Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
TGF-β is abundant in bone and a critical coupling factor in bone cells during bone remodeling.
Cancer frequently affects bone as primary bone cancer or during bone metastases.
TGF-β signaling in cancer has dual roles, as a tumor suppressor or a tumor and metastasis promoter.
TGF-β in cancer cells or in the microenvironment contributes to the progression of the disease.
Anti-TGF-β therapies, while efficient in preclinical models, have limited to no effect in patients.
The authors acknowledge financial support from the CONACYT (grants no. 272707 to PJC, and 241295 to PGJF) and the CICESE (grants no. 685-105 to PJC, and 685-102 to PGJF).
Our bodies are a piece of incredibly complex machinery, whether during the development to generate all of our cells, tissues, and organs starting from only two gametes, or later during the homeostasis to maintain their number, organization, and function. To achieve this, our cells need to exchange signals and integrate them to use the information in our genome appropriately. This fine-tuning is achieved, in part, thanks to vitamins, hormones, chemokines, or cytokines, and the signaling pathways they activate through their receptors. These signaling pathways are critical, and the mutations that accumulate in our DNA throughout our lives can cause their malfunction, leading to disorders and pathologies such as cancer. Among these signaling pathways, the transforming growth factor (TGF)-β signaling pathway requires particular attention, especially when it comes to cancer and cancer in bone.
The TGF-β signaling pathway is well conserved throughout the evolution of multicellular organisms, and almost all our cells can respond to TGF-β as they express its receptors. Thus, TGF-β has many functions, including the regulation of extracellular matrix (ECM) production, wound healing, immune response, bone development and remodeling, and cancer ( Figure 20.1 ).
Cancer is characterized by a loss of control in the proliferation and death of cells, leading to the formation of an invasive primary tumor from which cancer cells can disseminate throughout the body to form a secondary tumor or metastases [ ]. The growth of the primary tumor can cause the death of the patients when interfering with the function of the organ where it grows (i.e., intestinal or airways occlusion, liver failure). However, the formation of metastases causes most cancer-related deaths (>80%) when compromising the function of multiple organs. With an estimated 9.6 million deaths from cancer in 2020, it is among the leading causes of death in the World [ ]. Despite the comprehensive data of the International Agency for Research on Cancer (IARC) and the GLOBOCAN project, it is difficult to estimate the number of patients who will have cancer or cancer complications within their bones. Cancer cells can be found in the bones in case of primary cancer of the bone, occurring when a normal cell from the bone transforms into a cancer cell, or bone metastasis when cancer cells from a primary tumor in soft tissue disseminate to the bone. In the classification of GLOBOCAN, primary cancers of the bones, mostly osteosarcoma (OS), chondrosarcoma, and Ewing's sarcoma (EwS), are part of the “other specified sites” category that includes cancers like male breast cancer or cancers from the connective tissues and the eye [ ]. Bone metastases are usually found in patients with advanced forms of breast, prostate, or lung cancer with the highest prevalence. Based on this, it can be estimated that many cancer patients will have involvement in their bones at some point.
To address the situation of patients with cancer in bone and develop treatments that can alleviate their suffering or cure them, it is crucial to understand the molecular and cellular mechanisms of their disease. The skeleton is the main storehouse of TGF-β, deposited in the bone matrix and released during bone resorption. TGF-β is an essential regulator of bone physiology and has been associated with most of the skeletal complications of malignancy. Thus, TGF-β and TGF-β signaling have been recognized as potential targets for cancer treatment.
In this chapter, we will give an overview of the TGF-β signaling pathway and its role during bone development and remodeling. We will review the current knowledge on the contribution of TGF-β in multiple forms of cancer occurring in the bone—multiple myeloma, OS, chondrosarcoma, EwS, and giant tumor cell of the bone—and in bone metastases, as well as some effects of bone-derived TGF-β outside of the skeleton. We will also highlight the different possible anti-TGF-β strategies developed that could treat skeletal complications of malignancy.
TGF-β is a rare multifunctional growth factor that belongs to a structurally conserved family of proteins known as the TGF-β superfamily. The TGF-β superfamily includes TGF-βs, bone morphogenetic proteins (BMPs), activins, growth and differentiation factors (GDFs), inhibins, nodal, and anti-müllerian hormone. The superfamily is involved in regulating multiple cellular functions, including proliferation, differentiation, and apoptosis, and is essential during embryogenic development and to maintain homeostasis during adult life [ ]. The components of their signaling pathways are shared between them and have been identified in all metazoans, from humans to the warty comb jelly ( Mnemiopsis leidyi ), passing by the African clawed frog ( Xenopus laevis ), the fruit fly ( Drosophila melanogaster ), and the free-living roundworm ( Caenorhabditis elegans ) [ , ].
Three different isoforms of TGF-β have been found: TGF-β1, TGF-β2, and TGF-β3. TGF-β is secreted as a 390 amino acid latent precursor protein (pre-pro-TGF-β) that regulates its biological activity. The pre-pro-TGF-β consists of three parts: the signal peptides, the latency-associated peptide (LAP), and the mature peptide. The signal peptide contains a hydrophobic signal peptide, three N-linked glycosylation sites, and a proteolytic cleavage site. The first step in processing pre-pro-TGF-β is the cleavage of the signal peptide during the transit through the endoplasmic reticulum, resulting in two monomers dimerizing thanks to disulfide bridges at cysteine residues in the LAP and the mature peptide. Furthermore, the pro-region of TGF-β is glycosylated with the addition of mannose 6-phosphate residues during its transit through the Golgi apparatus. This modification is necessary for the proper secretion and activation of TGF-β [ , ].
The pro-region of TGF-β is cleaved by the convertase Furin, separating the LAP and the mature TGF-β, although both remain associated by noncovalent bonds. This association confers latency to the mature peptide forming the small latent complex, which can become associated with a latent TGF-β-binding protein (LTBP) to form the large latent complex [ ]. The LTBP is indispensable for the correct folding of TGF-β and maintenance of the large latent complex within the ECM after its secretion ( Fig. 20.1 ) [ ].
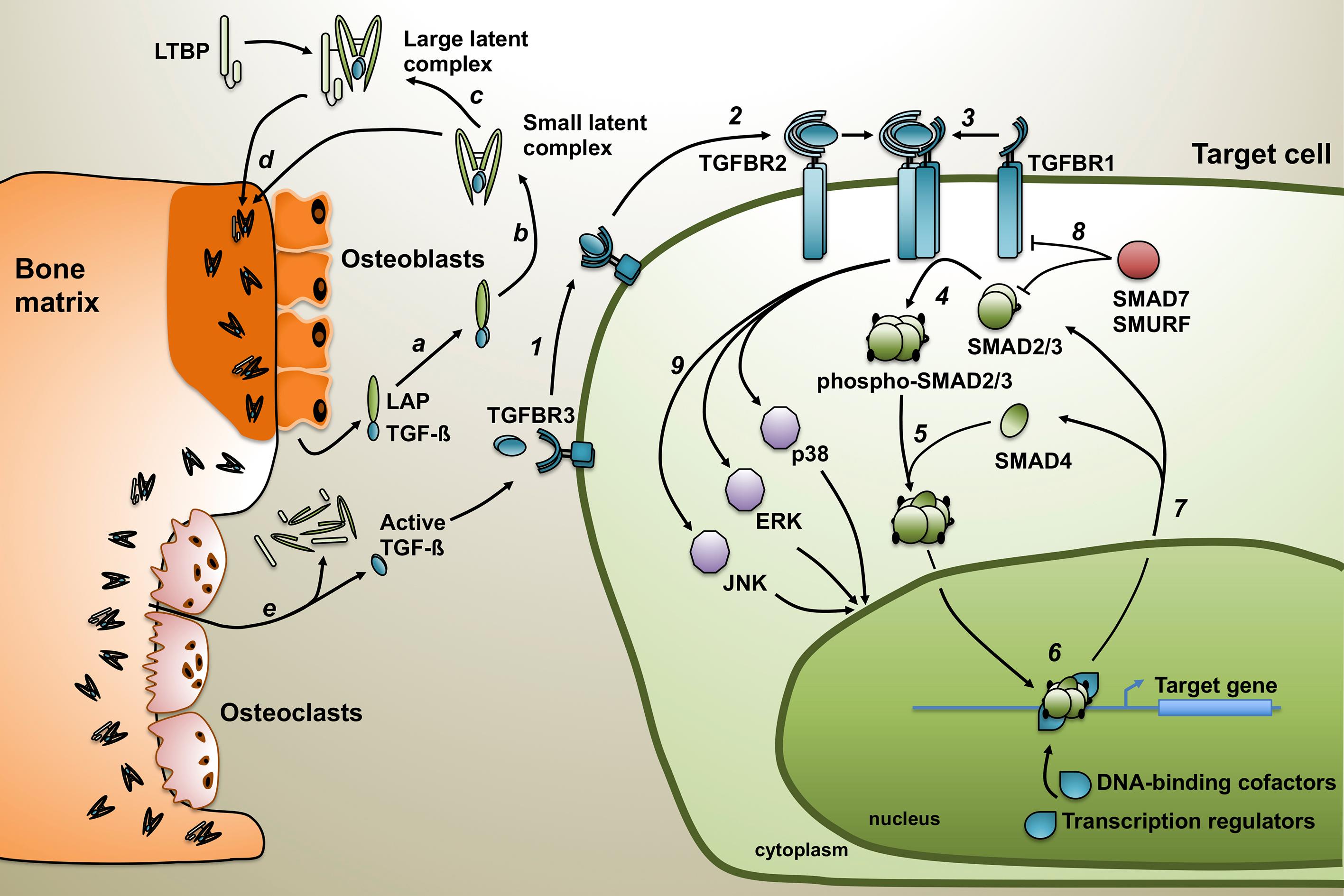
The bone matrix is the primary storehouse of TGF-β in the body. Actually, TGF-β was isolated from bovine demineralized bone for the first time and associated with cell differentiation for cartilage formation during endochondral bone formation [ ]. The presence of the three TGF-β isoforms has been characterized in bone, although TGF-β1 is the most abundant. While most of the cells secrete and produce TGF-β in its large latent complex form, bone cells are an exception. In bone organ cultures, osteoblasts produce large amounts of latent TGF-β that lacks the LTBP [ ]. Therefore, the small latent complex is the predominant form in the bone environment [ ].
TGF-β is released from the bone matrix and activated during osteoclastic bone resorption ( Fig. 20.1 ) [ ]. Active TGF-β can bind to the TGF-β type II receptor (TGFBR2) on the cell membrane, forming a complex that will recruit the TGF-β type I receptor (TGFBR1 or ALK5). Both receptors have a serine/threonine kinase domain in their cytoplasmic extremity. TGFBR2 phosphorylates and activates the TGFBR1 that initiates the intracellular signaling cascade. TGF-β is also having some co-receptors, the TGF-β type III receptor (TGFBR3), also called betaglycan, and endoglin. These co-receptors are membrane proteins that increase the efficiency and the specificity of TGF-β binding to the receptors of the TGF-β superfamily. TGFBR3 is a membrane proteoglycan that binds all TGF-β isoforms with high affinity. In its membranal form, it potentiates TGF-β signaling by presenting TGF-β to TGFBR2 [ ]. However, after proteolytic shedding, TGFBR3 can be soluble. It turns into a potent antagonist of TGF-β signaling by trapping TGF-β and preventing its binding to the receptor [ ]. TGFBR3 is widely distributed, being expressed in almost all the cells, except in endothelial cells where endoglin, a membrane glycoprotein, efficiently regulates TGF-β signaling [ ].
After the formation and activation of the heteromeric complex TGFBR2–TGFBR1, TGFBR1 phosphorylates and activates the TGF-β-specific intracellular signaling mediators, the receptor-associated SMADs (R-SMADs), SMAD2, and SMAD3 ( Fig. 20.1 ). Phosphorylation of the R-SMADs induces their release from SARA (SMAD anchor for receptor activation), a protein that maintains SMADs at the membrane. The phosphorylated SMAD2/3 complex then binds the co-SMAD, SMAD4, and translocates to the nucleus. R-SMAD proteins have two globular domains: MH1 that binds to DNA sequences, and MH2 that binds to other SMADs and regulators of the transcription, like the coactivators p300 and CBP or the co-repressors TGIF or SKI [ , ]. The nature of the genes regulated depends on the composition of the complex formed, which explains the variety of genes regulated and effects mediated by the TGF-β/SMAD signaling pathway [ ].
TGF-β can also activate other signaling pathways like mitogen-activated protein kinase (MAPK) signaling by inducing the phosphorylation of the extracellular signal–regulated kinases (ERK-1, ERK-2), p38, or the c-Jun amino-terminal kinase (JNK). This other signaling can be independent of or in cooperation with SMAD proteins [ ]. In bone, TGF-β induces p38 MAPK in monocytes but not in mature osteoclasts, promoting osteoclastogenesis. In contrast, continuous exposure to TGF-β decreases the expression of the receptor activator of NF-κB (RANK) and the formation of osteoclasts [ ]. TGF-β also activates monomeric G proteins or small GTPases, such as RHOA, RHOB, and CDC42 [ ]. The activation of these signaling pathways by TGF-β is associated with the induction of the epithelial-to-mesenchymal transition (EMT) [ ].
The skeletal system in human develops from the condensation of mesenchymal stem cells that have their origin in the neural crest or mesoderm. This process, known as skeletogenesis, starts around the fourth week of gestation and continues after birth until puberty. Bone formation occurs in two ways. The endochondral ossification occurs in long bones where a cartilage model is formed and then replaced by mineralized bone. In flat bones, the intramembranous ossification produces bone directly from mesenchymal cells without the prior formation of cartilage.
The skeleton is continuously active and requires the coordinated activities of bone-forming osteoblasts and bone-resorbing osteoclasts. These processes are orchestrated and coupled by systemic and local growth factors and their signaling pathways, including the TGF-β signaling pathways. It plays a central role during the temporal and spatial regulation of bone remodeling.
Genetically modified mice have been useful to determine the role of TGF-β during skeletal development. TGF-β1 knockout mice have shorter tibias and a significant decrease in the bone mineral density and elasticity of the long bones [ ]. TGF-β2 null mice have perinatal mortality and a wide range of defects in skeletal development of the limbs, the spinal column, the inner ear, and craniofacial bones [ ]. Mice lacking TGF-β3 exhibit defects in palatal shelf fusion, while no craniofacial abnormalities were observed [ ]. Interestingly, the phenotypes associated with the knockout of the different isoforms have little overlap, indicating noncompensating functions between the TGF-β isoforms. Genetically modified mice helped to establish that bone matrix properties correlate with the level of TGF-β signaling. A reduction of TGF-β signaling with the knockout of Smad3 enhanced the mechanical properties and mineral concentration of the bone matrix and the bone mass [ ].
TGF-β is a central component of the coupling between bone formation and resorption during bone remodeling [ ]. However, its role in bone remodeling is complex since it is context-dependent, both in time and space. In vitro , at early stages, TGF-β1 promotes bone formation through the chemotactic attraction of osteoblast. It stimulates osteoblast proliferation, as well as the production of ECM proteins and proteoglycans [ ]. In mice, the inactivation of TGF-β signaling in osteoblasts, using Smad3 or Smad4 knockout strategies, decreased bone mass, and bone formation [ ]. Such phenotypes were associated with TGF-β signaling effects during the early stage of osteoblast differentiation when TGF-β increased bone formation. In contrast, overexpression of TGF-β2 in osteoblasts led to progressive bone loss and increased bone fragility [ ]. Accordingly, inactivation of TGF-β signaling in osteoblasts in Tgfbr2 knockout mice or mice with osteoblasts overexpressing a dominant-negative form of Tgfbr2 lacking its cytoplasmic domain led to an increase of the trabecular bone volume and bone mass [ ]. Further, deletion of Tgfbr2 in osteoblasts increases the cell surface expression of the Pth-Pthrp receptor (Pth1r) and PTH signaling. Injection of PTH(7–34), a fragment of PTH that functions as an antagonist of the receptor, rescued the bone phenotype associated with Tgfbr2 knockout [ ]. These last results are consistent with the effect of systemic inhibition of TGF-β signaling with a TGF-β-neutralizing antibody, or SD-208, a small molecule inhibitor of the kinase domain of TGFBR1 that increased osteoblast differentiation and bone formation, as well as reduced osteoclast formation and bone resorption leading to an increase of the trabecular bone [ , ].
The effects of TGF-β on osteoclastogenesis and bone resorption are equally complex. Active osteoclasts can activate TGF-β, which attenuates bone resorption by impairing osteoclastogenesis and promotes bone formation through chemotactic attraction and stimulation of proliferation and differentiation of osteoblast precursors [ ]. TGF-β can inhibit and stimulate osteoclast differentiation depending on cell context via direct and indirect actions. TGF-β increases osteoprotegerin (OPG) secretion from osteoblasts and bone marrow stromal cells (BMSCs) and decreases osteoblastic production of RANKL (RANK ligand), which leads to reduced osteoclast differentiation [ , ]. However, OPG-neutralizing antibodies did not rescue the inhibition of the osteoclastogenesis induced by TGF-β, indicating that the effect is independent of OPG and more likely mediated by the decrease of RANKL [ ]. Accordingly, TGF-β inhibited osteoclast formation in the absence of OPG in cocultures of cells derived from Opg −/− mice, confirming that TGF-β is acting by limiting bioactive RANKL rather than increasing OPG secretion [ ]. Although these in vitro data suggest that TGF-β has a biphasic effect during osteoclastogenesis and bone resorption, data from genetically modified mice and mice treated with TGF-β inhibitors consistently show that TGF-β promotes osteoclastogenesis and bone resorption [ , , ].
Besides its role in bone, TGF-β has a critical role to play, whether during development or tissue homeostasis. In normal cells such as epithelial cells, it is a well-known inhibitor of proliferation by causing cell cycle arrest in the G 1 phase. TGF-β signaling pathway can prevent the proteasomal degradation of the cyclin-dependent kinase inhibitors p27 Kip1 that accumulates in the nucleus, and silencing of p27 Kip1 reversed the TGF-β induced growth inhibition [ ]. The SMAD complex can also induce the expression of p15 Ink4b and p21 Cip1 , other cyclin-dependent kinase inhibitors, when interacting with the DNA-binding cofactors and transcription factors FoxO1, FoxO3, and FoxO4, members of the forkhead box O family in keratinocytes [ ]. Activation of TGF-β signaling leads then to the inactivation of cyclin-dependent kinases, like CDK4 and G 1 arrest [ ]. Expression of the proto-oncogene MYC can reverse this effect. However, TGF-β can also induce a decrease in MYC levels, achieving a proliferation block [ ]. In addition to preventing proliferation, TGF-β can also induce apoptosis in both non-SMAD- and SMAD-dependent manners [ ]. In gastric epithelial cells, TGF-β increases the expression of BIM, a proapoptotic member of the Bcl-2 homology domain 3 (BH3)-only protein family, and the subsequent activation of BAX, Caspase-9, and Caspase-3 [ ]. Overexpression of the inhibitor SMAD7 reversed this mitochondrial apoptosis. Such control of cell survival is critical during development since mice lacking BIM or Caspase-9 developed gastric epithelial abnormalities associated with apoptosis defects [ ].
As an inducer of cytostasis and apoptosis, the TGF-β axis can act as a tumor suppressor when it comes to skin cancers and carcinoma (cancers arising from epithelial cells), such as breast carcinoma. In genetically modified mice that develop mammary carcinoma due to the expression of TGF-α (MMTV-Tgfa), the overexpression of TGF-β1 in the mammary epithelium (MMTV-Tgfb1) prevented the formation of tumors [ ]. Consistently, in MMTV-PyVmT mice that spontaneously develop breast tumors due to the expression of the oncogene Erbb2, disruption of the TGF-β axis when deleting Tgfbr2 increased the development and decreased the time for their detection [ ]. In patients, the reduced detection of TGFBR2 in biopsies correlated with increased aggressiveness for both in situ and invasive carcinomas [ ].
Different forms of disruption of the TGF-β signaling pathway have been identified in cancer cells, which could result from a selection to counteract the TGF-β tumor suppressor effect. In breast cancer patients, hypermethylation in the TGFBR2 gene leads to decreased or lack of expression of the receptor [ ]. In gastrointestinal cancers, inactivating mutations are often found. In colon cancer with microsatellite instability, mutations were found in the TGFBR2 gene of more than 90% of the samples [ ]. While TGFBR2 is mutated in less than 10% of pancreatic cancers, inactivating mutations of SMAD4 are found in 30%–50% of tumors analyzed [ , ]. Cancers that originate in bone, such as multiple myeloma or primary cancer of the bone, do not belong to the carcinoma category due to the lack of epithelial cells there. However, considering the large quantities of TGF-β in bone, it stands to reason that tumor cells originating in bone will have to be protected from this tumor suppressor effect too.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here