula (the spinoglenoid notch) to enter the infraspinatus fossa. Its course renders it particularly vulnerable to traction lesions at the suprascapular notch, at the spinoglenoid notch, and within the supraspinatus and infraspinatus fossae. The suprascapular nerve is dissected bluntly with finger dissection to the suprascapular notch to confirm its integrity. This is done even when the location of a lesion proximal to the upper trunk is obvious to ensure that a secondary (distal) lesion is not present. To perform the nerve transfer, the suprascapular nerve is transected at its origin from the upper trunk ( Fig. 34.18 ). If there is no scarring, it may be possible to dissect it further proximally and separate it from the upper trunk to increase the available length. The suprascapular nerve is coapted to the spinal accessory nerve without an intervening nerve graft.
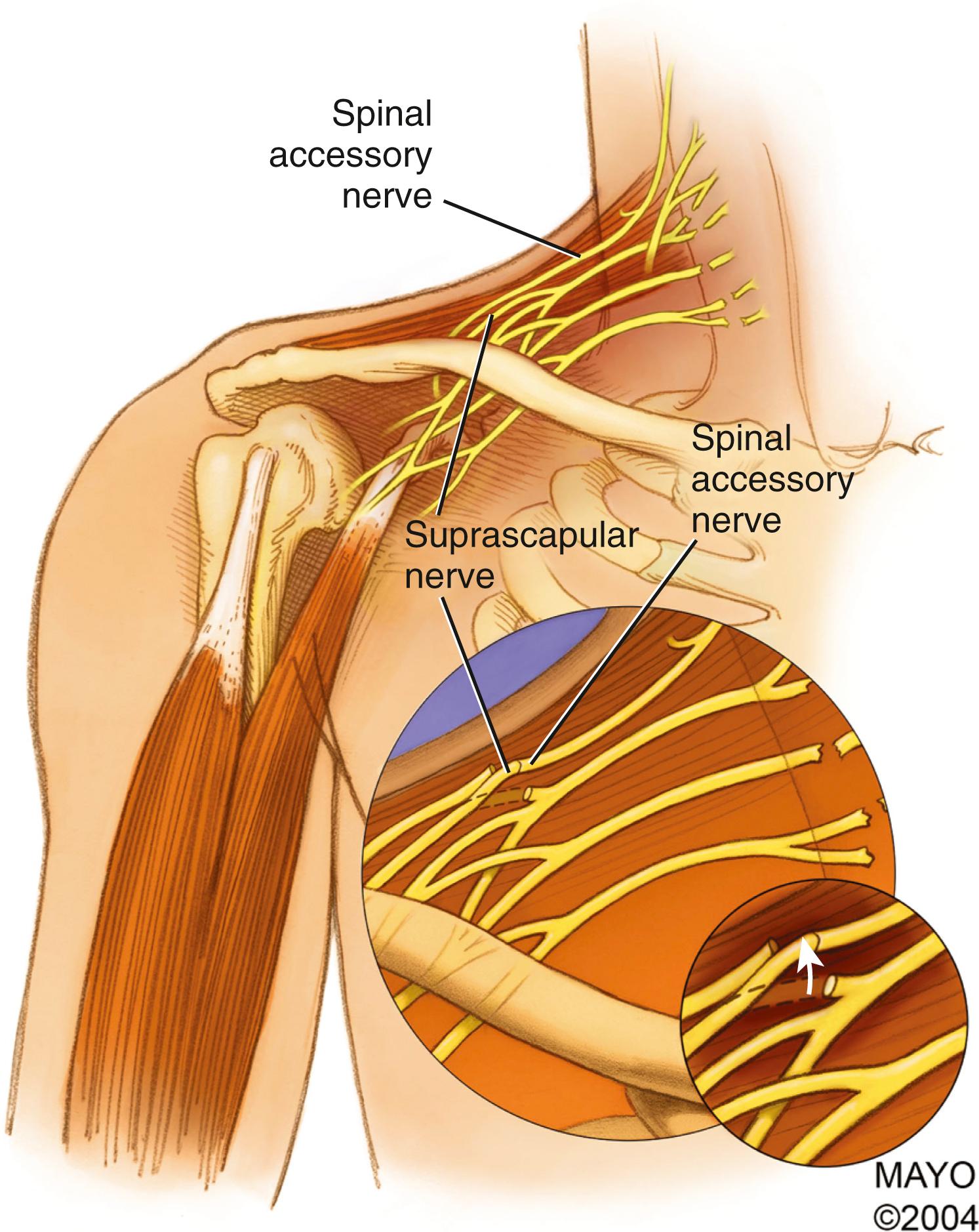
Others have proposed a posterior approach when the spinal accessory nerve is used to reinnervate the suprascapular nerve, particularly when a segmental injury to the suprascapular nerve at the suprascapular notch is suspected. The advantage of this approach is a more distal repair to a healthy portion of the suprascapular nerve. The disadvantage is perhaps a reduced number of myelinated axons in the more distal terminal spinal accessory nerve and the need for prone or lateral decubitus positioning to enable a posterior approach to the scapula. Both the spinal accessory and the suprascapular nerves can be exposed via a transverse incision at the superior border of the scapula. The suprascapular nerve is located midway between the superior angle of the scapula medially and the acromion laterally. After identifying the nerve at the suprascapular notch, the superior transverse ligament is divided, and the nerve is dissected as far proximally as possible. The spinal accessory nerve is located at a point 40% of the distance between the dorsal midline (spinous processes) and the acromion. It can be identified deep to the trapezius muscle, which is separated parallel to the course of its fibers. The spinal accessory nerve is divided as far distally as possible to achieve a coaptation without tension.
The expected outcome of transfer of the spinal accessory nerve to the suprascapular nerve for restoration of shoulder abduction is influenced by multiple factors, including the type of injury (C5-C6 versus pan-plexus injury) , and the number of transfers performed (concomitant axillary reinnervation or not). The range of outcomes reported in the literature is wide. A metaanalysis of the world literature reported that 92% of patients recovered some antigravity shoulder abduction with nerve transfer to the suprascapular nerve. Multiple other groups , , have reported a range of results, with mean degrees of abduction of between 45 and 122 degrees. Patients with an isolated C5-C6 palsy seem to have better outcomes than patients with a C5-C7 palsy, who seem to have better outcomes than those with a complete brachial plexus injury. , The function of the serratus
Most commonly used for C5-C6, C5-C7, and complete plexus injuries
Ideal for injuries that have occurred less than 6 to 9 months previously
Spare the most proximal branch of the spinal accessory nerve.
Sufficient distal dissection will permit direct transfer of the spinal accessory nerve to the suprascapular nerve without an intervening nerve graft.
Use a transverse skin incision over the distal clavicle.
Transfer only the middle and distal branches of the spinal accessory nerve.
Outcomes are less favorable in patients with C5-C7 or complete injuries.
Shoulder immobilization is maintained for 3 weeks.
EMG-documented reinnervation begins by 6 months postoperatively.
anterior muscle may play a role in these outcomes. Patients with partial innervation of the serratus anterior (from an intact C7 nerve) may achieve significantly better range of shoulder abduction and flexion because of better scapular stability. The variability in results may also be due to the difficulty and complexity of assessing the outcome of shoulder motion (either by range of motion or muscle grading). The outcome of transferring the spinal accessory nerve to the suprascapular nerve for restoration of external rotation is less frequently reported, but it varies from 0 to 118 degrees of external rotation, , depending on the injury pattern and the studies ( Fig. 34.19 ).
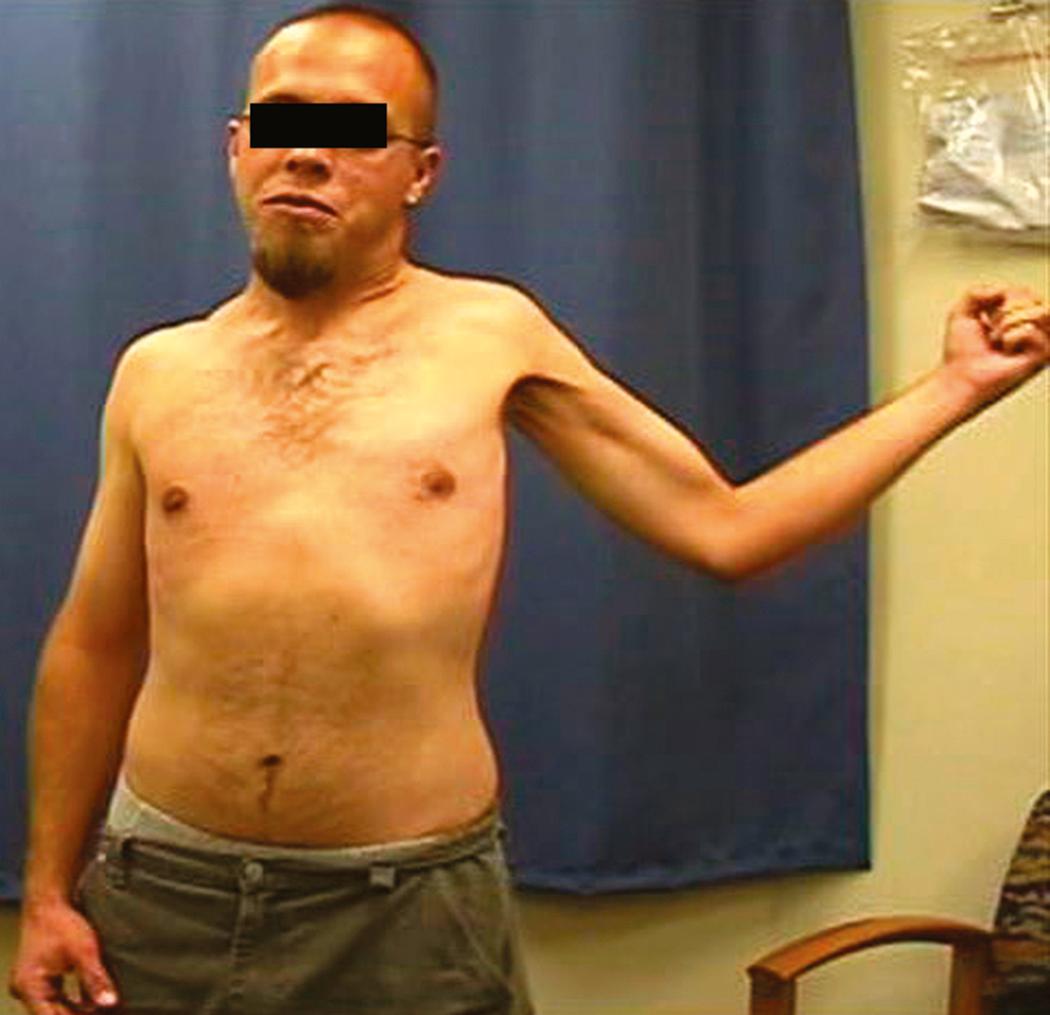
Transfer of the spinal accessory nerve to the musculocutaneous nerve is occasionally used to restore elbow flexion ( Fig. 34.20 ). However, we more frequently choose to transfer ulnar nerve fascicles (in C5-C6 injuries) or intercostal nerves (in pan-plexus injuries) to the motor branch of the biceps. Use of the spinal accessory nerve requires an interposition nerve graft, which may degrade results.
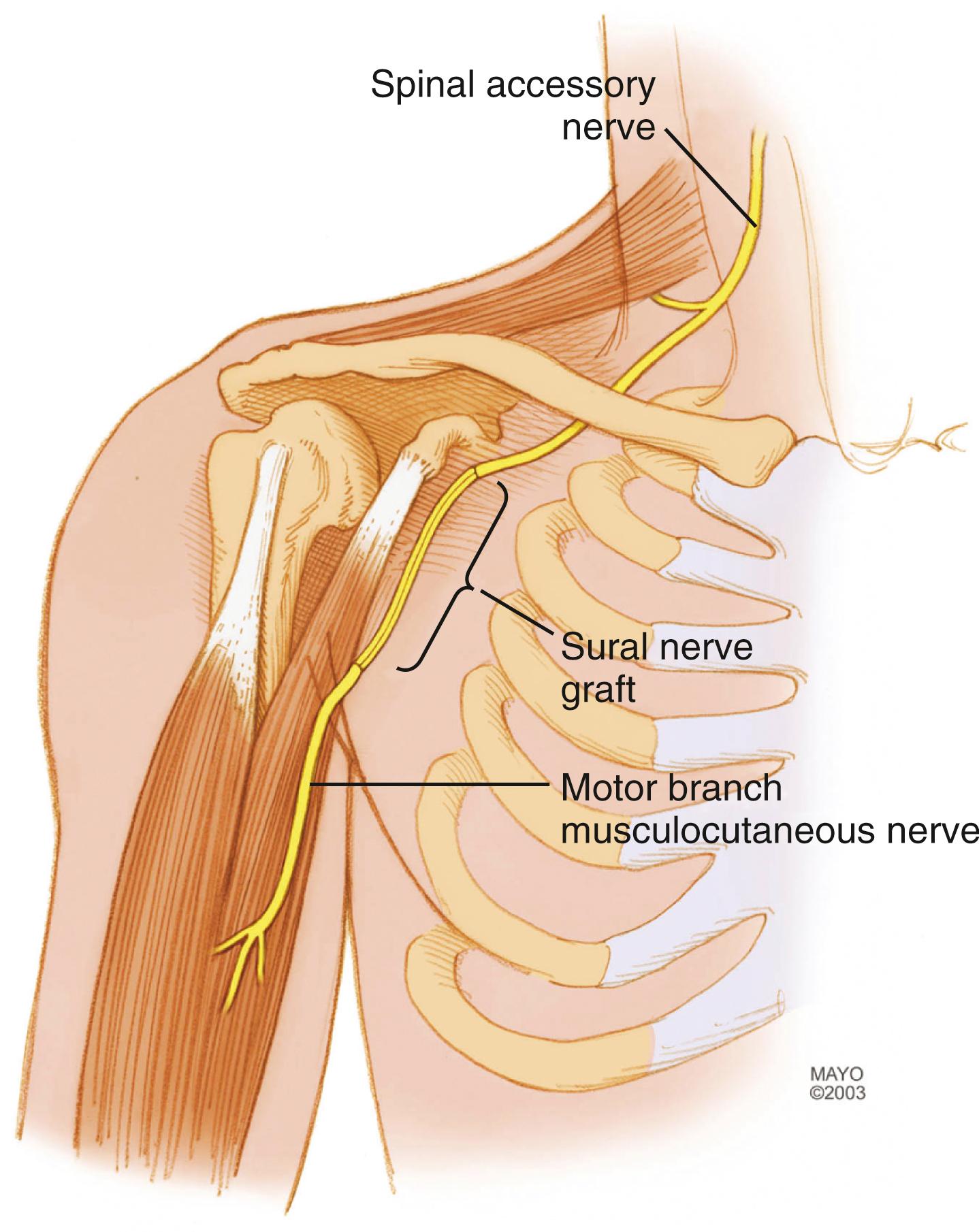
The musculocutaneous nerve may be exposed through an extended deltopectoral or a proximal arm skin incision. The musculocutaneous nerve originates from the lateral cord of the brachial plexus just distal to the coracoid process. After it pierces the coracobrachialis muscle, the nerve runs on the deep surface of the biceps. The musculocutaneous nerve then gives rise to the motor branch or branches to the biceps, which enter the muscle approximately 12 cm distal to the acromion. Two types of distribution of motor branches to the two heads of the biceps are found: a common trunk (pattern 1), which will divide into two branches (to the short and long heads of the biceps), and two separate nerve branches (pattern 2), the branch to the short head generally originating more proximally on the musculocutaneous nerve. Intercommunicating nerve fibers can be found between the motor branches. After traveling a distance within the short and long heads of the biceps (median distance of 1 and 2 cm, respectively, in pattern 1 or 3 cm for each branch in pattern 2), the main branches divide into terminal branches distributed throughout the muscle bellies. The musculocutaneous nerve then gives rise to the motor branch or branches of the brachialis muscle (≈17 cm distal to the acromion) and continues as the lateral antebrachial cutaneous nerve. Once the musculocutaneous nerve and its branches are identified and dissected, the nerve is cut just after it branches to the coracobrachialis muscle. A sural nerve graft (typically 9 to 10 cm long) is used to connect the spinal accessory nerve to the musculocutaneous nerve. The nerve graft is usually placed beneath the clavicle, coapted first to the spinal accessory nerve and then to the musculocutaneous nerve. The repair is performed with 9-0 or 10-0 nylon without tension under magnification with the operating microscope.
A metaanalysis of the outcome of transfer of the spinal accessory nerve to the musculocutaneous nerve for restoration of elbow flexion showed that 77% of patients achieved biceps strength of M3 and 29% achieved strength of M4. The results of spinal accessory nerve transfer (with an interposition graft) versus intercostal nerve transfer (without an interposition graft) for restoration of M3-strength elbow flexion were comparable (77% versus 72%), but significantly lower for restoration of M4-strength elbow flexion (29% versus 41%; P < .001).
In patients with preserved C8-T1 function, transfer of a functioning ulnar nerve motor fascicle for reinnervation of the biceps muscle has gained popularity because of its technical ease and reliability. , This technique has largely replaced the use of intercostal nerve transfers for reinnervation of elbow flexion in cases of C5-C6 and C5-7 palsy. It is often done in these groups of patients in conjunction with other nerve transfers, such as transfer of the spinal accessory nerve to the suprascapular nerve and the triceps motor branch to the axillary nerve for reconstruction of shoulder function. We and others have not recommended using in patients with C5-C8 injury due to suboptimal results (poorer elbow flexion and higher risk of downgrading ulnar nerve function).
The larger of the two terminal branches of the medial cord of the brachial plexus continues as the ulnar nerve, whereas the smaller terminal branch is the medial cord contribution to the median nerve. In the axilla and upper part of the arm, the ulnar nerve runs inferior and medial to the axillary artery. It passes anterior to the triceps muscle and enters the ulnar groove between the medial epicondyle of the humerus and the olecranon. In the upper half of the arm, the ulnar nerve has rich interfascicular connections and 6 to 10 fascicles, each of which contains both motor and sensory nerve fibers. According to Sunderland’s intraneural topography, the posteromedial fascicles contain the motor fibers to the forearm muscles and the anterolateral fascicles tend to contain the motor fibers to the intrinsic muscles of the hand.
Both the musculocutaneous and ulnar nerves are exposed through a longitudinal medial skin incision in the proximal upper part of the arm. The musculocutaneous nerve and its three branches (motor branch to the biceps, motor branch to the brachialis, and lateral antebrachial cutaneous nerve) are identified. After the type of motor branch to the biceps (common or separated branches pattern) is confirmed, the branch is divided sufficiently proximal to be long enough to reach the ulnar nerve ( Figs. 34.21 and 34.22 ). The ulnar nerve is identified and intraneural dissection is performed under loupe or microscope magnification. The ulnar fascicles are tested with a nerve stimulator, and the fascicles that innervate the flexor carpi ulnaris muscle, generally located posteromedially, are identified. Others that do not use nerve stimulation have not reported complications ; the ulnar nerve fascicles at this level are mixed and redundant, and all fascicles contain a mix of motor and sensory nerve fibers. Depending on the size of the motor branch to the biceps, one or two fascicles are selected. These chosen fascicles are transected distally under magnification and with enough length to approximate to the biceps motor branch without tension. They are coapted to the motor branch with 9-0 or 10-0 nylon epineural suture and reinforced with fibrin glue.
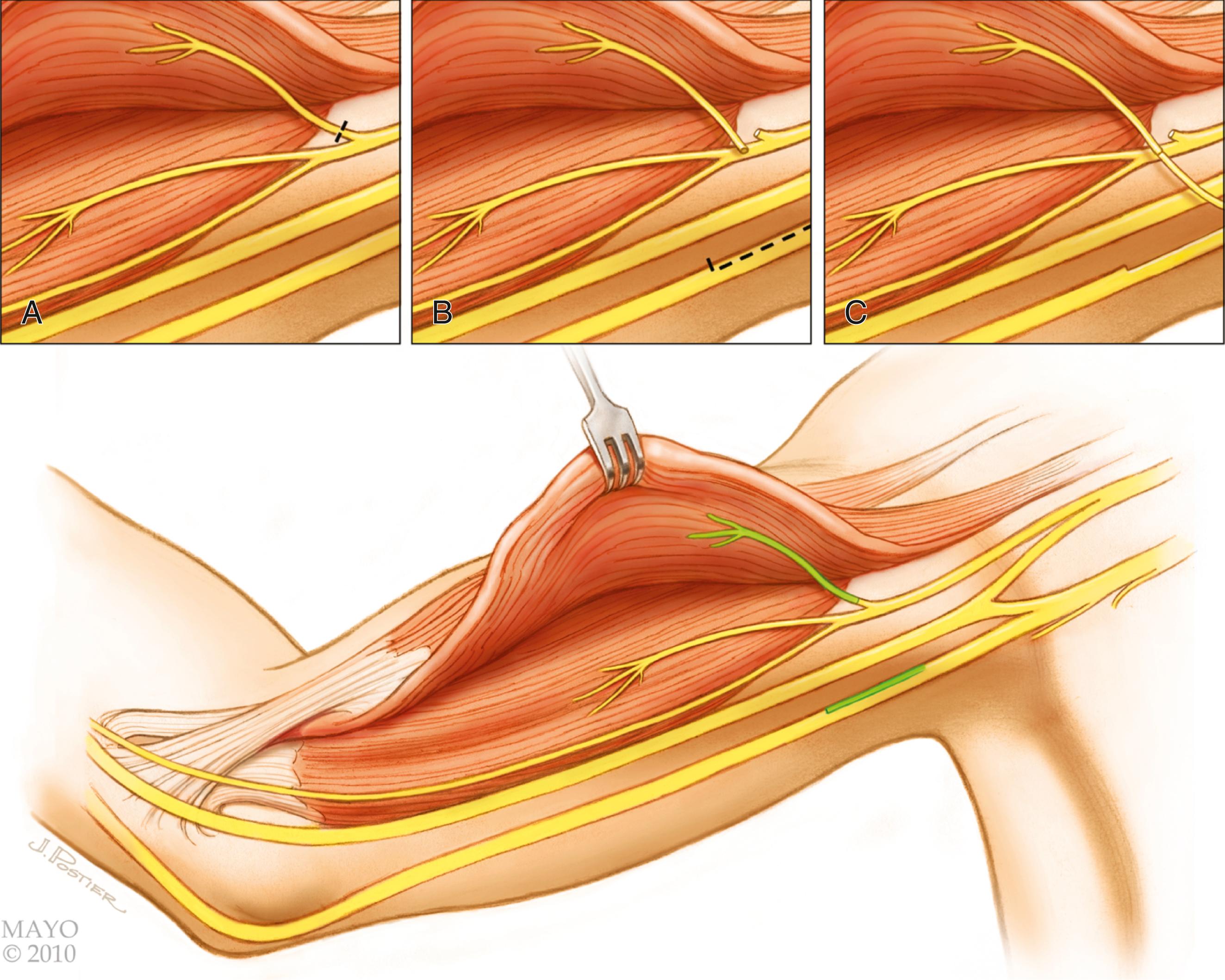
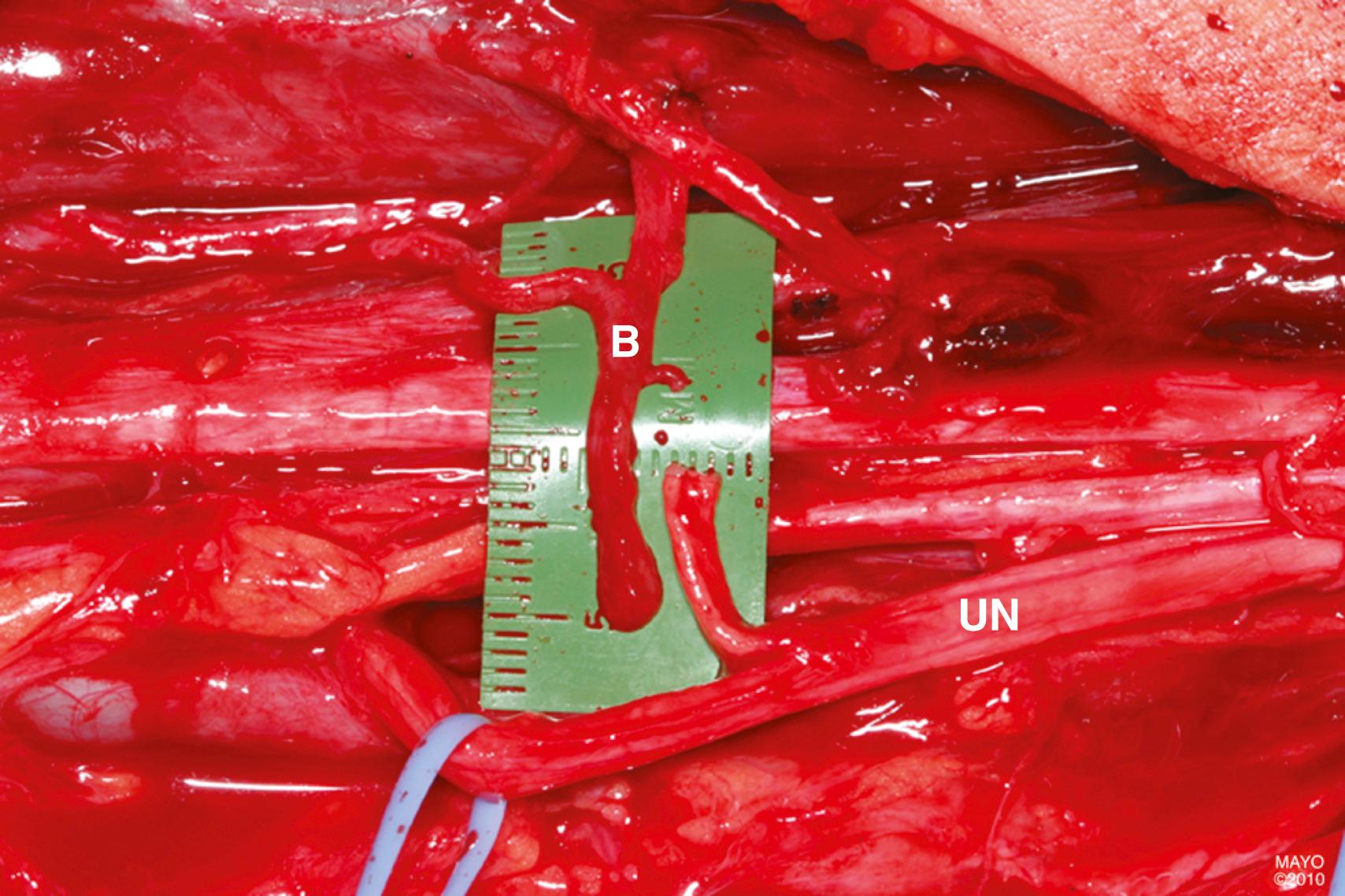
Patients with ulnar nerve distribution weakness and sensory deficit preoperatively are not candidates for this technique.
Some patients will experience transient numbness in the ulnar nerve distribution postoperatively. These sensory changes will improve over a period of days to months and are rarely permanent. Occasionally, patients will have transient muscle weakness of the ulnar-innervated hand intrinsic muscles. Few permanent deficits have been reported. Most patients gain powerful grip and pinch strength during the postoperative rehabilitation program; it may be better than their preoperative strength. Whether this is due to progressive muscle strengthening exercises or a tincture of time in a patient who is spontaneously recovering is unknown.
Most authors emphasize the quick reinnervation of the biceps muscle after this procedure as an important advantage. Many patients have good recovery of biceps contraction beginning several months after the procedure. More than 90% of patients achieved biceps strength of British Medical Research Council (BMRC) grade 3 or 4 ( Fig. 34.23 ).
In 2005, double-nerve fascicular transfer to restore elbow flexion was described in patients with upper pattern brachial plexus injury. , This procedure combines reinnervation of the biceps muscle with reinnervation of the brachialis muscle, the most powerful elbow flexor ( Fig. 34.24 ). Double-nerve transfer has improved outcomes in some series. , In patients with C5-C7 injury patterns, single-nerve transfer may be selected over a dual-nerve fascicle depending on the distribution of the hand and forearm weakness, and some authors have recommended not performing this technique in patients with C5-C7 injury. However, we and others have used it in patients with C5-C7 injury and achieved satisfactory recovery without adverse sequelae. The question of whether a single-nerve transfer versus a double-nerve transfer for elbow flexion is necessary has been the subject of recent publications. Our group compared the torque strength of a single-fascicle versus a double-fascicle nerve transfer to the biceps and brachialis motor branches, respectively. Overall, single-nerve transfers did not outperform double-nerve transfers, and the two types of transfers had similar torque strength. In a prospective comparative study, elbow flexion strength did not differ between groups treated with a single-fascicle or double-fascicle transfer. For this reason, the surgeon needs to carefully decide if the second fascicle transfer from the median nerve to the brachialis is necessary in their patients.
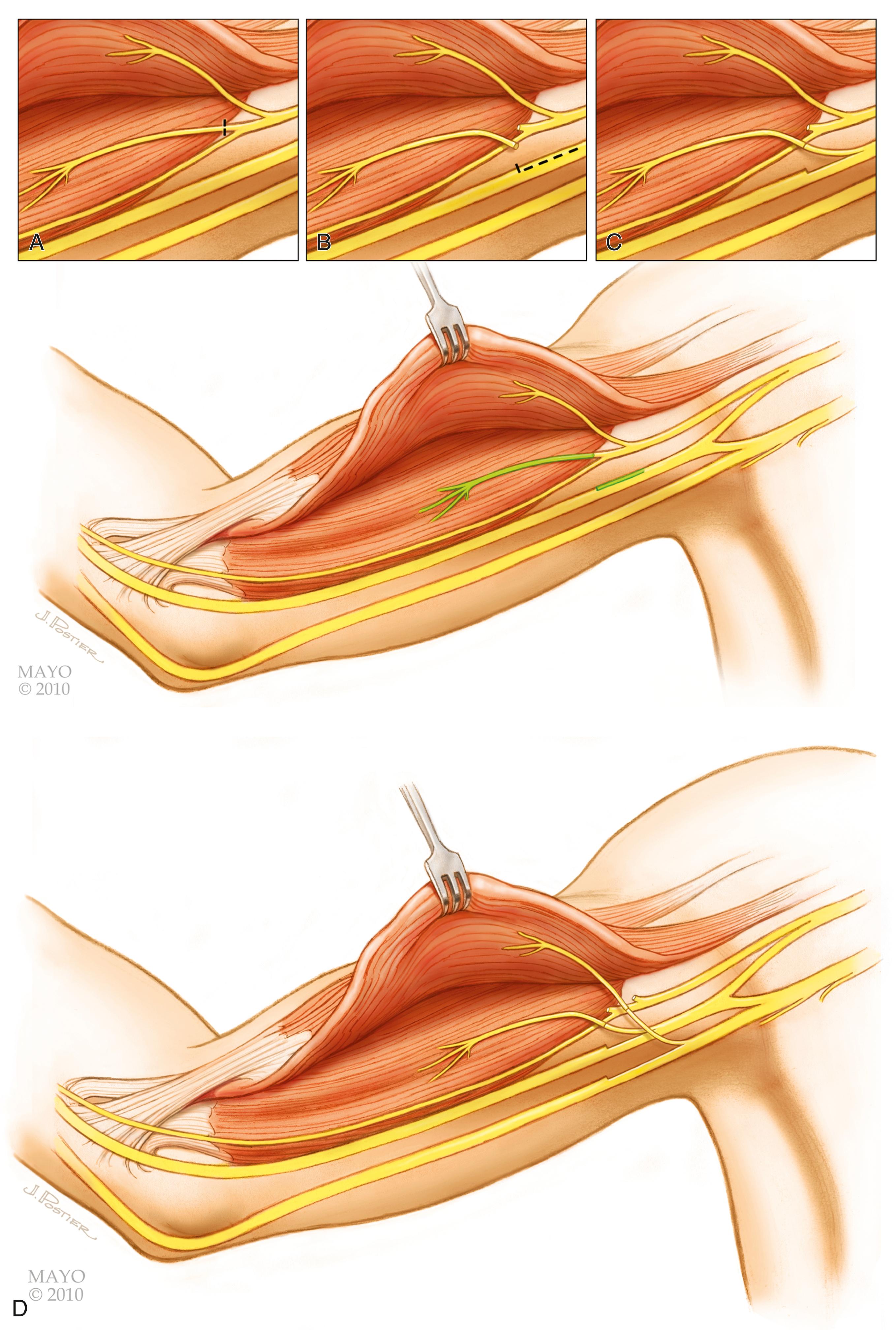
The same medial arm exposure described for the ulnar nerve fascicular transfer is used to expose the median nerve and the brachialis motor branch. As described previously, the musculocutaneous nerve is identified in the proximal part of the arm. The biceps motor branch is seen, and several centimeters more distally, the brachialis motor branch is identified along with the lateral antebrachial cutaneous nerve. Identification of the brachialis motor branch can be confirmed by seeing it enter the muscle. It is distinguished from the lateral antebrachial cutaneous nerve, which is larger and runs more superficially; gentle traction on the lateral antebrachial cutaneous nerve will tent the skin in the proximal part of the forearm. The brachialis branch can be dissected proximally up to the common stem, where the biceps branch originates. The median nerve is superficial to the brachial artery and vein; it is the largest of the nerves at this level. Intraneural dissection is performed under magnification, and several fascicles are isolated. We prefer to map the nerve with a portable stimulator to identify a fascicle producing wrist flexion (via the flexor carpi radialis [C6-7]), when it is functioning. Other groups have used fascicles innervating the flexor digitorum superficialis. The chosen fascicle is dissected over a segment long enough that when it is divided distally, it can be transferred and reach the brachialis motor branch. The nerves are coapted with epineural 9-0 or 10-0 nylon suture without tension and reinforced with fibrin glue.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here