Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Figure 4-1 lists the approximate concentrations of important electrolytes and other substances in the extracellular fluid and intracellular fluid. Note that the extracellular fluid contains a large amount of sodium but only a small amount of potassium. The opposite is true of the intracellular fluid. Also, the extracellular fluid contains a large amount of chloride ions, whereas the intracellular fluid contains very little of these ions. However, the concentrations of phosphates and proteins in the intracellular fluid are considerably greater than those in the extracellular fluid. These differences are extremely important to the life of the cell. The purpose of this chapter is to explain how the differences are brought about by the cell membrane transport mechanisms.
The structure of the membrane covering the outside of every cell of the body is discussed in Chapter 2 and illustrated in Figure 2-3 and Figure 4-2 . This membrane consists almost entirely of a lipid bilayer with large numbers of protein molecules in the lipid, many of which penetrate all the way through the membrane.
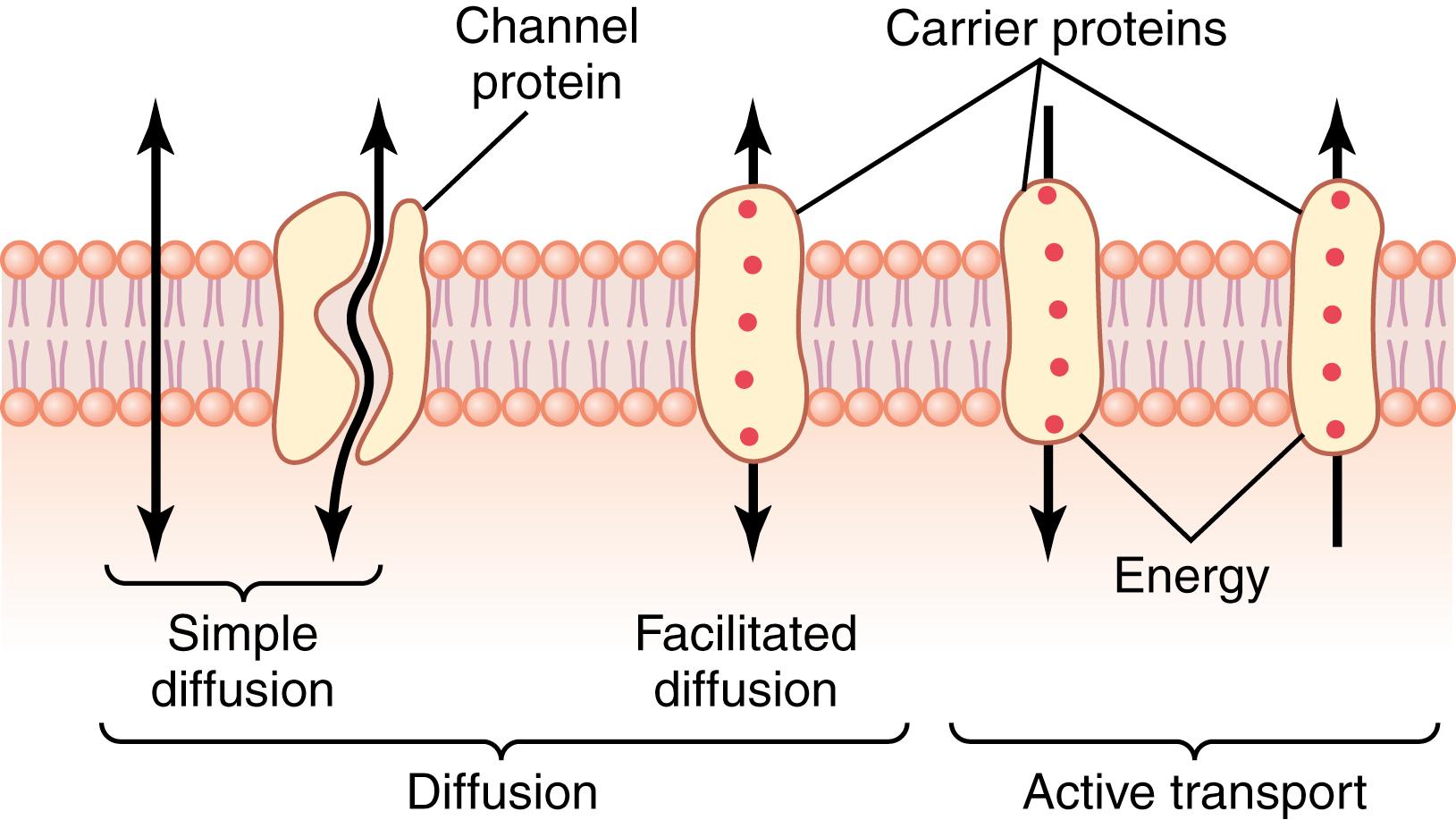
The lipid bilayer is not miscible with the extracellular fluid or the intracellular fluid. Therefore, it constitutes a barrier against movement of water molecules and water-soluble substances between the extracellular and intracellular fluid compartments. However, as shown in Figure 4-2 by the leftmost arrow, lipid-soluble substances can diffuse directly through the lipid substance.
The membrane protein molecules interrupt the continuity of the lipid bilayer, constituting an alternative pathway through the cell membrane. Many of these penetrating proteins can function as transport proteins. Some proteins have watery spaces all the way through the molecule and allow free movement of water, as well as selected ions or molecules; these proteins are called channel proteins. Other proteins, called carrier proteins, bind with molecules or ions that are to be transported, and conformational changes in the protein molecules then move the substances through the interstices of the protein to the other side of the membrane. Channel proteins and carrier proteins are usually selective for the types of molecules or ions that are allowed to cross the membrane.
Transport through the cell membrane, either directly through the lipid bilayer or through the proteins, occurs via one of two basic processes, diffusion or active transport.
Although many variations of these basic mechanisms exist, diffusion means random molecular movement of substances molecule by molecule, either through intermolecular spaces in the membrane or in combination with a carrier protein. The energy that causes diffusion is the energy of the normal kinetic motion of matter.
In contrast, active transport means movement of ions or other substances across the membrane in combination with a carrier protein in such a way that the carrier protein causes the substance to move against an energy gradient, such as from a low-concentration state to a high-concentration state. This movement requires an additional source of energy besides kinetic energy. A more detailed explanation of the basic physics and physical chemistry of these two processes is provided later in this chapter.
All molecules and ions in the body fluids, including water molecules and dissolved substances, are in constant motion, with each particle moving in its separate way. The motion of these particles is what physicists call “heat”—the greater the motion, the higher the temperature—and the motion never ceases, except at absolute zero temperature. When a moving molecule, A, approaches a stationary molecule, B, the electrostatic and other nuclear forces of molecule A repel molecule B, transferring some of the energy of motion of molecule A to molecule B. Consequently, molecule B gains kinetic energy of motion, whereas molecule A slows down, losing some of its kinetic energy. As shown in Figure 4-3 , a single molecule in a solution bounces among the other molecules—first in one direction, then another, then another, and so forth—randomly bouncing thousands of times each second. This continual movement of molecules among one another in liquids or gases is called diffusion.
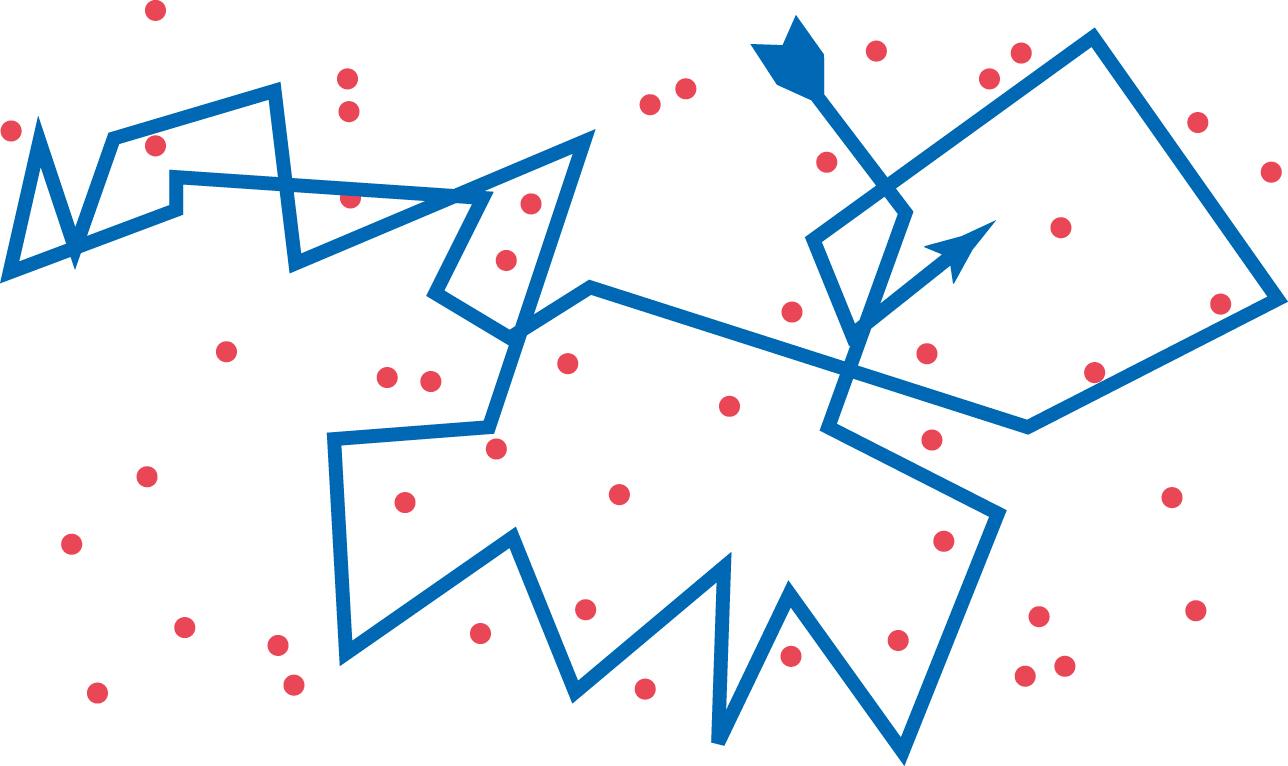
Ions diffuse in the same manner as whole molecules, and even suspended colloid particles diffuse in a similar manner, except that the colloids diffuse far less rapidly than molecular substances because of their large size.
Diffusion through the cell membrane is divided into two subtypes, called simple diffusion and facilitated diffusion. Simple diffusion means that kinetic movement of molecules or ions occurs through a membrane opening or through intermolecular spaces without interaction with carrier proteins in the membrane. The rate of diffusion is determined by the amount of substance available, the velocity of kinetic motion, and the number and sizes of openings in the membrane through which the molecules or ions can move.
Facilitated diffusion requires interaction of a carrier protein. The carrier protein aids passage of molecules or ions through the membrane by binding chemically with them and shuttling them through the membrane in this form.
Simple diffusion can occur through the cell membrane by two pathways: (1) through the interstices of the lipid bilayer if the diffusing substance is lipid-soluble; and (2) through watery channels that penetrate all the way through some of the large transport proteins, as shown to the left in Figure 4-2 .
The lipid solubility of a substance is an important factor for determining how rapidly it diffuses through the lipid bilayer. For example, the lipid solubilities of oxygen, nitrogen, carbon dioxide, and alcohols are high, and all these substances can dissolve directly in the lipid bilayer and diffuse through the cell membrane in the same manner that diffusion of water solutes occurs in a watery solution. The rate of diffusion of each of these substances through the membrane is directly proportional to its lipid solubility. Especially large amounts of oxygen can be transported in this way; therefore, oxygen can be delivered to the interior of the cell almost as though the cell membrane did not exist.
Even though water is highly insoluble in the membrane lipids, it readily passes through channels in protein molecules that penetrate all the way through the membrane. Many of the body’s cell membranes contain protein “pores” called aquaporins that selectively permit rapid passage of water through the membrane. The aquaporins are highly specialized, and there are at least 13 different types in various cells of mammals.
The rapidity with which water molecules can diffuse through most cell membranes is astounding. For example, the total amount of water that diffuses in each direction through the red blood cell membrane during each second is about 100 times as great as the volume of the red blood cell.
Other lipid-insoluble molecules can pass through the protein pore channels in the same way as water molecules if they are water-soluble and small enough. However, as they become larger, their penetration falls off rapidly. For example, the diameter of the urea molecule is only 20% greater than that of water, yet its penetration through the cell membrane pores is about 1000 times less than that of water. Even so, given the astonishing rate of water penetration, this amount of urea penetration still allows rapid transport of urea through the membrane within minutes.
Computerized three-dimensional reconstructions of protein pores and channels have demonstrated tubular pathways all the way from the extracellular to the intracellular fluid. Therefore, substances can move by simple diffusion directly along these pores and channels from one side of the membrane to the other.
Pores are composed of integral cell membrane proteins that form open tubes through the membrane and are always open. However, the diameter of a pore and its electrical charges provide selectivity that permits only certain molecules to pass through. For example, aquaporins permit rapid passage of water through cell membranes but exclude other molecules. Aquaporins have a narrow pore that permits water molecules to diffuse through the membrane in single file. The pore is too narrow to permit passage of any hydrated ions. As discussed in Chapter 28, Chapter 76 , the density of some aquaporins (e.g., aquaporin-2) in cell membranes is not static but is altered in different physiological conditions.
The protein channels are distinguished by two important characteristics: (1) they are often selectively permeable to certain substances; and (2) many of the channels can be opened or closed by gates that are regulated by electrical signals (voltage-gated channels) or chemicals that bind to the channel proteins (ligand-gated channels). Thus, ion channels are flexible dynamic structures, and subtle conformational changes influence gating and ion selectivity.
Many protein channels are highly selective for transport of one or more specific ions or molecules. This selectivity results from specific characteristics of the channel, such as its diameter, shape, and the nature of the electrical charges and chemical bonds along its inside surfaces.
Potassium channels permit passage of potassium ions across the cell membrane about 1000 times more readily than they permit passage of sodium ions. This high degree of selectivity cannot be explained entirely by the molecular diameters of the ions because potassium ions are slightly larger than sodium ions. Using x-ray crystallography, potassium channels were found to have a tetrameric structure consisting of four identical protein subunits surrounding a central pore ( Figure 4-4 ). At the top of the channel pore are pore loops that form a narrow selectivity filter . Lining the selectivity filter are carbonyl oxygens. When hydrated potassium ions enter the selectivity filter, they interact with the carbonyl oxygens and shed most of their bound water molecules, permitting the dehydrated potassium ions to pass through the channel. The carbonyl oxygens are too far apart, however, to enable them to interact closely with the smaller sodium ions, which are therefore effectively excluded by the selectivity filter from passing through the pore.
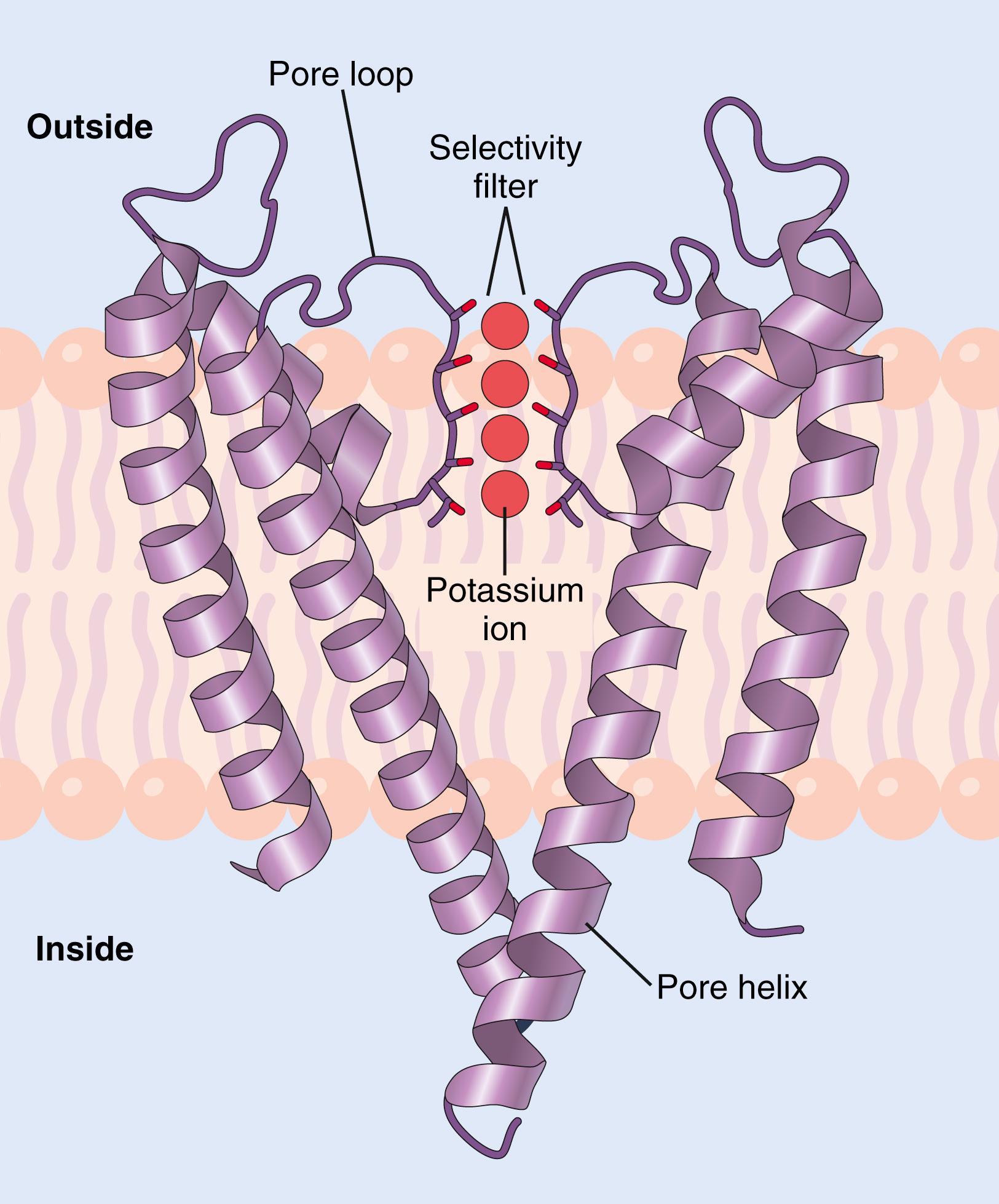
Different selectivity filters for the various ion channels are believed to determine, in large part, the specificity of various channels for cations or anions or for particular ions, such as sodium (Na + ), potassium (K + ), and calcium (Ca 2+ ), that gain access to the channels.
One of the most important of the protein channels, the sodium channel, is only 0.3 to 0.5 nanometer in diameter, but the ability of sodium channels to discriminate sodium ions among other competing ions in the surrounding fluids is crucial for proper cellular function. The narrowest part of the sodium channel’s open pore, the selectivity filter , is lined with strongly negatively charged amino acid residues, as shown in the top panel of Figure 4-5 . These strong negative charges can pull small dehydrated sodium ions away from their hydrating water molecules into these channels, although the ions do not need to be fully dehydrated to pass through the channels. Once in the channel, the sodium ions diffuse in either direction according to the usual laws of diffusion. Thus, the sodium channel is highly selective for passage of sodium ions.
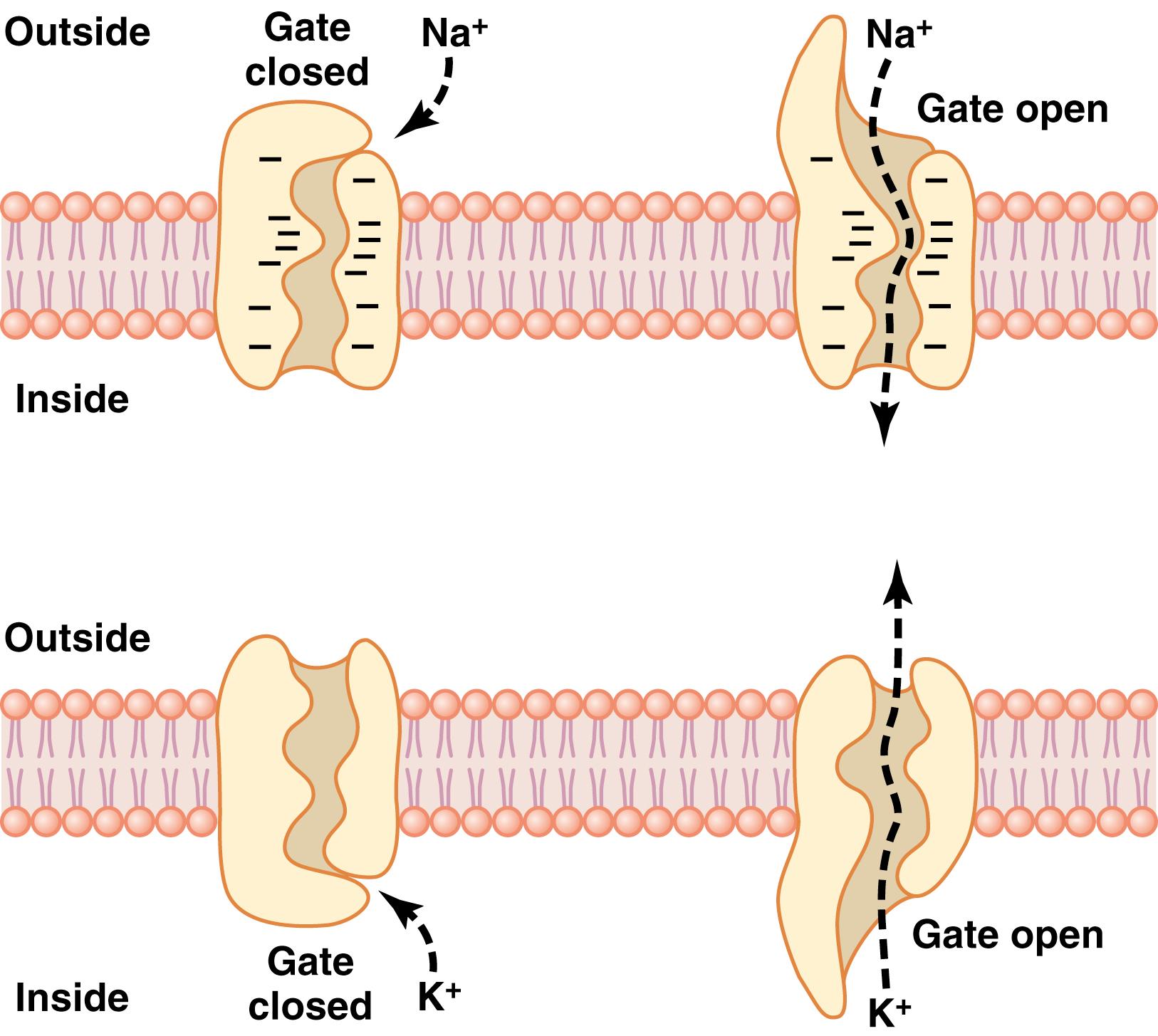
Gating of protein channels provides a means of controlling ion permeability of the channels. This mechanism is shown in both panels of Figure 4-5 for selective gating of sodium and potassium ions. Some of the gates are thought to be gatelike extensions of the transport protein molecule, which can close the opening of the channel or can be lifted away from the opening by a conformational change in the shape of the protein molecule.
The opening and closing of gates are controlled in two principal ways:
Voltage gating. In the case of voltage gating, the molecular conformation of the gate or its chemical bonds responds to the electrical potential across the cell membrane. For example, in the top panel of Figure 4-5 , a strong negative charge on the inside of the cell membrane may cause the outside sodium gates to remain tightly closed. Conversely, when the inside of the membrane loses its negative charge, these gates open suddenly and allow sodium to pass inward through the sodium pores. This process is the basic mechanism for eliciting action potentials in nerves that are responsible for nerve signals. In the bottom panel of Figure 4-5 , the potassium gates are on the intracellular ends of the potassium channels, and they open when the inside of the cell membrane becomes positively charged. The opening of these gates is partly responsible for terminating the action potential, a process discussed in Chapter 5 .
Chemical (ligand) gating. Some protein channel gates are opened by the binding of a chemical substance (a ligand) with the protein, which causes a conformational or chemical bonding change in the protein molecule that opens or closes the gate. One of the most important instances of chemical gating is the effect of the neurotransmitter acetylcholine on the acetylcholine receptor which serves as a ligand-gated ion channel . Acetylcholine opens the gate of this channel, providing a negatively charged pore about 0.65 nanometer in diameter that allows uncharged molecules or positive ions smaller than this diameter to pass through. This gate is exceedingly important for the transmission of nerve signals from one nerve cell to another (see Chapter 46 ) and from nerve cells to muscle cells to cause muscle contraction (see Chapter 7 ).
Figure 4-6 A shows two recordings of electrical current flowing through a single sodium channel when there was an approximately 25-millivolt potential gradient across the membrane. Note that the channel conducts current in an all-or-none fashion. That is, the gate of the channel snaps open and then snaps closed, with each open state lasting for only a fraction of a millisecond, up to several milliseconds, demonstrating the rapidity with which changes can occur during the opening and closing of the protein gates. At one voltage potential, the channel may remain closed all the time or almost all the time, whereas at another voltage, it may remain open either all or most of the time. At in-between voltages, as shown in the figure, the gates tend to snap open and closed intermittently, resulting in an average current flow somewhere between the minimum and maximum.
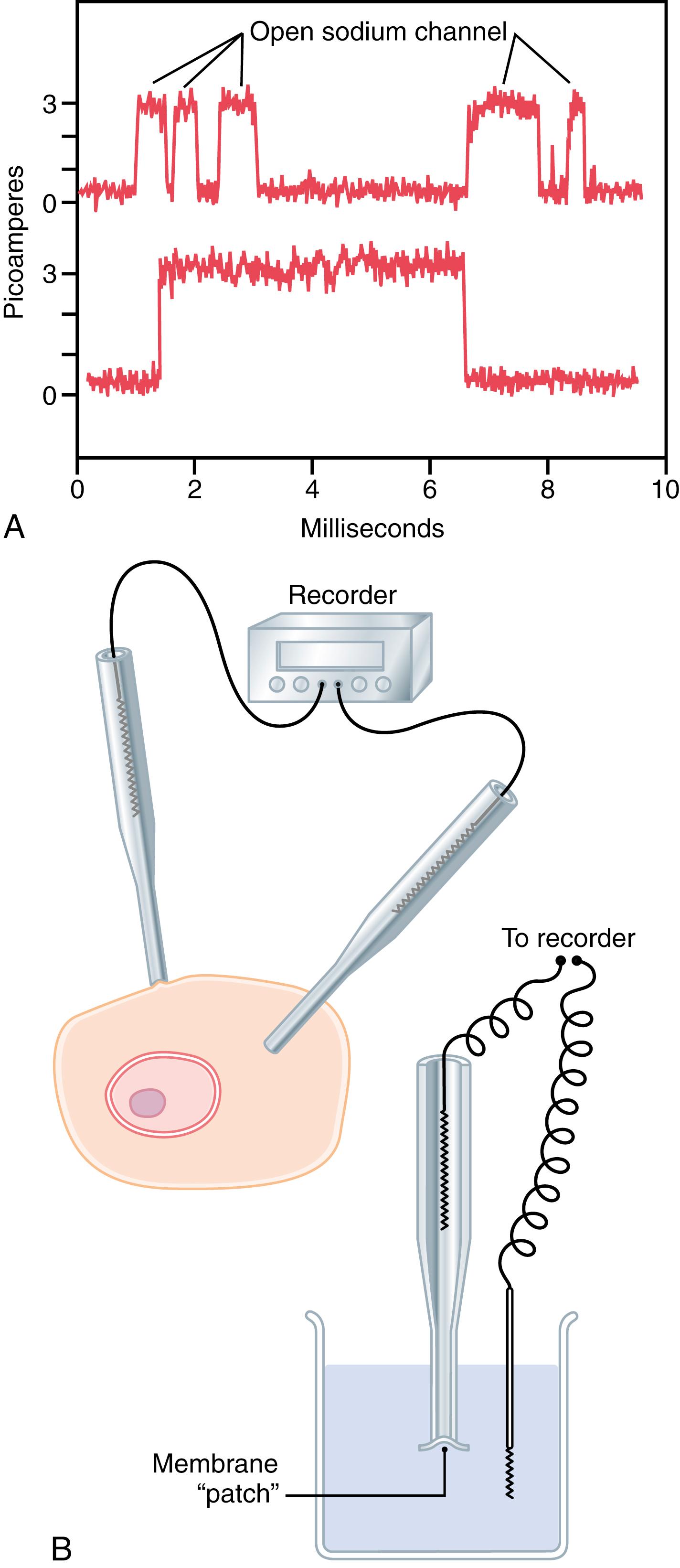
The patch clamp method for recording ion current flow through single protein channels is illustrated in Figure 4-6 B . A micropipette with a tip diameter of only 1 or 2 micrometers is abutted against the outside of a cell membrane. Suction is then applied inside the pipette to pull the membrane against the tip of the pipette, which creates a seal where the edges of the pipette touch the cell membrane. The result is a minute membrane “patch” at the tip of the pipette through which electrical current flow can be recorded.
Alternatively, as shown at the bottom right in Figure 4-6 B , the small cell membrane patch at the end of the pipette can be torn away from the cell. The pipette with its sealed patch is then inserted into a free solution, which allows the concentrations of ions both inside the micropipette and in the outside solution to be altered as desired. Also, the voltage between the two sides of the membrane can be set, or “clamped,” to a given voltage.
It has been possible to make such patches small enough so that only a single channel protein is found in the membrane patch being studied. By varying the concentrations of different ions, as well as the voltage across the membrane, one can determine the transport characteristics of the single channel, along with its gating properties.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here