Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The elbow functions to facilitate positioning of the hand in space; it serves as a mechanical lever through the forearm for lifting and as a means of force transmission. Motion at the joint is a combination of flexion, extension, and pronosupination that facilitates activities of daily living. Trauma to the elbow can be challenging to treat. The anatomy is complex, and the stability of the joint may be compromised by injury to the bones, ligaments, and soft tissue that confer stability. Moreover, the articular surfaces are unforgiving of small defects or malalignment, and the elbow has an unfortunate tendency toward stiffness after trauma and/or immobilization. This chapter provides information on the diagnosis and treatment of trauma to the elbow distal to the distal humerus.
Several cadaveric series have investigated the mechanisms that create various injuries about the elbow. Amis and Miller found that radial head and coronoid fractures resulted after forearm impact during elbow flexion of less than 80 degrees, whereas olecranon fractures occurred with direct blows at 90 degrees and distal humerus fractures occurred with injuries while the elbow was flexed greater than 110 degrees. Fitzpatrick and colleagues investigated the position of forearm rotation on injury patterns during axial load, finding that “terrible triad” injuries resulted from a forearm-pronated position, whereas most commonly, the supinated and axially loaded elbow resulted in a dislocation without fracture. In addition, rotation during the injury regardless of starting position resulted in the failure of differing structures. When the ulna internally rotated, the medial structures were disrupted first, whereas with external rotation, the lateral structures failed first.
Evaluation of the injured elbow begins with a history and physical examination. The mechanism of injury is sought, and details surrounding the injury and clues to associated injuries are assessed. Patients are queried about loss of consciousness and, in the setting of lacerations or abrasions, tetanus immunization status. Past medical history as it pertains to and influences current and future treatment is noted. Palpation and assessment of the limb proximal and distal to the elbow, particularly the wrist and forearm, is performed to exclude concomitant injury. The neurovascular status of the limb, including the status of the major peripheral, is documented. Plain film radiographs in multiple planes are obtained to evaluate for bony injury or dislocation. If the elbow has been dislocated, an attempt is made to reduce the elbow. Although reduction of dislocations is frequently performed by emergency room staff, when it is done by the orthopaedic surgeon, important information regarding the stability of the elbow can be obtained. In general, the elbow may be reduced by application of gentle traction through the forearm with the upper arm stabilized and with a downward counterforce applied to the upper arm. The elbow is flexed, and often a palpable clunk confirms reduction of the elbow ( Fig. 44.1 ).
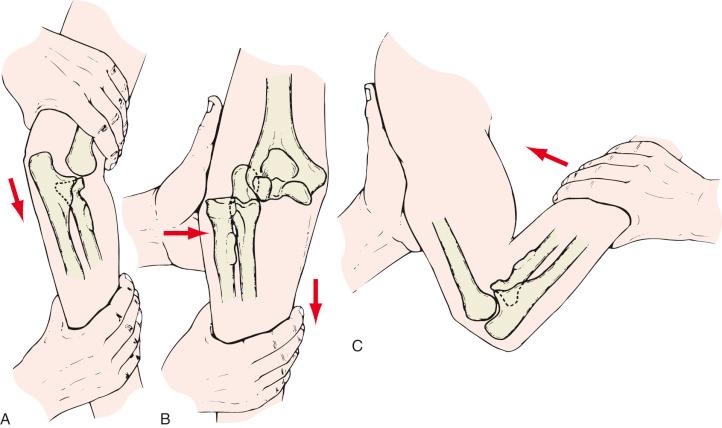
After reduction, the elbow is gently extended to assess the arc and positions of instability, if any. If the elbow is completely stable through the arc of flexion and extension and there are no fractures that require treatment, immediate motion is commenced. If, however, the elbow has a tendency to subluxate, the positions of instability are noted, and the elbow is immobilized in the position of stability. If operative treatment is otherwise not indicated, a hinged elbow brace with the stop placed 20 degrees short of the position of instability is used, allowing full flexion of the elbow. Gradually the elbow is mobilized and extended over a few weeks’ time to allow full motion.
Standard three-view radiographs (posterior-anterior [PA], lateral, and oblique) are obtained. In addition to injury films, postreduction views are obtained after reduction of any elbow dislocation. A radial head view is sometimes useful to assess any radial head pathology. Computed tomography (CT) scanning with the advent of two-dimensional (2-D) and three-dimensional (3-D) reconstructions has dramatically changed the evaluation of elbow trauma and improved our understanding of injury patterns and how to treat them. Notably, CT scanning is best obtained with the elbow in as near extension as possible, with the elbow centered in the gantry. This may be challenging in the setting of an elbow dislocation that tends to dislocate in extension or in patients who have a hard time placing the arm over the head.
Any fractures are noted and appropriate treatment instituted according to the subsequent sections.
Radial head fractures are typically readily diagnosed based on examination and plain film radiographs. Patients are typically tender over the lateral aspect of the elbow and may have pain with motion of the elbow, particularly in pronosupination. It is important to exclude more involved injuries to the elbow, such as concomitant fractures or ligament injuries, and exclude injuries to the forearm, such as the Essex-Lopresti lesion; thus the patient should be specifically queried regarding wrist pain and examined at the forearm and wrist.
Radial head fractures may be classified into one of three types, according to the Mason classification ( Fig. 44.2 ). A fourth type, representing a radial head fracture associated with an elbow dislocation, was added by Johnston.
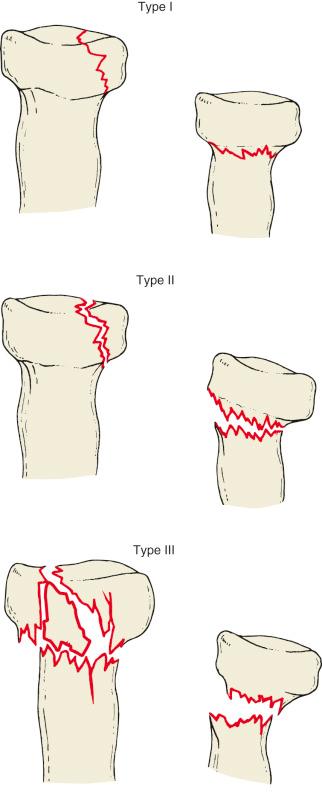
Mason type I fractures are minimally displaced or nondisplaced and have no mechanical block to motion.
Mason type II fractures involve more than 2 mm of displacement and more than a third of the radial head. Mason type III fractures are comminuted, multifragmented fractures that are likely irreparable based on preoperative radiographs.
Open injuries are treated with tetanus immunization, intravenous (IV) antibiotics, and débridement. Closed injuries are treated with reduction of any dislocation and splinting or placement of a sling for stable, minimally displaced injuries.
Options for management of radial head fractures include nonoperative treatment with early mobilization, fragment excision in the setting of a single fragment and/or a bony block to motion, open reduction and internal fixation (ORIF), radial head excision, or radial head replacement arthroplasty.
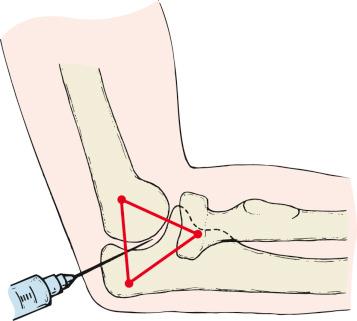
Patients who are seen within a few days of the injury may have an aspiration of the hematoma and the joint injected with local anesthesia. Frequently, the hemarthrosis and pain make it difficult to assess motion and bony blocks. The aspiration may be performed via the “soft spot,” the center of a triangle between the radial head, lateral epicondyle, and the olecranon tip, or via the anterolateral portal site just anterior and distal to the radiocapitellar joint. A 19-gauge needle and a 5- or 10-cc syringe are used to aspirate the hematoma, which is discarded. The syringe is exchanged for 1% lidocaine or other local analgesic mixture, which is placed into the joint. After this, which often helps improve pain, pronosupination may be assessed ( Fig. 44.3 ). Although the use of aspiration with or without infusion of local analgesic can certainly be of diagnostic value, several series have investigated whether aspiration improves pain or clinical results and have questioned the value of injecting local analgesics. In some series, aspiration and injection resulted in improved pain, whereas in another, no difference was noted. One recent investigation showed no differences between aspiration alone or aspiration with infusion of local analgesic.
All type I fractures and many type II fractures may be treated nonoperatively provided there is not a bony block to motion and the articular surface is reasonably congruent, with an anticipated high rate of satisfactory outcomes.
Aspiration may be performed with or without infusion of local analgesic, particularly if there is a question with respect to motion permitted by the fracture.
If nonoperative treatment is employed, the patient is offered a sling for comfort and asked to mobilize within a week. Patients are followed radiographically and clinically to ensure motion is coming along and further displacement does not change the treatment recommendations. Within approximately 4 to 6 weeks, most patients’ acute pain has resolved and motion arc has normalized or nearly normalized.
Fragment excision may be considered in the setting of small fragments that are less than 25% of the head, which are too small, osteoporotic, or comminuted for fixation, and do not articulate with the proximal radial ulnar joint. The elbow should remain stable before and after fragment excision.
Radial ORIF is indicated in the setting of type II and fixable type III fractures and in the setting of radial neck injuries. Care should be taken to ensure that patients are not better served by either nonoperative treatment or radial head replacement. Recent series have highlighted that although ORIF has a high success rate across series, in some patients (type II fractures), the outcomes seem similar to nonoperative treatment with the disadvantage of a higher complication rate and cost.
In addition, for many type III fractures, radial head replacement may more reliably produce satisfactory outcomes and may represent a better option. In particular, the presence of three or more fragments in the setting of an unstable fracture of the radial head has a poorer prognosis, and if stable secure fixation cannot be achieved, radial head excision or replacement may be a superior option.
Fixation may be performed using screws or plate-and-screw constructs. Any hardware must be placed in the “safe zone,” or the region that does not articulate with the proximal radial ulnar joint. This represents the lateral region of the radial head and neck when the forearm is in a neutral position; alternatively, it can be identified as the area bounded by the region between the Lister tubercle distally and the radial styloid.
The choice of fixation type is ideally lower-profile screw fixation, provided that stable fixation may be achieved. Plate fixation is frequently followed by a need for hardware removal and poorer motion compared with screw fixation. For type III fractures that preoperative radiographs suggest are irreparable based on the amount of comminution or the presence of multiple fragments, replacement arthroplasty or excision may be considered. Excision is undesirable in the setting of instability of the elbow and should be avoided in such situations. In addition, in association with longitudinal instability of the forearm, such as the Essex-Lopresti injury, radial head excision is to be avoided.
The radial head is an eccentric dish-shaped structure with a variable offset from the neck and angled 15 degrees oriented away from the radial tuberosity. These anatomic features have implications for fracture fixation or prosthetic replacement. Likewise, the proximal radioulnar articulation needs to be considered in reconstructive or replacement procedures. The cartilage of the radial head represents an arc of about 280 degrees about the rim of the radius. Hardware placement should avoid the articulating region, which can be visually identified as a thickened area of cartilage. In addition, the safe zone for hardware placement has been defined as within a 90-degree arc bounded by the Lister tubercle and the radial styloid distally or simply the lateral aspect of the radial head when the forearm is in neutral.
The two most commonly used approaches to the radial head include the Kocher interval between the extensor carpi ulnaris (ECU) and anconeus and the common extensor splitting approach. The Kocher interval is far away from the region of the posterior interosseous nerve, but the lateral ulnar collateral ligament (LUCL) is at risk with this approach. The lateral collateral ligament (LCL) complex includes a coalescence of several structures, including the most important stabilizing structure, the LUCL, the annular ligament, and the accessory lateral collateral ligament. The LUCL arises from the anterior inferior lateral epicondyle and inserts upon the crista supinatoris of the ulna. If the Kocher approach is used, during dissection, the surgeon attempts to preserve the ligament and enter the joint anterior to it. This approach is most useful if the LUCL has already been compromised by trauma or in the setting of lateral elbow instability for purposes of LUCL reconstruction.
An alternative exposure is to split the origin of the extensor digitorum communis (EDC) at the lateral epicondyle ( Fig. 44.4 ). This approach preserves the LUCL, but at the distal aspect of the surgical dissection, the posterior interosseous nerve (PIN) may be encountered. The PIN arises as the continuation of the deep radial nerve near the radiocapitellar joint. It then passes about the lateral aspect of the radius and through the supinator, which it innervates. To help protect the PIN during this approach, the forearm may be pronated, which results in the PIN falling more distally away from the surgical field of dissection. The surgeon should keep in mind that the PIN typically may be found between 2 and 5 cm distal to the radiocapitellar joint, depending on the size of the patient and position of forearm rotation.
In general, the authors prefer supine positioning for the approach to the radial head. We also prefer general anesthesia because positioning may be uncomfortable in the awake patient, and the nerves may be immediately assessed in the postoperative period with general anesthesia as opposed to regional. The patient is placed supine and the arm prepped from fingertips to axilla. An arm table may be used, or the arm may be placed across the patient's chest. Alternatively, an arm board is very helpful to use so that the advantages of both positions may be exploited. The arm board may be rotated inward during part of the case or left at a right angle to facilitate exposure. It is also extraordinarily easy to rotate the arm board out of the field and allow for easy use of the mini-fluoroscopy unit.
A sterile tourniquet is applied and later inflated to 200–250 mmHg to provide hemostasis during the case. A mini-fluoroscopy unit is used to image during the case.
The authors prefer a lateral-based incision for exposure of isolated radial head or lateral-sided injuries. If it is thought that medial and lateral structures need to be addressed, an alternative is a single posterior incision; however, our preference is to make separate lateral- and medial-based incisions to limit the potential soft tissue dissection and potential for seroma formation under large skin flaps.
A gently curved incision is made centered over the lateral epicondyle and radial head. Subcutaneous nerves are identified and preserved, and the muscular and tendinous origins of the extensor apparatus are exposed.
If the radial head fracture is accompanied by a concomitant LUCL injury, the approach exploits the ligament injury to perform the arthrotomy. With gentle probing, if it is not already readily apparent, the tissue defect over the lateral epicondyle and path of the LUCL avulsion can be identified. This represents the Kocher approach between the ECU and anconeus ( Fig. 44.5 ). It is unlikely to injure the PIN with this approach because it is distant from the surgical dissection site, but if the collateral ligament is intact, the ligament may be at risk of iatrogenic injury. Thus this approach is not preferred unless the collateral ligament has avulsed.
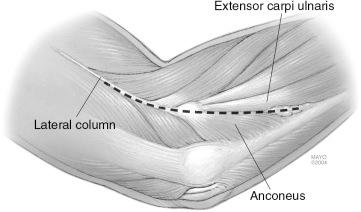
When the collateral ligament is intact but a radial head fracture needs to be addressed, we prefer an approach that splits the EDC origin in line with its fibers. The forearm is kept in pronation, which rotates the PIN farther away from the operative dissection field. The radial head is exposed by this capsulotomy, and the fracture is identified.
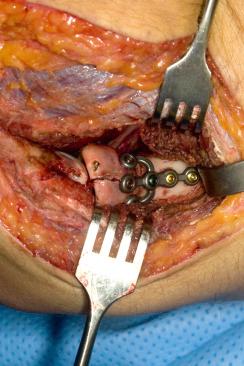
After exposure, the fracture is then assessed, and it is determined if it is amenable to fixation or best served by radial head excision or arthroplasty. Although it may be technically possible to fix some fractures, fixation may be tenuous, and some fractures may be more appropriately treated with arthroplasty. A few recent series suggest that type III and some type II fractures that undergo fixation do poorly in the setting of fracture-dislocations, comminution, or in conjunction with three or more fracture fragments.
Provisional fixation may be obtained with small Kirschner wires (K-wires) and towel clips or small, pointed bone-reduction clamps. Ideally, small screws or plates are placed for definitive fixation. Screw fixation is preferred due to the lower profile and improved motion postoperatively; in addition, there is a high rate of hardware removal with the use of plates. Occasionally bone grafting may be helpful.
If the radial head is to be replaced, care should be taken to avoid placing too large an implant. The native radial head or its fragments are assembled on the back table and help to define the proper size of implant to place. It should be remembered that the eccentric inner dish shape of the native radial head is the guide for the appropriate size for the implant, thus choosing a smaller implant than the entire radial head. The lesser sigmoid notch may be used as a guide but is less reliable than the native radial head. In general, the radial head should sit within 2 mm of the proximal radioulnar joint.
Most implant systems have modularity, allowing one to choose appropriate head, neck, and stem sizing to re-create a semblance of normal anatomy. Available radial head implants include fixated devices secured into the radial neck and shaft with cement or by a press-fit mechanism; alternatively, they can be intentionally loose, smooth-stemmed implants that are intended to act as a smooth spacer. Bipolar implants that insert firmly into the radial shaft by a fixed mechanism are available, with an articulating head–neck junction to allow motion. The major head choices are anatomic (with a fixated stem) or nonanatomic (with a smooth mobile stem). To date, little information is available regarding measurable advantages of one implant over another. However, when anatomic designs are used, it is important to place these prostheses in an anatomically accurate position.
Pitfalls in the setting of radial head surgery include excision of a radial head in the setting of unrecognized Essex-Lopresti injury or ligament injury. The radial head is an important stabilizer to the elbow in the setting of ligament instability or longitudinal instability of the forearm, and either repair or replacement is required in such settings. Intraoperatively, the “radial pull test” may be used to assess the competence of the forearm stabilizers (the interosseous membrane [IOM] and the triangular fibrocartilage complex [TFCC]). After the radial head is resected, 20 lb of longitudinal traction is applied at the radial neck while the wrist is examined with fluoroscopy. If migration of the radius occurs as evidenced by ulnar positive variance and a change in this variance of more than 3 mm, then the IOM is likely torn. If more than 6 mm is seen, then both the IOM and the TFCC are likely torn, and all of the forearm longitudinal stabilizers are gone. In this setting, radial head replacement and stabilization of the distal radial ulnar joint and the forearm are recommended.
One common complication associated with prosthetic complication is “overstuffing of the radial head” with too large an implant. This can lead to overloading of the joint space and maltracking of the joint with accelerated articular wear of the joint and pain. This is particularly problematic in the setting of concomitant ligament injury. If the fragments of the native radial head are available, it is easiest to attempt to match the size of the native head, realizing that the metallic radial head should match the inner diameter of the dish of the radial head. Other complications can be related to implant choice. Care should be taken when anatomic radial head designs are placed because nonanatomic placement of an intentionally anatomic, fixed design can lead to abnormal tracking and wear on the capitellum. In addition, in the setting of longitudinal instability of the forearm, bipolar-type implants should be avoided because the articulating joint at the neck may allow escape of the implant with axial loading.
Pitfalls and complications in radial head fixation include an attempt to fix a radial head that might be better served by replacement arthroplasty, hardware prominence that may restrict motion (particularly common in the setting of plate fixation of fractures), and hardware issues associated with impingement at the proximal radioulnar joint or into the radiocapitellar joint. Attention to avoiding the articular surfaces as described in the fracture fixation section can help reduce this risk. Likewise, patients should be counseled preoperatively about the potential need for hardware removal, particularly in the setting of radial head and neck fractures fixated with plates.
Intraoperative problems include difficulty fixing a fracture and the need for conversion to radial head implant. It is therefore a good idea to have implants available for all anticipated possibilities. In general, we suggest having available a variety of small screws, plate and screw devices, and a radial head implant and being prepared to repair the collateral ligaments via bony tunnels or suture anchors. In addition, in the setting of gross instability, the surgeon should be prepared to proceed in a stepwise fashion to repair injured structures so that the patient will leave the operating room with a stable elbow. Typically, in the setting of instability, we address radial head fractures, coronoid fractures (if the fracture is large enough for fixation), and the LUCL. The elbow is assessed throughout the arc of motion for stability, and gentle-stress-view fluoroscopic scans are taken. If this fails to restore stability, then the medial collateral ligament (MCL) may be addressed. If the elbow remains grossly unstable, a static external fixator may be placed. The pins are placed under direct visualization to avoid injury to major peripheral nerves. The fixator is placed such that the elbow is maintained in the joint for a period of 4 weeks. Typically, it is removed in the operating room. First, the bar is disconnected, and the elbow is examined under anesthesia for instability. If it remains unstable, the bar is reconnected; however, in most cases, the elbow is now stable, and the fixator is disassembled.
Postoperatively, it is ideal if the radial head fixation is stable enough to permit early motion. If ligament injury is absent and the injury represents an isolated radial head fracture treated with stable fixation or implant arthroplasty, we typically place the patient in a splint and sling for comfort for a few days and initiate gentle range-of-motion (ROM) exercises within a few days. If there has been an associated ligament injury, such as an LUCL and/or MCL that has been repaired, then the patient is generally immobilized in a splint at 90 degrees in pronation (for LUCL repair), supination (for MCL repair), or neutral (if both have been repaired) and subsequently allowed to start early flexion-extension motion provided stability permits it, with avoidance of terminal extension and forearm rotation. Pronosupination is permitted when the elbow is flexed greater than 90 degrees. At the 6-week mark, if full motion has not yet been achieved, one can consider nighttime extension splinting.
The role of and compliance with heterotopic ossification (HO) prophylaxis regimens remain subjects of discussion. In patients who can tolerate nonsteroidal antiinflammatory drugs (NSAIDs) and have no renal or gastrointestinal (GI) contraindications, indomethacin sustained-release 75 mg daily for several weeks may play a role, particularly in the setting of radial head replacement and in those who may be prone to HO (head injury, history of HO). Other factors associated with HO formation include the presence of bony debris, hematoma, increasing duration of surgery, and dissection.
Use of external-beam irradiation (700 cGy in a single dose within 24 hours of surgery) as HO has been described, but its use is reserved for selected cases because a recent series suggests increased nonunion risk.
Complications include loss of motion, failure to heal, and HO.
A high rate of satisfactory outcomes may be seen after nonoperative treatment of type I and many type II fractures. In one series, patients who developed problems were adequately treated with late radial head excision.
Patients treated with ORIF of radial head fractures tend to have improved radiographic findings (i.e., less degenerative changes than those with similar fractures treated nonoperatively), but they also have a high rate of satisfactory outcomes, as do patients treated with radial head replacement arthroplasty. Radiographic signs of degenerative changes after ORIF or replacement are not uncommon but are usually asymptomatic.
For many type III fractures, radial head replacement may more reliably produce satisfactory outcomes over radial head ORIF, especially in the presence of three or more fragments or ligament instability.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here