Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Solid organ transplantation is a lifesaving and life-enhancing treatment for many patients with organ failure. For many patients with end-stage renal failure (ESRF), kidney transplantation improves quality of life (QOL) and confers survival benefits ( EBM 26.1 ). Liver, heart and lung transplantation can be truly lifesaving, as there are few alternatives. The two main obstacles to transplantation are overcoming the recipient’s immune response and a shortage of donor organs.
Solid organ transplantation requires the removal of an organ from one individual with cessation of circulation in the donor, storage, transport and transplantation into another individual. Restoration of the blood supply in the recipient gives rise to activation of the recipient’s immune system based on two stimuli: cellular damage stimulates the innate immune response, and differences between donor and recipient cell surface molecules activate the adaptive immune response by recognition of nonself proteins.
Cellular damage occurs due to ischaemia and subsequent return of the blood supply, which is known as ischaemia reperfusion injury (IRI). Such cell damage and death are associated with the release of molecules known as danger-associated molecular patterns (DAMPs), which are recognised by cells of the innate immune system via their receptors, pattern recognition receptors (PRRs), as signals of cell injury. The innate immune system has two arms: the soluble and cellular arms ( EBM 26.2 ).
Soluble arm: This complement system is a series of protein kinases, which are sequentially activated to culminate in the formation of the membrane attack complex. Components of this complex are inserted into the cell membrane of the graft, disrupting its integrity and causing cell breakdown or lysis.
Cellular arm: This comprises several mainly white blood cells, such as neutrophils, which are recruited early in the process, entering the graft and producing cytokines. Macrophages play an important role in the process of IRI, not only causing direct cell damage, but also priming the recipient immune system for activation of the adaptive immune system.
IRI affects outcome and may result in:
Delayed primary graft function
Increased acute rejection rates
Reduced long-term graft survival
To initiate an adaptive immune response, the graft must express antigens recognised by the recipient as foreign, and these may include ABO blood group antigens and histocompatibility antigens, known as human leucocyte antigens (HLA). Although ABO compatibility is required in the majority of transplants, this is easily managed by careful patient selection. HLA incompatibility is more common, as there is a large degree of variation between individuals in terms of their HLA, or ‘self’ antigens, and so it is more challenging to match individuals. This is particularly relevant in kidney transplantation.
The adaptive immune response occurs in two stages: the afferent arm includes presentation of donor antigen to recipient T cells, T-cell receptor binding and costimulation, leading to T-cell activation. The efferent arm describes the sequence of events that occurs because of T-cell activation.
Antigen presentation: Donor major histocompatibility antigens (MHC) are recognised as foreign (allorecognition) by recipient T cells following presentation upon donor (direct) or recipient (indirect) antigen-presenting cells, such as dendritic cells and B cells ( Fig. 26.1 ).
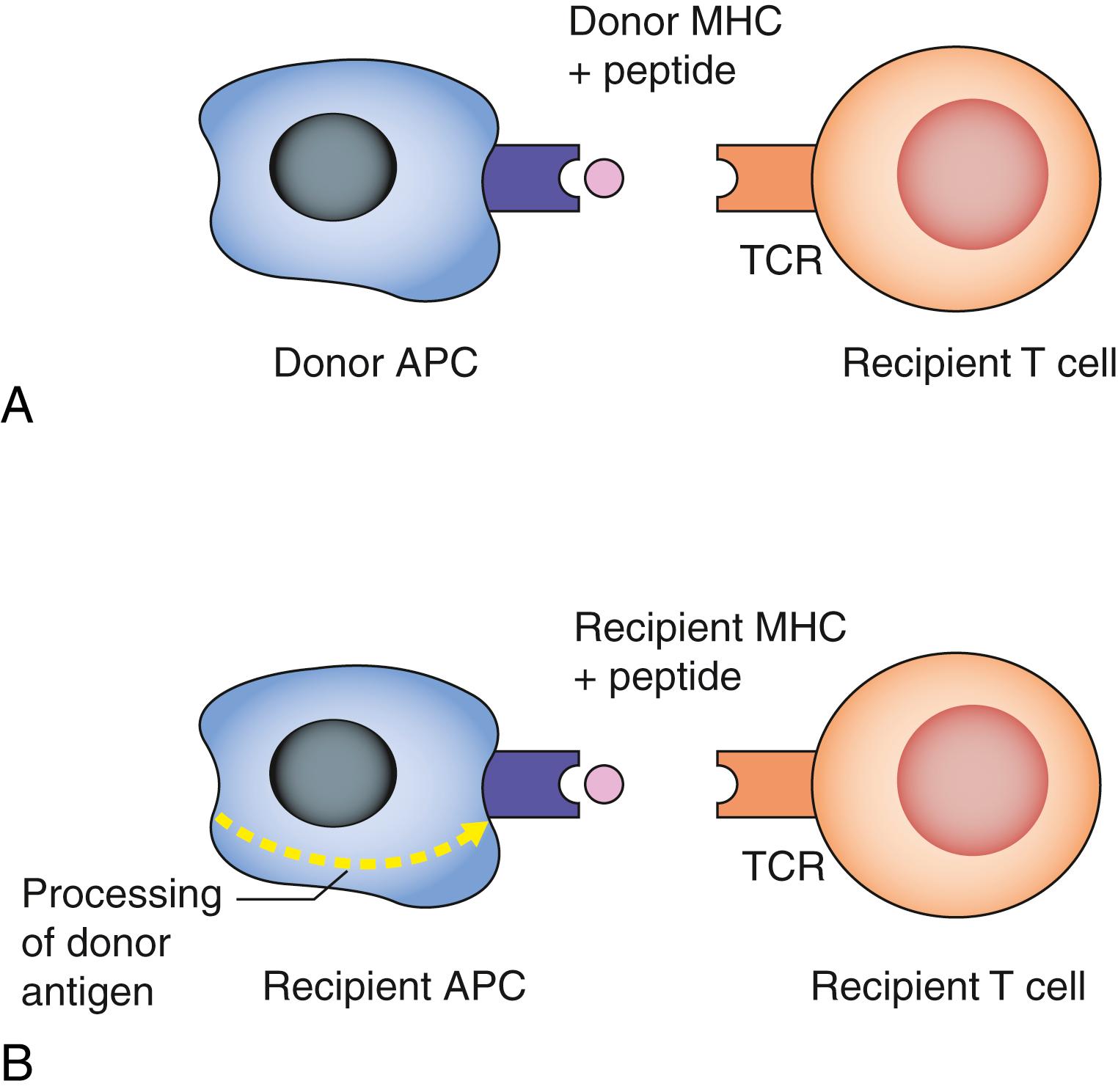
T-cell receptor binding and costimulation: MHC antigen binding to T-cell receptors in the presence of costimulatory molecules can result in a number of possible outcomes, depending on the costimulatory stimulus, and these include T-cell activation, apoptosis (programmed cell death) or anergy (lack of response to antigen or antibody).
T-cell activation: Following their clonal expansion, T cells differentiate into helper (CD4 + ) and effector (CD8 + ) cells that secrete cytokines and kill target cells. Engagement of CD4 + T (helper) cells plays a central role in initiating and amplifying the rejection response.
B-cell activation: Traditionally seen as purely antibody-producing cells, it is clear that B cells contribute to the alloimmune response in a variety of additional ways: as antigen-presenting cells and through cytokine production. Complex interactions between CD4 + T cells and
Renal transplantation has lower mortality than dialysis
The survival benefit of transplantation increases with time
Transplantation offers better QOL than dialysis
IRI presents clinically as delayed graft function (DGF)
DGF is associated with increased risk of graft loss at 3 years
Patients with DGF have higher serum creatinine in long-term follow up
DGF is associated with an increase in risk of acute rejection
B cells also lead to the production of donor-specific antibodies that play a crucial role in antibody-mediated rejection. If a patient has been previously exposed to foreign HLA molecules, for example, through pregnancy, blood transfusions or a previous transplant, they may have developed antibodies to those proteins, known as immunologic memory. This leads to a rapid antibody-mediated response and damages the transplanted organ.
Donor organ damage can be mediated via cellular or antibody-mediated (humoral) mechanisms. The latter depends on B lymphocyte maturation and the production of complement-activating antibodies. The former, also known as delayed-type hypersensitivity (DTH), involves cytotoxic T cells, natural killer (NK) cells, macrophages and neutrophils.
It is becoming increasingly clear that the immune response is a dynamic balance of activating and regulatory cells, and the contribution of regulatory T and B cells is of particular interest. Regulatory T cells (Tregs) are best characterised as CD4+CD25+ and depend on the transcription factor foxp3. Tregs suppress some of the inflammatory cytokines, such as IL-2, and produce IL-10. Less is known about regulatory B cells, but they are of interest in our understanding of transplant tolerance or immune nonresponsiveness.
Results from the presence of preformed cytotoxic antibodies directed against donor HLA or AB antigens
Avoidable through blood group and tissue matching
Apparent following removal of the vascular clamps as the donor organ becomes swollen and discoloured
Leads to graft destruction within 24 hours.
Occurs in approximately 20% transplants, most commonly in the first 6 months
Associated with immune cell infiltration, predominantly T cell-mediated, and can be treated with high-dose intravenous methylprednisolone
Diagnosed on renal transplant biopsy and classified according to Banff 2017 diagnostic criteria ( Table 26.1 ).
| Category 1 | Normal biopsy or nonspecific changes |
| Category 2 | Antibody-mediated changes Active antibody mediated rejection (ABMR) Chronic active ABMR |
| Category 3 | Borderline changes Suspicious for acute T-cell-mediated rejection |
| Category 4 | Acute T cell mediated rejection Chronic active T-cell-mediated rejection |
Caused by circulating antibodies that react to donor endothelium
Diagnostic criteria for AMR are
histologic evidence of acute injury,
evidence of antibody interaction in the kidney, e.g., complement fragment 4d (C4d) positivity and
circulating antibody
Treatment is more challenging and usually involves plasma exchange and intravenous immunoglobulin.
Occurs after 6 months and often leads to a progressive decline in, and eventual loss of, organ function
Multifactorial aetiology: immune-mediated injury, IRI, toxicity from immunosuppressive agents and viral infections
Characterised histologically by cellular atrophy and fibrosis; there are currently no therapeutic strategies to combat it.
To minimise the risk of rejection, tests are undertaken by histocompatibility scientists to optimise the match between donor and recipient.
Prior to adding patients to the transplant waiting list, potential recipients are typed for class I and class II HLA antigens; this is known as tissue typing
Most allocation schemes for kidney transplantation internationally place a high priority on donor and recipient HLA matching, as the better match the kidney is to the recipient, the better the outcome
Newer technologies, such as single antigen bead testing (SAB), allow identification of specific antibodies produced by the recipient in response to pregnancies, previous transplants and blood transfusions, and this allows more thorough tissue matching.
A crossmatch is undertaken in some transplant patients immediately prior to renal transplantation to ensure that there is no reactivity between donor and recipient cells.
Flow cytometry crossmatch (FC-XM) is a sensitive test, which, if positive, may represent an increased risk of rejection
Review of recipient’s antibody profile with SAB testing allows for more accurate prediction of rejection risk
Virtual crossmatch: In patients with a known antibody profile who are at low immunologic risk, it is possible to predict a negative crossmatch and thus proceed without waiting for the tests to be performed. This saves approximately 4 hours of cold ischaemia.
The challenge is to minimise the risk of graft rejection with as few side effects as possible. Various strategies are adopted: induction therapy, maintenance immunosuppression and treatment of rejection. The mechanisms of action of the common immunosuppressive drugs are outlined in Fig. 26.2 .
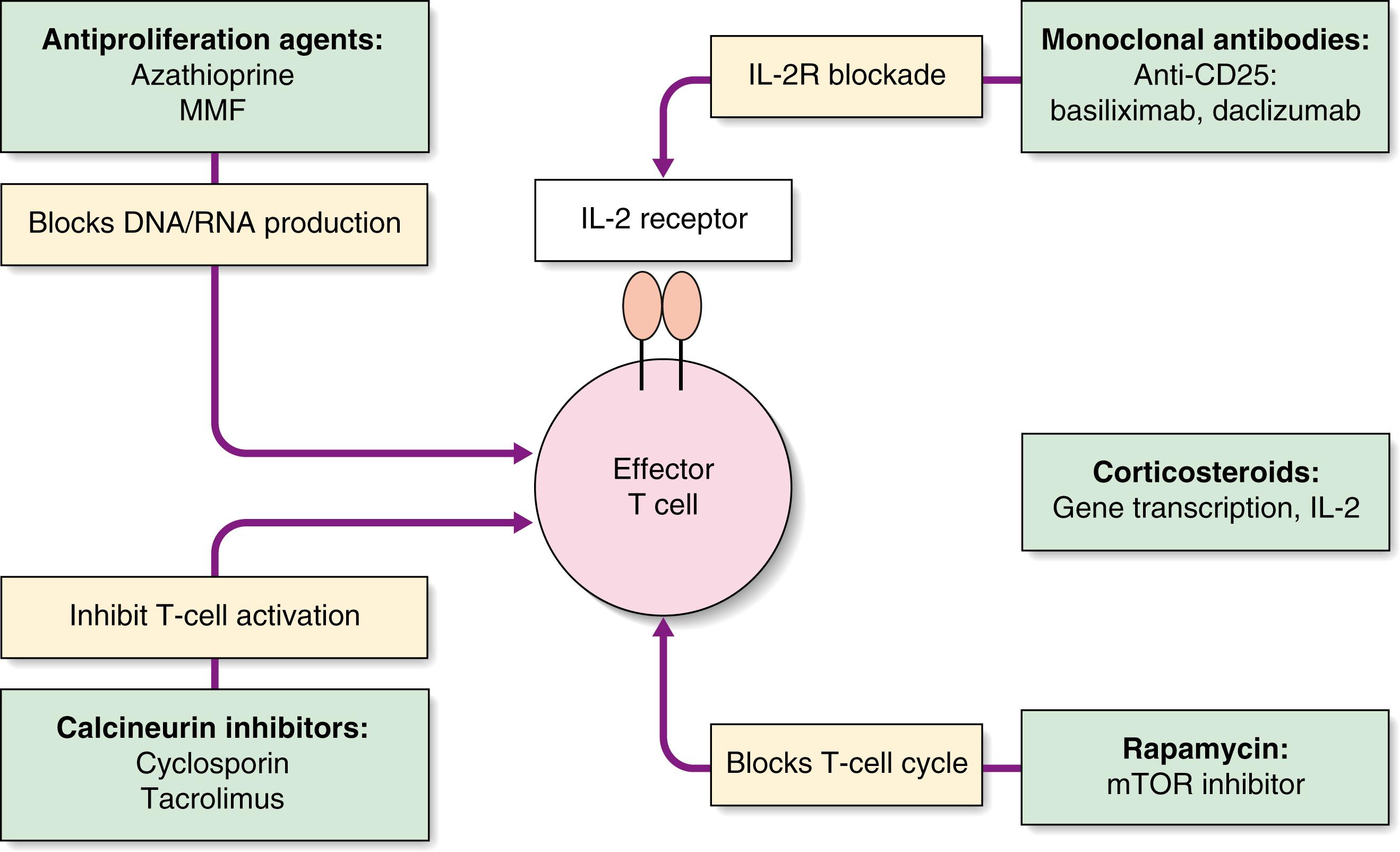
Play an important role in induction and maintenance and are the first-line treatment for acute rejection
Side effects of steroids are numerous and are responsible for many of the long-term complications of immunosuppressive therapy ( Table 26.2 )
|
Several groups have explored reduction of the steroid load through various strategies:
Steroid withdrawal, undertaken some time after transplantation
Steroid avoidance, in which steroids are stopped a few days postoperatively
Steroid free
Whilst the benefits of reduced steroid use have been observed in these studies, steroid free and steroid withdrawal are associated with an increased incidence of biopsy-proven acute rejection at 12 months posttransplant.
Prodrug for mycophenolic acid (MPA), which prevents lymphocyte activation by inhibiting DNA formation
Has replaced azathioprine in many renal transplant patients, following the publication of several randomised trials that demonstrated reduced treatment failure at 6 months postkidney transplant
Side effects include gastrointestinal disturbances, leucopenia, thrombocytopenia and anaemia.
Led to significantly improved 1-year outcome in liver and renal transplant patients compared with cyclosporin
3C Study Collaborative Group. AM J Transplant 2018:18(6):1424–1434.
Adapted from Muduma G, Saunders R, Odeyemi I, Pollock R. Plos One 2016: 11(11) .
Tacrolimus offers improved mortality and hypertension rates compared with ciclosporin
Ciclosporin gives lower risk of new onset diabetes after transplantation versus tacrolimus
Sirolimus gives increased risk of rejection and infection with no improvement in transplant function compared with tacrolimus
Is now the mainstay of many immunosuppressive regimens
Side effects include nephrotoxicity, neurotoxicity, diabetes and alopecia ( EBM 26.3 ).
Antibody therapies may be used as induction therapy peritransplantation or for treatment of acute rejection.
Antibody therapies may be depleting or nondepleting:
Depleting antibodies: Target cells are removed from the peripheral blood, including rabbit antithymocyte globulin (ATG) and alemtuzumab. They may be used as induction therapy or to treat steroid-resistant rejection.
Nondepleting antibodies: In which the function of the target cells is affected, e.g., the interleukin-2 receptor antibody, basiliximab. This is used as induction therapy, reducing acute rejection rates, with few side effects.
Belatacept: Acts through T-cell costimulatory blockade and has been introduced in clinical trials with the aim of reducing requirement for calcineurin inhibitors
Bortezomib: Acts as a proteasome inhibitor, inhibiting the production of antibody-producing plasma cells; it is used in antibody-incompatible transplants, to reduce the level of circulating donor-specific antibody and in cases of refractory antibody-mediated rejection
Eculizumab: Understanding the key role of complement in mediating antibody damage has led to the development of the complement inhibitors such as the monoclonal antibody, eculizumab. It has been used to prevent antibody-mediated rejection and to reduce the risk of recurrent atypical haemolytic uraemic syndrome (aHUS) posttransplant.
The risk of infection is related to the dose of immunosuppression and is therefore greatest early after transplantation. Bacterial infections are most common during the first month. Viral infections are most common between 1 and 6 months and, of these, cytomegalovirus (CMV) is the most clinically relevant. The risk of CMV disease is reduced using antiviral agents such as valganciclovir prophylactically. Opportunistic infections with protozoa and fungi are also important, and most patients receive up to 6 months’ cotrimoxazole prophylaxis against Pneumocystis jirovecii.
The risk of developing skin cancer is particularly high, with squamous cell carcinoma being 20 times more common in transplant patients than in the normal population. Posttransplant lymphoproliferative disorders (PTLDs) are usually related to infection with Epstein–Barr virus and are associated with a high risk of developing B-cell lymphoma. Treatment of PTLD involves reduction in immunosuppression and, in some cases, chemotherapy.
The immune response to a transplanted organ is largely mediated by T cells, and these are the target for immunosuppressive therapy.
Acute rejection is seen in up to 50% of grafts, and episodes are treated with high-dose steroids.
Chronic allograft damage has a multifactorial aetiology and results in significant graft loss over the months and years following transplantation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here