Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Adverse reactions following blood transfusion reflect immunologic, pathophysiologic, and microbiologic events. This chapter presents information about transfusion-associated viral, bacterial, parasitic, and prion infections and discusses a number of emerging agents including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Transfusion-transmitted infection risk mitigation through blood donor screening and blood testing strategies are presented. The boxed discussions provide insights into interventions aimed at reducing risk from known and emerging threats and new technologies for reducing or eliminating microbial contamination. Red cell, platelet, and plasma transfusions represent important therapeutic modalities for appropriately selected patients. Awareness of the hazards of transfusion and the rate at which these events occur should enable physicians to better determine the benefit/risk ratios when prescribing transfusions.
The hepatitis viruses can be classified according to their predominant modes of transmission, parenteral and enteric, with the parenterally transmitted agents, hepatitis B virus (HBV) and hepatitis C virus (HCV) dominating concerns about transfusion transmission because many individuals unknowingly infected with these agents become asymptomatic chronic carriers and may make blood donations.
HBV is a deoxyribonucleic acid (DNA) virus in the family Hepadnaviridae . The infectious virion is occasionally referred to as the Dane particle and has surface and core components, surface antigen (HBsAg) and core antigen (HBcAg), respectively. Epitopes on the viral surface provide a basis for epidemiologic studies and consist of the HBsAg “a,” d/y, and w/r determinants. However, this approach is being replaced by genotyping and DNA sequencing methods. Recombinant vaccines containing the “a” determinant confer protective immunity to a high proportion of vaccinees and are dramatically altering the incidence and prevalence of HBV infection in the general population where they are in wide use and consequently also impact the frequency of HBV infection in the donor population.
The average incubation period (the time from infection to liver enzyme elevation and symptomatic hepatitis) is 59 days (range, 5 to 12 weeks) but may be as long as 6 months. Symptoms, which occur in 30% to 50% of infected persons aged 5 years and older, include fatigue, anorexia, nausea, vomiting, jaundice, dark urine, light stools, arthralgias, rashes, vasculitis, and glomerulonephritis. The risk for progression to chronic infection is inversely related to age at infection. HBV infection becomes chronic in more than 90% of infants, 25% to 50% of children 1 to 5 years of age, and fewer than 5% of older children and adults. Approximately 5% of the US population has serologic evidence of prior HBV infection (antibodies against HBcAg [anti-HBc] reactive). From the mid-1980s with increasing immunization in early childhood, the incidence of acute HBV infection has decreased. In 2017, 46 states reported over 3400 cases of acute HBV to the US Centers for Disease Control and Prevention (CDC), or 1.1 case per 100,000 population. Due to underreporting and asymptomatic cases that are not diagnosed and reported, estimates of the actual number of acute cases in 2017 is over 22,000. An additional 13,300 cases of chronic HBV cases were reported and approximately 862,000 people are estimated to be living with chronic HBV infection. Approximately 1700 deaths occurred in 2017 resulting from hepatitis B and its complications.
Based largely on data from parenteral exposures of health care workers, HBV is 100 times more infectious than human immunodeficiency virus (HIV) and 10 times more infectious than HCV. The predominant mode of transmission to adults and adolescents is through sexual contact. Forty percent have infected partners, 15% are males having sex with other males, injecting drug users account for 14% of cases, and one-third have no identifiable risk. A recent study of risk factors in blood donors found to be infected with HBV found that of 292 infected donors surveyed, the characteristics most frequently associated with infection were living abroad or having immigrated to the United States (51% vs. controls at 6%), a family member infected with hepatitis (15% vs. 2%), having been in a jail or detention for 3 nights or more (19% vs. 5%), and having taken illegal drugs (20% vs. 12%).
HBsAg is detectable in blood approximately 4 weeks (30 to 60 days) after infection. Subsequently, immunoglobulin M (IgM) anti-HBc antibodies appear coincident with symptom onset. High viral titers present at that time decline subsequently. HBsAg persists transiently in acute infections for up to 4 months (average 63 days). More recent data using extremely sensitive HBsAg assays for blood donation screening show the mean period of HBsAg duration to be 43 days. Antibodies against HBsAg (anti-HBs) develop subsequently and are generally thought to protect against reinfection although recent studies on blood donors have identified a few breakthrough cases of infection. These cases were mainly caused by HBV genotypes differing from that of the vaccine strain and were mild and self-limited. Such donations were not detected by HBsAg blood donation screening but required more sensitive HBV DNA screening.
Some anti-HBc–positive and HBsAg-negative individuals have circulating HBV DNA, and this pattern defines so-called occult HBV infection (OBI). In rare cases, OBI may be accompanied by anti-HBs, usually at levels below 200 mIU per mL. Donors with OBI may transmit HBV via blood transfusion, but the frequency of such infection is low, and seems to be absent if anti-HBs are present. Although anti-HBs usually confers immunity to reinfection, sufficient virus remains in the liver to transmit HBV following liver transplant from anti-HBs–positive donors through reactivation under intense immunosuppression.
It should be noted that the FDA has eliminated the requirement to ask blood donors about a history of viral hepatitis, effective May 2016. The detection of HBV DNA is performed by nucleic acid testing (NAT) in minipools of 6 to 16 donations each as now required per Food and Drug Administration (FDA) Guidance, Use of Nucleic Acid Tests on Pooled and Individual Samples from Donors of Whole Blood and Blood Components, including Source Plasma, to Reduce the Risk of Transmission of Hepatitis B Virus ( www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/default.htm , October 2013) and made final in updates to the Code of Federal Regulations. The risk for HBV transmission per unit in the United States has recently decreased substantially as a result of very sensitive HBsAg testing, the implementation of NAT, and a recent decline in the incidence of HBV infections among donors undoubtedly because of universal vaccination ( Table 118.1 ). Before NAT implementation, most contemporary HBV transfusion-transmitted infections were attributable to blood donations from asymptomatic donors during acute infection preceding the development of detectable HBsAg. Early in infection, HBV replicates relatively slowly with a doubling time of approximately 2.6 days. The current window period between infection and the detection of HBV DNA by NAT ranges from 30 to 38 days depending on the infectious dose, which, early in infection, is estimated to be between 1 and 10 copies per mL. Since the implementation of HBV NAT in the United States, there have been no documented cases of breakthrough HBV transfusion transmissions. The per unit residual risk for donations to the American Red Cross was estimated to be 1 per 843,000 to 1 per 1,208,000 in 2013 and 1 per 1,529,000 in 2015 to 2016 using an 18.5 day window period and weighted for all donors (first-time and repeat). Residual risks of 1 per million using the same methods are documented from large multicenter national studies for 2018 to 2019.
| Pathogenic Agent or Disease | Average Estimated Risk Per Unit |
|---|---|
| Hepatitis A | Rare |
| Hepatitis B | 1 per million |
| Hepatitis C | 1 per 2 million |
| Human immunodeficiency virus | 1 per 1.6 million |
| Human T-lymphotropic viruses 1, 2 | 1 per 3.3 million |
| Cytomegalovirus |
|
| Parvovirus B19 | Rare |
| West Nile virus | Rare |
| Malaria | 0–3 cases per year |
| Babesiosis |
|
| Chagas disease | 20 transfusion-associated cases reported |
| Creutzfeldt-Jakob disease |
|
| Bacterial Contamination: | |
| Red cells | 1 per 30,000 |
| Septic reactions | 1 per 500,000–1 per 10 million |
| Platelets | 1 per 3000–1 per 8000 |
| Septic reaction | 1 per 107,000 |
| Emerging Infections: | Risk unknown |
|
|
There is evidence that some HBsAg assays will not detect all HBsAg and HBc genetic variants of HBV. Because all blood donations are screened by HBV NAT, HBsAg, and anti-HBc, it is unlikely that any mutant strain of HBV would go undetected in the United States. Among 12.8 million blood donors to the American Red Cross during 2009 to 2011, 1 per 9337 were infected with HBV. Of these, 1090 were positive for HBsAg, with or without HBV DNA (1 per 11,736), and only 5 were identified solely by NAT in minipools (1 per 2.55 million). However, more recent data, also from the American Red Cross, demonstrate the HBV DNA detection rate of seronegative donations by NAT to be 1 per 770,000 donations. NAT on individual donation aliquots has the potential to reduce the risk for transfusion-transmitted HBV further by detecting newly infected donors slightly earlier than either minipool-NAT or HBsAg tests licensed for blood donor screening, but the additional cost of individual donation-NAT in the United States would be high with very little to no demonstrable clinical benefit.
Hepatitis D virus (HDV) was originally called the delta agent. It is a defective ribonucleic acid (RNA)–containing passenger virus that requires active synthesis of HBsAg to act as a “helper” for assembly of HDV virions. As many as 10% of HBV infections are accompanied by HDV worldwide. Its prevalence is very low in the United States but higher in intravenous drug users. HDV superinfection of chronic HBV carriers is associated with worsened chronic sequelae and with fulminant hepatitis. Screening for HBV acts synergistically to prevent transfusion-associated HDV cases by identifying donors that are co-infected with HBV and HDV. There has never been an HBV-HDV–related transfusion transmission reported.
HCV is an RNA virus in the family Flaviviridae , genus Hepacivirus . There are six genotypes that share similar epidemiology, pathogenesis, and natural histories. In the United States, genotype 1 causes 75% of infections, genotypes 2 or 3 causing 15% to 20% of infections, and genotypes 4, 5, and 6 causing less than 5% of infections. Genotype 1 responds relatively poorly to traditional treatment regimens using pegylated interferon combined with oral doses of ribavirin compared with genotypes 2 and 3; the overall cure rate for all genotypes using this regimen was no better than 40% to 50%. HCV protease inhibitors (direct acting antivirals) are materially improving these rates. Two oral regimens of Harvoni and Viekira Pak are now the standard of care in the United States, and result in a sustained virologic response of greater than 90% ( https://www.cdc.gov/hepatitis/statistics/2017surveillance/index.htm#hepatitisB , Viral Hepatitis–2017 Surveillance).
HCV is distinguished by a low rate of recognized acute infection and by a high rate of chronic infection, with substantial morbidity and mortality over long periods of observation as a result. The CDC estimates that there were 2.4 million people living with chronic HCV infections in 2017.
The most common source of HCV acquisition is intravenous drug use. The prevalence of HCV in US adults (20 to 59 years old) with any history of illegal intravenous drug use is greater than 45%. Other risks include blood transfusion before donor serologic screening began in 1990, exposure in health care settings, including through dialysis, among infants born to HCV-infected mothers, and tattoos in unregulated settings. In a large series, 15% to 30% of patients report no risk factors. Vertical transmission occurs in 3% to 7% of infants of mothers with active infections. In contrast to HBV, sexual transmission is an inefficient route of infection and much less often reported but was found to be the most likely mode of transmission of HCV among HIV-infected men who have sex with men (MSM) in New York City. In 2012 the CDC recommended that individuals born during 1945 to 1965 have one-time HCV screening since among all persons living with HCV infection, about 75% were born during this time. Such individuals have a 3% prevalence, which is five times higher than the prevalence seen in adults born in other years. Among blood donors, 0.03% have confirmed-positive HCV test results. There have been studies on risk factors among HCV-infected blood donors, and despite policies requiring deferral of injection drug users, such use is the most common risk factor. Of 316 blood donors followed who were HCV-confirmed positive, risks identified via a retrospective questionnaire revealed risks versus control donors of illegal drug use (37% vs. <1% for controls), followed by jail or detention (57% vs. 5%), history of a blood transfusion before screening (18% vs. 7%), and living in a household with someone having hepatitis (15% vs. 2%).
At most 20% to 30% of newly infected persons develop recognizable symptoms during acute HCV infection. Some 20% of infected individuals clear their infection over a relatively short initial period, remaining antibody-positive but RNA-negative. Chronic infection develops in 75% to 85% of persons infected after 45 years of age and in 50% to 60% of those infected as children or young adults. Chronic HCV infection progresses to cirrhosis in 15% to 30% over 30 years of observation. Hepatocellular carcinoma occurs in 1% to 4% per year in those with cirrhosis. HCV is among the most prevalent causes of chronic hepatitis, cirrhosis, and primary liver cancer in the developed world and is the most common indication for liver transplantation in the United States, resulting in around 2400 procedures annually.
A single positive anti-HCV result cannot distinguish between acute and chronic HCV infection or between current or cleared infection. In 2012 laboratory criteria for the confirmation of anti-HCV reactivity were modified to add one specific assay including RNA detection, using a supplemental anti-HCV assay (as available) or a single value above a specific threshold on the screening test. The risk for posttransfusion HCV infection declined progressively with the introduction of surrogate markers for non-A, non-B hepatitis in the 1980s (alanine aminotransferase [ALT] and anti-HBc) and of serologic testing for HCV antibodies in May 1990, followed sequentially by improved serologic testing and NAT. The seronegative-window period for the first-generation HCV antibody test extended to 6 months from infection but was reduced to 82 and 70 days with second- and third-generation assays, respectively. NAT further reduced the window period to approximately 7 days. The risk per unit declined from an estimated 1 per 276,000 units to 1 per 2,600,000 units from 2007 to 2016 and in 2018 to 2019 in larger multicenter national studies to 1 per 2 million weighted for all donations from both first-time and repeat donors.
Like HBV, in the United States, testing for HCV RNA has used NAT in small minipools combining aliquots from 16 to 24 donations (currently 6 to 16). Loss of test sensitivity because of sample pooling was tolerable given the rapid increase or burst of HCV viremia before antibody seroconversion (estimated doubling time of 10.8 hours in that period during the 40- to 50-day window period) and the high titer of viremia that remains preceding antibody seroconversion. However, the rest of the world (except the United States, Canada, the United Kingdom, and most of Germany) has implemented NAT for screening individual donation samples intended for transfusion. The HCV RNA yield by NAT in seronegative donations using data from the American Red Cross is 1 per 270,000 donations.
As an alternative to NAT assays, enzyme immunoassays (EIAs) have been developed that detect HCV HBcAg in serum or plasma, either as an individual analyte in parallel with antibody assays or as HCV antigen-antibody combination assays. These tests reduce the pre-seroconversion window period and were adopted in some developing countries. They are less sensitive than HCV NAT, are not approved for blood donor screening in the United States, and use has decreased significantly. Since the implementation of NAT, reports of transfusion-transmitted HCV are very infrequent.
The high seroprevalence of HCV at the onset of serology screening in the early 1990s, the prolonged interval between infection and clinical manifestations, and the relatively high rate of HCV clinical sequelae prompted blood collection facilities and hospitals to conduct “look-back” notification of previous recipients of blood given by donors found on subsequent donations to be HCV infected. Look-back was subsequently made mandatory in companion rules from FDA and the Centers for Medicare and Medicaid Services. In general, HCV look-back programs found half or fewer of targeted transfused individuals alive but were able to find both seropositive and RNA-positive recipients who were unaware of their status. This population has benefited significantly from identification and subsequent treatment with the advent of curative direct-acting antivirals.
The hepatitis A virus (HAV), a nonenveloped picornavirus, genus Hepatovirus , is transmitted predominantly by the fecal-oral route, with an average incubation period of 28 days (range, 15 to 50 days) with signs or symptoms persisting for less than 2 months. The incidence of HAV infection in the United States fell by 76% between 1997 and 2003 after the recommendation for targeted immunization of members in high-risk communities. Populations at risk include those in areas where extended community outbreaks occur and children living in states that have high and intermediate rates of disease, staff and residents of closed communities, close personal contacts of cases, the staff and parents of children in daycare centers, and those with common-source exposure to infected food or water. For many sporadic cases there is no recognized source. HAV is self-limited with no chronic carrier state, but approximately 10% to 15% of infected individuals develop a more prolonged or relapsing illness. It is the most frequent cause of hepatitis among children under 11 years of age.
Transfusion-related transmission, although rare, is caused by a blood donation from a recently infected, asymptomatic, viremic individual. The peak viremia occurs 2 weeks before onset of jaundice or elevation of hepatocellular enzymes and persists for a median period of 42 days (range, up to 59 days). The virus is quite resistant to many inactivation procedures, including the pathogen-reduction procedures being developed for cellular blood components (e.g., licensed psoralens or investigational riboflavin, both with ultraviolet [UV] irradiation) and fresh-frozen plasma (solvent/detergent and methylene blue). Transmission by clotting-factor concentrates treated with the solvent/detergent pathogen-reduction process occurred in the 1990s, but not thereafter. Plasma for further manufacture is routinely screened for HAV RNA by pooled NAT.
A 120-day deferral is recommended after exposure to HAV during community outbreaks to prevent transfusion transmission. Screening for HAV is not done for donations of blood for transfusion.
While the average number of annual HAV infections reported to CDC in recent years has declined substantially compared to 2000, fluctuations have occurred in the last 20 years due to large outbreaks. After a long downward trend, the first increase between 2012 and 2013 (1562 and 1781 reported cases, respectively) was due to a large multi-state outbreak associated with pomegranate arils imported from Turkey. Between 2015 and 2016, the reported cases again increased by 44.4% from 1390 in 2015 to 2007 cases in 2016. The 2016 increase was due to two hepatitis A outbreaks, each of which was linked to imported foods. Substantial increases in incident cases of hepatitis A occurred in 2017 and 2018 (3366 and 12,474 reported cases, respectively) due to ongoing outbreaks reported to CDC among people who use drugs and people experiencing homelessness as well as outbreaks among MSM.
The hepatitis E virus (HEV) is a small, nonenveloped single-stranded RNA virus in the Hepeviridae family. HEV was first recognized in the 1980s in Afghanistan among soldiers with unexplained hepatitis. There is a single serotype but at least four genotypes with differing geographic distributions and epidemiologic patterns. Genotypes 1 and 2 are generally associated with large, water-borne (fecal-orally transmitted) outbreaks in less developed tropical countries. Illness is usually self-limited but can be lethal in pregnant women, their fetuses, and patients with chronic liver disease. Genotypes 3 and 4 appear to be animal viruses that result in zoonotic infection of humans, most often through consumption of inadequately cooked pork products. Genotype 3 seems to be widely distributed and is present in developed countries, whereas genotype 4 seems to be more common in certain Asian countries. Transfusion-related transmission, mostly of serotype 3, has been well-documented in Japan, France, England, the Netherlands, and Spain.
Recent studies suggest a wide range of seroprevalence rates, but some of the variability may be attributable to the differences in performance characteristics of the tests used and some to dietary habits. Most studies indicate a cohort effect, with prevalence rates increasing with age. Transfusion infectivity is logically associated with the presence of detectable viral RNA in the plasma and the frequency of this finding varies between 1 in 1000 to 1 in 10,000 donations.
In a large study in England, 225,000 donations were tested and 79, or 1 in 2848, were found to be positive when tested for HEV RNA. For 43 of these, recipient tracing was possible and 18 (42%) showed evidence of transfusion-transmitted infection. A North American study screened 101,489 donations from Canada and the United States for HEV RNA and reported 14 positive donors, or 1 in 7300, all with low viral loads (ranging from 20 to 3080 copies/mL) consistent with a prior US study finding 7.7% of donors anti-HEV positive but only two of approximately 19,000 that were RNA positive. Although the Canadian prevalence was fourfold higher than in the United States, quantitative and risk-based analysis found testing to not be cost effective. A current broader concern is the finding that highly immunosuppressed patients (such as solid-organ transplant recipients) do develop chronic HEV infections with long-term clinical sequelae, although these have not been specifically linked to infection via transfusion. While testing is standard in some European countries and Japan, where incidence rates are higher and transfusion transmissions well documented, in the United States and Canada routine testing is currently not recommended due to the absence of recognized transfusion transmission.
Cases of posttransfusion hepatitis only rarely if ever occur but there remains speculation that undiscovered hepatitis agents exist. A small but consistent percentage of community-acquired hepatitis cases test negative for known hepatitis viruses, some cirrhosis is classified as “cryptogenic,” an etiologic agent for hepatitis-associated aplastic anemia ( Chapter 31 ) eludes description, and the cause of some cases of acute liver failure remains elusive. Several candidate agents have been proposed as non–A-E hepatitis viruses. None of these agents have been shown to be pathogenic and are instead likely commensal and nonpathogenic.
GBV-C (initially called hepatitis G virus) is a flavivirus with no confirmed disease association that is transmitted parenterally, including frequently by transfusion. Of interest, GBV-C infection may delay progression of disease in those co-infected with HIV, which has led to studies of the interactions between these viruses.
From 1% to 4% of US blood donors are viremic compared with 15% to 20% of intravenous drug users who have detectable GBV-C RNA. Infection occurs frequently among those infected with HCV and HIV. More people have antibodies against the E2 envelope protein, in the absence of RNA, suggesting viral clearance. GBV-C has not been shown to cause liver disease or other morbidities, and hence there is no consideration of donor screening at this time. GBV-C is now referred to as a human pegivirus. A second human pegivirus has recently been described associated with HCV likely as a result of an acute parenteral co-infection event, but again, has not been associated with hepatitis or any pathology. This virus was identified by next generation sequencing. Likely other such commensal agents will continue to be identified by the use of sophisticated molecular techniques ( Chapter 3 ).
The torque teno virus (TTV) complex is a genetically diverse group of nonenveloped DNA viruses in the family Circoviridae , which was discovered in 1997. They cause viremia, and they are transmitted by transfusion, but they cause no recognized liver disease or other clinical illness.
SEN virus (SENV), another member of the Circoviridae , was described using degenerate polymerase chain reaction (PCR) primers while working with TTV. After an initial report associating SENV variants in two patients with transfusion-associated non–A-E hepatitis, subsequent epidemiologic studies have failed to link SENV with clinical hepatitis.
The HIVs type 1 and type 2 (HIV-1 and HIV-2) are retroviruses of the family Retroviridae , genus Lentivirus , and the etiologic agents of the acquired immunodeficiency syndrome (AIDS). They are enveloped viruses with two linear, positive-sense RNA molecules, 9.2 kb in length. The predominant transmission mechanisms are sexual, perinatal, and parenteral. An acute retroviral syndrome may be seen around 21 days after infection, although this period may range from 5 to 70 days and may involve fever, lymphadenopathy, and rash. If untreated, the incubation period for full-blown AIDS is measured in years. Molecular characterization divides HIV-1 into three groups: group M (main), group O (outliers), and group N (non-M/O). Group M subtype B infections predominate in the United States; only 3% of HIV-positive blood donors have non-B strains. Very rare group O infections have been detected in the United States among patients who were born, lived, or had sexual contact in endemic regions of West and Central Africa. HIV-2 infected persons in the United States are rare (less than 1% of HIV cases diagnosed annually) and have, for the most part, been infected after heterosexual transmission in West African emigrants or residents. HIV-2 disease requires a longer time to evolve and is less severe than that from HIV-1 There have been five confirmed cases of HIV-2 infection among blood donors in the United States.
In 2016 CDC estimated that a total of 1.1 million people in the United States 13 years or older were living with HIV infection, approximately 1 per 300 Americans, with 14% of those unaware of their infection. Of the approximately 38,000 new diagnoses in 2018, the highest number of new infections occurred in those 25 to 29 years old (20%), in predominately African Americans (40%). MSM continue to bear the greatest burden of HIV infection among all racial and ethnic groups. Although MSM represent 6% of the US population, in 2018 MSM accounted for nearly 82% of all new HIV infections in males and over 65% of all new HIV infections. Antiretroviral therapy for HIV treatment has improved dramatically since the advent of combination therapy in 1996, even against multidrug resistant viruses. HIV-associated morbidity and mortality have significantly been reduced so that HIV treatment has converted HIV infection to a chronic, versus a fatal, disease.
Antibody testing of blood donors detects both HIV-1 and its variants and HIV-2. Antibody testing for HIV-1 began in 1985, with the addition of HIV-2 in 1992 (although HIV-1 tests before that time detected most HIV-2 infections arising from over 60% sequence homology between the two viruses) and for HIV-1 group O in 2006.
Both serologic testing and NAT are performed on every blood donation. First-generation antibody tests had a window period (time between infection and detection) of 45 days on average but has decreased significantly as tests became more sensitive leading to less than a 20-day window period. HIV-1 NAT, implemented in 1999 (initially in duplex tests with HCV performed in pools of 6 to 16 donations), detects RNA at a minimum concentration of approximately 5 copies per milliliter (50% lower limit of detection) leaving about a 9-day window period of risk in which an infected donor could donate and not be detected by any test in current use. Rare genetic variants of HIV may escape NAT detection when nucleotide sequence changes affect NAT primer or probe binding sites, but the vast majority of these infections will be detected serologically. Further, tests are now designed to detect two or more separate sequences representing different viral regions. Currently, the frequency of confirmed-positive test results for antibodies to HIV is 1.65 per 100,000 donations (1:61,000). Almost all of these are also RNA-positive as the finding of an infected donor with low-level RNA levels is very rare (on the order of 2%). One donation per 2.1 million is confirmed positive for RNA in the absence of antibody (NAT yield).
To eliminate donations in the window period between exposure and test positivity, each blood donor is asked at each donation about exposure risks using questions developed in the early to mid-1980s, subsequent to the first reports of AIDS in hemophiliacs and transfusion recipients. These initial interventions targeted blood donations from homosexually active men and intravenous drug users, substantially reducing the transmission risk between 1983 and 1985. Five clusters of transfusion transmissions have been documented subsequent to the introduction of NAT, three before 2002 and one in 2008. It is of interest to note that in two cases, although the transfused plasma component transmitted HIV, the corresponding red cell component did not. Modeling suggests a residual risk for HIV transmission persists at approximately one transmission per 1.5 million donations, because NAT cannot detect HIV infection during the immunosilent 9-day eclipse phase between infection and test reactivity. Processing and quarantine procedural errors do not now appear to be a risk for transmission of HIV via transfusion. Fourth-generation tests combine HIV-1/2 antibody and HIV antigen detection and detect almost 90% of infections detected by NAT. Their role is expanding worldwide even in countries that use HIV-1 NAT. In 2014 the CDC published revised guidelines for laboratory diagnosis of HIV infection ( https://doi.org/10.15620/cdc.23447 ).
Recent studies have shown that the predominant risk factors for HIV infection among 149 male infected blood donors surveyed retrospectively continues to be a history of MSM behavior (62% in cases vs. 2% in controls) or of a male having sex with an HIV-positive individual (26% in cases and none in controls). Of note, 50% of MSM activity occurred within the last 12 months. Although in the past, intravenous drug use was also a prominent risk factor, this has decreased in prominence (24% in cases vs. 5% in controls).
In 2015, the Transfusion-Transmissible Infections Monitoring System (TTIMS) was established by the FDA, National Heart, Lung and Blood Institute (NHLBI), and the Office of the Assistant Secretary of Health (OASH) to establish a comprehensive monitoring program for transfusion-transmissible infections in the United States. Of primary focus for the first years of the program was the changing prevalence and incidence rates of HIV (as well as HCV and HBV) before and after MSM policy changes enacted by US blood centers following guidance changes by the FDA. Recent reports from the system show no marked changes in prevalence, incidence, residual risk, or recency of HIV infection during the 4 years surrounding policy changes, accounting for over 27.5 million donations from 9.4 million donors. In April 2020, in response to the SARS-CoV-2 pandemic, the US FDA updated their 2015 guidance reducing the deferral period of MSM from 1 year since last MSM activity, as outlined in the 2015 guidance, to 3 months since last activity. The TTIMS program will continue to monitor changes in screening and testing for TTIs following this policy change as well.
Current issues of concern related to HIV deferrals and testing include individuals on anti-retroviral therapy and those on post-exposure and pre-exposure prophylaxis. Initial reports show that individuals on ART or PrEP are part of the US donor population and that improved strategies need to be developed to encourage disclosure of these therapies and decrease test-seeking behaviors.
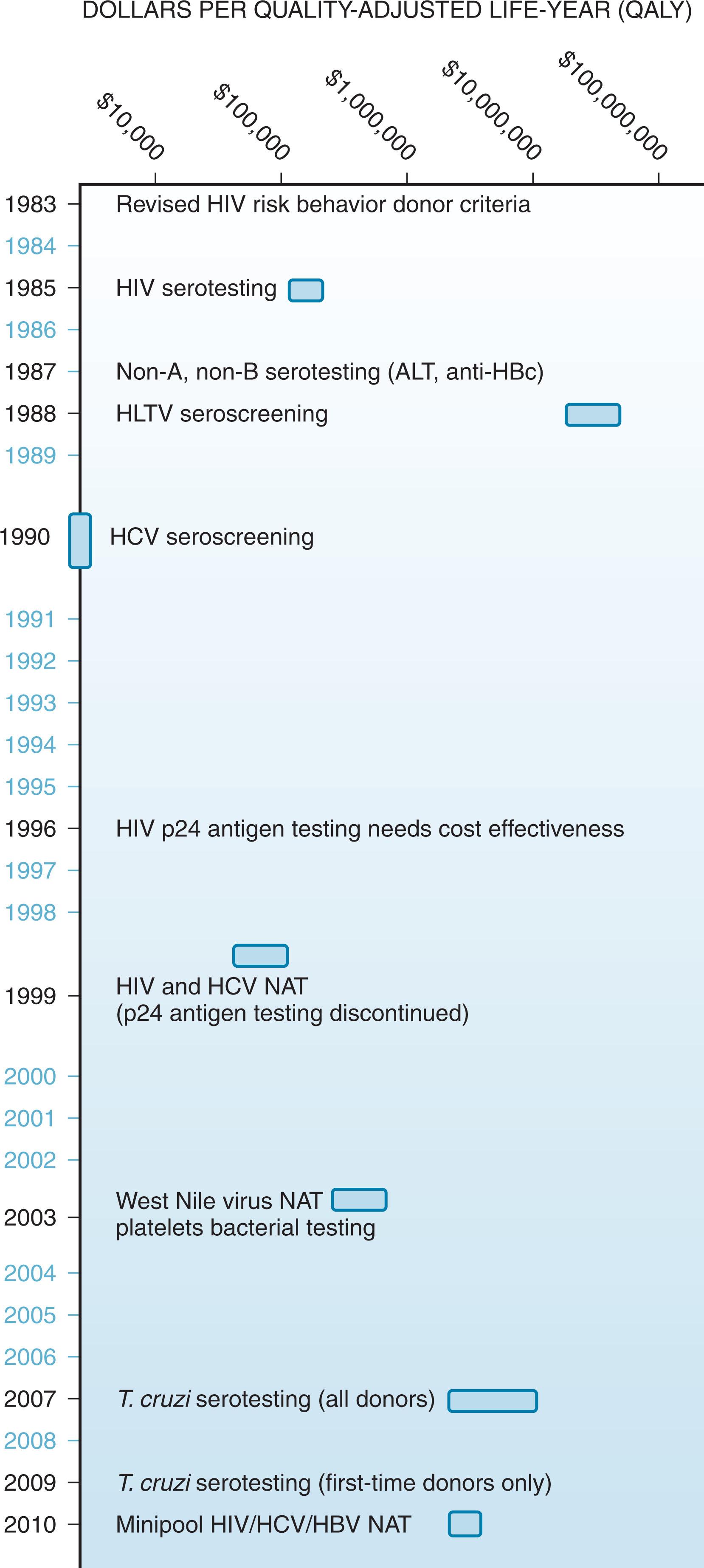
Most consider the blood supply in the developed world to be at its highest historical safety level. This reflects incremental improvements in donor selection and history screening, blood testing, and process control that span four decades. For years, blood collection professionals and government regulators formulated blood safety policy decisions in an apparent aim to achieve a zero-risk blood supply. In part, this reflects the perceived delayed response of the transfusion medicine community in the early 1980s to the emergence of HIV in the blood supply and the recognition of the scope and severity of post-transfusion non-A, non-B hepatitis (subsequently hepatitis C [HCV]) following that. It reflects also the “dread fear” associated with transfusion-associated HIV. This reaction is seen when devastating, unpredictable, and stigmatizing events threaten potential victims who have little ability to escape the risk. This fear was validated by numerous transfusion-related HIV cases. It was amplified by widely publicized lawsuits, indictments, and criminal convictions of health ministers and policy makers in the 1980s and 1990s ( l’affaire du sang contaminé in France addressing HIV and Canada’s Royal Commission of Inquiry on the Blood System into blood collection agencies’ response to non-A, non-B hepatitis risk) ( Fig. 118.1 ). (See box on Blood Safety Decision Making .)
Human T-lymphotropic virus-1 (HTLV-1) and HTLV-2 are closely related delta retroviruses with 60% to 70% sequence homology and shared tropism for T-lymphocytes. In contrast to HIV, HTLV is rarely present in cell-free plasma and shows little active replication in infected humans. HTLV-1 is distributed worldwide, with endemic foci in southern Japan, the Caribbean, certain parts of South America, Africa, the Middle East, and Melanesia. HTLV-2 is endemic among Amerindians in both North and South America and African Pygmies. An epidemic of HTLV-2 infections has occurred over the past 40 to 50 years among intravenous drug users in the United States, Brazil, and Europe. Transmission of both HTLV-1 and HTLV-2 occurs by parenteral exposures, sexual contact, and by vertical transmission from mother to child during pregnancy and breastfeeding.
Diseases associated with HTLV-1 infection include adult T-cell leukemia/lymphoma (ATL), HTLV-associated myelopathy/tropical spastic paraparesis (HAM/TSP), lymphocytic pneumonitis, uveitis, polymyositis, and arthritis. HTLV-2 does not appear to cause hematologic malignancies but has been associated with HAM/TSP and linked to a higher rate of common infections such as acute bronchitis, pneumonia, and urinary tract infections, suggesting a subtle immunomodulatory effect of the virus.
ATL occurs in only 1% to 5% of infected persons following a latent period of decades. The illness is characterized by malignant lymphocytosis and leukemia, lymphadenopathy, hepatomegaly, abnormal liver function test results, splenomegaly, skin lesions, bone lesions, and hypercalcemia ( Chapter 89 ). HAM/TSP occurs in approximately 2% of individuals infected with HTLV-1 and HTLV-2. Patients with transfusion-associated HAM/TSP develop neurologic symptoms rather more rapidly, at a median of 3.3 years after transfusion. This illness is characterized by slowly progressive chronic spastic paraparesis, lower limb weakness, urinary incontinence, impotence, sensory disturbances, low back pain, hyperreflexia, and impaired vibration sense.
Blood donor screening for HTLV-1 antibodies began in 1988, and more sensitive combination HTLV-1/2 assays that detect close to 100% of HTLV-2 infections were introduced in 1998. The prevalence of HTLV confirmed-positive donors decreased approximately 10-fold during the 1990s and continues to decrease to the current value of 0.002%. The rate is three-fold higher in female compared with male donors. Incident infections are rare in repeat donors, an observation that has led to one-time testing of donors in some European countries, but an incidence of 3 per million donor years of follow up is felt by some to be too high for adoption of this strategy in the United States. Cell-free components such as plasma and cryoprecipitate do not transmit HTLV, and less than 30% of infected cellular components transmit. The residual risk for transfusion-associated HTLV infection using contemporary serologic testing is approximately 1 per 3.3 million donations, but since the initiation of testing using these contemporary tests, there has not been a breakthrough HTLV infection by transfusion. The contribution of blood-donor serologic testing to this low residual risk is confounded by the effect of effective leukoreduction that reduces HTLV-1 copy numbers in red blood cell (RBC) concentrates by up to 6 logs, to below the infectious dose of 10 7 to 10 8 infected cells per unit (one of a number of arguments used in support of universal leukoreduction of blood components).
The deferral, notification, and counseling of healthy blood donors after a repeatedly reactive screening test for HTLVs has been problematic, and tens of thousands have been affected since screening started. The vast majority of such tests are false positive; a licensed confirmatory test is now available and will greatly improve this situation.
Not surprisingly, from the 1980s until now, donor deferrals and blood-testing interventions have been rapidly, successively, and additively implemented for emerging and theoretical risks. Collection facilities introduced antibody testing to the HBcAg of HBV (anti-HBc) and ALT testing as surrogates for non-A, non-B hepatitis, HIV-1 p24 antigen testing, then NAT for hepatitis C and HIV and subsequently HBV, extensive deferrals for the risks attending transmissible spongiform encephalopathies (TSEs), NAT for West Nile virus (WNV), and antibody testing for Trypanosoma cruzi , among many others. The cost-benefit estimates for some of these interventions exceeded generally accepted thresholds by orders of magnitude, but this did not deter their adoption. In general, the implementation of these measures was undertaken in practice and in cost by the blood providers, but it is unlikely that this reactive approach can be sustained in the current health care–reform environment and in the face of declining funding for blood providers, at least in the United States.
After HIV entered the blood supply, application of a stringent adaptation of the precautionary principle (originally promulgated for environmental protection, not transfusion safety) pushed decision making toward avoidance of all risks. The precautionary principle promotes implementation of measures to mitigate risk even if evidence of a risk is incomplete. It is supposed to be tempered by proportionality; that is, any measures adopted are to be proportional to the risk and with those used in similar circumstances, but some have argued that this has not been the case with blood safety measures, at least by the metric of cost-benefit. Nevertheless, although in potential conflict with evidence-based decision making, this approach resonated with policy advocates charged with transfusion safety. In contrast, when the risk for transfusion transmission of variant Creutzfeldt-Jakob (vCJD) disease (the human form of bovine spongiform encephalopathy, BSE) emerged as theoretical, modeling was used to balance the perceived risk against the impact of extensive donor deferrals on the adequacy of the blood supply and to arrive at a policy decision. Some argue that the magnitude of risk does not justify the stringency of the donor deferral policy, given the small risk in a country that was not BSE-endemic or lacked any BSE-contaminated materials in their food supply, but the process was the first to attempt to balance risk with adequacy. Subsequently, vCJD was shown to be transfusion transmissible.
While screening donors for a risk such as vCJD does not have a large negative impact on availability of donations because of its distant impact on most US blood donors, universal screening for other disease agents is not feasible or warranted. For example, screening all donations for T. and Zika virus based on geographic exposure or theoretic local exposure can cause significant reduction in the number of collections without adding much value. Universal donation screening for T. cruzi antibodies has been replaced with one-time testing with the assumption that a negative test remains consistent throughout the donor’s life. Zika screening was challenging as regions of the United States became endemic for the disease after its introduction during the pandemic in 2015 to 2016. Following the pandemic, Zika virus testing (by NAT) was reduced from universal individual donation testing to minipool NAT using a model very similar to that used for WNV.
Hemovigilance programs, such as the Serious Hazards of Transfusion (SHOT) in the United Kingdom and others in Canada, France, and a smaller program in the United States, have emerged, supplying evidence about a much broader range of transfusion hazards than just infections. For example, a data-driven decision to minimize plasma transfusions from potentially alloimmunized female donors resulted in a dramatic reduction in transfusion-related acute lung injury (TRALI) in the United Kingdom, and studies in the United States have reproduced this finding. These systems provide an opportunity for monitoring the risks and benefits of new initiatives, (e.g., proactive pathogen-reduction). Pathogen-reduction processes (see box on Pathogen Reduction ) offer the opportunity to abrogate most of the residual risk for all of the historically important transfusion-transmitted viral infections, bacterial contamination of platelets, babesiosis and malaria contaminated red cells, WNV infections, and Chagas disease. Pathogen-reduction could eliminate the often lengthy, reactive, iterative paradigm of emergence of a new pathogen in the population, recognition of a material threat to transfusion recipients, development of donor-deferral strategies followed by development and refinement of test systems that has characterized our historical approach. Critically, broadly active pathogen-reduction processes offer a layer of protection against unsuspected emergence of new agents. If already in use, they would need only to be validated as active against a new agent or appropriate model agents. The challenge with this approach is broad-based availability (all components) and wide-scale acceptance, the impact on product quality, and the potential long-term toxicities that may not be apparent in premarketing clinical trials or implementation to date.
More recently, as cost pressures for health care increase, transfusion professionals are questioning whether the zero-risk paradigm remains relevant. A consensus conference held in Toronto in October 2010 addressed concerns about “safety at any cost” and inconsistent decision-making practices affecting the blood supply. This initiative has led to a definitive effort to establish a framework for risk-based decision-making under the direction of the Alliance of Blood Operators ( www.allianceofbloodoperators.org/abo-resources/risk-based-decision-making/rbdm-framework.aspx ). The process involves risk identification, risk assessment, risk management, and risk communication, along with an assessment of risk tolerance, in the context of full and open communication with stakeholders. Risk will never be zero, and there is now a realization that cost considerations, politics, ideology, and public opinion cannot be ignored.
Human herpesviruses (HHVs) are enveloped, structurally complex double-stranded DNA viruses that cause common infectious diseases. Primary infection is followed by lifelong carrier states and the possibility of reactivation. They are classified in three subfamilies, Alphaherpesvirinae, Betaherpesvirinae , and Gammaherpesvirinae , of which the latter two contain the herpesviruses that are of greatest concern from a transfusion medicine standpoint. Members of the Alphaherpesvirinae subfamily, herpes simplex viruses (HSVs) and varicella-zoster virus (VZV), are rarely, if ever, associated with transfusion-transmitted infections. Transfusion-transmitted cytomegalovirus (CMV) is well recognized. Transmissions of Epstein-Barr virus (EBV) and HHVs 6 to 8 by blood are virtually nonexistent in the United States because of the use of primarily leukocyte-depleted (leukoreduced) blood products.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here