Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Intraoperative transesophageal echocardiography (TEE) plays a valuable role in interventional cardiac procedures, when used to guide transseptal puncture, evaluation of left atrial thrombus, closure of paravalvular leaks, transcatheter valve deployment, and during removal of transvenous lead extractions. Real-time three-dimensional (3D) TEE can be displayed to both the interventionalist and the echocardiographer for guidance and intraprocedural monitoring.
Precise evaluation of left ventricular function and synchrony may be characterized by measurements of tissue velocity and deformation in one or two dimensions. Myocardial strain is the systolic deformation of a myocardial fiber normalized to its original length, while strain rate expresses the speed of deformation. Doppler tissue imaging uses the Doppler signal while speckle-tracking imaging uses the two-dimensional speckled pattern of myocardium to calculate strain, strain rate, velocity, and displacement.
Intracardiac tumors are rare; when a cardiac mass is identified, it is frequently a thrombus or vegetation.
Hypertrophic cardiomyopathy is the most commonly inherited cardiomyopathy and often manifests with asymmetric thickening of the septum and dynamic outflow tract obstruction (>30 mm Hg gradient considered significant). Myocarditis can manifest clinically as a fulminant (hyperacute) course that demonstrates thickened, edematous myocardium with severely reduced left ventricular or biventricular ejection fraction. The patient is often critically ill with a good chance for recovery if supported through the initial process.
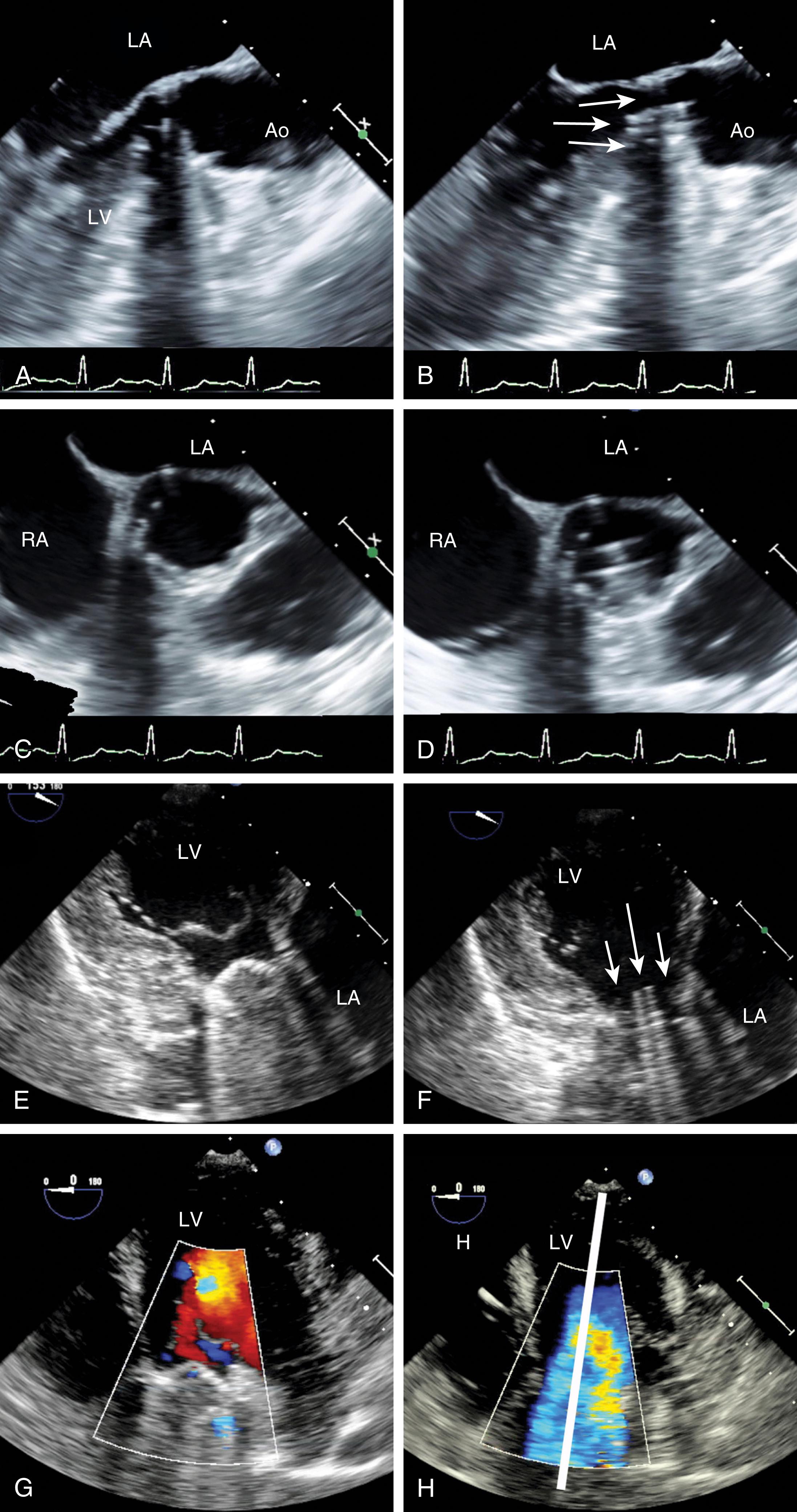
Dimensions of the aortic annulus should be measured in systole from leading edge to leading edge, whereas measurements of the aortic root and ascending aorta should be taken at end-diastole. Measuring from leading edge to leading edge produces dimensions similar to inner edge computed tomography and magnetic resonance imaging measurements.
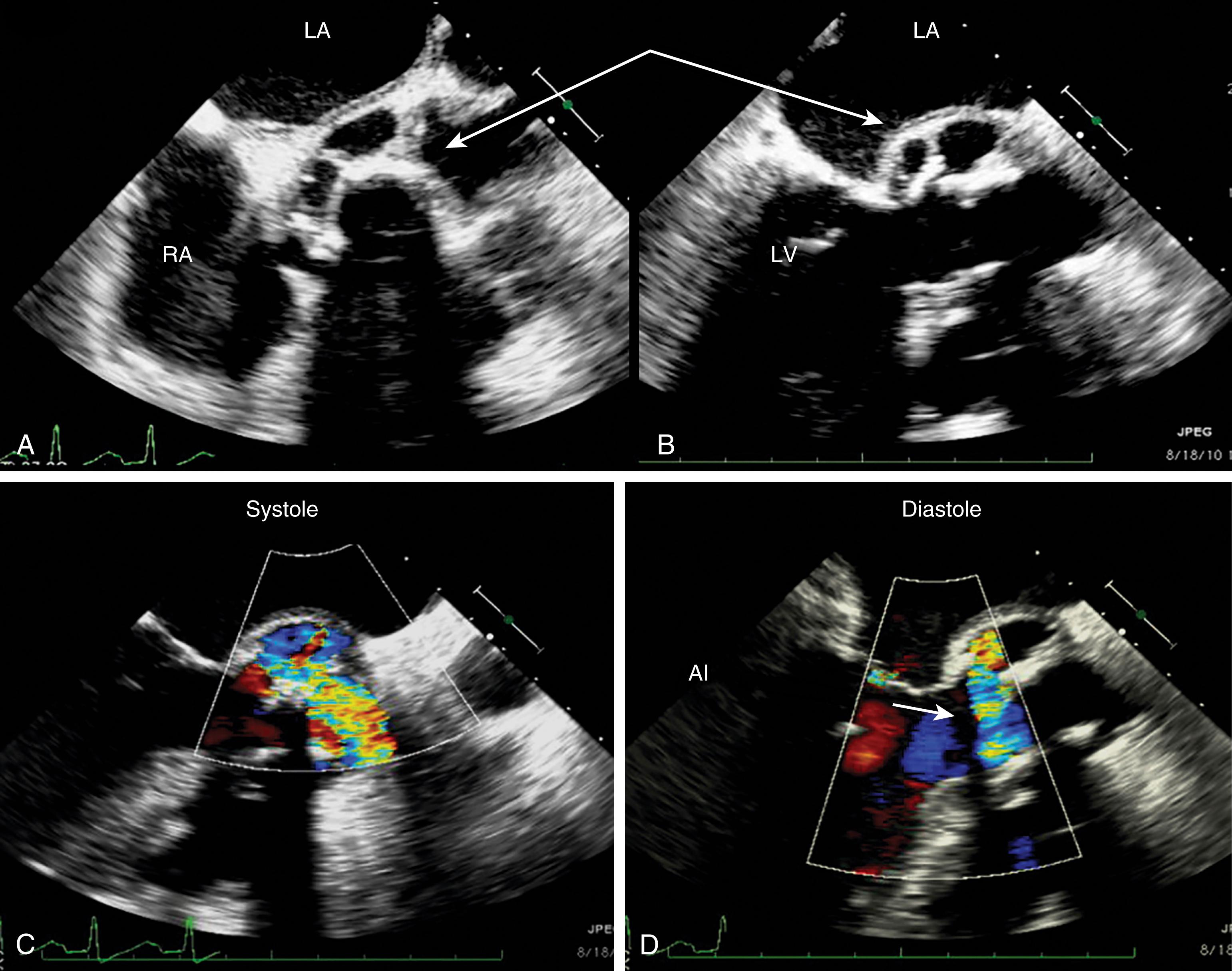
The diagnosis of cardiac tamponade is pathophysiologic and requires both the presence of a pericardial effusion and an increase in pericardial pressure that limits ventricular filling. Echocardiographic findings that confirm the diagnosis of cardiac tamponade are early systolic right atrial collapse and diastolic right ventricular collapse when the pericardial pressure exceeds chamber pressure.
The use of both transthoracic echocardiography and TEE across the perioperative spectrum, such as in presurgical testing clinics, postanesthesia care units, and the critical care arena, can be accomplished by the development of a clinical perioperative echocardiography consult service. Quality assurance, ongoing education, and the implementation of appropriate use criteria are critical pieces in forming such a service.
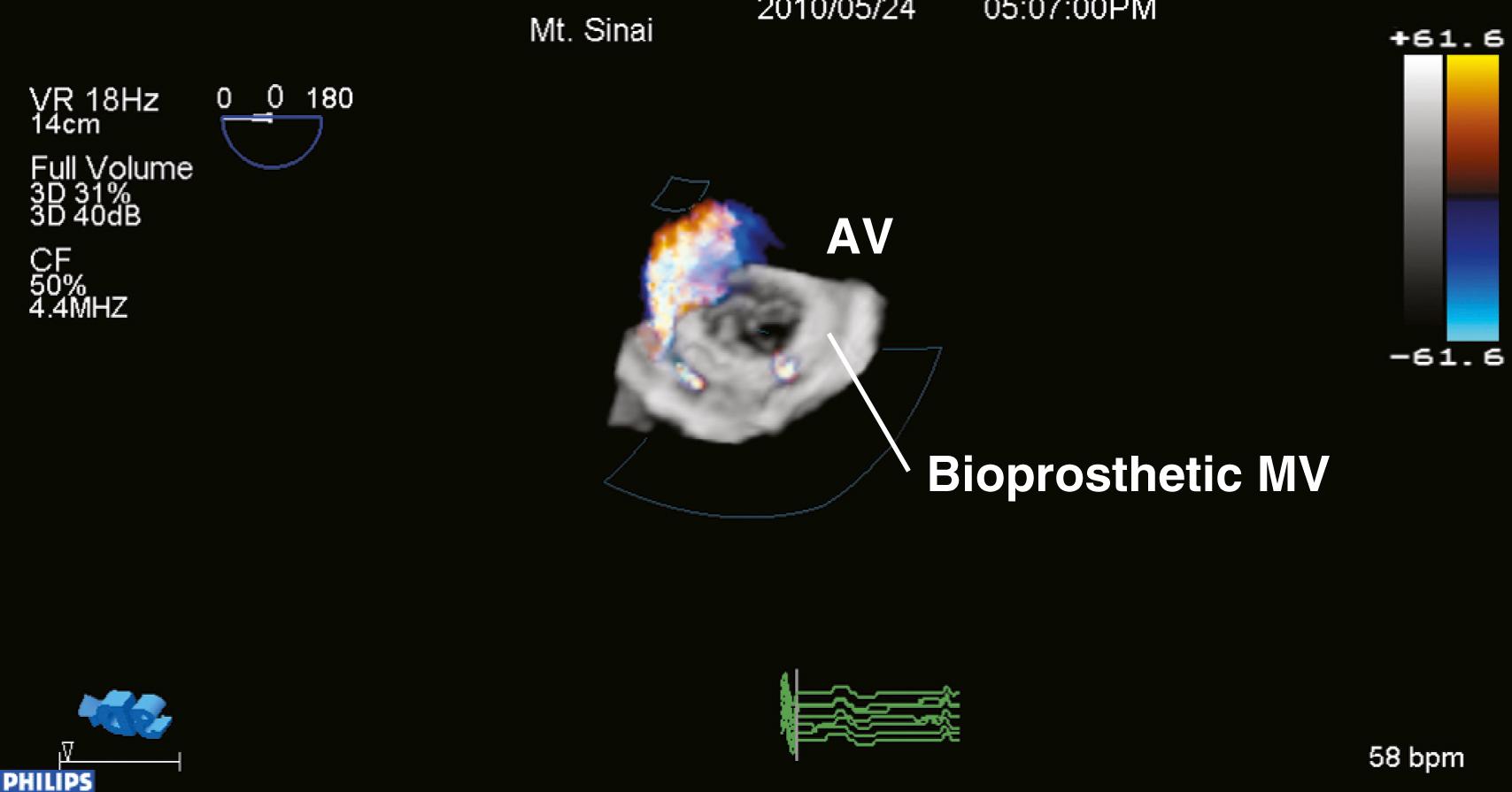
Real-time, 3D TEE offers improved insight for diagnosing valve defects and predicting the success of surgical mitral valve repair.
A thorough echocardiography evaluation of valve positioning and description, evaluation of leaflet mobility, spectral Doppler flow profile, and color flow Doppler assessment should be performed with all prosthetic valves. Abnormal leaflet mobility, color flow, or Doppler profile should alert the echocardiographer to prosthetic valve dysfunction.
Perioperative echocardiography use, such as in presurgical testing clinics, postanesthesia care units, and the critical care arena, has led many in cardiac anesthesiology to provide echocardiography evaluation outside of the cardiac operating suite. Some centers have developed perioperative echocardiography clinical services. Rescue echocardiography has been shown to change medical and surgical decision-making, and many programs are expanding their resources to include ultrasound assessment in rapid response teams and in noncardiac surgery.
Training in both transthoracic echocardiography (TTE) and TEE is becoming more prevalent. While certification in perioperative TTE does not yet exist for anesthesiologists as it does for TEE, many critical care and cardiac anesthesiologists are learning the skill and implementing it in their clinical department and receiving critical care ultrasound certification. Perhaps the most difficult task in developing a clinical service starts with the education and training of a “critical mass” of experts in perioperative echocardiography. Defining the team, establishing training requirements, certification needs, and credentialing of physicians to lead the service are time-consuming, costly, and yet crucial for success. Institutional support to train physicians and provide competency assessment and quality assurance programs must be solidified prior to the development of the clinical service.
Portable echocardiography or “laptop” machines allow for fast echocardiography assessment in an unstable or critically ill patient. Handheld ultrasound devices, while simple to use and store, do not currently provide Doppler assessment that is needed in rescue echocardiography for hemodynamic evaluation or valve assessment.
Perioperative patients that require either TTE or TEE examinations are often unstable or undergoing surgical evaluation, and different physician providers from various specialties need access to echocardiography reports and images for clinical decision-making. Digital transfer of images into a hospital echocardiography database is critical, as well as standard reporting of examinations performed. Reporting should be fashioned to agree with recommendations from the American Society of Echocardiography where appropriate and the Intersocietal Accreditation Commission. ,
Perioperative echocardiography can be implemented in several different anesthesia care arenas including preoperative assessments of patients undergoing moderate- to high-risk noncardiac surgery, rescue echocardiography (evaluation of unstable surgical patients in the operating rooms or recovery/critical care units), and assessment of patients undergoing urgent cases with unknown cardiac status. In a paper by Shillcutt and colleagues, on the utilization of echocardiography, the most common indications for both transthoracic and TEE performed in noncardiac surgery were hypotension (49% of cases), history of coronary artery disease (16% of cases), and history of heart failure (12% of cases). Multiple studies suggest that perioperative echo influences medical decision-making.
Billing and current procedural terminology (CPT) codes for perioperative echo are published by the Centers for Medicare and Medicaid Services and include codes for placement of the TEE probe, obtaining images and analyzing the data. Modifiers are included if the echo is performed by the same anesthesiologist providing anesthesia.
Although multiplane two-dimensional (2D) images can be acquired easily with modern TEE probes by simply rotating the image plane electronically from 0 to 180 degrees, the echocardiographer stitches the different 2D planes together, creating a “mental” three-dimensional (3D) image. Transmitting this “mental” image to other members of the surgical team can be challenging. By directly displaying a 3D image, cardiac anatomy and function is assessed more rapidly and communication between the echocardiographer and the cardiac surgeon is facilitated.
Three-dimensional echocardiographic (3DE) imaging is also useful for the evaluation of cardiac chamber volumes by avoiding geometric assumptions, assessments of regional left ventricular wall motion and quantification, and volumetric evaluation of regurgitant lesions and shunts.
Three-dimensional echocardiography uses a special matrix array of thousands of piezoelectric crystals within the transducer head. This 3D imaging matrix array, as opposed to conventional 2D imaging transducers, not only has columns in a single one-dimensional (1D) plane but also has rows of elements. Instead of having a single column of 128 elements, the matrix array comprises more than 50 rows and 50 columns of elements ( Fig. 11.1 ). Each crystal can be independently activated, focused, and steered to generate an ultrasound beam in the x, y or z axis to generate a 3D pyramidal volume. 3D image acquisition can occur live or gated (timed to the electrocardiogram [ECG] over a number of heartbeats).
Notably, 3DE is subject to the same laws of acoustic physics as 2D echocardiography (2DE). Artifacts such as ringing, reverberations, shadowing, and attenuation occur in 3D, as well as 2D. The product of frame rate, sector or volume size, and imaging resolution equals a constant. By increasing the requirements of one of these variables, a decrease in one or both of the others will occur. Modern ultrasound devices are equipped with incredible computing power, enabling them to display large 2D sectors while still maintaining excellent image resolution and high frame rates. Unfortunately, this does not apply for real-time instantaneous 3D imaging. The large amount of data that must be acquired and processed requires the echocardiographer to reduce sector size to maintain adequate resolution and frame rate. The rate-limiting factor in 3D imaging is no longer processing power but the speed of ultrasound in tissue.
The classic 20 views of 2D TEE are not required in 3DE because entire volumetric data sets can be spatially orientated and cropped by the echocardiographer. Recently, the American Society of Echocardiography published guidelines detailing the acquisition and display of cardiac anatomy by 3D TEE. These views explain the “surgical views” for display intraoperatively.
Because the limiting factor in 3DE is no longer processer performance, but the speed of sound traveling through tissue during “live” scanning, the size of this sector should be limited to guarantee adequate image resolution and frame rate. If larger sectors are required, the constraint of transmit time of ultrasound is overcome by stitching gated beats together, which enables wider volumes to be generated while maintaining frame rate and resolution. Several modes of 3DE are described in the following subsections.
In this mode, a 3D volume pyramid is obtained. The image shown in this mode is real time. Just as in live 2D imaging, manipulations of the TEE probe (eg, rotation) lead to instantaneous changes in the image seen on the monitor ( Fig. 11.2 ).
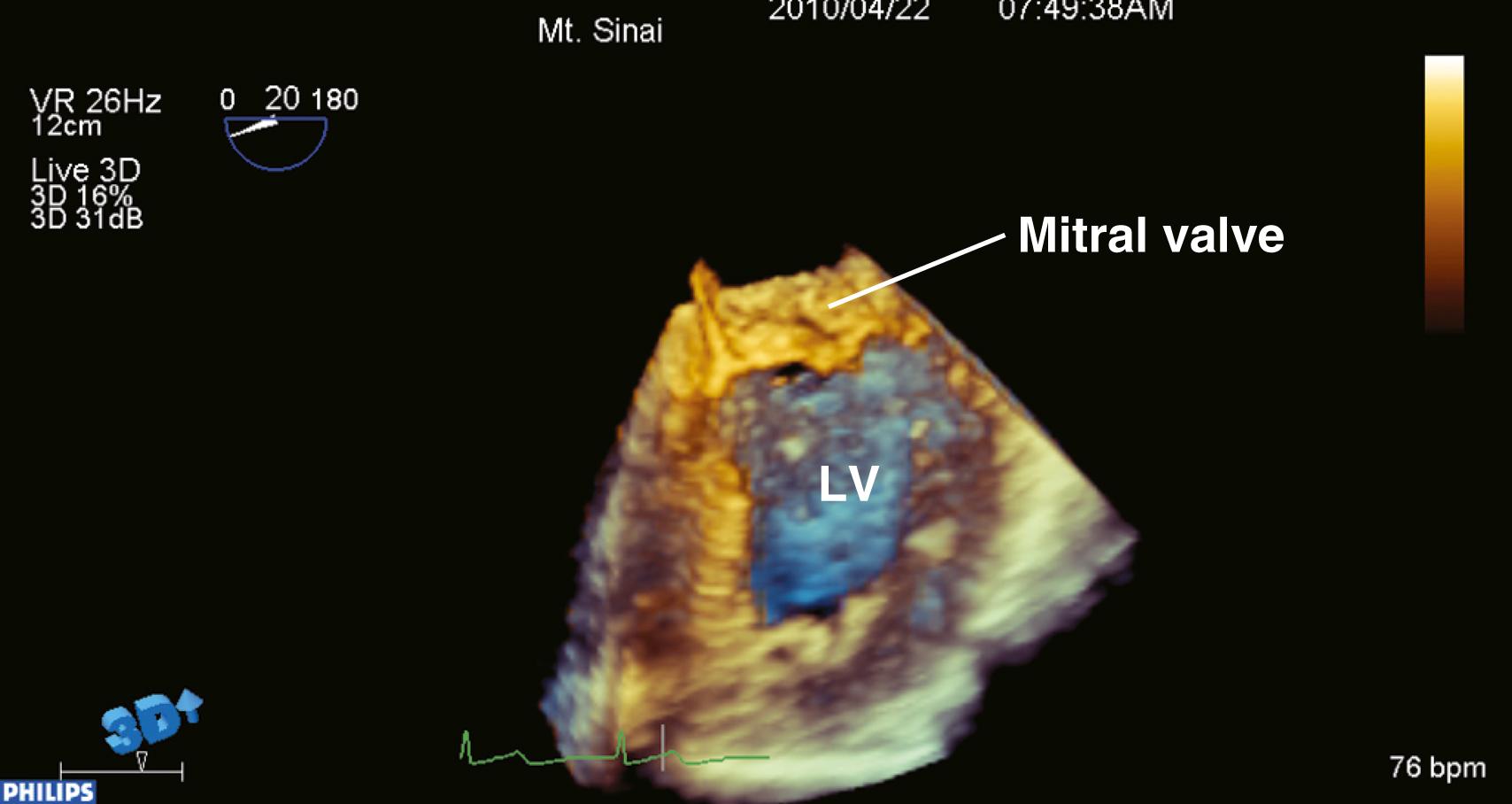
If only a specific region of interest (ROI) requires imaging, the “zoom mode” can be used. A typical example for this mode would be the mitral valve (MV) apparatus. The 3D zoom mode displays a small magnified pyramidal volume that may vary from 20 × 20 degrees up to 90 × 90 degrees, depending on the density setting. This small data set can be spatially orientated at the discretion of the echocardiographer. A key advantage to this mode is that the real-time 3D images are devoid of rotational artifacts, as are commonly encountered with ECG-gated 3D acquisitions ( Fig. 11.3 ).
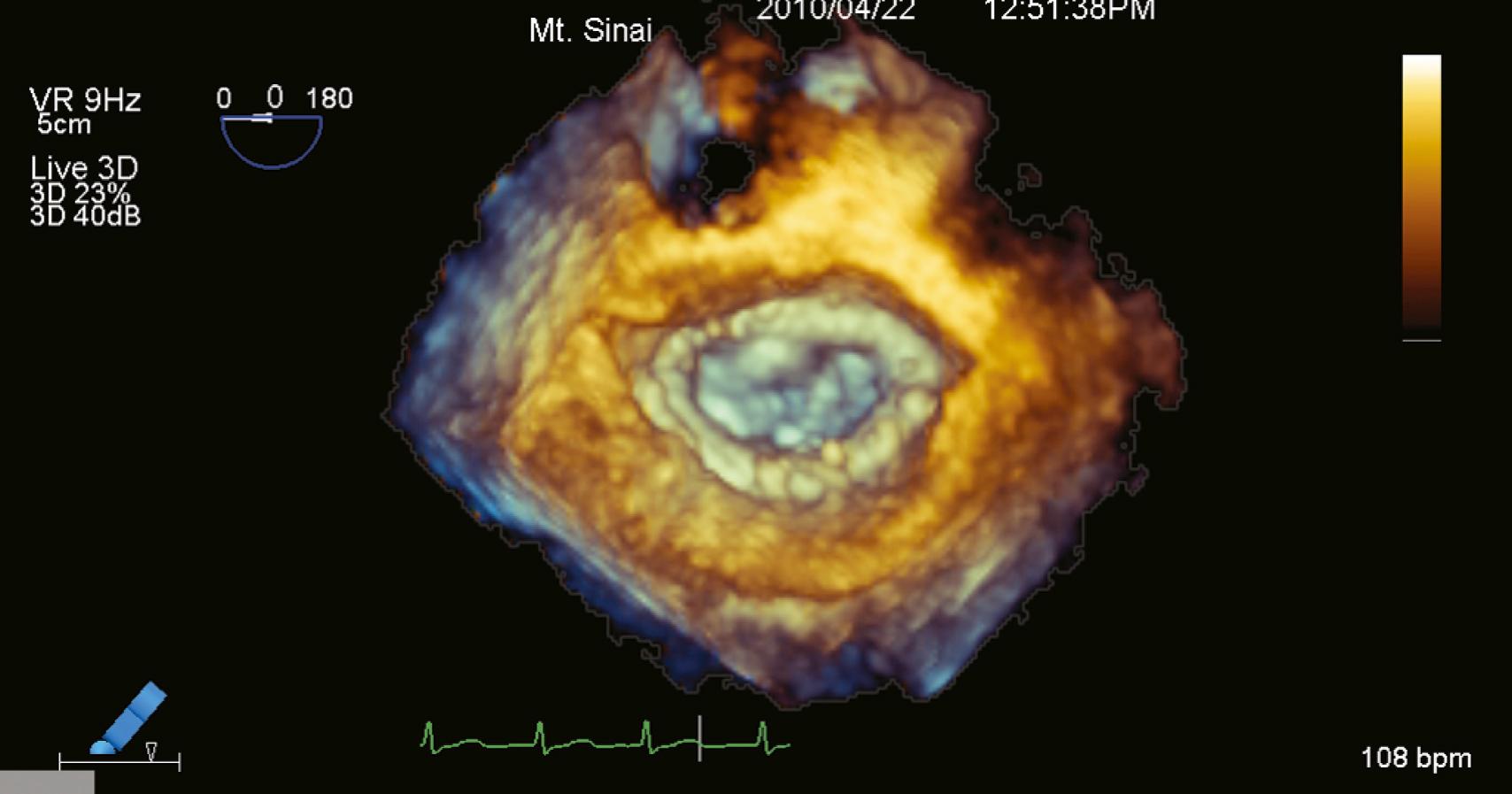
Because of insufficient time for sound to travel back and forth in large volumes while maintaining a frame rate greater than 20 Hz with reasonable resolution in live scanning modes, one maneuver to overcome this limitation entails stitching four to eight gates together to create a “full-volume” mode. These gated “subvolumes” represent a pyramidal 3D data set as would be acquired in the live 3D mode. This technique can generate more than 90-degree scanning volumes at frame rates greater than 30 Hz. Increasing the gates from four to eight creates smaller 3D subvolumes; this can be used to maintain frame rates and/or resolution as the volumes (pyramids) become larger ( Fig. 11.4 ).
Unfortunately, as with any conventional gating technique, patients with arrhythmias are prone to motion artifacts when the individual data sets are combined. The acquired real-time 3D data set subsequently can be cropped, analyzed, and quantified using integrated software in the 3D operating system (QLAB; Philips Healthcare, Andover, MA; Fig. 11.5 ).
Because of the large amount of data that must be acquired with 3D color Doppler mode, a gating method must be utilized similar to that of the full-volume mode; however, because of the large amount of data required, 8 to 11 beats need to be combined to create an image. Jet direction, extent, and geometry easily can be recognized using this technique. The strength of this methodology lies in its ability to quantitate severity of regurgitant lesions; 3D quantification of mitral regurgitation (MR) correlates better than 2D imaging, when using angiography as the gold standard. In an experimental setting, 3D quantification was more accurate (2.6% underestimation) than 2D or M-mode methods, which had the tendency to underestimate regurgitant volumes (44.2% and 32.1%, respectively; Fig. 11.6 ). Recent guidelines from the American Society of Echocardiography for the noninvasive evaluation of native valvular regurgitation recommend 3D to locate valve pathology, calculate left ventricle (LV)/right ventricle (RV) volumes, and measure effective regurgitant orifice area (EROA) and quantitation of flow and regurgitant volume by 3D color flow Doppler (CFD).
Three-dimensional imaging of the LV gives volume and ejection fraction (EF) measurements that are independent of geometric assumptions of the LV shape, accurate, and reproducible. The mitral annulus and LV apex are used as landmarks and semiautomated software helps with edge detection. A full-volume data set is optimized to ensure it is on axis and tracing the LV borders is used for volume and wall motion assessment.
The RV is a complex, crescent-shaped structure that does not lend itself easily to geometric assumptions as its LV counterpart and has been the Achilles heel to 2D imaging. Because of the fact that numerous reports have linked RV function to prognostic outcome in a variety of cardiopulmonary diseases, it would be of great interest to quantify its function echocardiographically.
A preliminary report showed that 3DE marginally underestimated RV volumes when compared with cardiac magnetic resonance, and that correlation to cardiac magnetic resonance–measured volumes was as good as that obtained by cardiac computed tomography. While 3D right ventricular ejection fraction (RVEF) may be more reliable, currently there is insufficient data to demonstrate its clinical value. Further research in this field will undoubtedly lead to increased understanding of perioperative RV function.
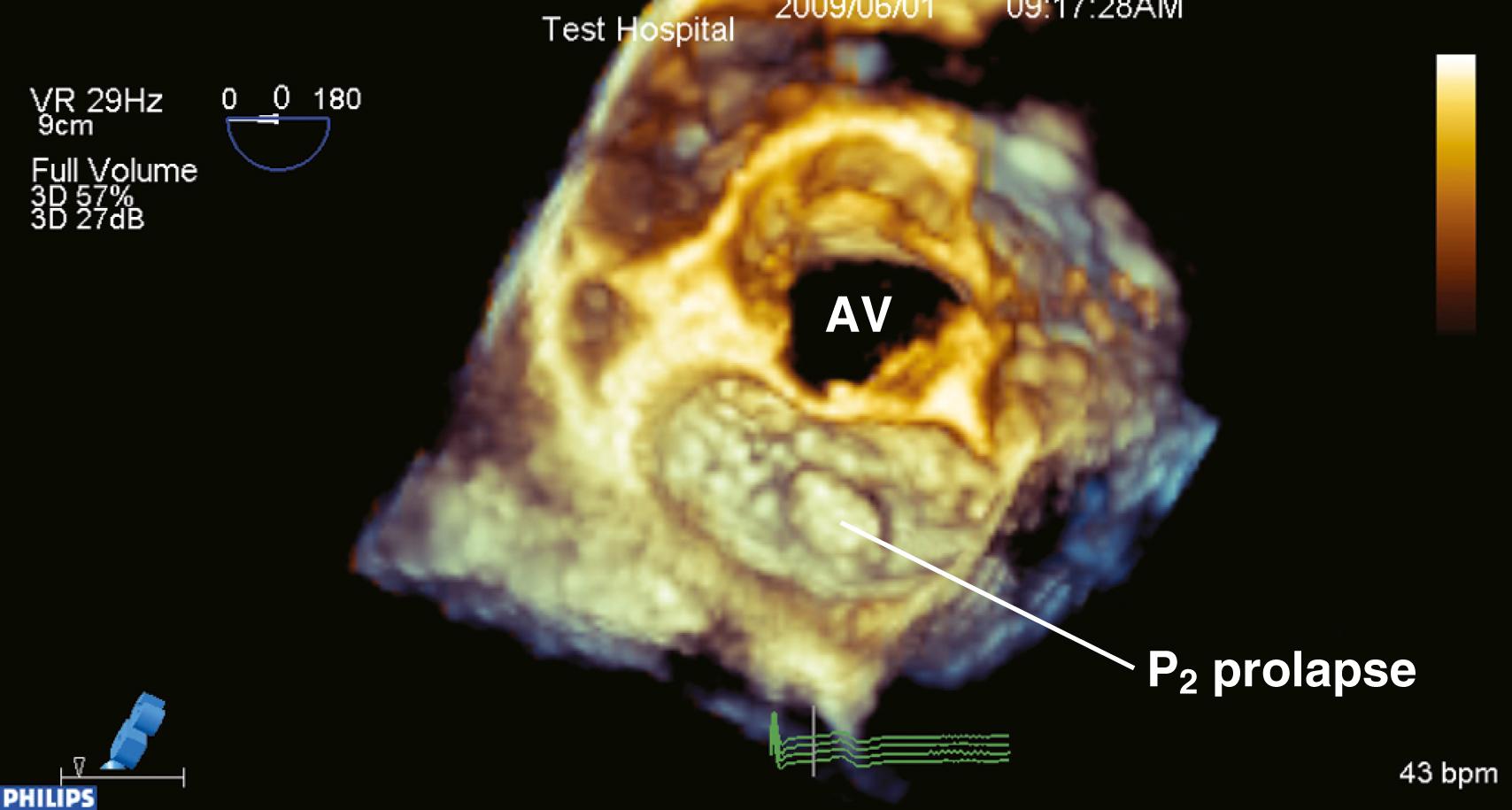
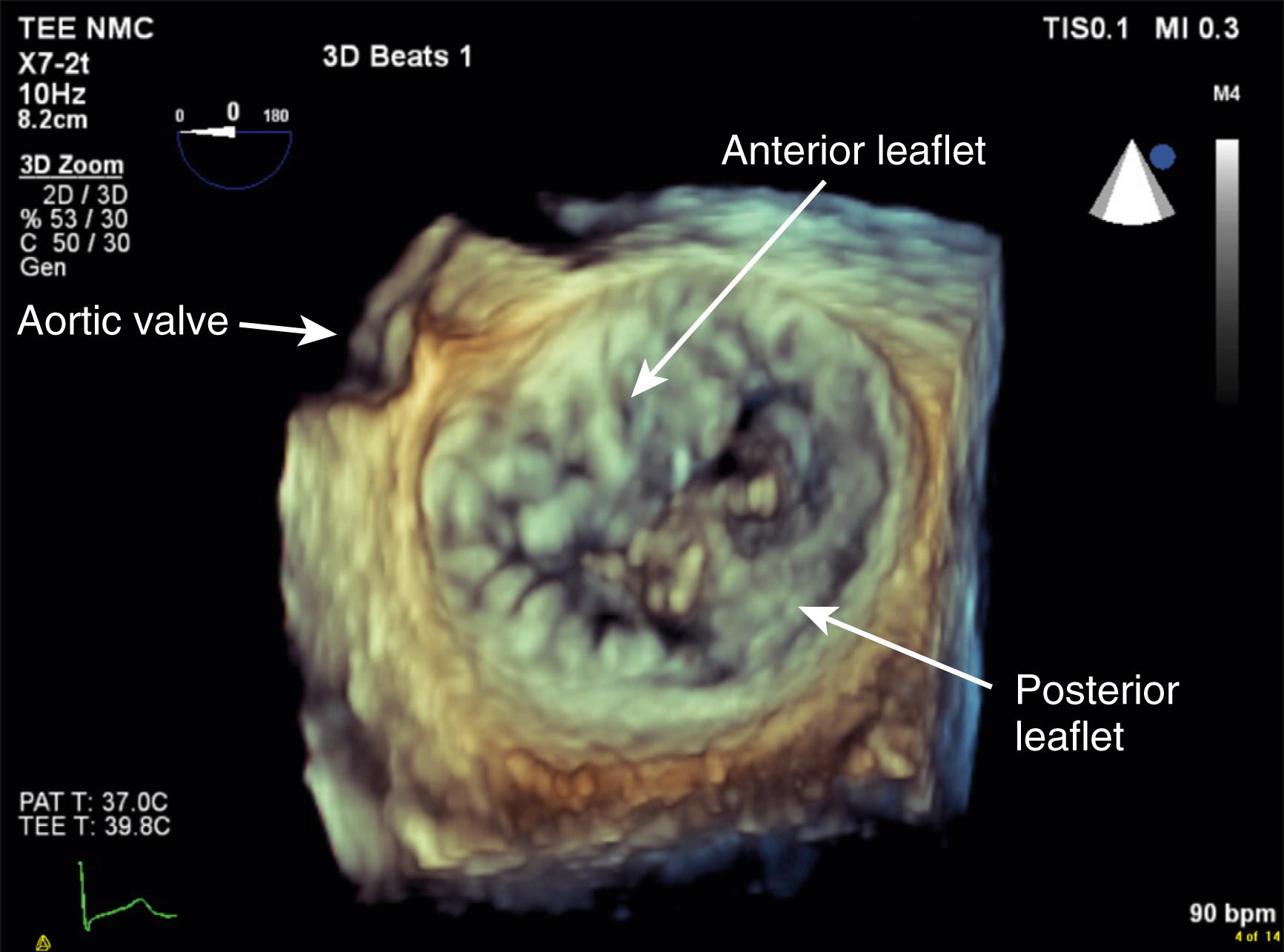
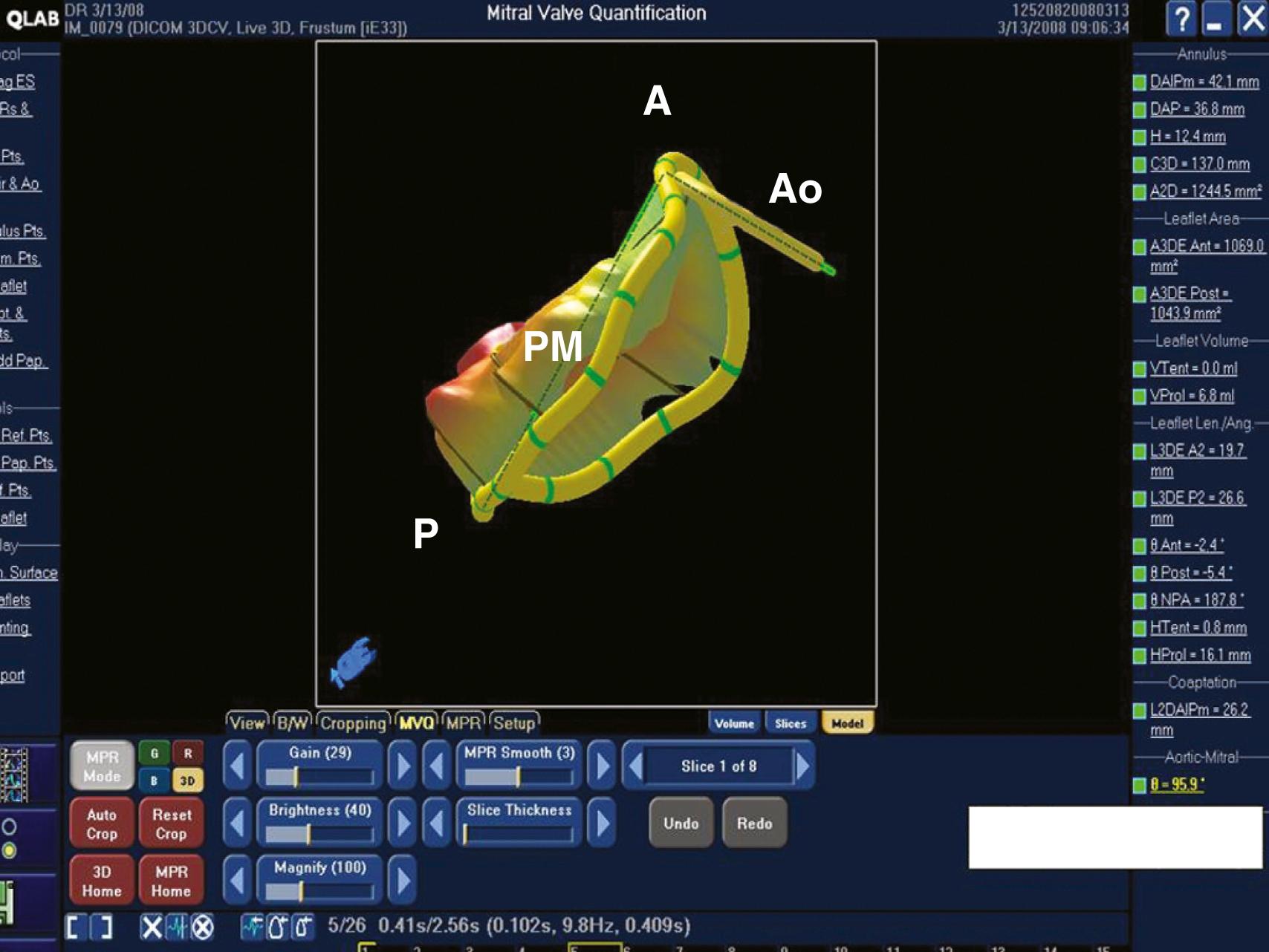
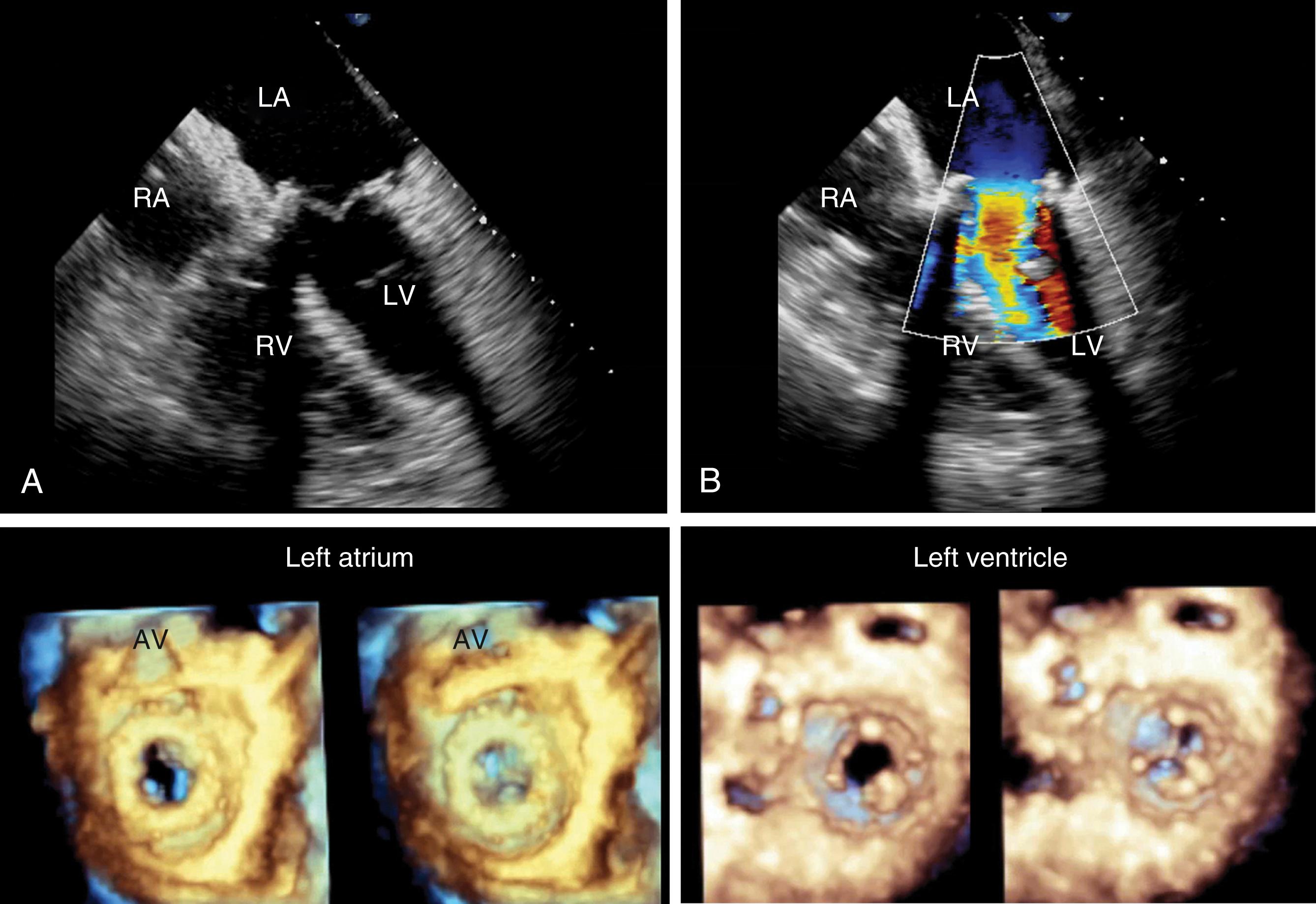
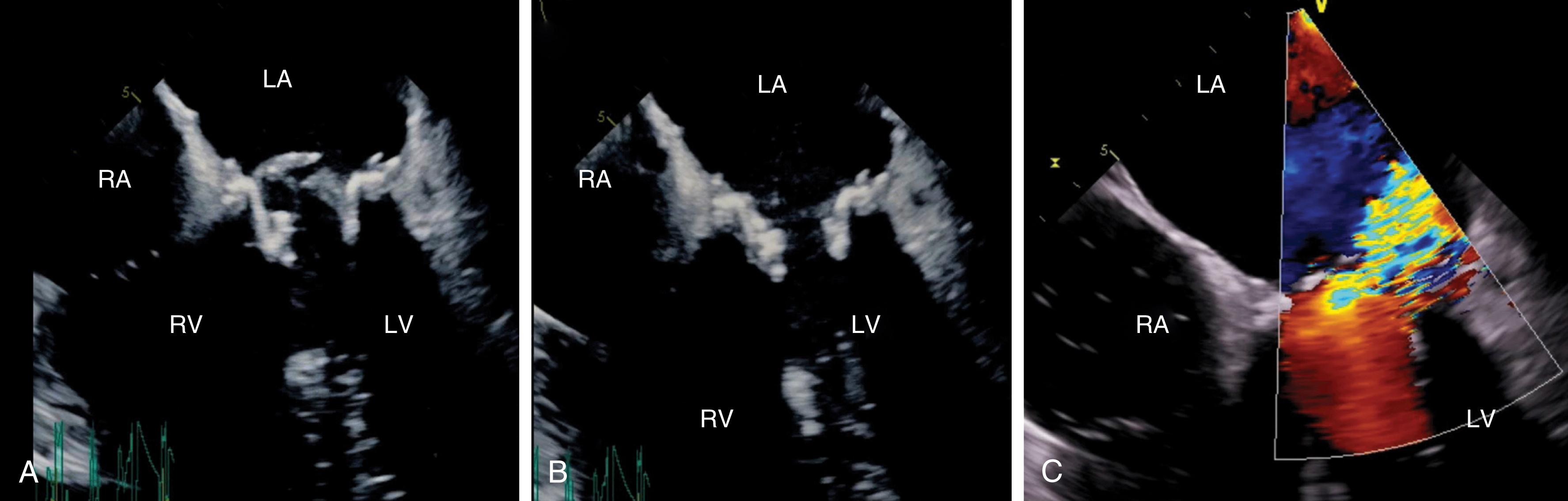
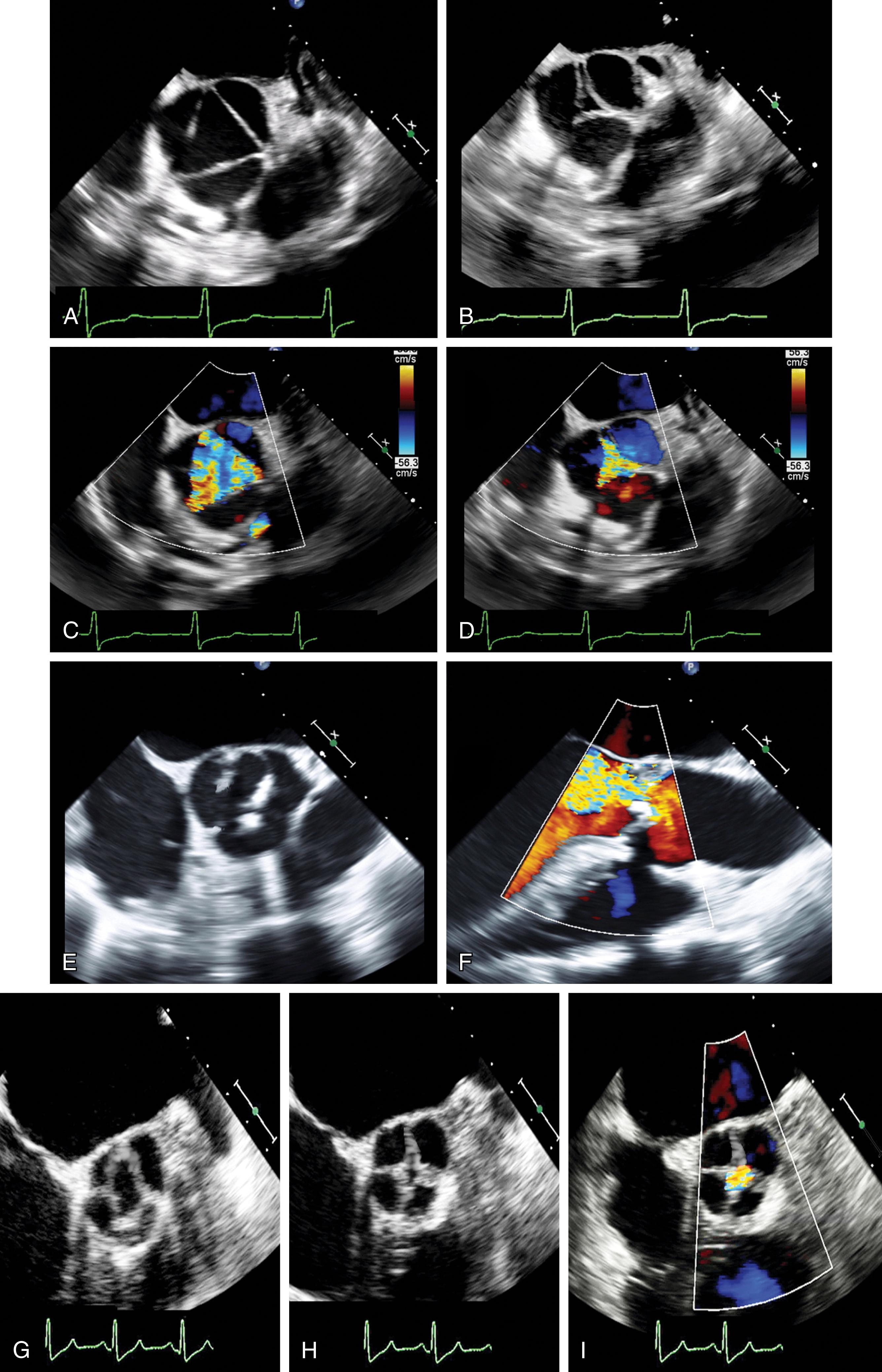
To ensure a high success rate of MV reconstruction, the cardiac anesthesiologist must have a detailed understanding of and insight into the mechanism responsible for regurgitant lesions to identify these echocardiographically. The MV apparatus can best be viewed by utilizing the 3D zoom mode. The data block should be spatially orientated to view the MV from the left atrial (LA) perspective, with the aortic valve (AV) positioned at the top of the monitor (12 o’clock). This orientation is commonly referred to as the “surgeon’s view” ( Fig. 11.7 ). This mode is especially useful in patients with atrial fibrillation, an arrhythmia frequently encountered in patients with MV disease, because it represents a live and instantaneous imaging mode not influenced by gating artifacts.
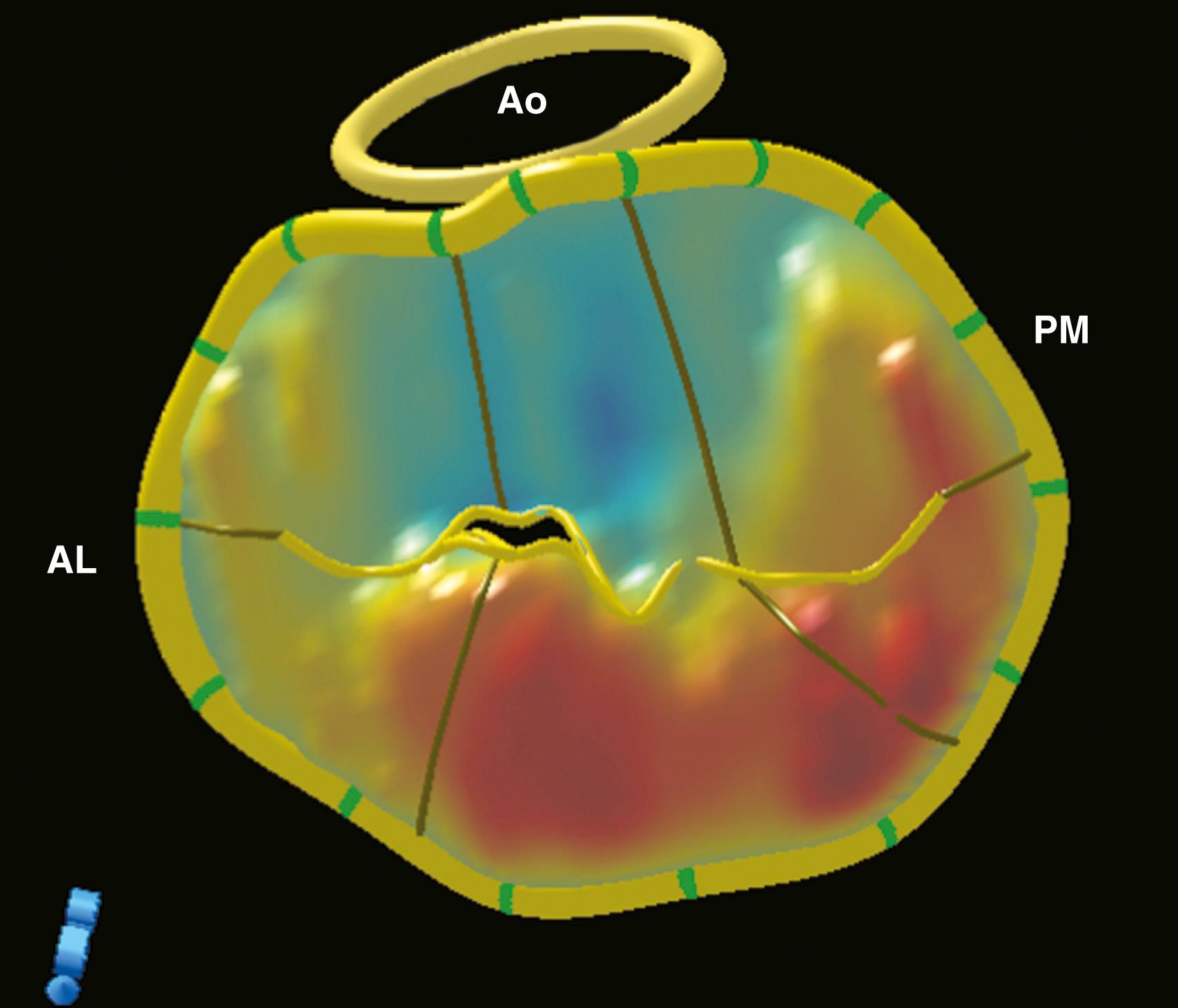
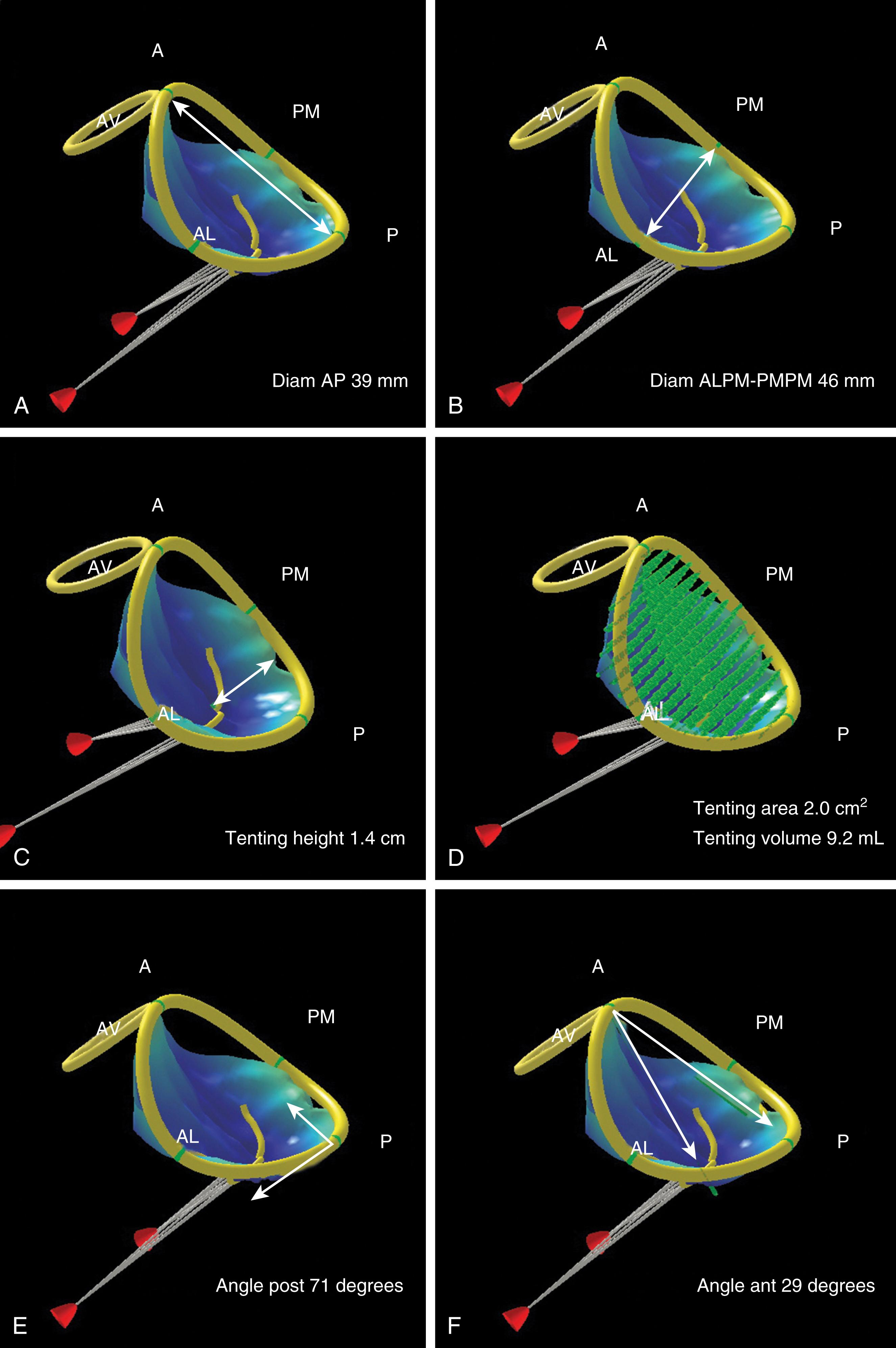
Although a comprehensive 2DE examination can identify the mechanism of regurgitation in most cases, 3DE can not only identify the mechanism but also provide information in regards to annular and leaflet geometry, which cannot be obtained by 2D imaging. Measurements that can be easily obtained include (1) the major anatomically oriented 3D axes of the annulus, anteroposterior (A-P) and anterolateral-posteromedial (AL-PM) diameters, as well as annular height; (2) 3D curvilinear leaflet lengths and areas of all segments (A1, A2, A3, P1, P2, P3); (3) total and functional anterior and posterior leaflet surface areas; and (4) the angle between the AV annulus and the MV annulus (aortomitral angle). A narrow angle should alert the imager to the possibility of systolic anterior motion (SAM) during the postrepair period ( Fig. 11.8 ).
Unlike the MV, acquiring high-quality images of the AV and tricuspid valve (TV) represents a more difficult undertaking. The explanation lies, on the one hand, on the thinner leaflet tissue that generally comprises both the AV and TV and, on the other hand, on the orientation of the tissue as a reflector. Because these factors result in weaker acoustic signal strength, the 3D volume renderer is more apt to tag these as transparent and render the voxels as blood, ie, invisible. Caution must be taken by the echocardiographer not to misdiagnose these imaging artifacts as perforations. Both the AV and the TV should be displayed in the surgical view, as seen in Figs. 11.9 and 11.10 , respectively. A description of these views can be found in this text’s companion textbook, Perioperative Transesophageal Echocardiography: A Companion to Kaplan’s Cardiac Anesthesia .
Echocardiography has many roles and applications that provide healthcare professionals with invaluable information about patients with new murmurs, arrhythmias, thromboembolic events, and/or heart failure. For patients with suspected valvular dysfunction, echocardiography is the method of choice for the detection, diagnosis, and subsequent follow-up evaluations. , , An understanding of valvular dysfunction and resultant hemodynamic and cardiopulmonary effects is crucial to patient care.
The same principles and techniques used in the assessment of native valves apply to the echocardiographic assessment of prosthetic valves; the latter are much more demanding because of specific differences in flow patterns, expected Doppler velocities, and regurgitant flows. In addition, artifacts produced by prosthetic materials may obstruct imaging, requiring the echocardiographer to view the prosthesis from multiple angles.
Whereas the evaluation of native and prosthetic valves is primarily focused on the valve itself, a comprehensive examination using all imaging mode from multiple echocardiographic windows and angles helps discern between primary and secondary valve dysfunction, and the degree of compensation or decompensation.
A baseline knowledge of normal native valvular anatomy, flows, and function is important to allow one to diagnose dysfunction. Antegrade blood flow across normal functioning aortic and pulmonic valves is laminar, centrally directed, with an asymmetric, triangular-shaped profile, characterized by a fast-rise flow velocity, peaking around one-third of the ejection duration. Antegrade flow patterns across the MV and TV consist of low velocity with an early diastolic peak followed by a near equal or smaller late diastolic peak occurring after atrial contraction appearing like an “M.” More than 70% of patients with normal native valve function display trace or mild mitral, tricuspid, and pulmonary valve (PV) regurgitation without obvious defect. By contrast, only about 15% of patients display trace or mild aortic insufficiency (AI) in the absence of disease.
Native valves are described by the number of leaflets, thickness, calcification, presence of masses, leaflet mobility, patency, and competence. For the dysfunctional valve, the presentations (acute vs chronic), the finding of secondary cardiac changes, and evidence of decompensation help determine and guide therapeutic options and plans. Causes of dysfunction can be specific for the valve and described as either primary or secondary, and acute or chronic ( Tables 11.1 and 11.2 ). , ,
| Acute Stenosis | Chronic Stenosis | |
|---|---|---|
| Aortic |
|
|
| Mitral |
|
|
| Tricuspid |
|
|
| Pulmonary |
|
|
| Valve | Acute Regurgitation | Chronic Regurgitation |
|---|---|---|
| Aortic |
|
|
| Mitral |
|
|
| Tricuspid |
|
|
| Pulmonary |
|
|
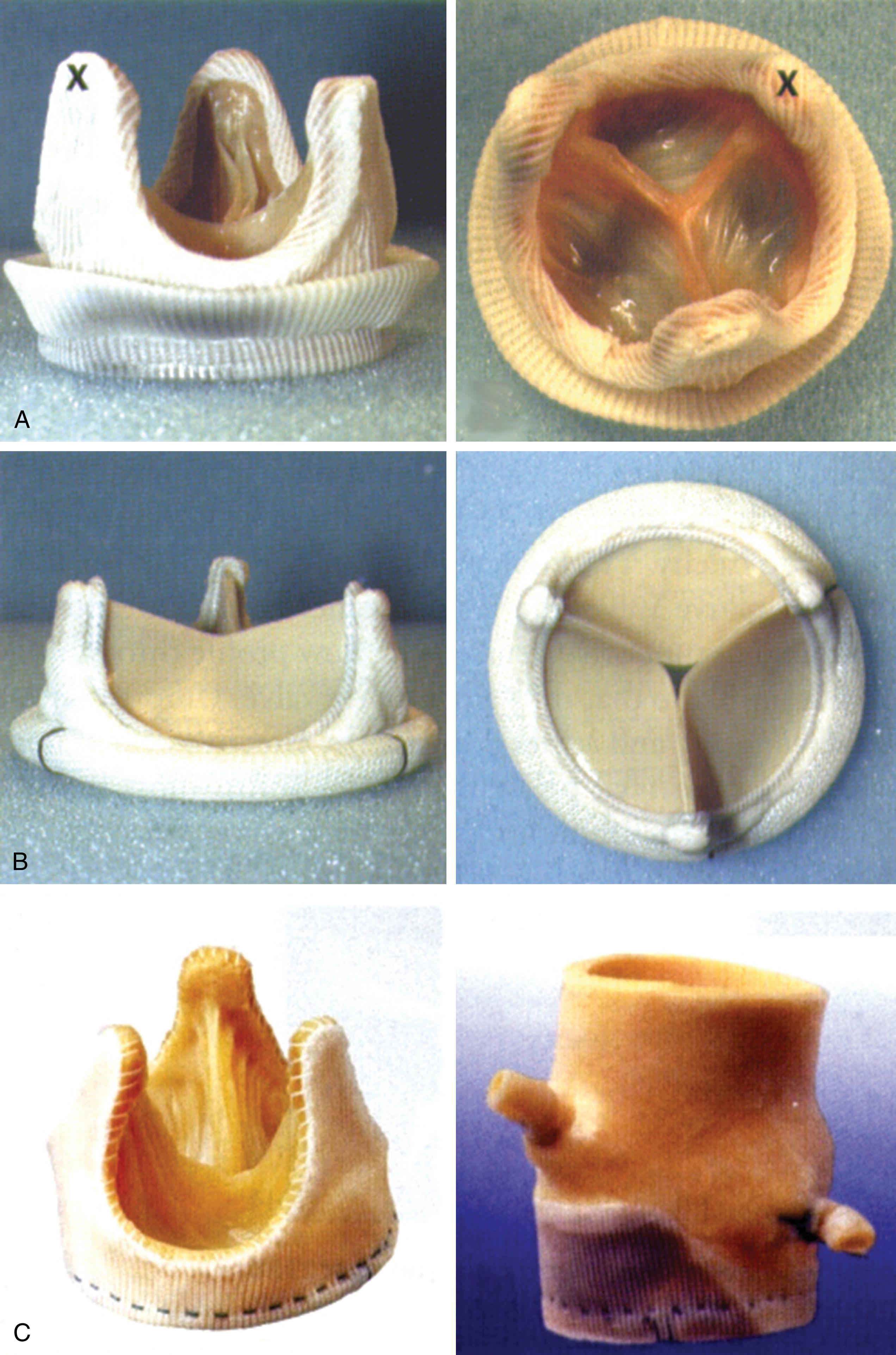
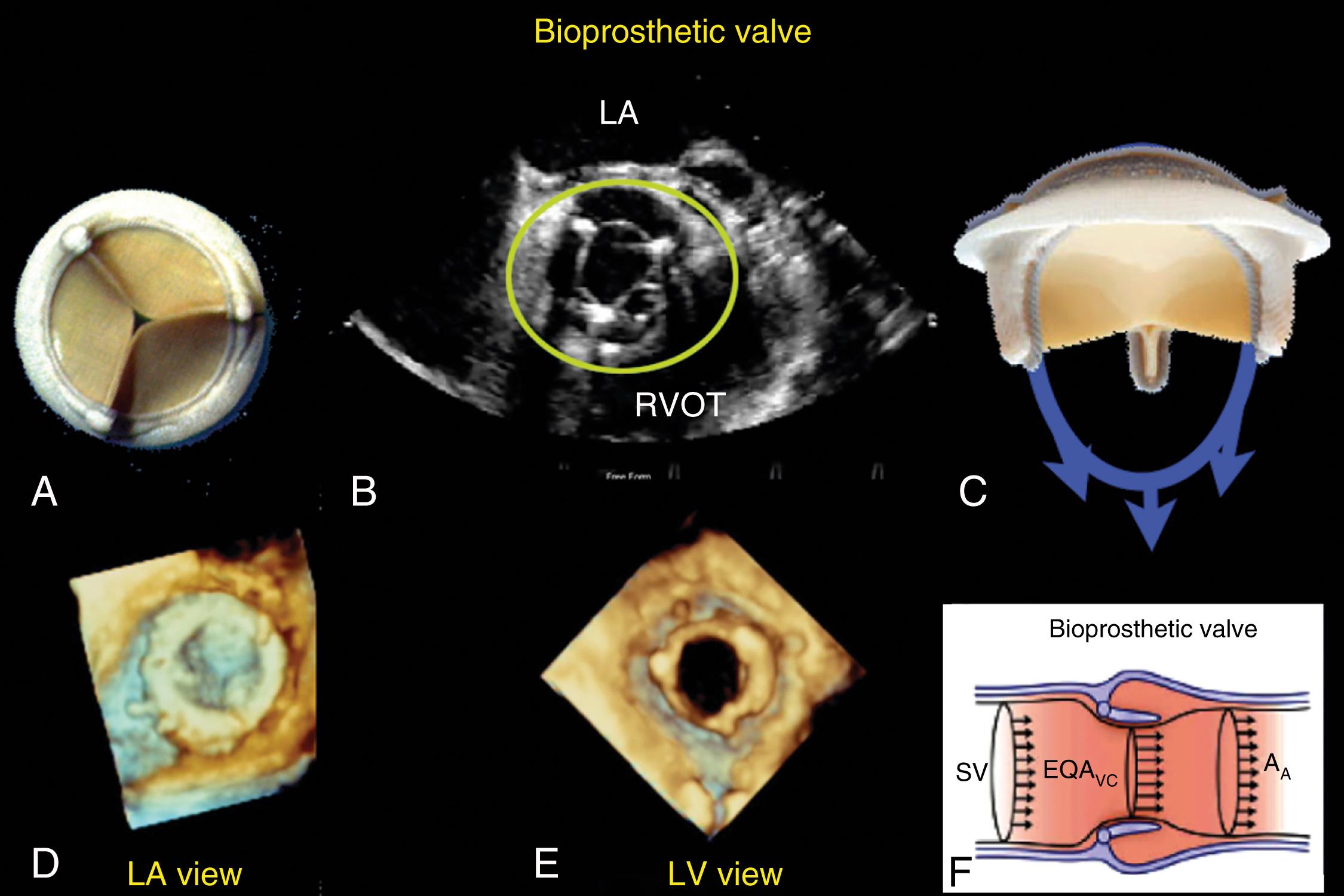
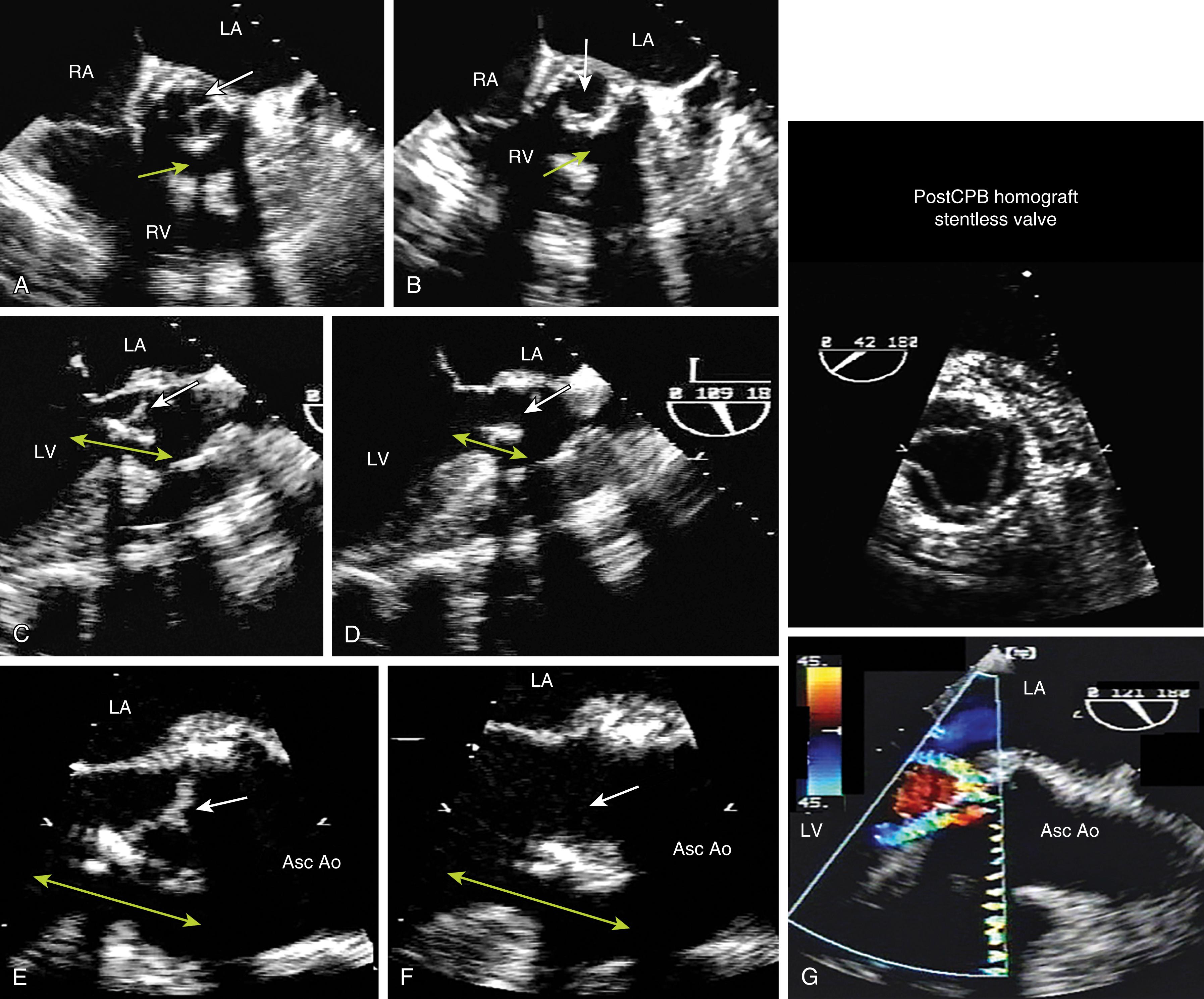
Current prosthetic valves are grouped as biologic or mechanical. , The manufacturer’s valve sizing refers to the external diameter of the sewing ring, not the actual diameter of the effective orifice space. Bioprosthetic valves do not require long-term anticoagulation ( Fig. 11.11 ) and are composed of three biologic cusps (leaflets) sewn into the outer fabric, which open to create a circular orifice with a flow pattern that is similar to the native valve when in the aortic position. Bioprosthetic valves may be stented or stentless, the former having the leaflets sewn into a fabric-covered metal supporting strut. Stentless valves are either a preparation of animal aorta or sculpted pericardial leaflets without any added strut support which was meant to overcome stress and tissue failure associated with the stent, while providing a larger effective orifice area (EOA) compared to its stented counterpart.
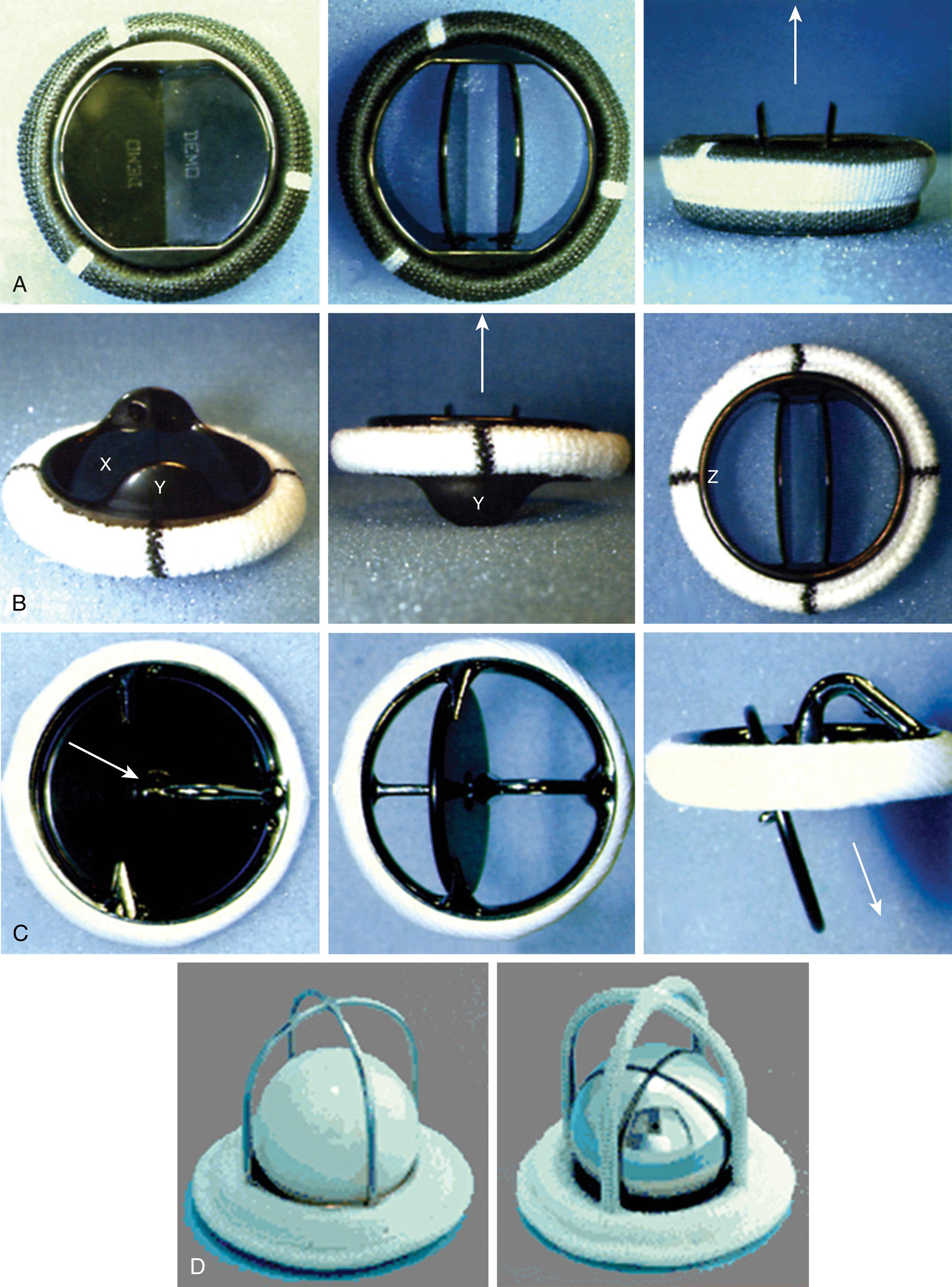
A variety of mechanical valves ( Fig. 11.12 ) have been implanted. The most commonly implanted mechanical valve is a bileaflet valve in which two semicircular disks rotate around supporting struts attached to the valve housing. These open to form two large lateral orifices and a smaller central orifice. Several other types of mechanical valves were implanted in the past and will uncommonly be encountered in patients. The ball-in-cage valve houses a spherical occluder contained within a metallic cage. This occluder fills the cage orifice in the closed position but rides above the orifice during antegrade flow. Single tilting disk valves contain a single circular disk controlled by a metal strut that opens at an angle to the annulus plane ( Boxes 11.1 and 11.2 ).
Paravalve
Calcification
Abscess
Dehiscence
Leaflet pathology: excess mobility
Abscess
Tears
Degeneration
Endocarditis
Bioprosthetic valves: leaflet perforation/destruction
Mechanical valves: interference with valve opening/closing
Leaflet pathology: restriction/distortion
Suture
Thrombus
Pannus
Preserved leaflet valve apparatus interfering with closure of mechanical leaflets
Stuck leaflet
Prosthetic valve stenosis
Stuck leaflet
Thrombus
Pannus
Nonvalvular obstruction
Dehiscence
Obstructing retained native tissue
Suture causing restriction of leaflet
Subvalvular obstruction (aortic and pulmonary valves)
Systolic anterior motion (aortic and pulmonary valves)
Supraannular narrowing (aortic and pulmonary valves)
Doppler issues/artifacts/errors: normal prosthetic valve function
Sampling of other flow (ie, sample mitral regurgitation during assessment of the aortic valve)
Subvalvular obstruction
High cardiac output
Significant aortic valve insufficiency
Patient-prosthesis mismatch
Compliance issues (net changes) affecting pressure half-time
Compared with a normal native valve, normally functioning prosthetic and repaired valves in the same space have a smaller EOA and higher antegrade pressure gradients. Knowledge of manufacturers’ publish data on the flow characteristics of each specific prosthetic valve and its various sizes is useful to discern normal from abnormal function ( Table 11.3 ).
| Diameter (mm) | Peak Gradient (mm Hg) | Mean Gradient (mm Hg) | Effective Orifice Area (cm 2 ) |
|---|---|---|---|
| Stented Bioprosthetic Aortic Valve | |||
| 19 | 32–44 | 24–26 | 0.8–1.2 |
| 21 | 25–28 | 17–21 | 1.1–1.5 |
| 23 | 21–29 | 12–16 | 1.3–1.7 |
| 25 | 16–24 | 9–13 | 1.9 |
| 27 | 19–22 | 6–12 | 2.2 |
| 29 | 18–22 | 10–12 | 2.8 |
| Stentless Aortic Valve | |||
| 19 | 12–13 | 1.2–1.3 | |
| 21 | 17–40 | 7.5–18 | 1.2–1.6 |
| 23 | 18–29 | 7–18 | 1.6–2.2 |
| 25 | 14–28 | 5–17 | 1.6–2.3 |
| 27 | 26 | 4.7–18 | 1.9–2.7 |
| 29 | 24 | 4 | 2.4 |
| Bileaflet Mechanical Aortic Valve | |||
| 16 | 40–50 | 25–30 | 0.6 |
| 17 | 30–40 | 20–25 | 0.9–1.0 |
| 19 | 30–40 | 15–20 | 0.9–1.2 |
| 21 | 25–30 | 13–20 | 1.2–1.4 |
| 23 | 19–25 | 11–20 | 1.4–1.8 |
| 25 | 17–23 | 9–12 | 1.9–2.2 |
| 27 | 14–20 | 8–11 | 2.3–2.5 |
| 29 | 10–20 | 6–9 | 2.8–3.1 |
| 31 | 10–15 | 5–10 | 3.1 |
| Bileaflet Mechanical Mitral Valve | |||
| 25 | 10 | 4 | 1.8–2.7 |
| 27 | 8–11 | 3–4 | 1.8–2.9 |
| 29 | 8–10 | 5 | 1.8–2.3 |
| 31 | 8–12 | 4–5 | 2.0–2.8 |
| 33 | 8–9 | 4–5 | 3.0 |
| Stented Bioprosthetic Mitral Valve | |||
| 25 | 10–15 | 5.9–6.3 | 2.0–2.4 |
| 27 | 9.5–16 | 5.4–6.2 | 2.0–2.6 |
| 29 | 5–13 | 3.6–4.6 | 2.4–2.6 |
| 31 | 4–13.5 | 2.0–5.0 | 2.3–2.4 |
| 33 | 12.8 | 3.8 | 3.4 |
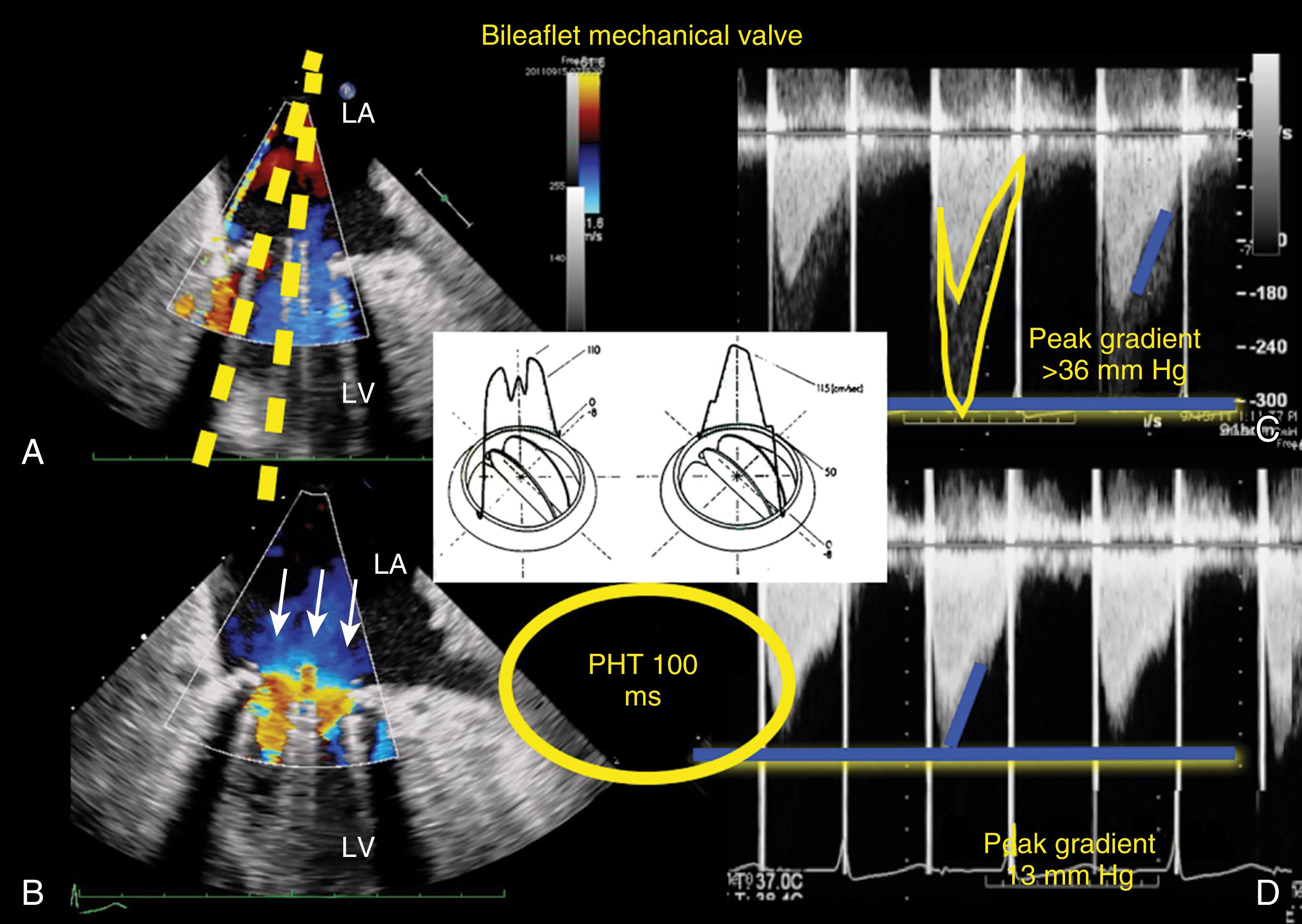
Whereas Doppler flow profiles across normal native, most repaired, and bioprosthetic valves are consistent for flow moving through a single orifice, flow profiles across multiple orificed mechanical valves and some repaired valves (eg, Alfieri repair, MitraClip repair) are more complex ( Figs. 11.13 and 11.14 ). Flow and pressure gradient through each orifice are dependent on orifice size, geometry, and stroke volume (SV).
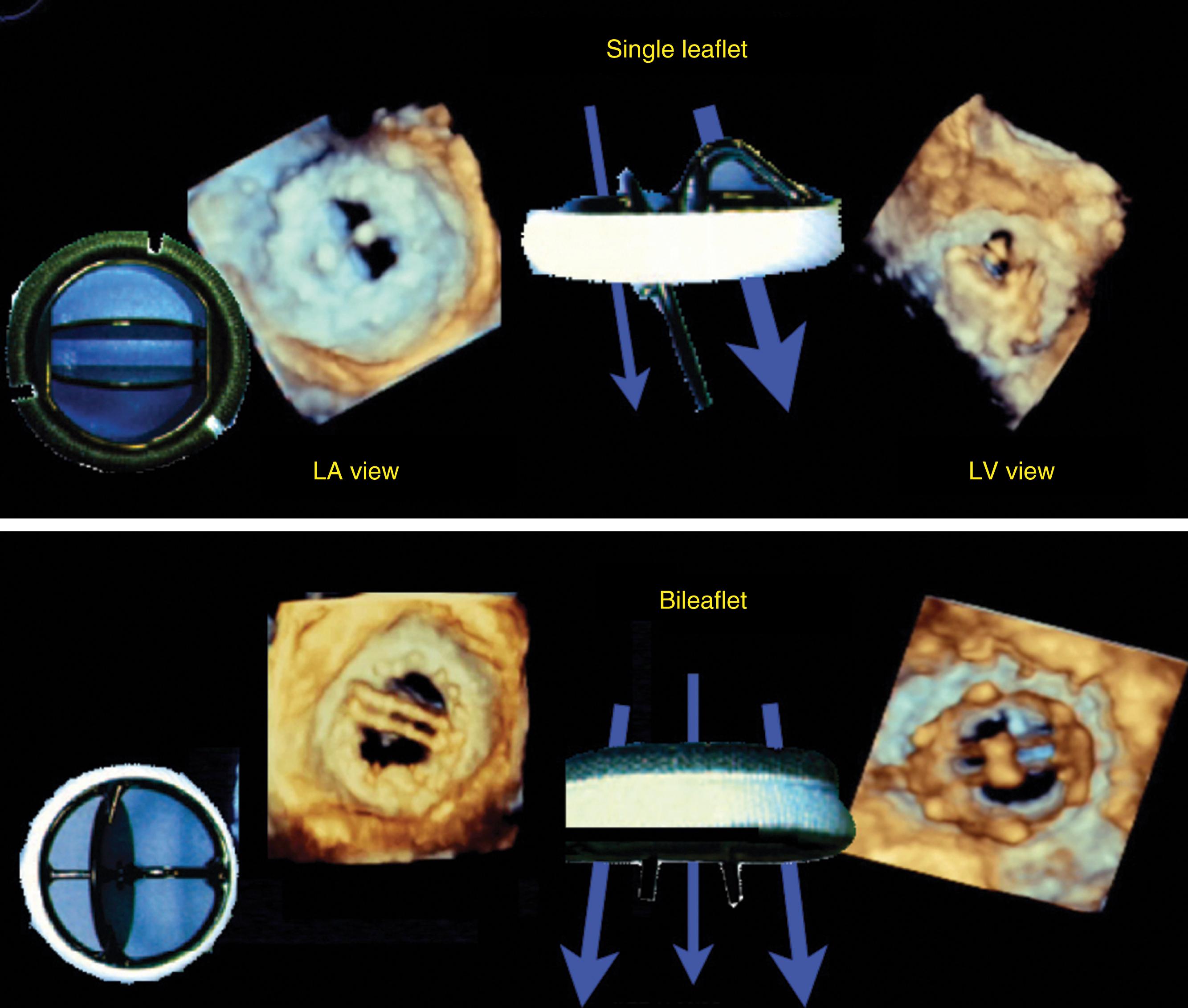
The bileaflet prosthetic valve has two semicircular disks which opens to form two larger semicircular lateral orifices and a smaller slit-like central orifice ( Figs. 11.13–11.15 ) , , In the open position, CFD shows antegrade flow through two larger lateral orifices and a small central orifice. A higher velocity, less dense, outer flow profile (outer envelope) may reflect due to local acceleration forces through the central orifice. In the mitral position, this flow velocity has also been thought to represent distal flow accelerations or artifact. For the bileaflet mechanical mitral prosthesis, the denser, lower velocity, inner flow profile better represents valve function. , ,
Given that pressure gradients vary depending on SV and orifice size and are specific for native, prosthetic, and repaired valves, determining the valve EOA adds useful information regarding function. Indexing the valve areas to individual body surface area (BSA) allows an assessment of the valve relative to the patient’s size. A relatively small valve area (relative to the BSA) is called patient-prosthesis mismatch (PPM). PPM is more commonly described and discussed in the context of AV surgery, and is associated with short- and long-term adverse outcomes. A recent investigation into intraoperative Doppler interrogation of surgical AV replacements demonstrated that intraoperative gradients are less valuable than the type and size of a prosthetic valve in predicting postoperative PPM. Elevated gradients therefore are important in identifying valve disfunction as opposed to identifying PPM.
Mechanical valves have normal washing jets engineered into their design ( Fig. 11.16 ). , , These flows occur within the annulus and are thought to prevent the formation of thrombi at sites of blood flow stasis. These jets are small (vena contracta [VC] <3 mm) and low velocity, typically have a uniform color pattern (minimal aliasing), and do not extend far from the valve plane. Bioprosthetic valves tend to have small central regurgitant jets. All paravalvular regurgitant jets are considered abnormal. However, regurgitant jets less than 3 mm in width are not likely to pose a long-term problem, especially in the absence of clear disease. Ideally, a repaired valve will have no regurgitant jet, however, due to multiple variables, the postrepair examination should yield mild or less regurgitation.
Antegrade flow across a single tilting disk prosthetic valve is characterized by a major and a minor orifice. CFD indicates nonlaminar and asymmetric flow as blood accelerates along the tilted surface of the open disk. In the closed position, a single tilting disk normally demonstrates a regurgitation jet directed away from the sewing ring starting at the edge of the major orifice. When a bileaflet mechanical valve is imaged in the closed position, multiple washing jets of regurgitation are normally seen in the plane parallel to the leaflet-opening plane. All the regurgitant jets are located inside the sewing ring, with two jets originating from where the leaflets meet the housing apparatus, and a third central jet originating where the leaflets meet each other.
Three-dimensional imaging with CFD highlights why simpler 2D assessments may not be accurate ( Figs. 11.17–11.19 ). Specifically, geometric assumptions required for several of the equations and principles are not always accurate. Visualizing the flow profile on three levels—pre-valve, valve, and post-valve—displays the characteristics of the valve orifice and flow through it. The recent advancements in 3D imaging allow a more accurate area measurement using planimetry (see Fig. 11.19 ).
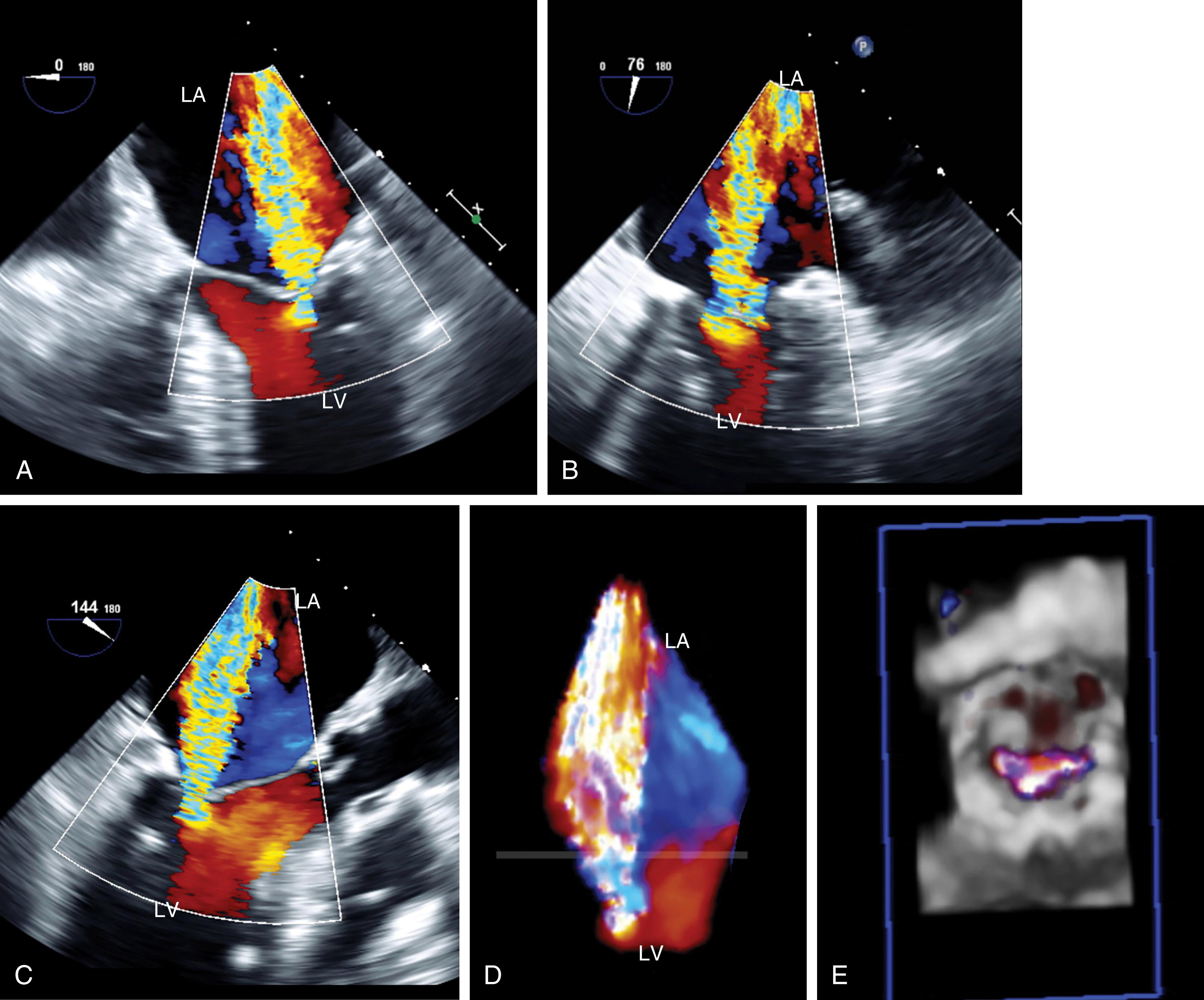
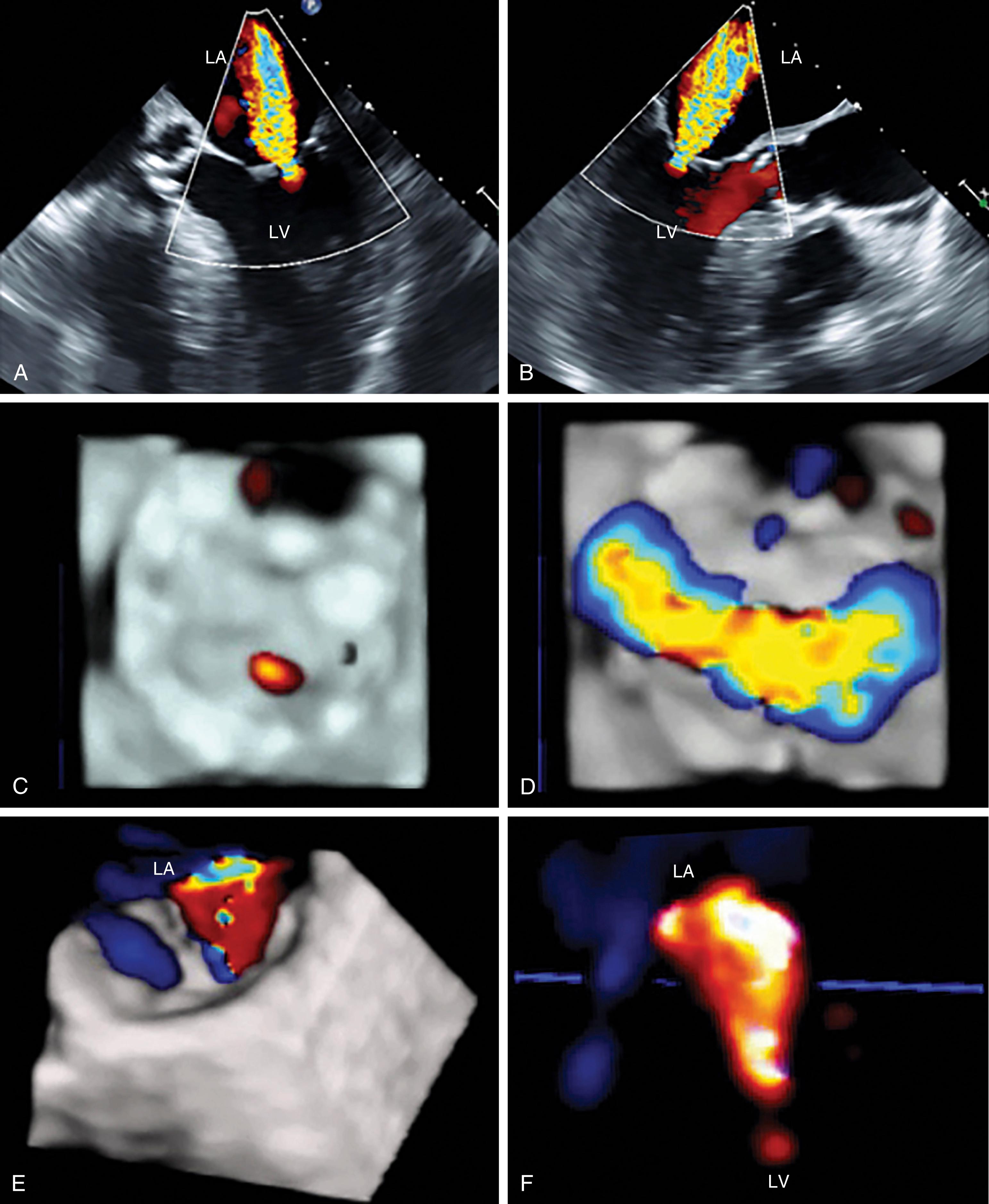
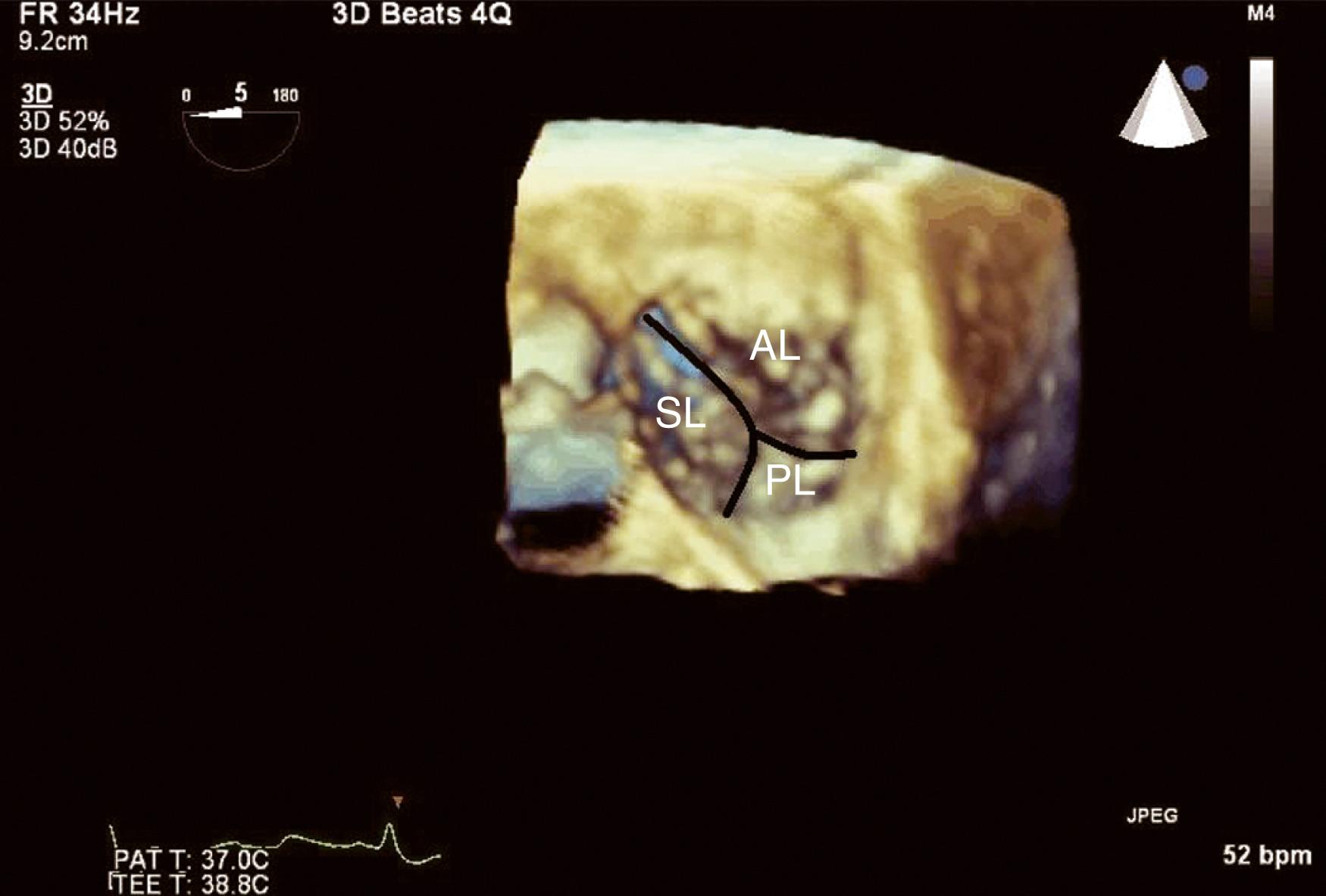
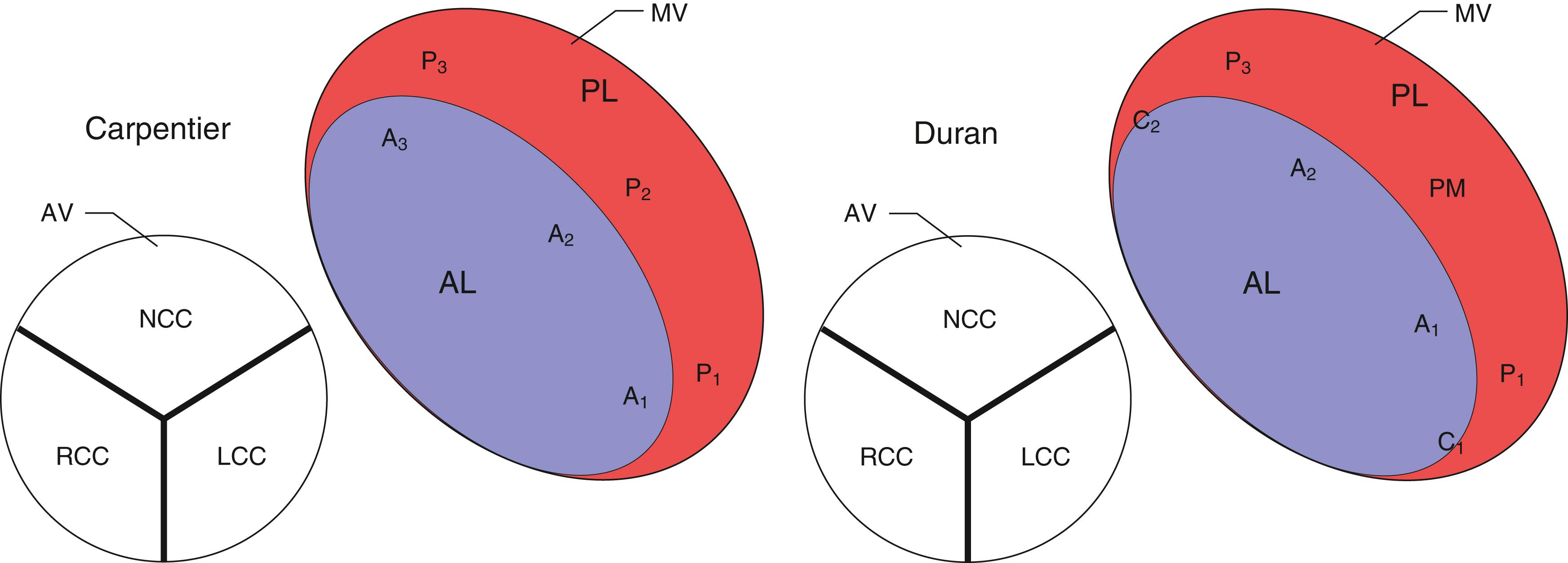
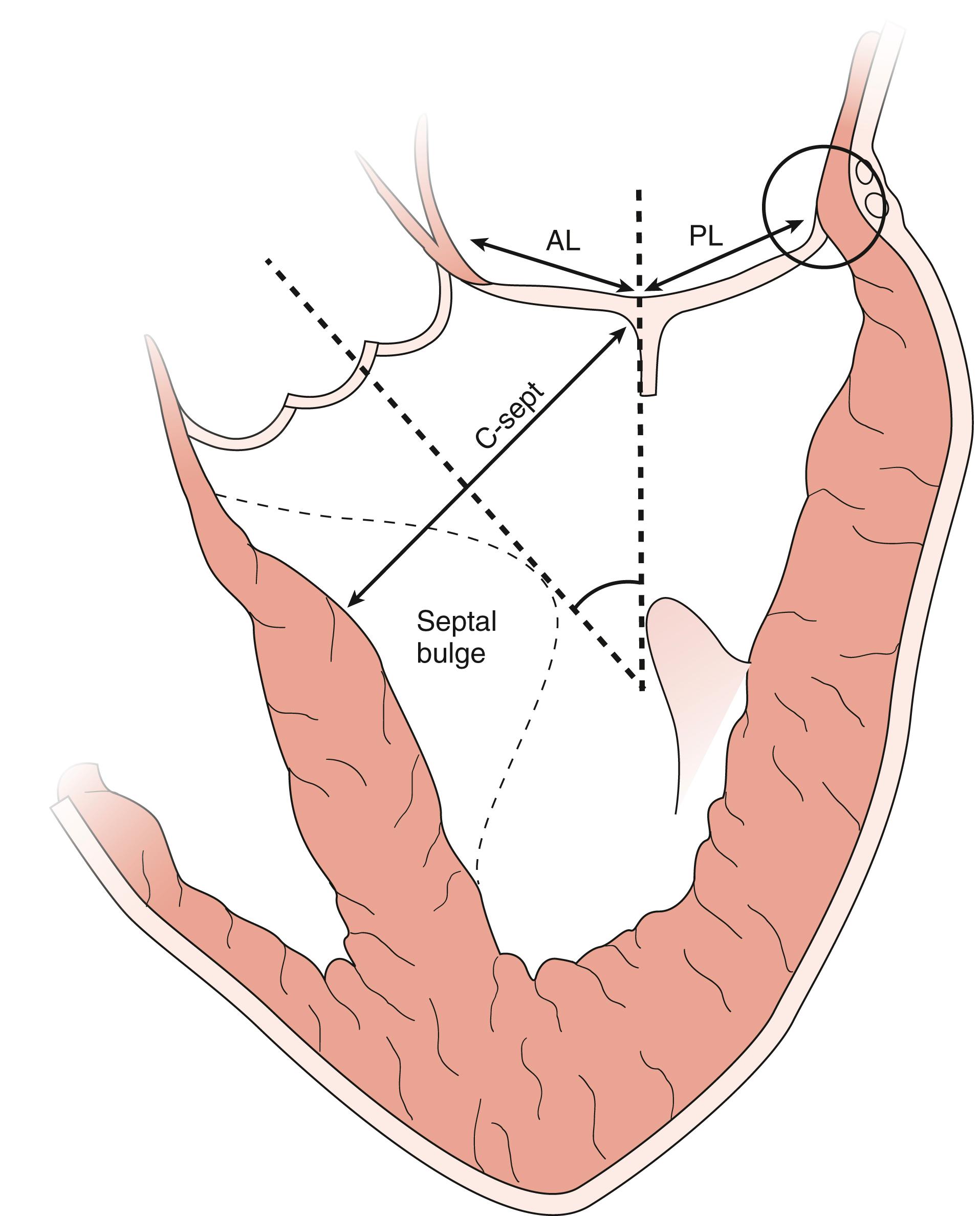
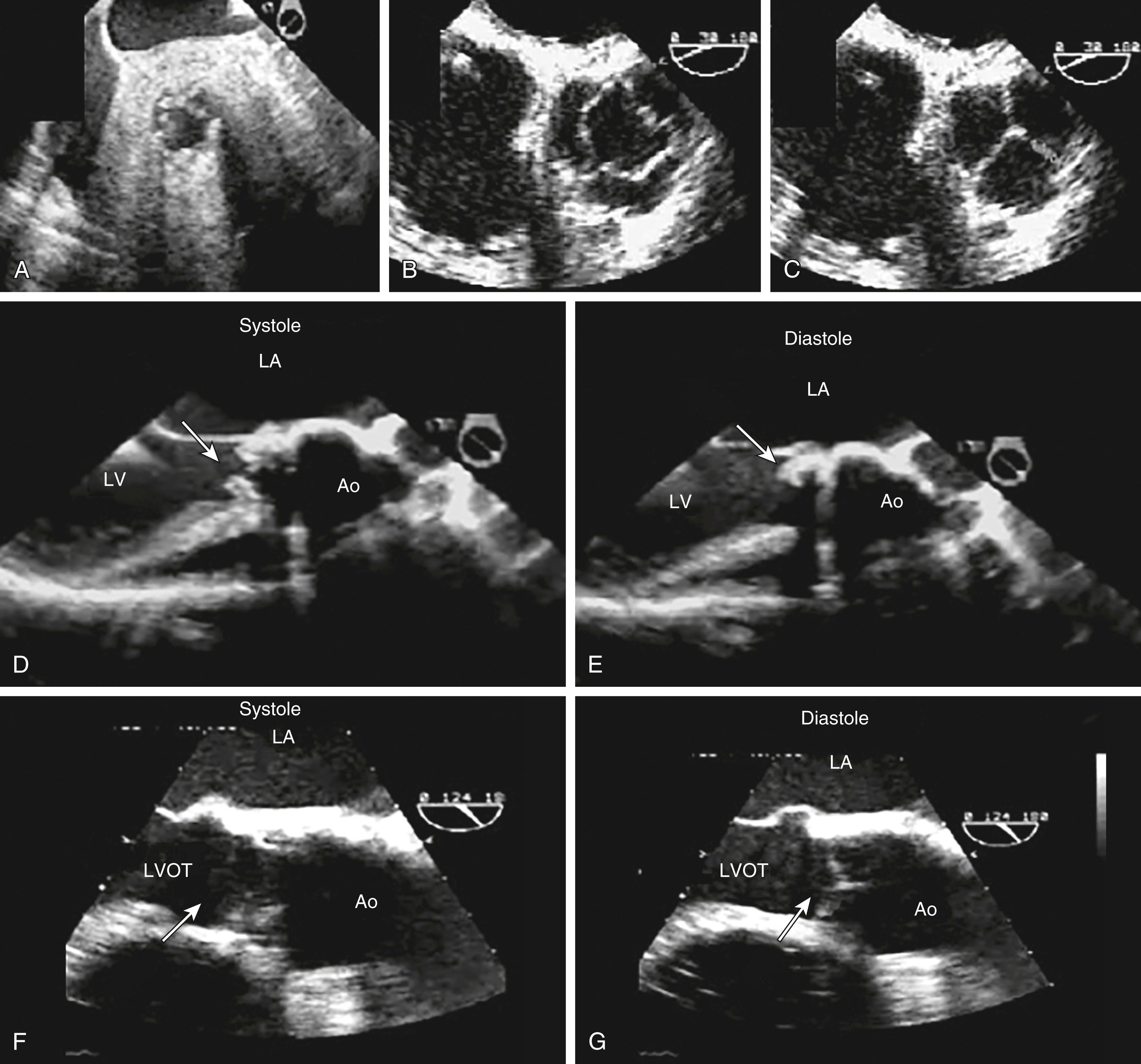
The anatomic and functional features of MV have been reviewed extensively (see Chapter 10 ) and understanding of MV function and failure has contributed to the success of MV repair. , The normal MV is a thin bileaflet (each < 2 mm thick) valve, of which the anterior and posterior leaflets insert into their respective annuli. Each of the leaflets is divided into segments or scallops, which have been defined by Carpentier and Duran ( Fig. 11.20 ). Both investigators describe the posterior leaflet as having three scallops or segments: anterolateral and superior (P 1 ), middle (P 2 ), and posteromedial and inferior (P 3 ). Duran divides the anterior leaflet into two larger segments (anterior [A 1 ] and posterior [A 2 ]) and two smaller commissural segments (C 1 and C 2 ). By contrast, Carpentier describes the anterior leaflet with relation to the posterior leaflet; A 1 , A 2 , and A 3 lying in the same respective planes. Although the posterior annulus covers two-thirds of the annular circumference, the posterior leaflet covers only one-third of the valve area. By contrast, the anterior leaflet is responsible for two-thirds of the valve area (see Fig. 11.20 ). Functionally, it appears that the posterior leaflet provides a coapting surface for the larger anterior leaflet. During coaptation the leaflets overlap by at least 6 mm and the base of the coaptation point lies within 6 mm of the annular plane but not above it. The importance of the leaflet heights or lengths and coaptation point is seen in their effect on blood flow direction into and out of the LV. , During ventricular diastole, the anterior leaflet is concave to the left atrium, directing blood flow toward the ventricular apex along the posterolateral wall ( Fig. 11.21 ). Subsequently, before and during ventricular contraction, blood flow is directed toward the left ventricular outflow tract (LVOT) along the anteroseptal wall. During this latter time period, the anterior leaflet is convex toward the left atrium enlarging the LVOT just before and during systolic ejection. This pattern of blood flow sets up a vortex with energy that drives flow up and toward the AV with minimal interference and maximum efficiency (see Fig. 11.21 ). Other anatomies and flow directions are less efficient.
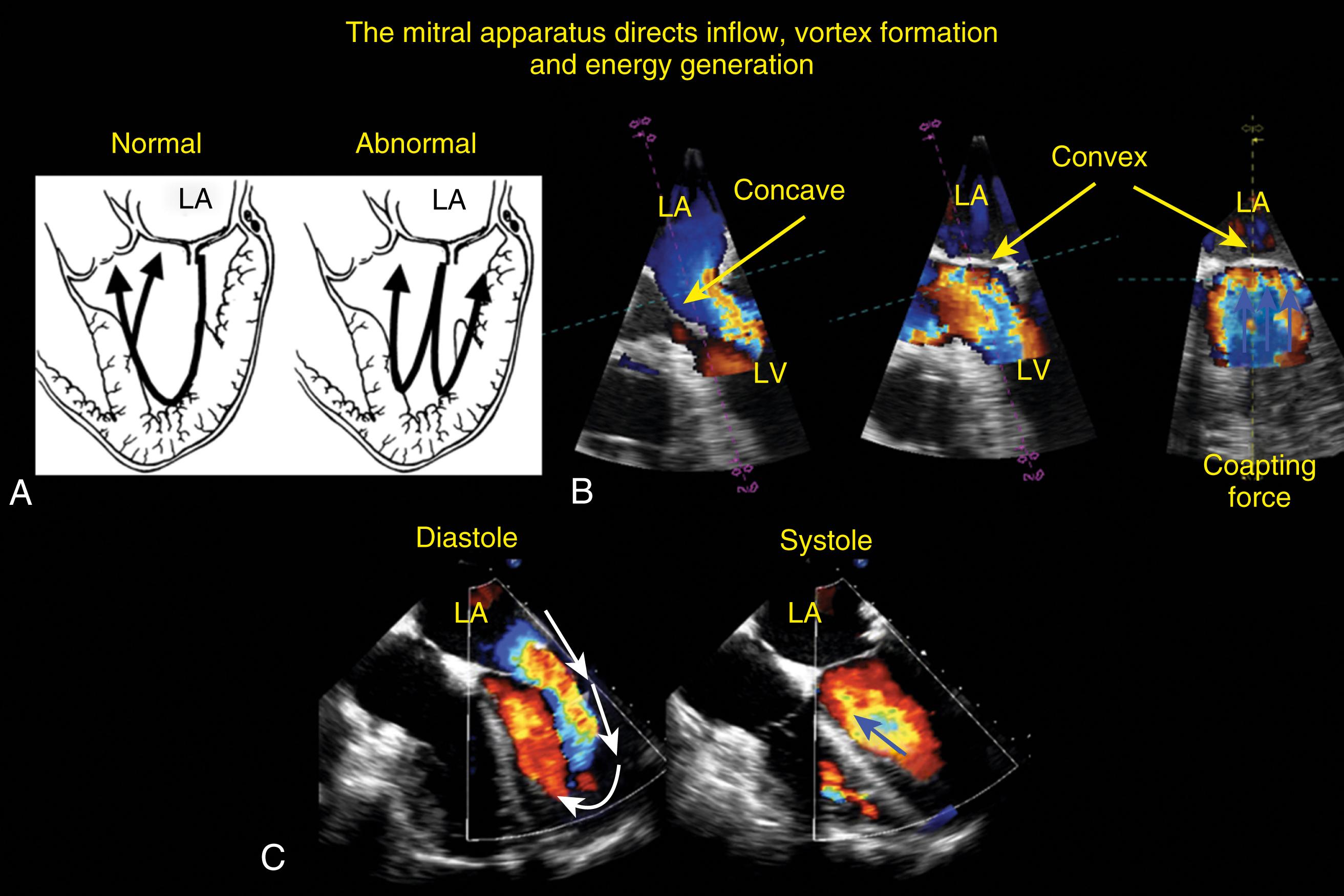
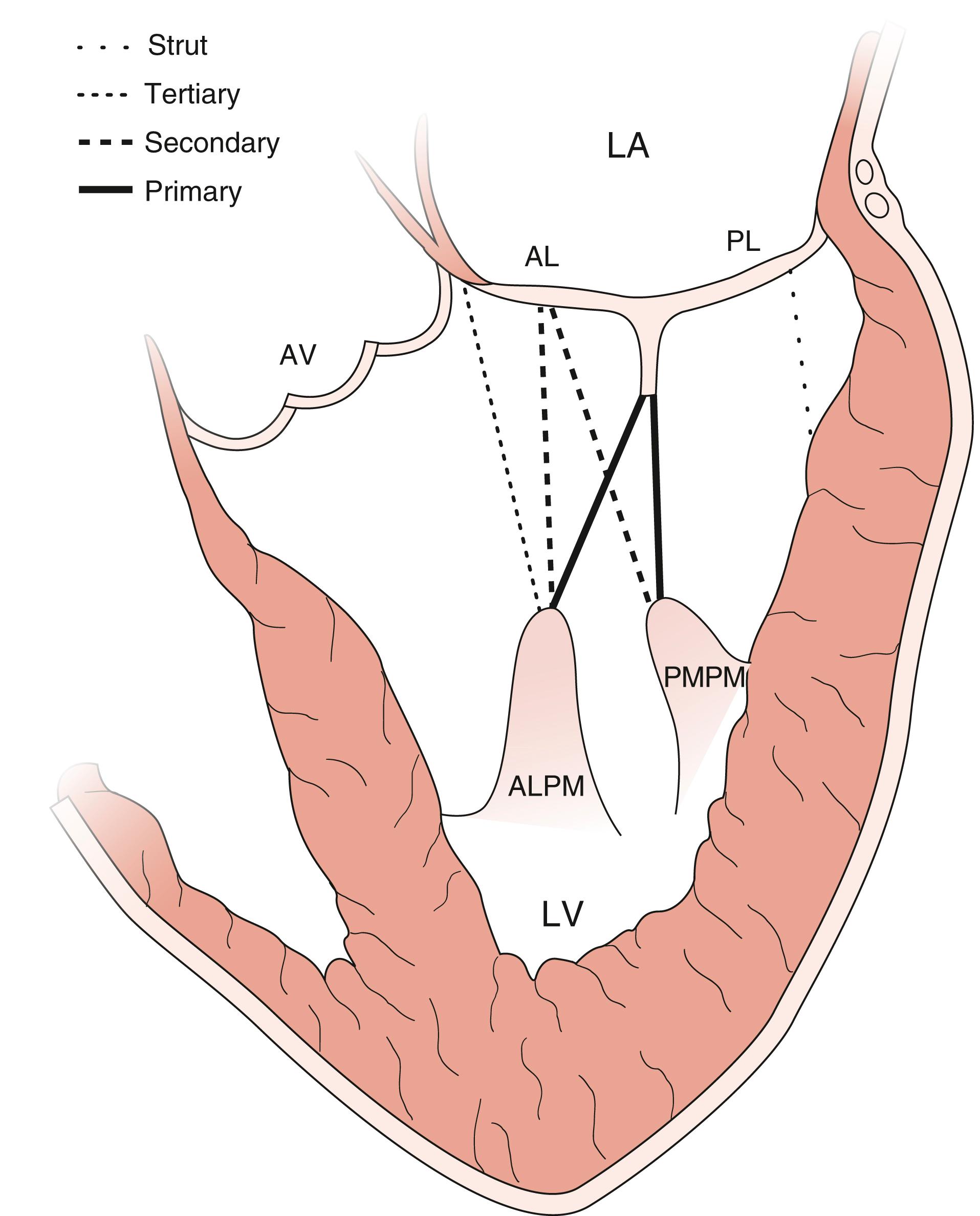
The chordae connect the mitral leaflets to the two papillary muscles (anterolateral and posteromedial) and ventricular walls, and create important interactions between the mitral apparatus and the ventricular myocardium ( Fig. 11.22 ). The anterolateral papillary muscles connect to the more anterior and lateral portions of each leaflet and the posteromedial papillary muscle connects to the more posterior and medial portions. The primary purpose is to prevent leaflet prolapse or flail by creating tension during papillary muscle contraction (i.e., ventricular systole). There are multiple types of chordae. While the primary chordae prevent prolapse, other secondary, tertiary, and quaternary chords connect areas of the mitral leaflets to the ventricular walls. These chordae help maintain both function and shape of the ventricle.
The normal mitral annulus is a 3D saddle-shaped dynamic structure ( Fig. 11.23 ). Its shape and position change throughout the cardiac cycle to optimize forward flow into the ventricle, prevent regurgitant flow, and accommodate ventricular systolic outflow. During diastole the mitral annulus is more planar and forward toward the ventricular cavity opening up wide and directing flow toward the posterolateral ventricular wall creating a vortex and vortical forces, resulting in flow toward the apex and then anteroseptal toward the LVOT prior to systolic ejection. In conjunction with the end of diastole and onset of systole, the mitral annulus contracts posterolaterally toward the membranous portion and superiorly toward the atrium, both actions increasing size of the LVOT, increasing its reservoir capacity prior to ejection, and minimizing interactions between systolic outflow and the mitral leaflets.
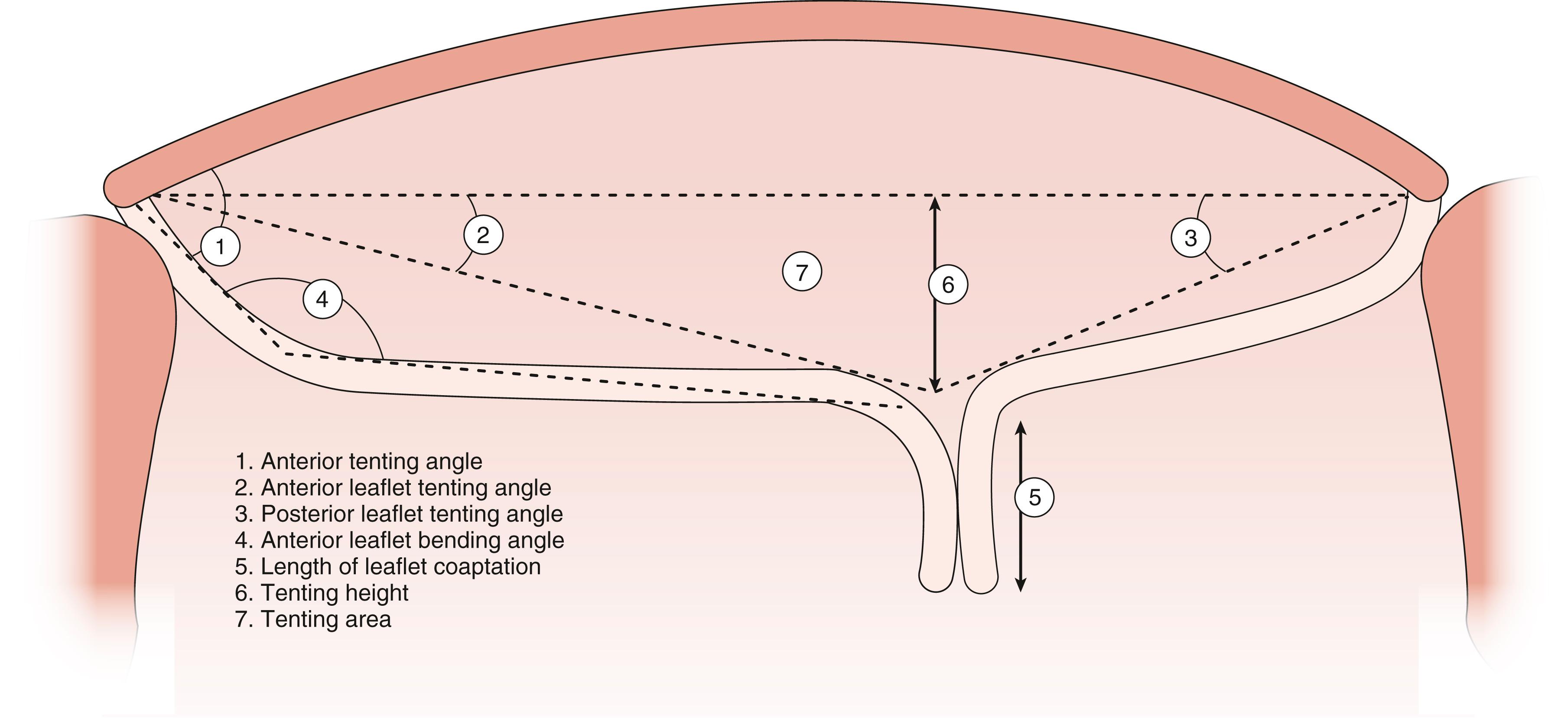
![Figure 11.23, Annular changes. Schematic shows three measures of the mitral annulus and how they change during the cardiac cycle. The black dashed arrows represent the annular dimensions at end-diastole. During ventricular systole the anteroposterior distance (DAPsyst) decreases and moves laterally (ie, away from the left ventricular outflow tract [LVOT]). The intercommissural line (DAL-PM) decreases and moves posterior. The annular height increases and moves the anterior portion of the annulus away from the LVOT. Altogether, the mitral annular area declines during ventricular systole and moves away from the LVOT to allow for unhindered systolic outflow. Ah , Annular height; ALPM , anterolateral papillary muscle; AV , aortic valve; DAP , anterior posterior diameter; diast , diastole; PMPM , posteromedial papillary muscle; syst , systole. Figure 11.23, Annular changes. Schematic shows three measures of the mitral annulus and how they change during the cardiac cycle. The black dashed arrows represent the annular dimensions at end-diastole. During ventricular systole the anteroposterior distance (DAPsyst) decreases and moves laterally (ie, away from the left ventricular outflow tract [LVOT]). The intercommissural line (DAL-PM) decreases and moves posterior. The annular height increases and moves the anterior portion of the annulus away from the LVOT. Altogether, the mitral annular area declines during ventricular systole and moves away from the LVOT to allow for unhindered systolic outflow. Ah , Annular height; ALPM , anterolateral papillary muscle; AV , aortic valve; DAP , anterior posterior diameter; diast , diastole; PMPM , posteromedial papillary muscle; syst , systole.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/TransesophagealEchocardiographyAdvancedEchocardiographyConcepts/22_3s20B9780323829243000119.jpg)
It is important to know the details of the surgical procedure including what kind of valve (bioprosthetic vs mechanical) was placed or how the valve was repaired. For valve repairs, information regarding annulus type (flat vs saddle-shaped, full vs partial), and leaflet resection, repair, or augmentation helps echocardiographers understand what they see during imaging. , , ,
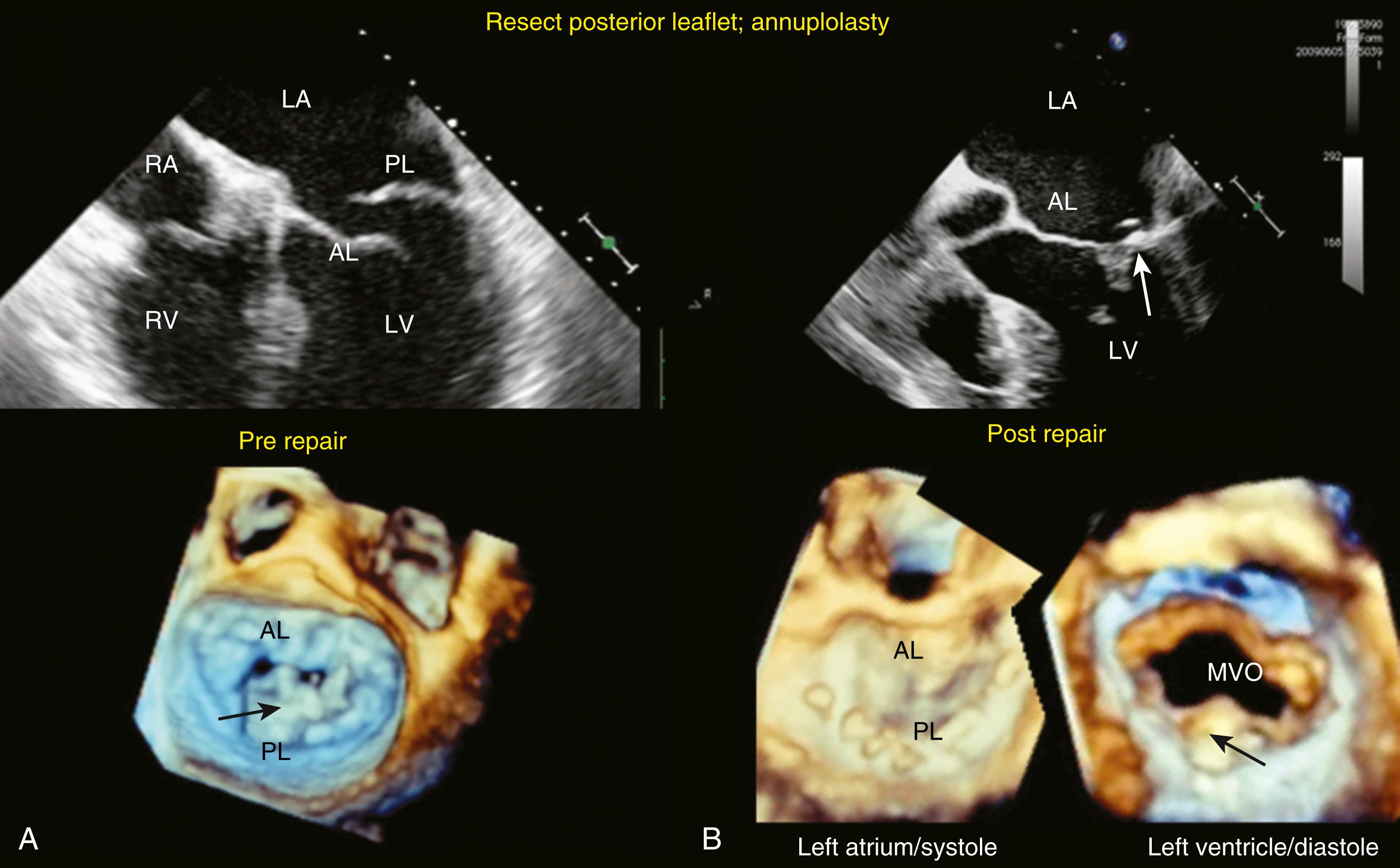
The repaired valve relies mostly on a normally mobile anterior leaflet that is adequate in size to cover the coapting area, while the posterior leaflets provide a coapting surface ( Figs. 11.24 and 11.25 ). , The anterior leaflet is generally preserved or, when necessary, repaired, while the abnormal posterior leaflet scallops are either resected or repaired. Variations of the latter include repair (neochordae) or folding of the posterior leaflet to provide a larger coapting surface and a larger valve area (see Figs. 11.24 and 11.25 ). The Alfieri repair is currently a much less common alternative repair procedure that reduces or prevents prolapse. , It is classically performed centrally, resulting in a figure-of-eight opening; however, it may also be performed peripherally along the commissures to form a dual figure-of-eight orifice in diastole ( Fig. 11.26 ). The relatively recent application and expanding role of the MitraClip procedure, a percutaneous “Alfieri” procedure, in which a metal clip apposing two opposing leaflet edges provides options for high-risk cases as a solution for high-risk surgical cases ( Fig. 11.27 ). For all echocardiographic assessments, recording of the patient’s hemodynamic status improves the perspective at the time of evaluation.
![Figure 11.24, Before and after valve repair. The folding leaflet plasty is a repair technique that reduces posterior leaflet (PL) height without resecting the leaflet. Suture is placed on the edge of the prolapsing scallop, passed below (left ventricle [LV] side) the leaflet and then through the respective annular segment. The leaflet is pulled down and folded over itself to produce a significant coapting surface for the anterior leaflet. (A) Patient with a flail PL. (B) and (C) Same patient postrepair image obtained during LV systole (syst) and diastole (diast) , respectively. (B) shows the coaptation zone (yellow arrow) created by the folding plasty, which can be seen in (C) ( blue surround that is pointed to by the yellow arrow ). (D) and (E) Path of repair suture (blue arrow) , until (F) leaflet is folded (blue box). (G) Annuloplasty ring supporting the repair. The lower series of images are three-dimensional views of the repair. The black arrow in the first image on the left shows the flail PL and torn chord (thin black arrow) , while the next three show the repair with the folded PL highlighted by the black arrows . AL , Anterior leaflet; LA , left atrium. Figure 11.24, Before and after valve repair. The folding leaflet plasty is a repair technique that reduces posterior leaflet (PL) height without resecting the leaflet. Suture is placed on the edge of the prolapsing scallop, passed below (left ventricle [LV] side) the leaflet and then through the respective annular segment. The leaflet is pulled down and folded over itself to produce a significant coapting surface for the anterior leaflet. (A) Patient with a flail PL. (B) and (C) Same patient postrepair image obtained during LV systole (syst) and diastole (diast) , respectively. (B) shows the coaptation zone (yellow arrow) created by the folding plasty, which can be seen in (C) ( blue surround that is pointed to by the yellow arrow ). (D) and (E) Path of repair suture (blue arrow) , until (F) leaflet is folded (blue box). (G) Annuloplasty ring supporting the repair. The lower series of images are three-dimensional views of the repair. The black arrow in the first image on the left shows the flail PL and torn chord (thin black arrow) , while the next three show the repair with the folded PL highlighted by the black arrows . AL , Anterior leaflet; LA , left atrium.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/TransesophagealEchocardiographyAdvancedEchocardiographyConcepts/23_3s20B9780323829243000119.jpg)
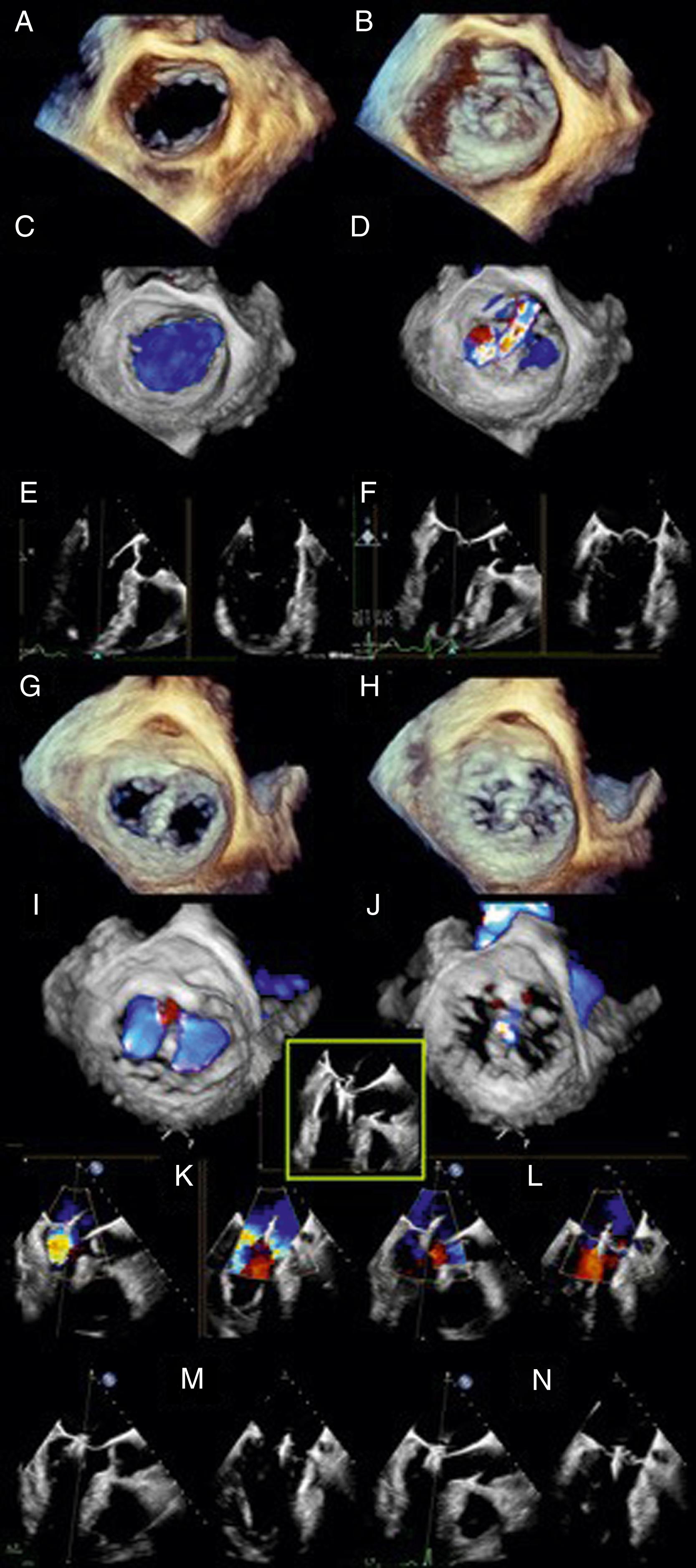
![Figure 11.26, Alfieri repair. (A) and (B) The case of a bileaflet prolapse or myxomatous disease is used to demonstrate the Alfieri repair, in which a running suture ( small black arrow in [D] and [F] and middle schematic [E]) is classically placed centrally but may also be placed peripherally. The centrally placed suture creates a figure-of-eight orifice as compared to (A–C) the single orifice. (D–F) After Alfieri repair. Three-dimensional views from (D) the left atrial and (F) ventricular perspectives show a figure-of-eight orifice. (E) Two-dimensional image shows the color Doppler display of two separate jets. AL , Anterior leaflet; LA , left atrium; LV , left ventricle; PL , posterior leaflet; RA , right atrium; RV , right ventricle. Figure 11.26, Alfieri repair. (A) and (B) The case of a bileaflet prolapse or myxomatous disease is used to demonstrate the Alfieri repair, in which a running suture ( small black arrow in [D] and [F] and middle schematic [E]) is classically placed centrally but may also be placed peripherally. The centrally placed suture creates a figure-of-eight orifice as compared to (A–C) the single orifice. (D–F) After Alfieri repair. Three-dimensional views from (D) the left atrial and (F) ventricular perspectives show a figure-of-eight orifice. (E) Two-dimensional image shows the color Doppler display of two separate jets. AL , Anterior leaflet; LA , left atrium; LV , left ventricle; PL , posterior leaflet; RA , right atrium; RV , right ventricle.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/TransesophagealEchocardiographyAdvancedEchocardiographyConcepts/25_3s20B9780323829243000119.jpg)
The 2D and Doppler examinations are accomplished from a number of echocardiographic windows, with emphasis on midesophageal (ME) windows (0–150 degrees), to analyze the native valve before surgery, and the repaired or prosthetic valve after ( Figs. 11.26 , 11.28–11.30 ). , , , Transgastric (TG) views complement imaging of the valve, allowing a short-axis perspective, and are also useful to visualize and interrogate, using Doppler, the LVOT ( Figs. 11.30–11.32 ).
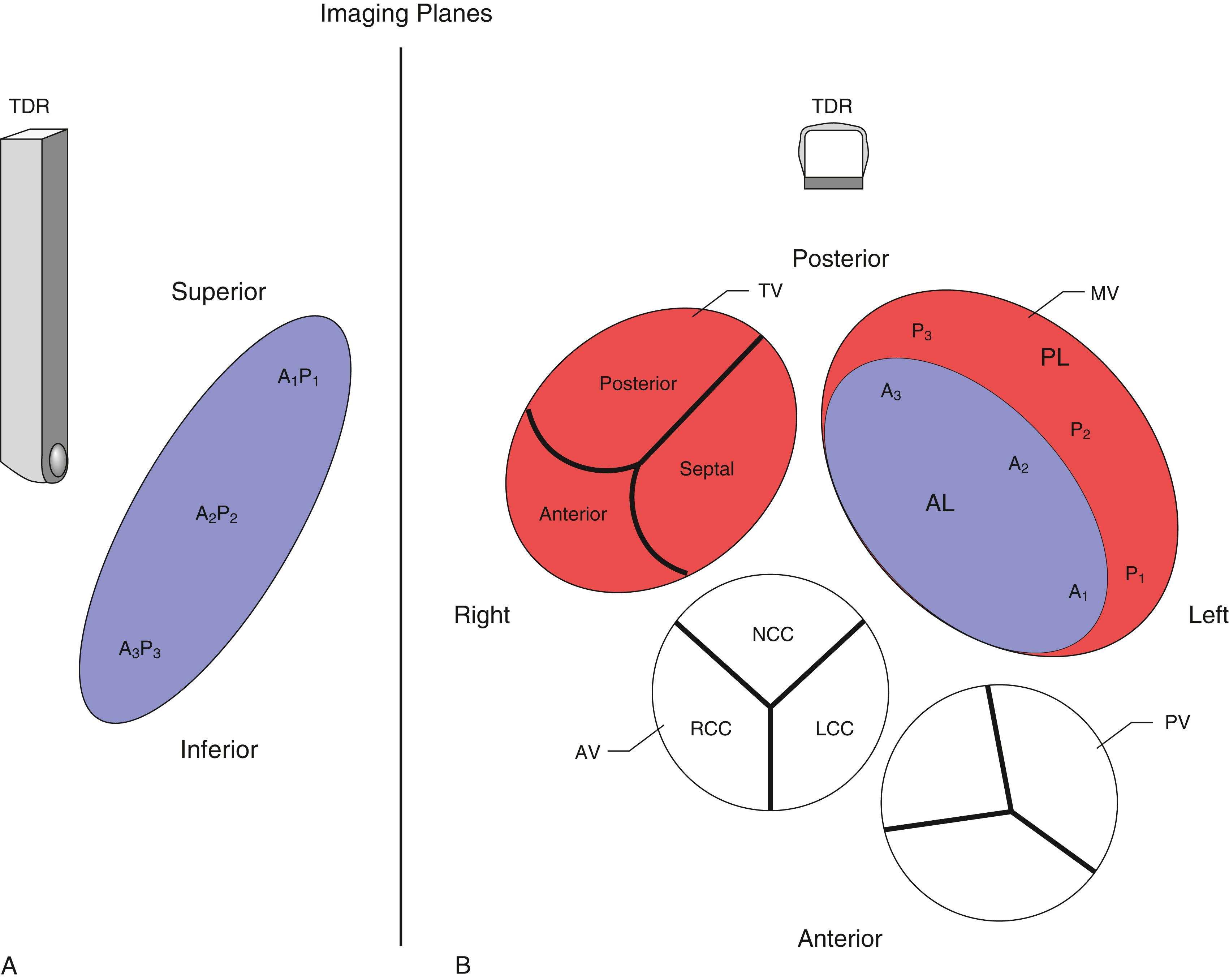
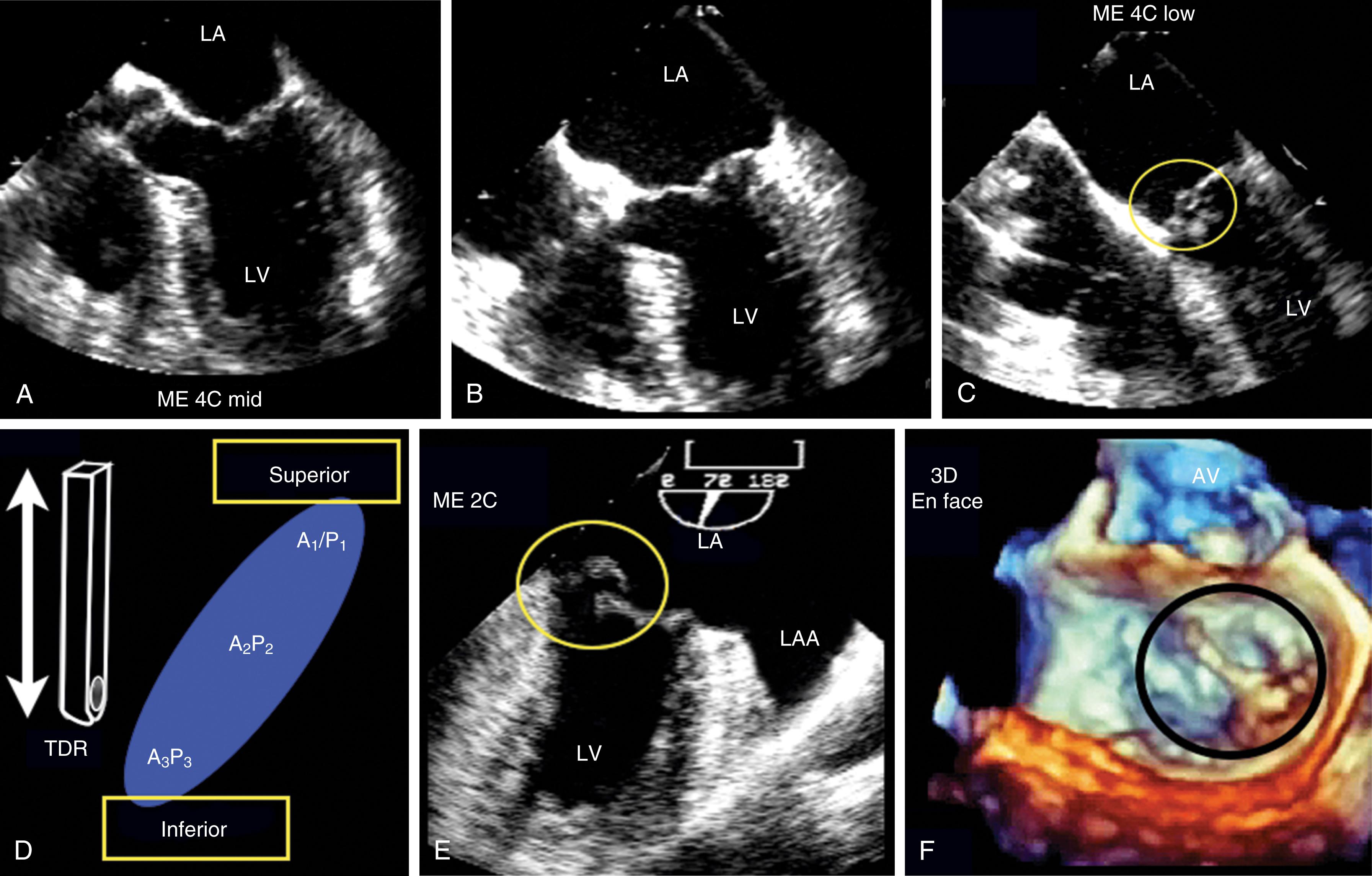
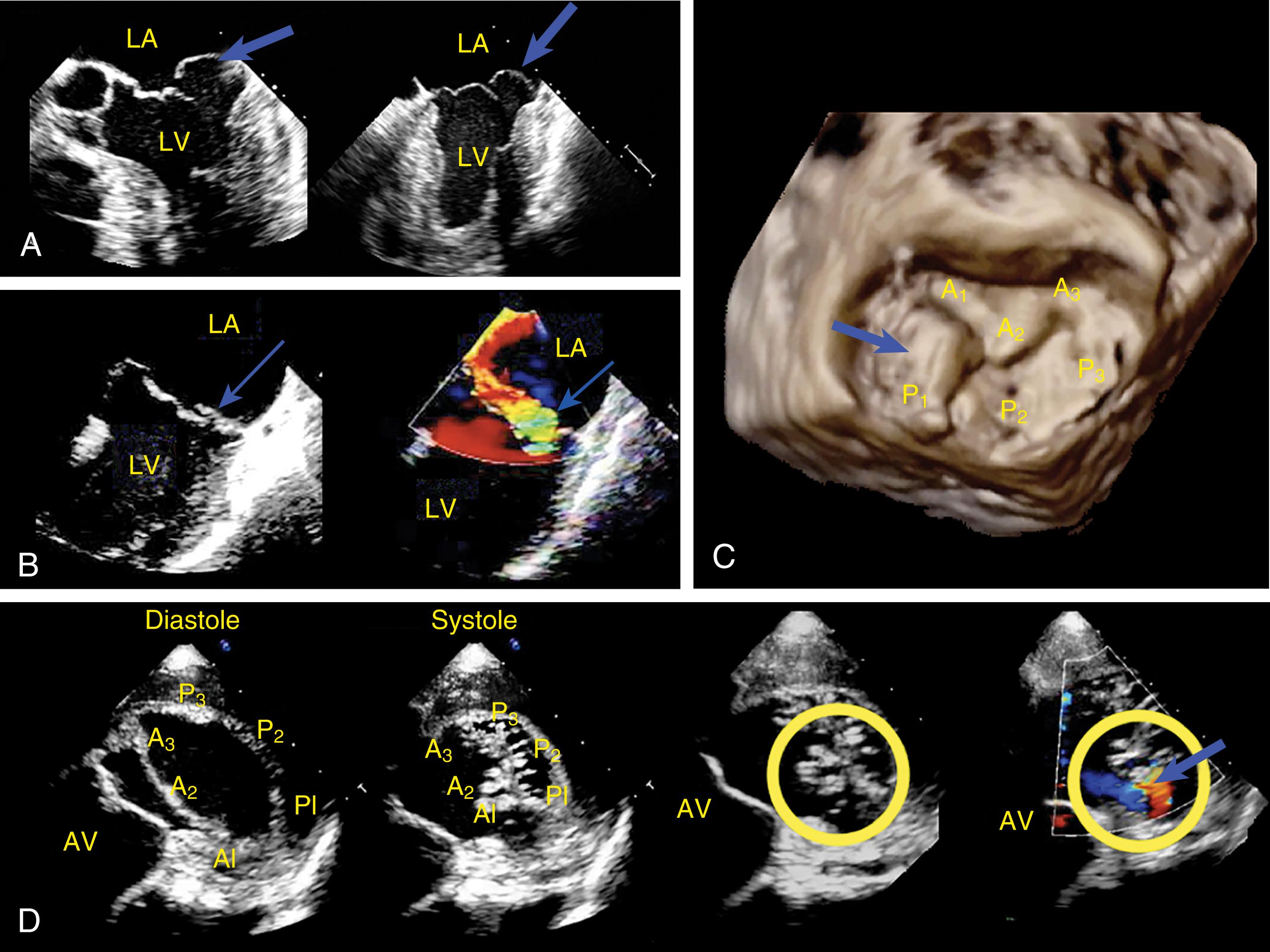
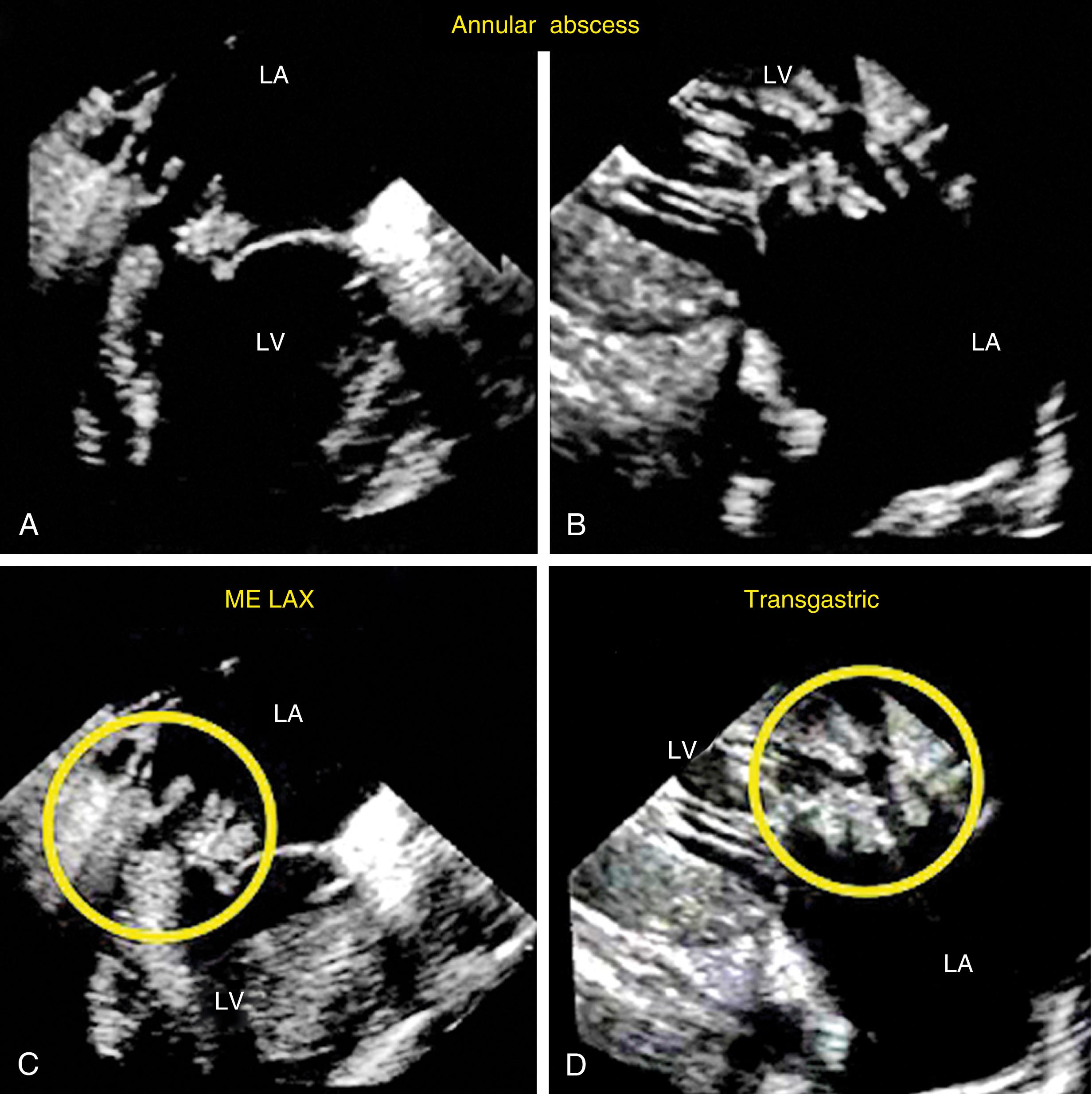
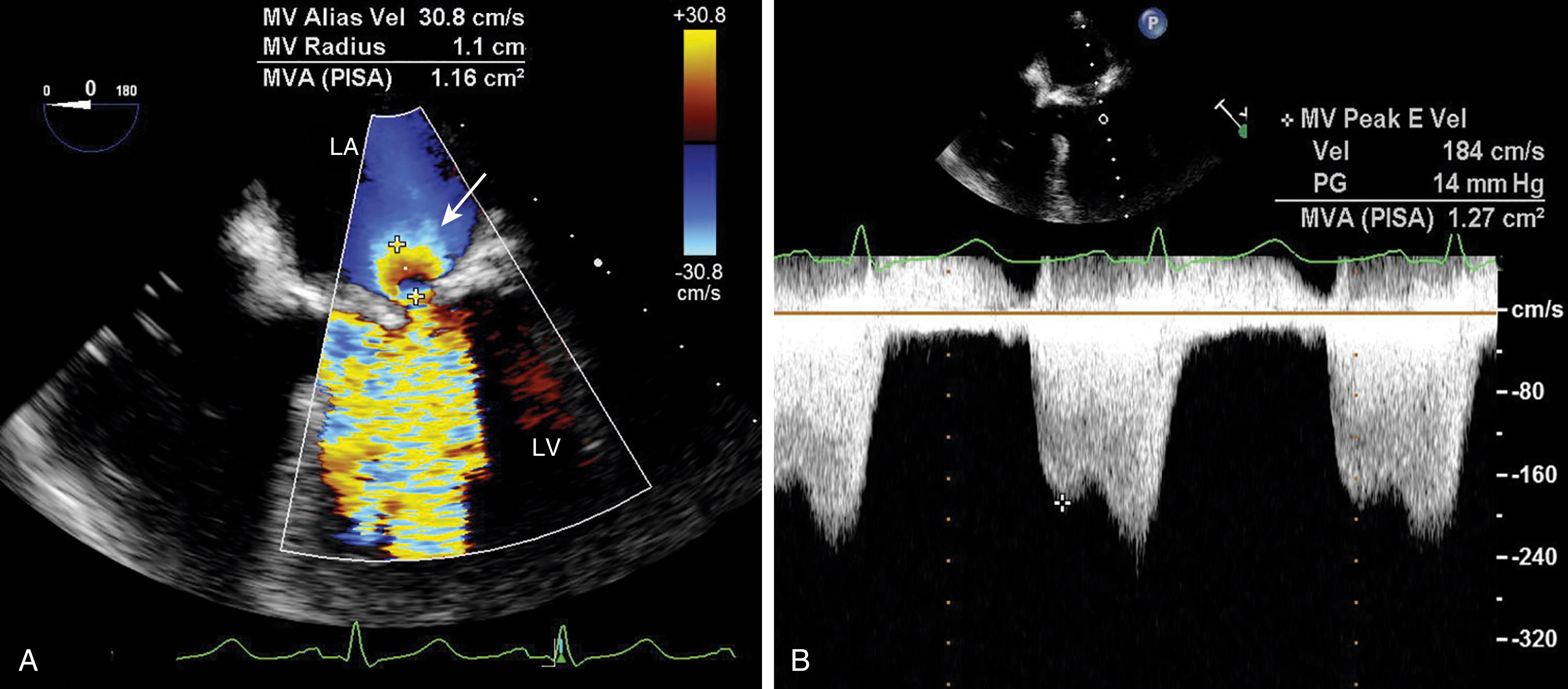
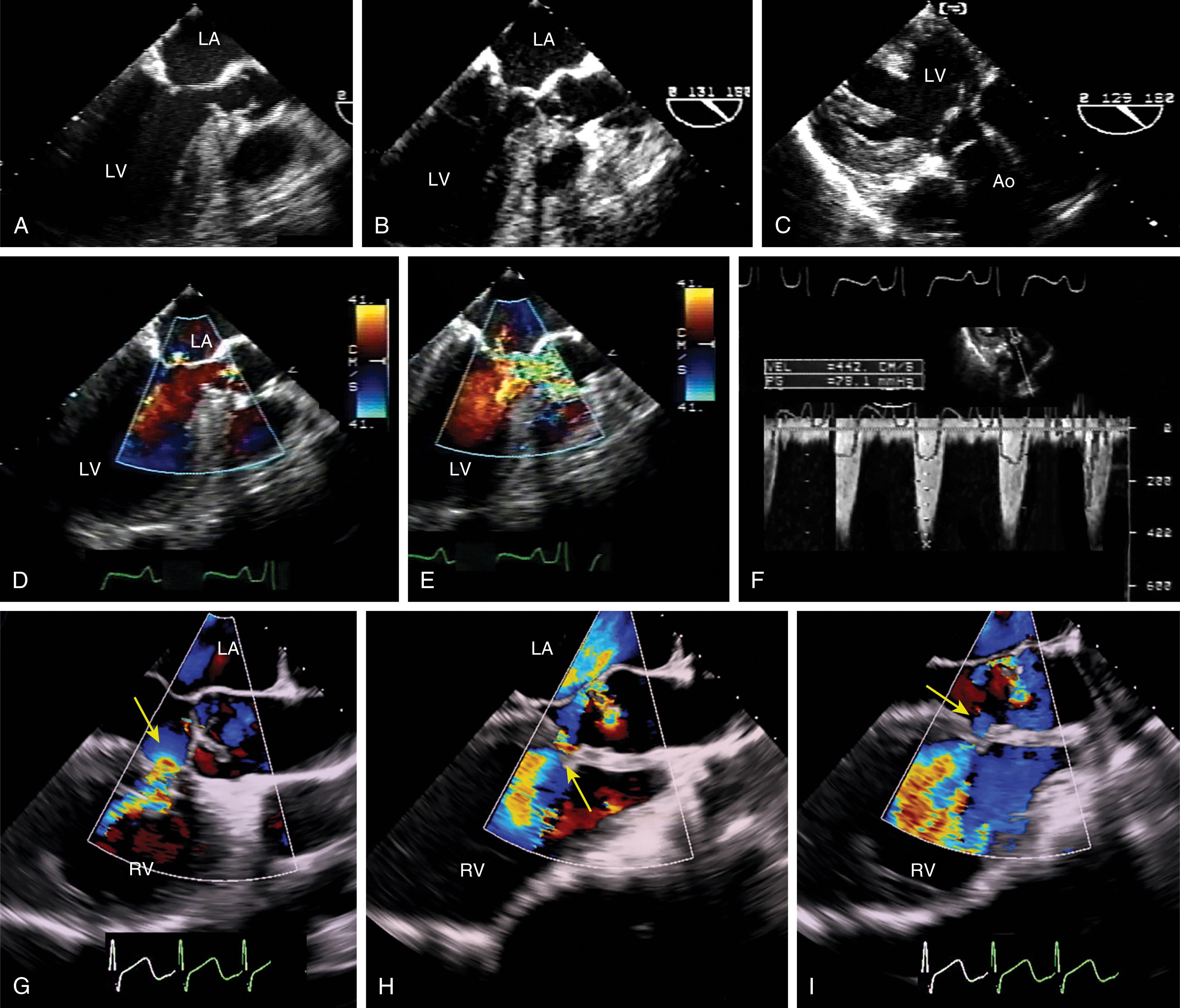
Evaluation includes assessment of thickness, and any associated mobile structures, the latter of which may include chordae, masses, fractured calcium deposits, and suture materials. Imaging of the subvalvular apparatus identifies thickening, calcifications, mobile or flail of the chordae and/or papillary muscles. The valve should appear stable (i.e., no rocking) and secured in the surrounding tissues. Hypoechoic spaces or lucency of the surrounding tissues may suggest infection or abscess, dehiscence, or rupture ( Fig. 11.33 ).
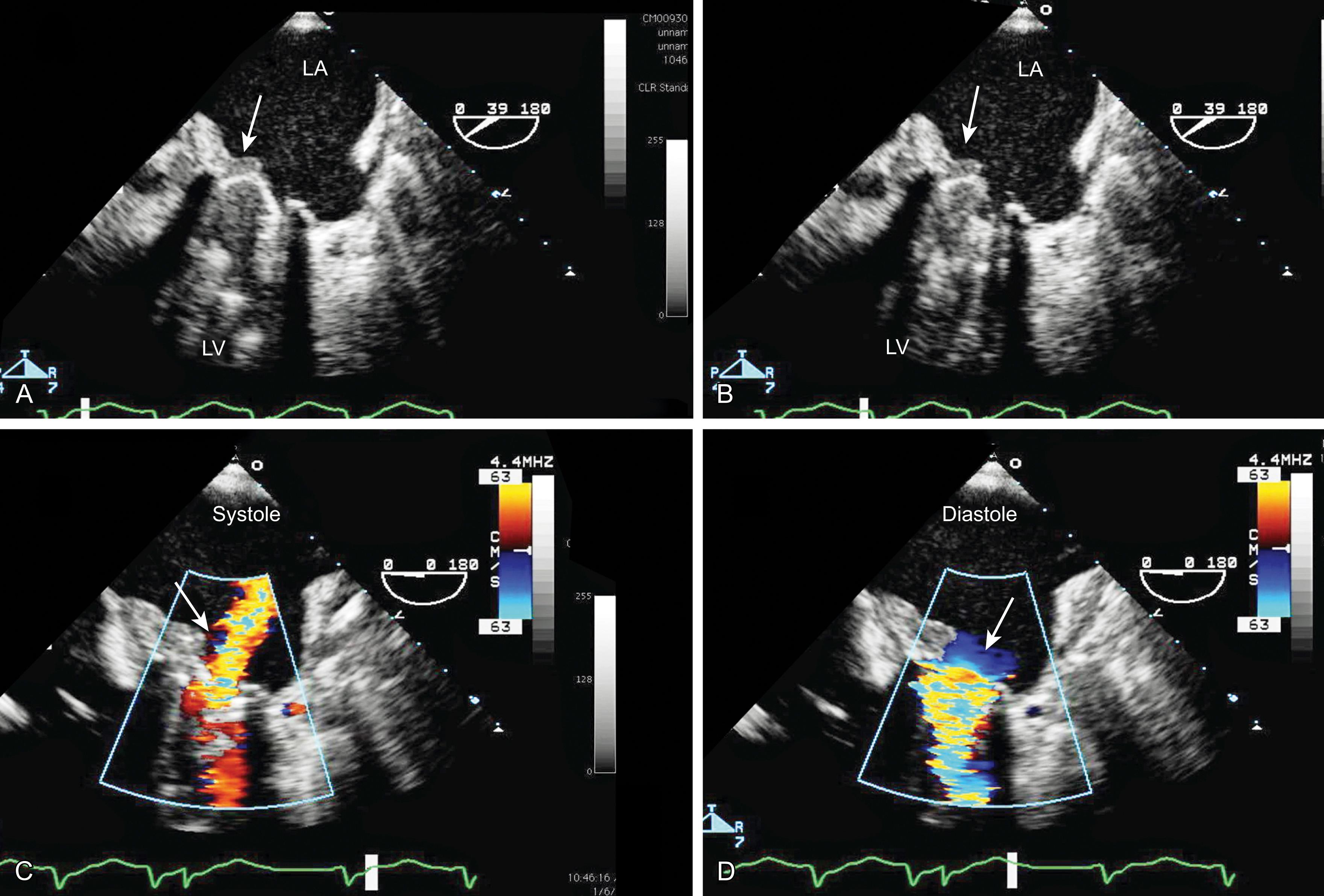
For mechanical prosthetic valves, imaging perpendicular to the leaflets allows seeing the opening and closing of the leaflet edges while parallel imaging facilitates viewing of signature regurgitant jets ( Figs. 11.13, 11.16, 11.34–11.37 ). Specific concerns regarding bioprosthetic valves include positioning of the struts in the LVOT potentially causing obstruction to systolic outflow ( Fig. 11.38 ).
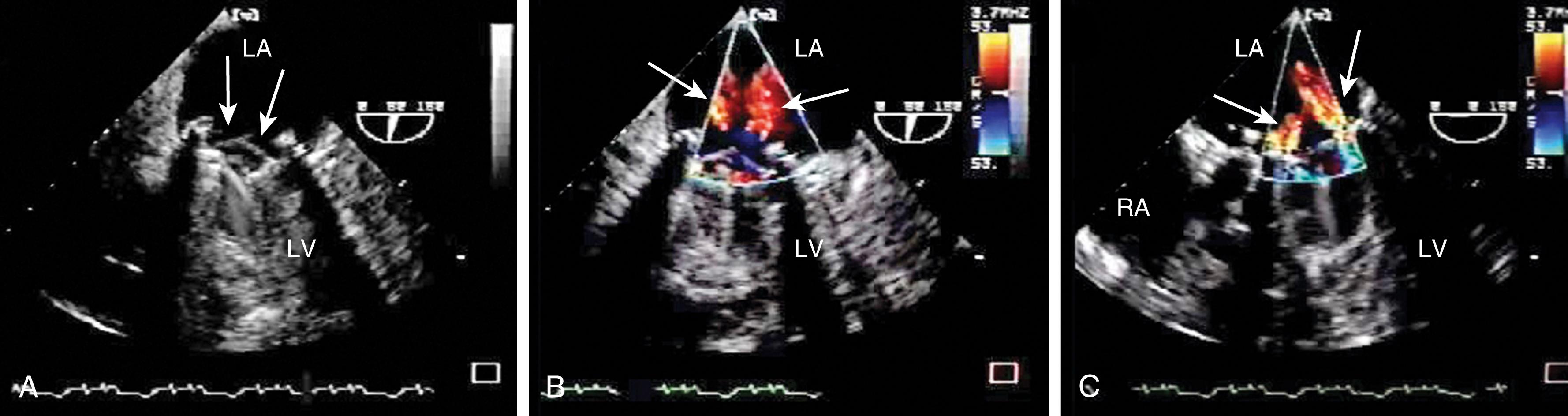
The Doppler examination assesses, both qualitatively and quantitatively, the presence, direction, and width of normal and abnormal blood flows of the native, prosthetic, and repaired valves. In general, abnormal flows may appear ‘turbulent’ in that they exceed the Nyquist limit or aliasing velocity. The ME windows offer the best proximity of the valve to the transducer aligning blood flow and the ultrasound beam. Although qualitative CFD assessments may suggest elevated velocities based on the presence of aliasing, this may or may not indicate abnormal flow. Further quantitative analysis should be performed to clarify the flow.
Different prosthetic valves have signature flow profiles. Bioprosthetic valves allow a large central forward flow and, for some prostheses, a small central regurgitant jet (see Figs. 11.34–11.37 ). Mechanical valves consist of two or three forward jets and one or two small regurgitant jets (see Fig. 11.37 ).
Flow across the repaired valve depends on the repair technique. Typically, a large singular jet directed toward the posterior and lateral walls toward the apex followed by flow toward the LVOT along the anterior and septal wall is seen, which is not dissimilar to the normal native valve (see Fig. 11.21 ). , Alternatively, a central Alfieri repair results in a figure-of-eight orifice and provides two forward moving jets of flow (see Fig. 11.26 ).
MR occurs as a result of dysfunction of one or more of the components of the mitral apparatus ( Table 11.4 ). MR can be grouped into three types: those with normal leaflet mobility (type 1) (see Fig. 10.79 ; ), those with excessive leaflet mobility (type 2) (see Fig. 10.75 ; ), or those with restrictive leaflet mobility (type 3). , The latter group can be further distinguished based on the timing of the cardiac cycle to which leaflet mobility is impaired. Type 3a involves restriction during the diastolic phase or during both systole and diastole as seen in rheumatic disease with involvement of leaflet and chordae (see Fig. 10.76 ; ). Type 3b involves restriction during the systolic phase of the cardiac cycle as seen in patients with functional MR as a result of either myocardial ischemia/infarction or cardiomyopathy during which tethering and/or remodeling has occurred (see Fig. 10.77 ; ). The types of MR are summarized in Fig. 10.78 .
| Mild | Moderate | Severe | |
|---|---|---|---|
| Angiographic grade | 1+ | 2+ | 3–4+ |
| Color Doppler jet area (cm 2 ) | <4 or <25% of LA area | >7 or >50% of LA area | |
| Vena contracta width (cm) | <0.3 | 0.3–0.69 | >0.7 |
| Regurgitant orifice area (cm 2 ) a | <0.20 | 0.20–0.49 | ≥0.50 |
| Regurgitant volume (mL) a | <30 | 30–60 | >60 |
| Regurgitant fraction (%) a | <30 | 30–50 | >50 |
| Pulmonary venous flow | Systolic dominance | Systolic blunting | Systolic flow reversal |
| Secondary findings | LAE, LVE, LVSD, PHTN | ||
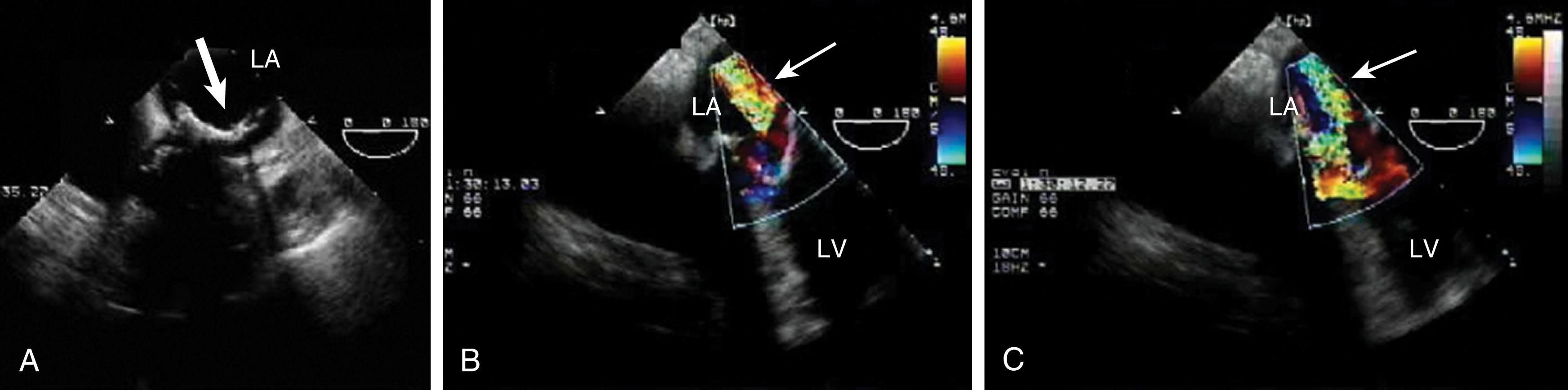
Type 1 MR can be seen in the presence of annular dilation or leaflet perforation. In the former, the annulus is more likely to dilate along the posterior and lateral annular areas as these are mostly muscle and membranous tissues. As the annulus dilates, it becomes more circular and flatter. The regurgitant jet may be centrally or eccentrically directed. The greater the amount of annular dilation (diameter >5 cm) and dysfunction, the less likely that valve repair will provide long-term freedom from valve replacement. Leaflet perforation occurs in the presence of endocarditis and is more commonly found on the anterior leaflet.
For type 2 MR, the leaflets are described by their systolic excursion relative to the annular plane. Prolapse is defined, during two-dimensional imaging from the long-axis widow, as a greater than 2-mm excursion above the annular plane with the leaflet tip pointing down. , Flail is similarly described except with the leaflet tip/edge pointing above the annular plane. Excessive leaflet mobility is often accompanied by redundant or torn chordae or, in the case of myocardial infarction (MI) or trauma, a torn or ruptured papillary muscle. Regurgitation caused by degenerative or myxomatous mitral disease is associated with either prolapse or flail of an isolated scallop or multiple scallops involving one or both leaflets. Most commonly, the posterior leaflet is involved with the middle scallop (P 2 ) being involved in more than 40% to 50% of the cases. , , Next in incidence, the middle scallop of the anterior leaflet (A 2 ) is involved. Both leaflets are involved in up to 40% of cases. The delineation of the scallops is important in determining the appropriate surgical procedure and whether a repair is feasible.
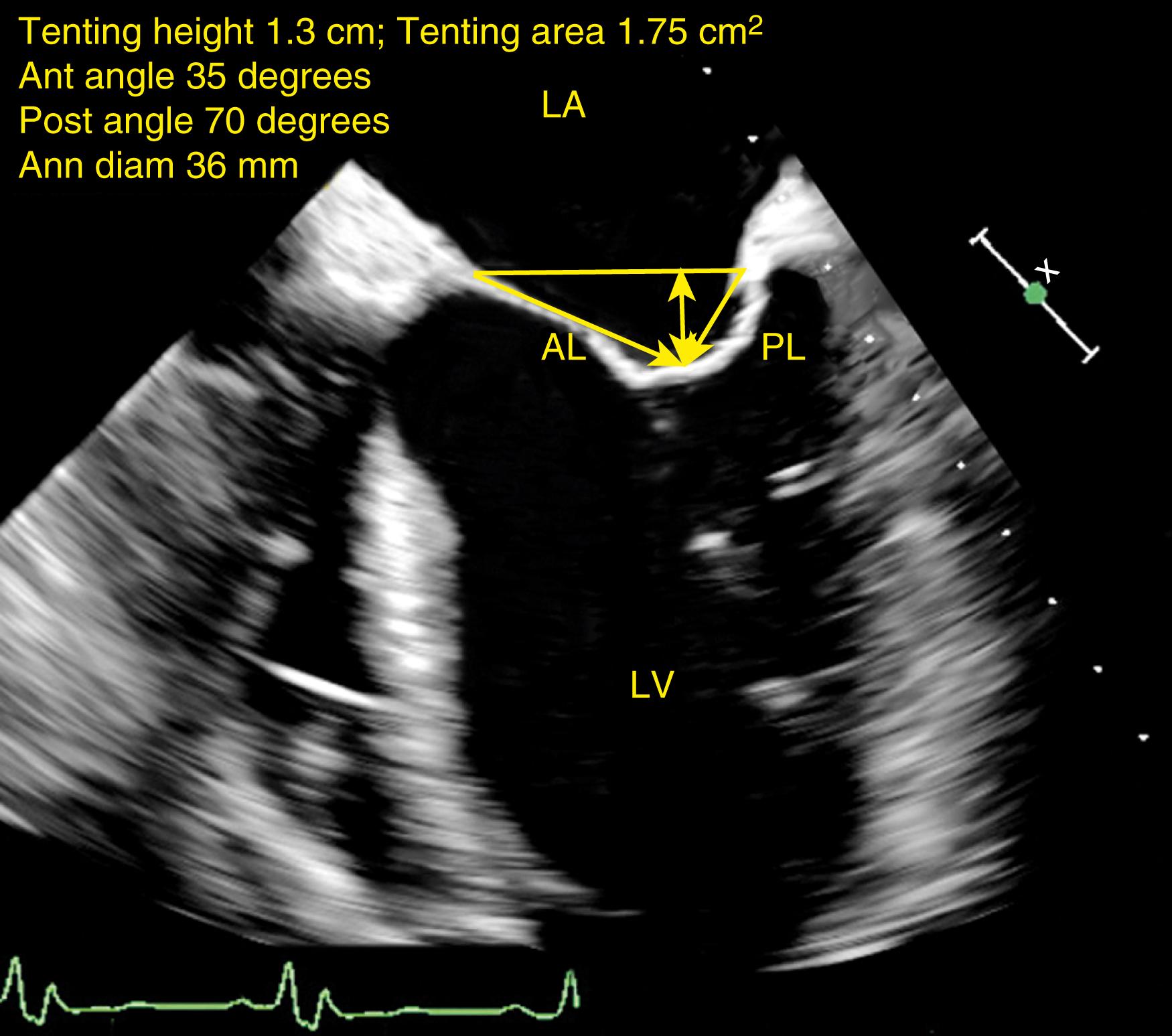
Type 3b MR seen with dilated cardiomyopathy (DCM) and/or ischemic cardiomyopathy may include normal appearing leaflets; however, reduced systolic return is decreased as a result of tethering. Quantitative assessments help to determine the presence and extent of tethering and may predict outcome after valve repair. These assessments include tethering height and area, and angles of coaptation ( Figs. 11.39 and 11.40 ). Tethering heights greater than 1 cm or tethering areas greater than 1.6 cm 2 are suggestive of severe long-standing changes with greater left ventricular dysfunction, dilation, and remodeling ( Figs. 11.40 and 11.41 ). With greater chronicity, leaflet compliance and mobility decline as well.
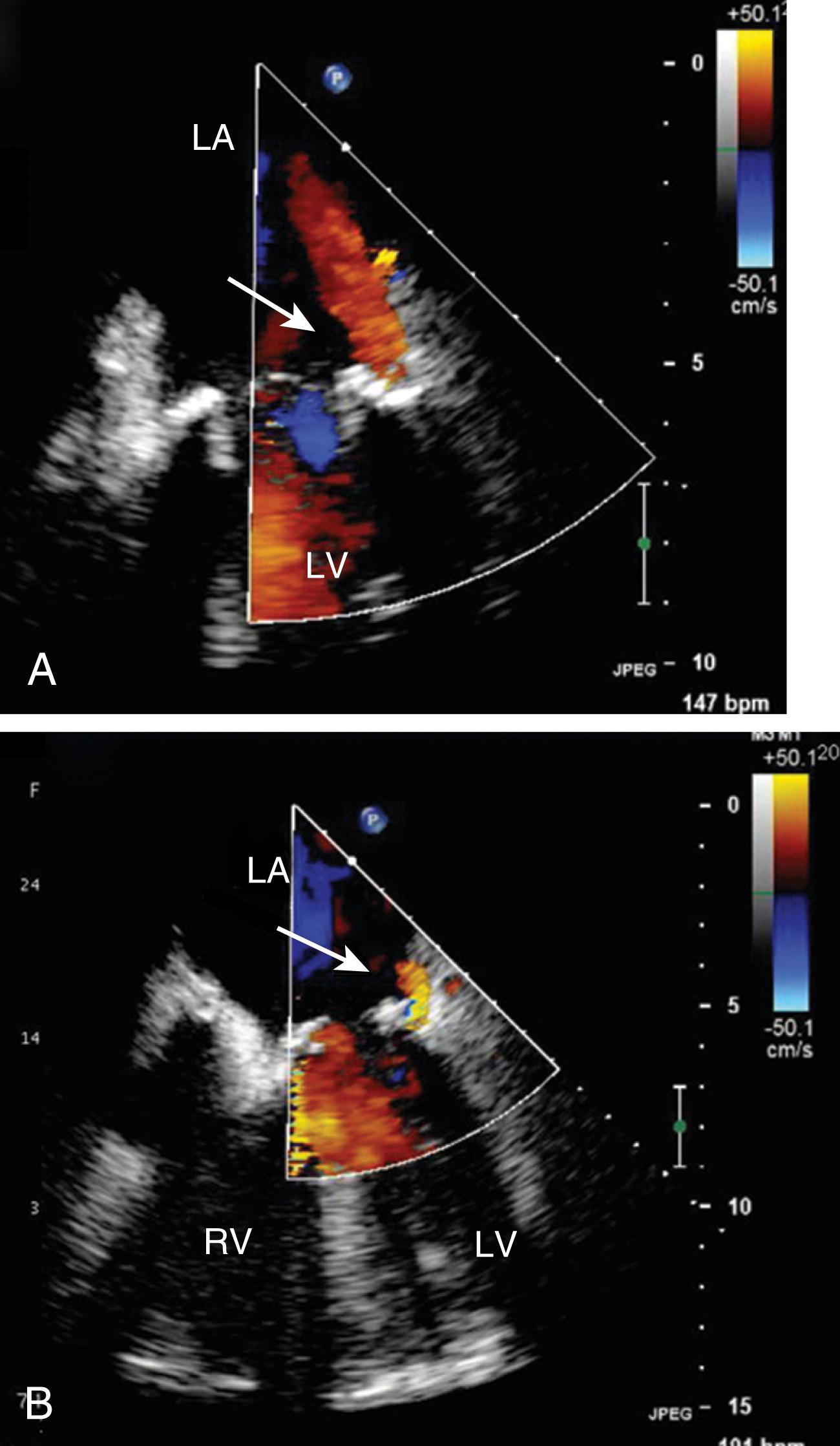
Restriction of abnormal leaflets (type 3a) is seen with rheumatic valves or after radiation therapy. In these cases, the valve leaflets, with or without subvalvular tissues, may be thickened and/or shortened. Although rheumatic leaflets (anterior leaflet) may appear like a hockey stick during diastole, this finding is not necessary for the diagnosis to be considered.
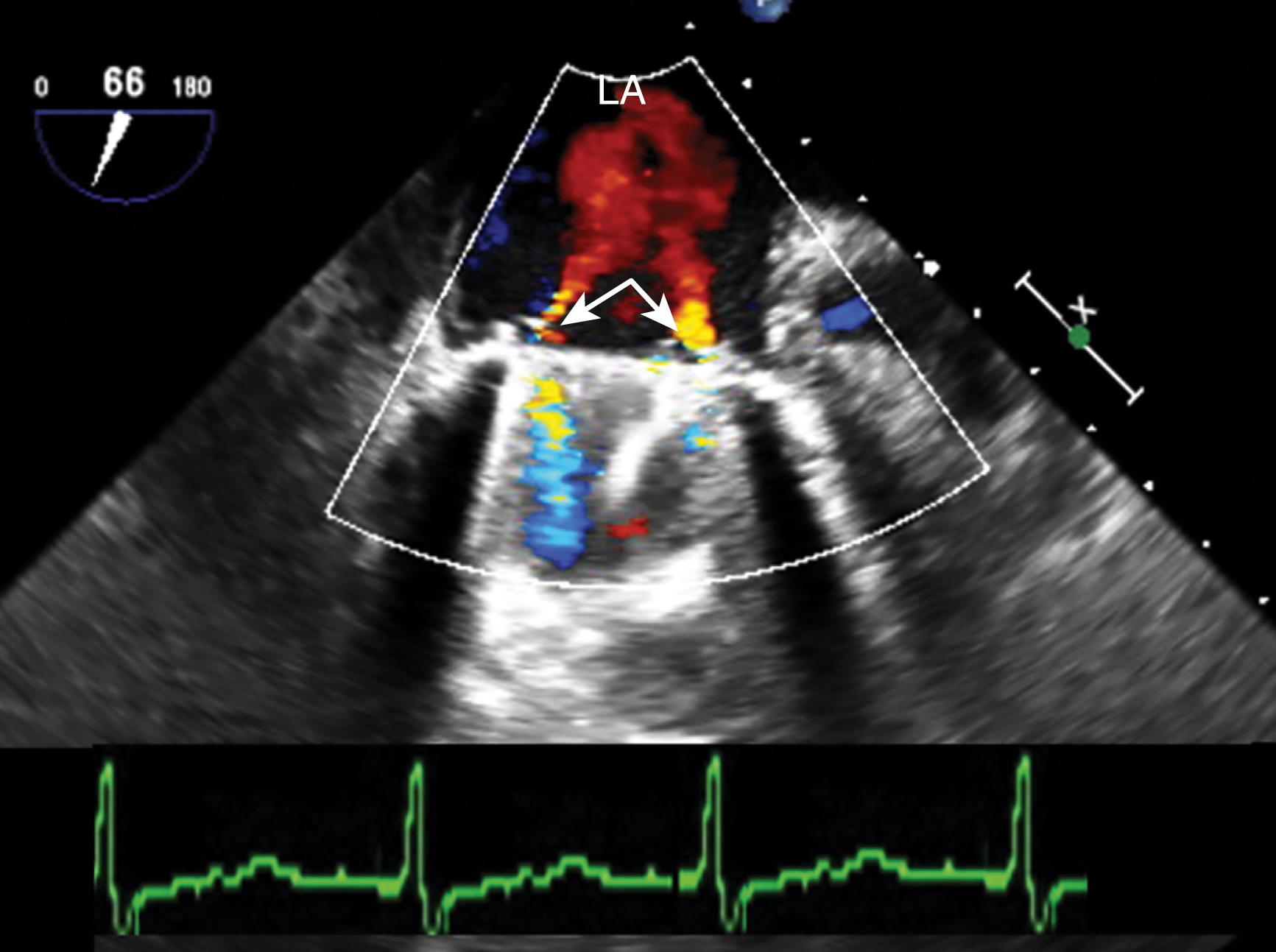
Prosthetic valves are associated with a normal signature of expected regurgitant jets and should be differentiated from abnormal flows (see Figs. 11.16, 11.31, 11.35–11.37 ). Normal or nonproblematic regurgitant jets are less than 3 mm in width and do not extend far from the valve. All paravalvular regurgitant jets are considered abnormal. However, regurgitant jets less than 3 mm in width are not likely to pose a long-term problem, especially in the absence of clear disease ( Fig. 11.42 ). Regurgitant jets greater than 3 mm in width and/or eccentrically directed away from the middle of the valve carry a greater risk of MR progression and/or reintervention ( Fig. 11.43 ). These abnormal flows can result from a number of causes, including leaflet restriction or excess mobility, endocarditis, or annular dehiscence ( Figs. 11.44–11.46 ).
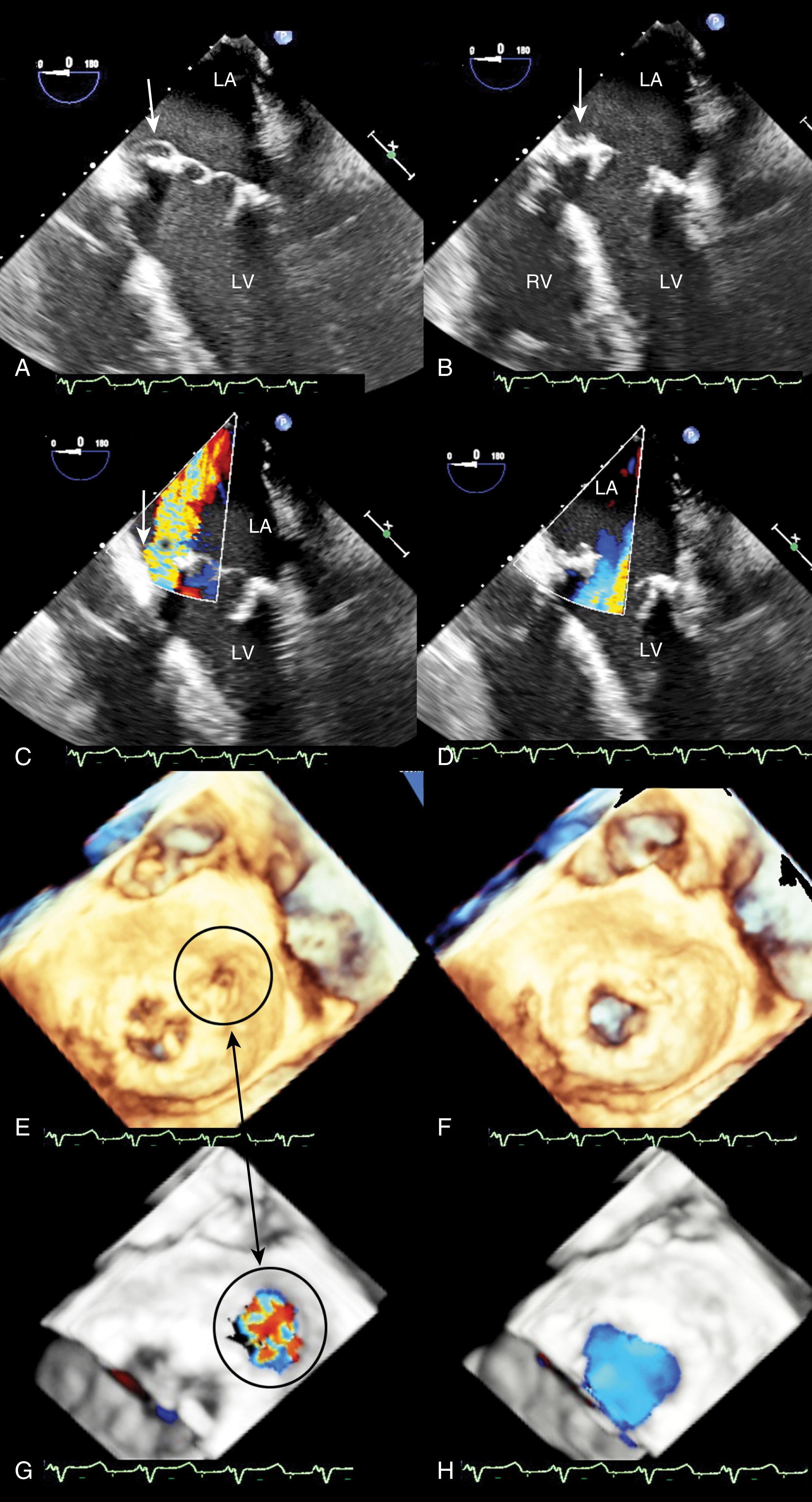
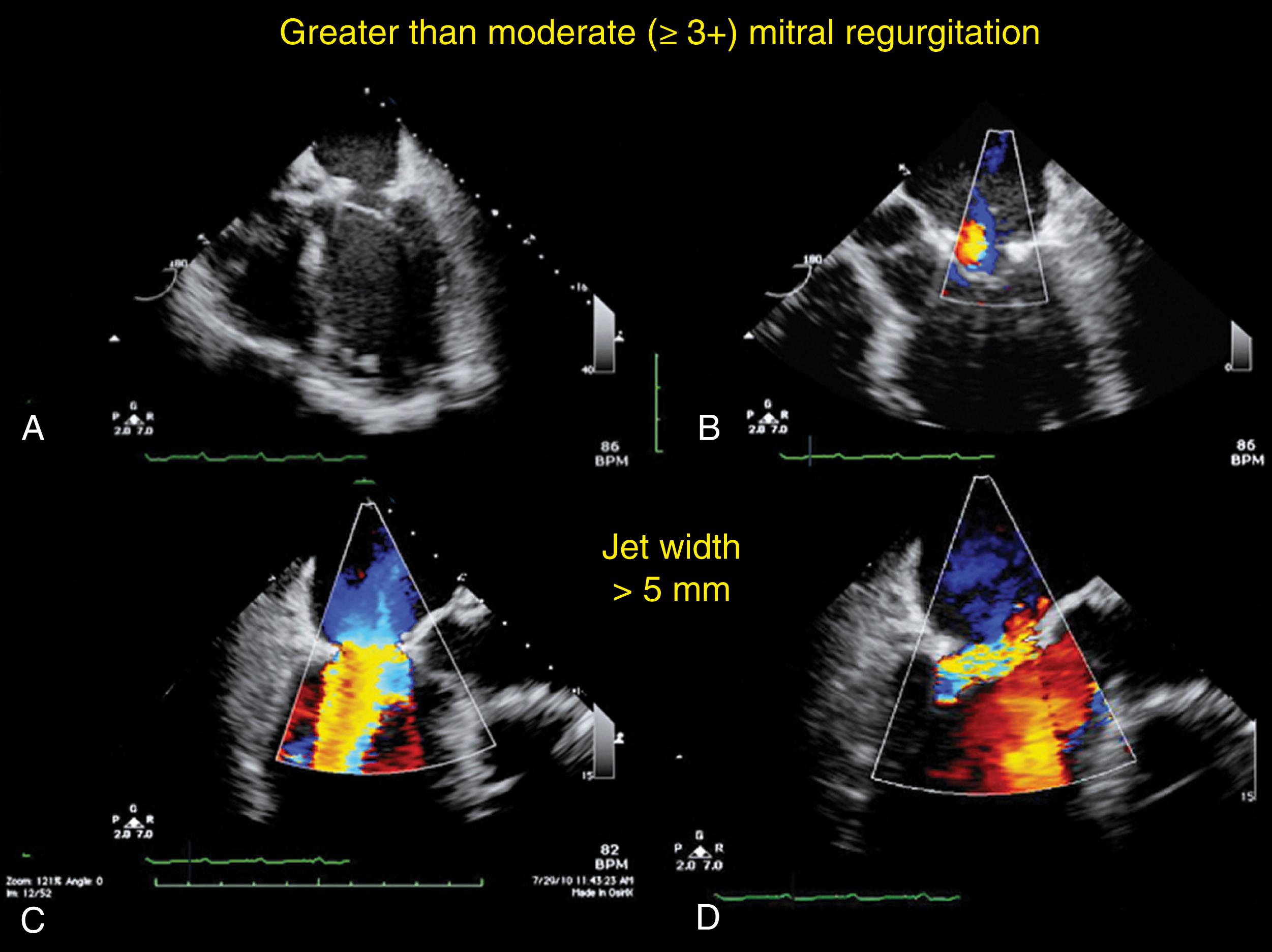
Repair of the MV generally is applied to regurgitant valves. , Although the goal is to eliminate regurgitation, mild or less is acceptable, while more than mild increases the risk of MR progression and reoperation (see Fig. 11.46 ). The location and direction of the regurgitant jet depends on the pre-repair dysfunction and the repair technique. Determining the cause of MR after repair is important to decide whether additional repair is feasible or if replacement is warranted.
MR is also described by its acuity, which is related to the level of compensation or decompensation and clinical presentation. In the acute setting, LA and LV compliances have not adapted, resulting in elevated chamber pressures, pulmonary hypertension, and pulmonary edema. RV dysfunction follows as a result. Chronic MR is initially compensated for and includes ventricular hypertrophy and increased EF to maintain forward flow. The increased left ventricular ejection fraction (LVEF) is the result of a lower total resistance as a result of the backward ejection into the pulmonary vascular bed. A normal LVEF in the presence of severe MR is greater than 60%. With continued or worsening MR, ventricular dilation, contractile dysfunction, and increased chamber pressures coincide with clinical decompensation. LA dysfunction, pulmonary hypertension, and, finally, right heart dysfunction occur. Arrhythmias reflect these cardiac changes and decompensation.
Two-dimensional analysis of the native valve helps determine feasibility and technique to repair the valve. For stenotic valves, the examination helps the practitioner determine whether valvuloplasty is a viable option.
Measurements guiding valve repair of the regurgitant valve include annular dimensions, leaflet lengths (anterior leaflet, posterior leaflet), coaptation height (tenting height or area), various angles of coaptation, and relations between the leaflets (coaptation) and the ventricular cavity, or specifically, the ventricular septum (see Figs. 11.39–11.41, and 11.47 ). , Data may help the practitioner predict risk of postrepair complications such as SAM and LVOT obstruction (see Fig. 11.47 ).
Predictors of repair failure differ for patients with functional or ischemic MR versus those with degenerative disease. , , For all patient types, severe annular dilation (>50 mm) represents prolonged MV dysfunction (i.e., end-stage disease) and a greater risk of repair failure. For functional or ischemic MR, the greater the amount of pre-repair tethering such as a tenting height >1.0 cm or area >1.62 cm 2 , and/or a posterolateral coaptation angle greater than 45 degrees is predictive of greater recurrence of moderate or greater MR after 6 months ( Figs. 11.40, 11.41, 11.48 ). Adverse ventricular remodeling predicts repair failure and is indicated by inferior and lateral wall motion abnormalities, dilated LV (LV end-diastolic diameter >65 mm; LV end-systolic diameter >50 mm, or LV end-systolic volume >75 mL/m 2 ), and/or a sphericity index higher than 0.7. Although repair of papillary muscle rupture has been reported, such cases are generally managed with valve replacement.
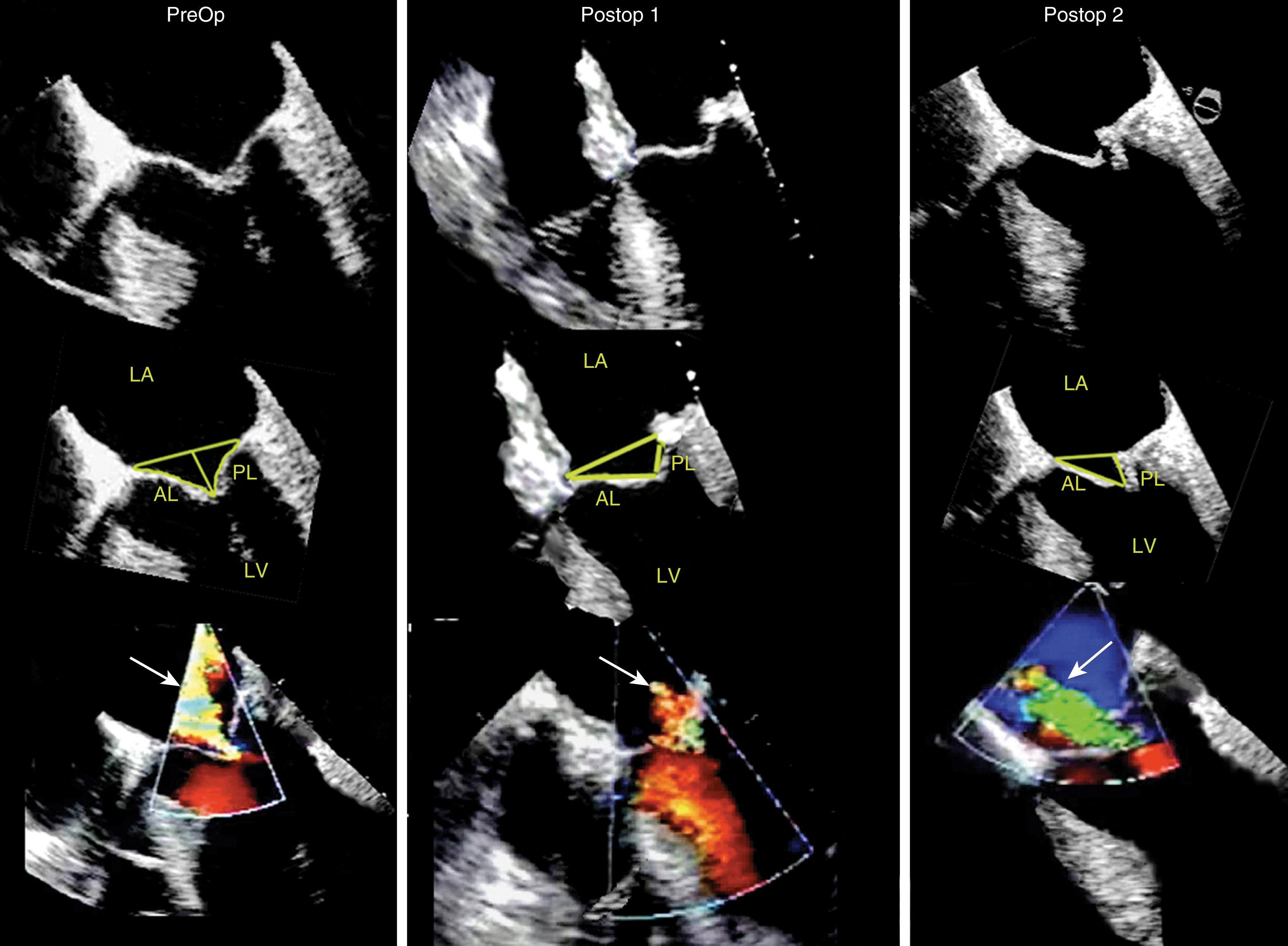
Predictors of a more complicated repair and failure for degenerative valve dysfunction include involvement of multiple scallops, anterior leaflet disease, bileaflet disease, and endocarditis with leaflet destruction. , In addition, severe MR in the presence of a normal annular diameter, or short anterior (<26 mm) and posterior (<10 mm) leaflets present a risk of repair failure and post-repair stenosis. These latter predictors reflect the importance of identifying something repairable and of having adequate leaflet heights prior to and after the repair.
Other predictors of difficult repair or adverse outcome include MR due to rheumatic valvitis, radiation therapy; severe calcification of either the valve, the annulus, and/or the subvalvular apparatus; and atrialization of the coaptation points ( Figs. 11.49 and 11.50 ). , , The former three reflect restricted tissues, an absence of repairable tissues, or the lack of adequate leaflet lengths. The latter reflects an end-stage degenerative state.
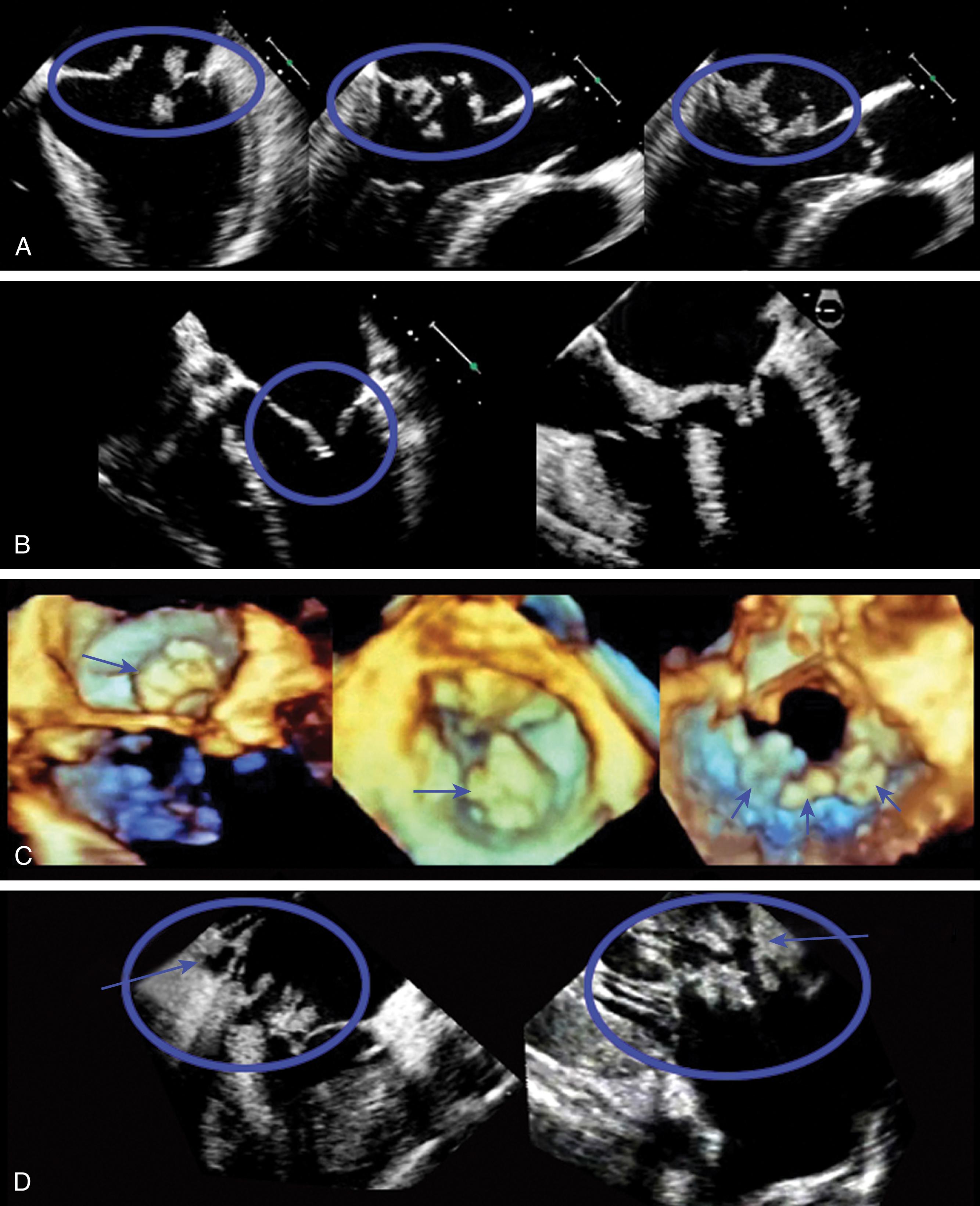
Assessing severity of regurgitant flow in the native or the prosthetic MV is similar (see Table 11.4 ). The examination includes the detection, location, determination of etiology, and severity of MR. A general comprehensive cardiac assessment helps determine etiology of MR, secondary cardiac effects of MR, and therapeutic decisions to manage MR. Contractile dysfunction (LVEF <60%), LA and LV chamber dilation (LV end-systolic volume >70 mL/m 2 ), elevation in left-sided heart pressures, the presence of pulmonary hypertension, and right-sided heart dysfunction suggest decompensated MR.
The stability of the valve determines the integrity of the surrounding tissues. A valve that appears to rock or is surrounded by a hypoechoic between the prosthesis and surrounding tissues might suggest infection or abscess or dehiscence. Dehiscence for an MV prosthesis is more likely to happen along the posterior and lateral annular areas where surgical exposure is less and concern exists over interfering with left circumflex coronary arterial flow (see Fig. 11.45 ).
The Doppler examination identifies regurgitant jets and determines whether or not they are within normal range for a native valve or a normal signature for a prosthetic valve. Eccentric jets are considered abnormal. Pulsed-wave (PW) Doppler can “map” out the valve and has largely been replaced by CFD, which is based on PW. Qualitatively, continuous-wave (CW) Doppler yields a regurgitant profile along the Doppler beam. Significant MR will appear as a dense holosystolic profile.
Severity assessment reflects the regurgitant orifice area (ROA) either indirectly or directly. Measurement of the regurgitant jet area during PW or CFD is neither specific nor sensitive enough to diagnose the severity of MR. Jet areas of eccentric jets underestimate severity, whereas those of central jets may overestimate severity. However, a regurgitant jet that has a large area (>7 cm 2 vs <4 cm 2 ) extending toward the posterior wall (>50%) or covering more than 50% of the LA area is considered moderate or greater. , , , This determination is further supported by the presence of flow convergence just below the regurgitant orifice. Moderate or worse MR is suspected by a PW Doppler profile of the pulmonary vein showing blunting or reversal of the systolic component (see Fig. 10.83 ).
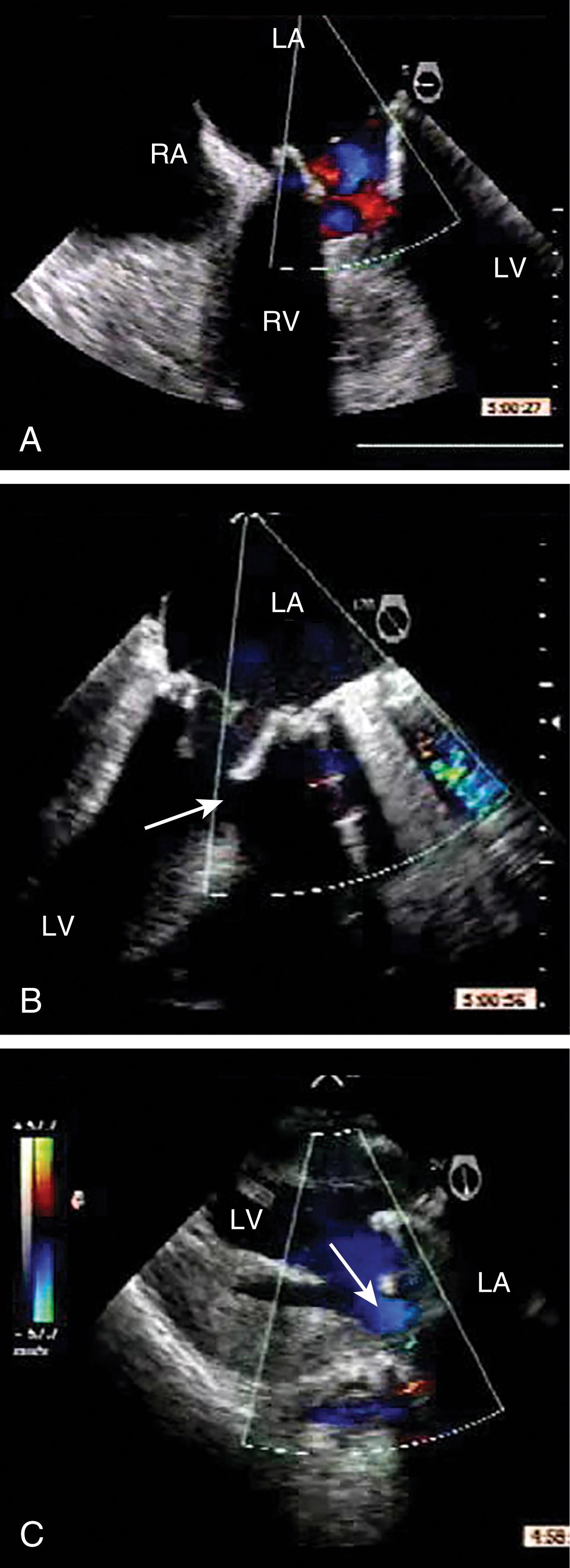
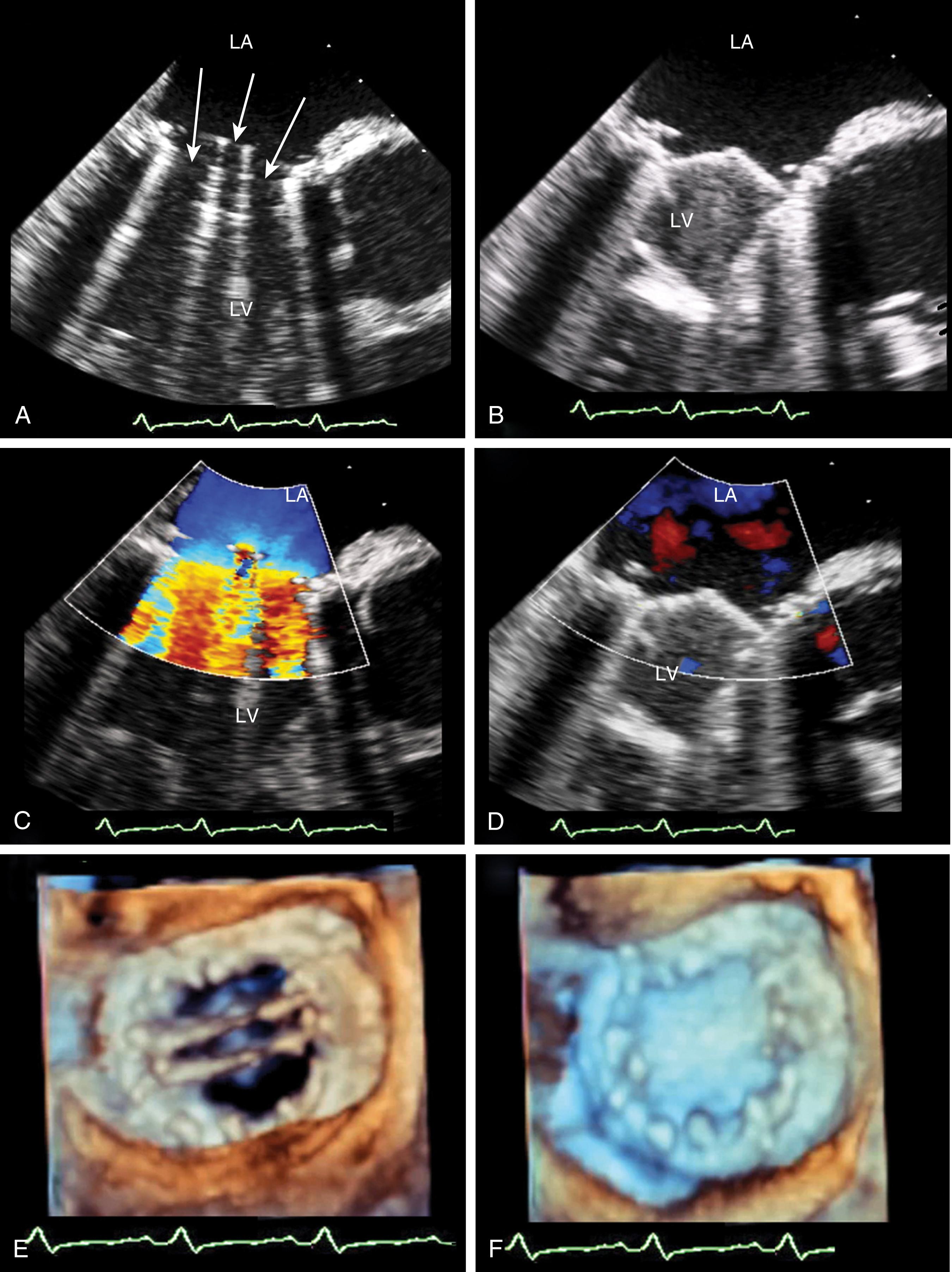
Quantitative assessments include measurements of the VC width, ROA, regurgitant volume, and regurgitant fraction, which are detailed in Chapter 10 . The latter three are obtained using the flow convergence or PISA (proximal isovelocity surface area) method or the continuity equation. Although the VC measurement is relatively easy to obtain, its accuracy is mainly useful for either mild or severe MR, but it varies for moderate degrees of MR. , , It is also important to measure the width of the VC perpendicular to the regurgitant jet and not in parallel (see Fig. 11.51 ). For the native valve, the VC should be obtained either in the ME four- or five-chamber or long-axis windows, but not in the ME two-chamber or bicommissural window, as the latter has been shown to overestimate MR severity. A VC width less than 3 mm or greater than 6 mm is consistent with mild and severe MR, respectively. , ,
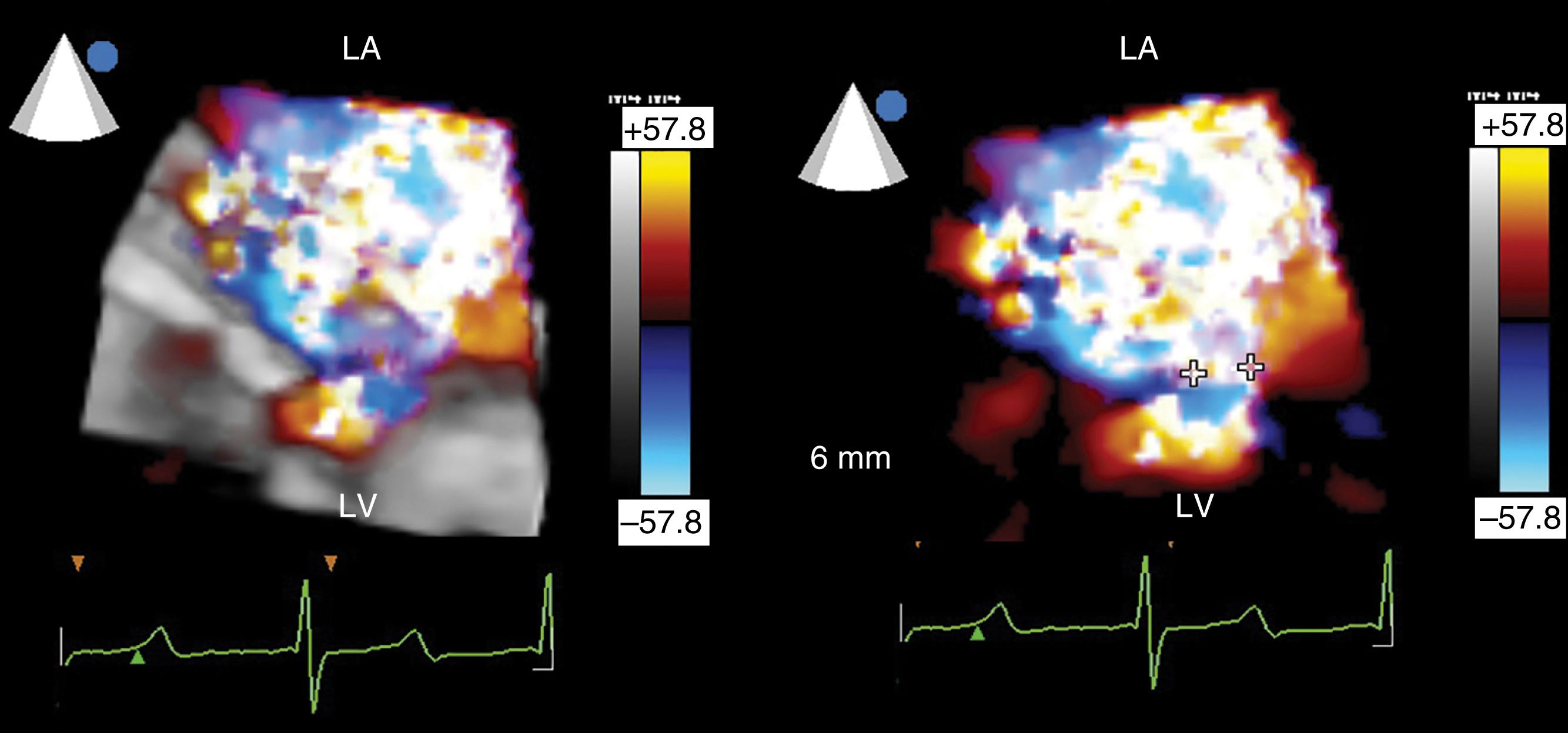
![Figure 11.51, Three-dimensional (3D) image of the regurgitant orifice. (A–C) Two-dimensional appearances of the same mitral regurgitant jet from three different midesophageal (ME) echocardiographic windows: four-chamber (4C) , two-chamber (2C) , and long-axis (LAX) , respectively. Vena contracta measurements from the ME4C and MELAX views are more accurate correlates of mitral regurgitation (MR) severity as they measure perpendicular to the MR jet, while the ME2C window views parallel to the MR jet. (D) This is confirmed using 3D imaging of the regurgitant jet during diastole (left) and systole (middle) from the left ventricular (LV) perspective. The shape of the regurgitant orifice, during systole on the right , is elliptical. The implications are that the effective regurgitant orifice is not circular and the flow convergence (proximal isovelocity surface area [PISA]) is not hemispherical as the principle behind the equation assumes it to be, creating errors in the PISA method. 3D imaging, when feasible, allows a direct measure of the regurgitant orifice area shown in black (right) . Figure 11.51, Three-dimensional (3D) image of the regurgitant orifice. (A–C) Two-dimensional appearances of the same mitral regurgitant jet from three different midesophageal (ME) echocardiographic windows: four-chamber (4C) , two-chamber (2C) , and long-axis (LAX) , respectively. Vena contracta measurements from the ME4C and MELAX views are more accurate correlates of mitral regurgitation (MR) severity as they measure perpendicular to the MR jet, while the ME2C window views parallel to the MR jet. (D) This is confirmed using 3D imaging of the regurgitant jet during diastole (left) and systole (middle) from the left ventricular (LV) perspective. The shape of the regurgitant orifice, during systole on the right , is elliptical. The implications are that the effective regurgitant orifice is not circular and the flow convergence (proximal isovelocity surface area [PISA]) is not hemispherical as the principle behind the equation assumes it to be, creating errors in the PISA method. 3D imaging, when feasible, allows a direct measure of the regurgitant orifice area shown in black (right) .](https://storage.googleapis.com/dl.dentistrykey.com/clinical/TransesophagealEchocardiographyAdvancedEchocardiographyConcepts/50_3s20B9780323829243000119.jpg)
The flow convergence method or PISA can be used qualitatively or quantitatively. , , , , The absence of a large flow convergence (especially with Nyquist limit of <20 cm/s) supports the determination of moderate or less MR. The corollary is that the presence of a flow convergence at a Nyquist limit greater than 40 cm/s is consistent with moderate or severe to severe MR. An ROA greater than 40 mm 2 and/or a regurgitant volume greater than 60 mL is consistent with severe MR. The accuracy of the flow convergence method assumes that the PISA shells are hemispherical and the ROA is circular. Three-dimensional imaging shows that these assumptions are not always correct (see Figs. 11.17–11.19, 11.51 ). A benefit of 3D color imaging is to allow direct measure of the ROA by planimetry.
Simplifications of the flow convergence method can be made if the Nyquist limit is set at 40 cm/s and it is assumed that the peak MR velocity is 5 m/s. If this were the case, then the ROA is equal to:
A dimensionless index is the ratio of the time velocity integral (TVI) of the MV to that across the AV. In the absence of significant AI, a value less than 1 or greater than 1.4 is consistent with mild and severe MR, respectively.
Data have been published to predict outcome for varying degrees of MR. Severe MR is defined by an effective ROA greater than 40 mm 2 and is associated with early and late mortality. , Whereas severe MR requires therapy, it is less clear how milder degrees of MR should be managed. In the same study, an effective ROA (20–39 mm 2 ) was also associated with a worse outcome, but it was not significant until after 2 to 3 years. ,
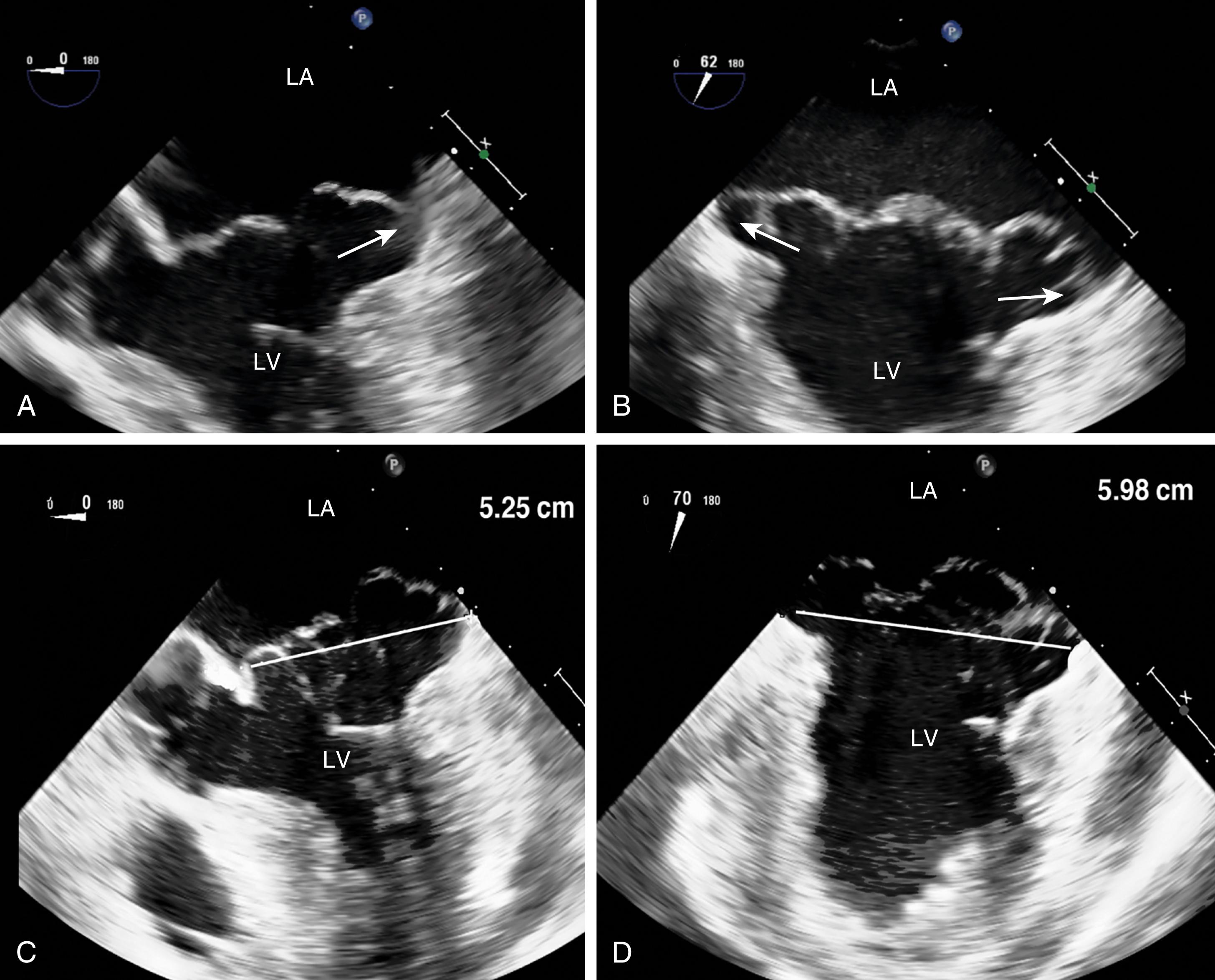
In the presence of severe symptomatic MR, surgery should be performed (see Figs. 11.52 and 11.53 ). For an asymptomatic patient with severe MR, surgery should be considered if a durable repair is likely. This is further supported if LV decompensation is present (LVEF <60%; LV end-systolic diameter > 40 mm) or if it is refractory to medical therapy. For patients with moderate MR, surgery should be considered if that patient is presenting for other cardiac surgical procedures, especially if it is thought that repair is feasible and to provide long-term reduction in MR (>5–10 years).
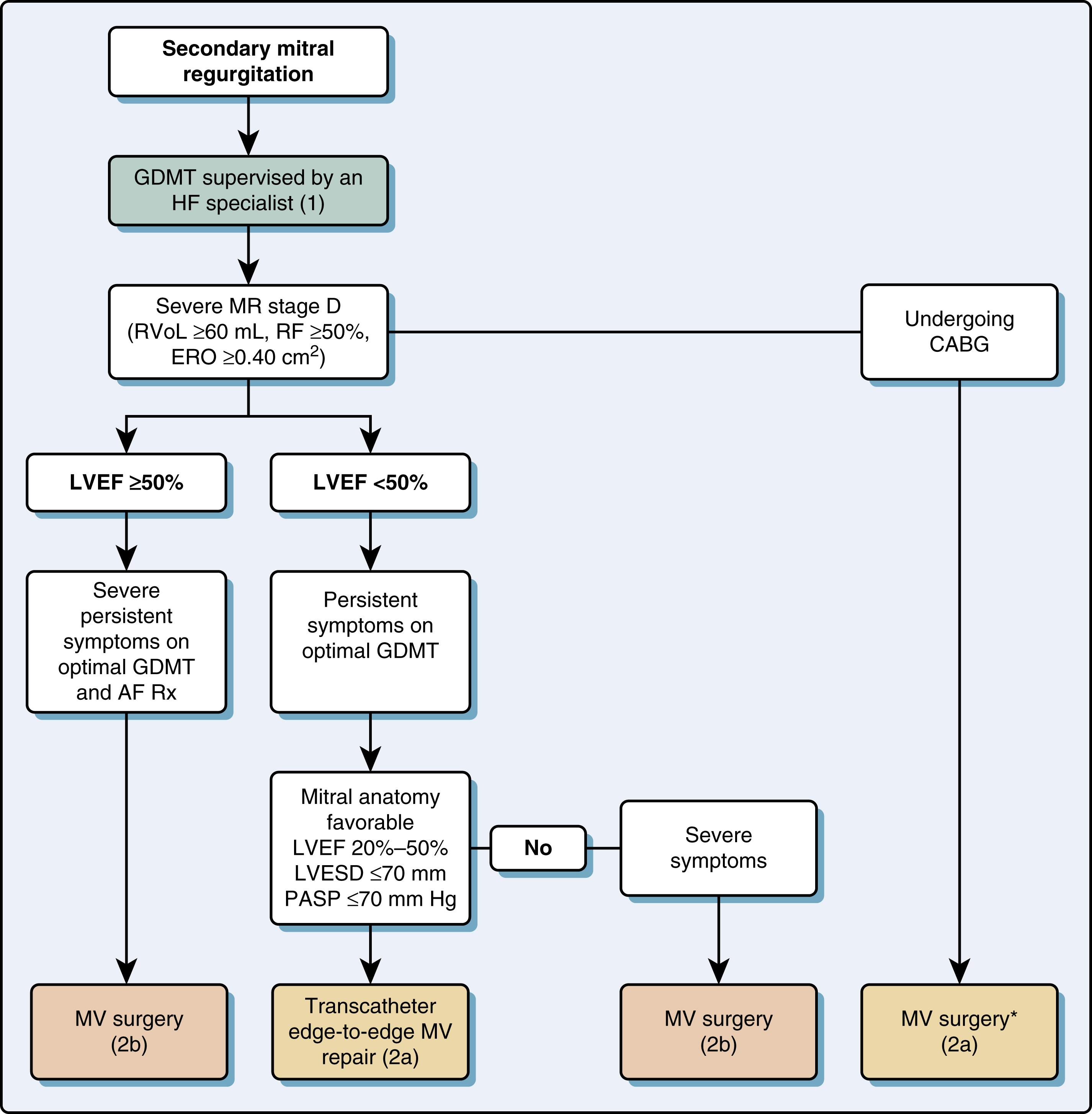
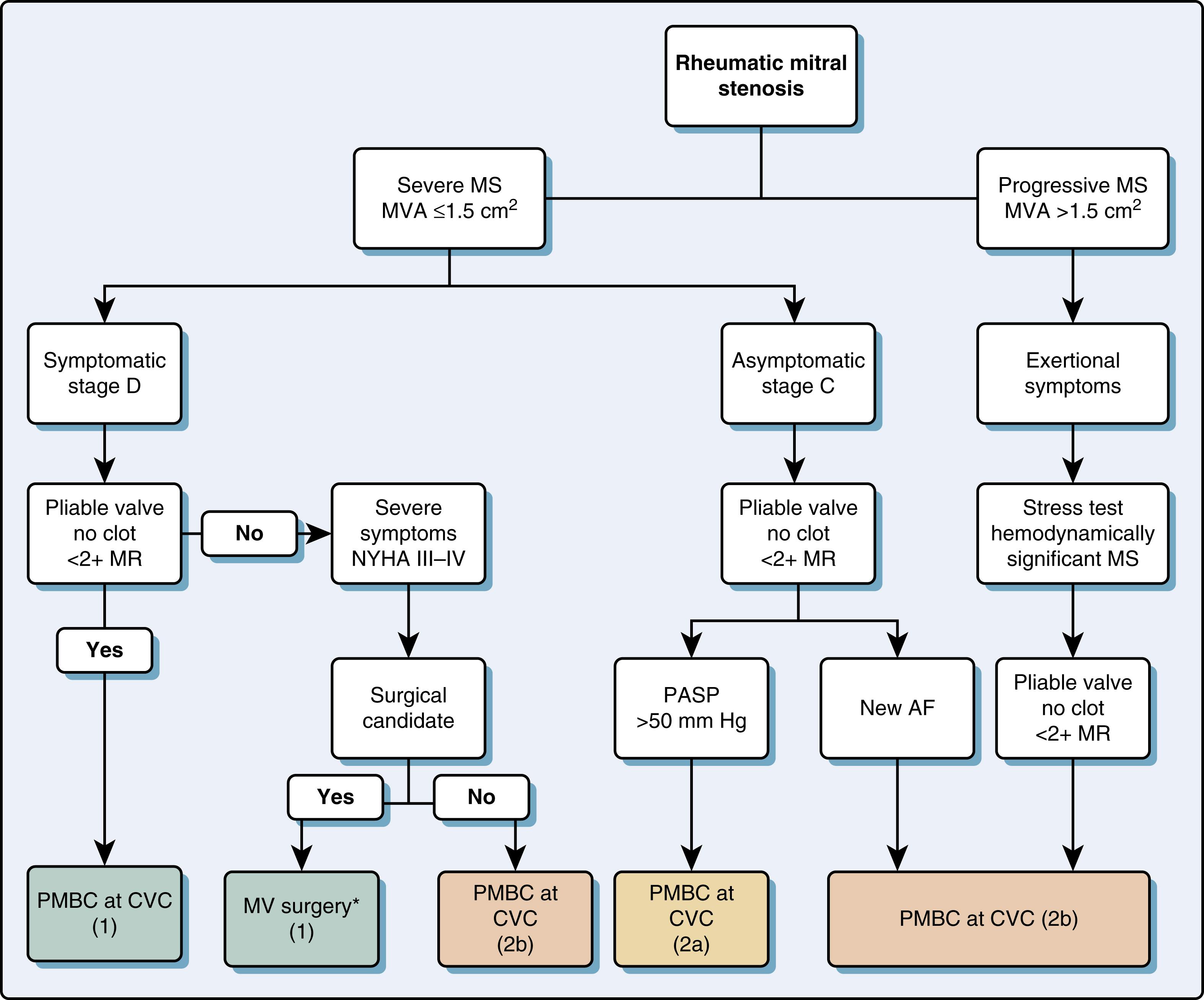
The normal mitral valve area (MVA) ranges from 4 to 6 cm 2 and transmitral flow velocities are less than 1 m/s ( Table 11.5 ). The more common causes of mitral stenosis (MS; MVA <2.0 cm 2 ) include rheumatic stenosis, calcific MS, and, less commonly, congenital MS. The echocardiographic examination incorporates 2D and Doppler technologies to diagnose and assess for the presence and severity of MS (see Figs. 11.54 and 11.55 ).
| Mild | Moderate | Severe | |
|---|---|---|---|
| Peak jet velocity (m/s) | <1.8 | 1.8–2.2 | ≥2.2 |
| Peak pressure gradient (mm Hg) | <12 | 12–18 | >18 |
| Mean pressure gradient (mm Hg) | <6 | 6–10 | >10 |
| MV area (cm 2 ) pressure half-time continuity equation planimetry | >2.0 | 1–2 | <1.0 |
| MV area index (cm 2 /m 2 ) | <1.25 | ||
| Velocity ratio (TVI mv /TVI lvot ) | <2.2 | 2.2–2.5 | >2.5 |
| Pressure half-time (ms) | <130 | 130–200 | >200 |
| Secondary findings | LAE, PHTN, RAE, RVE, TR | ||
Rheumatic valvitis causes commissural fusion, chordal shortening/fusion, and leaflet thickening, all complicated by calcification of the structures. The leaflet tips become fused. While stenosis is more common in the rheumatic MV, the leaflet and chordal abnormalities can also be associated with significant MR (Carpentier type IIIa). In addition, involvement of other cardiac tissues and valves is not uncommon, including AV insufficiency and tricuspid regurgitation (TR). Calcific mitral degeneration mainly affects the mitral annulus followed by leaflet base calcification and uncommonly results in stenosis unless associated with leaflet thickening.
Patients with MS may be asymptomatic or may have varying degrees of LV and RV failure depending on the severity and chronicity of the disease. With greater decompensation, forward flow across the valve and into the LV declines, while LA pressure size and function deteriorates. With time, the pulmonary vascular pressures rise, pulmonary edema occurs, and significant right-sided heart afterload, dilation, and dysfunction follow. Patients experience fatigue, hemoptysis, and right-sided heart failure. TR may result either from primary rheumatic involvement and/or secondary to pulmonary hypertension. Atrial fibrillation and stroke may be initial presenting signs, the latter, in part, resulting from development of atrial thrombus, 90% of which occur in the left atrial appendage (LAA).
The assessment of the stenotic MV assesses etiology, severity, and the need for and timing of invasive procedures, the latter being either valvuloplasty or valve replacement. The Wilkins score was proposed and now used to predict outcome after valvuloplasty. The score includes assessment of leaflet thickening, mobility, and calcification, as well as subvalvular thickening, each of which is scored from 1 to 14. A score less than 8 suggests that the valve is more amenable to valvuloplasty as compared to a score higher than 8. Variations on this score as well as others have been proposed, including one based on 3D imaging. Findings consistently show that the greater the amount of thickening, calcification, and immobility, the worse the outcome after valvuloplasty.
Assessing forward flow across the prosthetic MV is similar to that for the native valve. As described earlier, different prostheses have different signature forward flow profiles. It is expected that normal transvalvular velocities and calculated gradients will be higher than that normal native valve in the same position. The more information known about specific prosthesis, the easier it is to distinguish between normal and abnormal. Prosthetic valve stenosis is caused by leaflet restriction, thickening, and/or thrombosis (see Figs. 11.56 and 11.57 ).
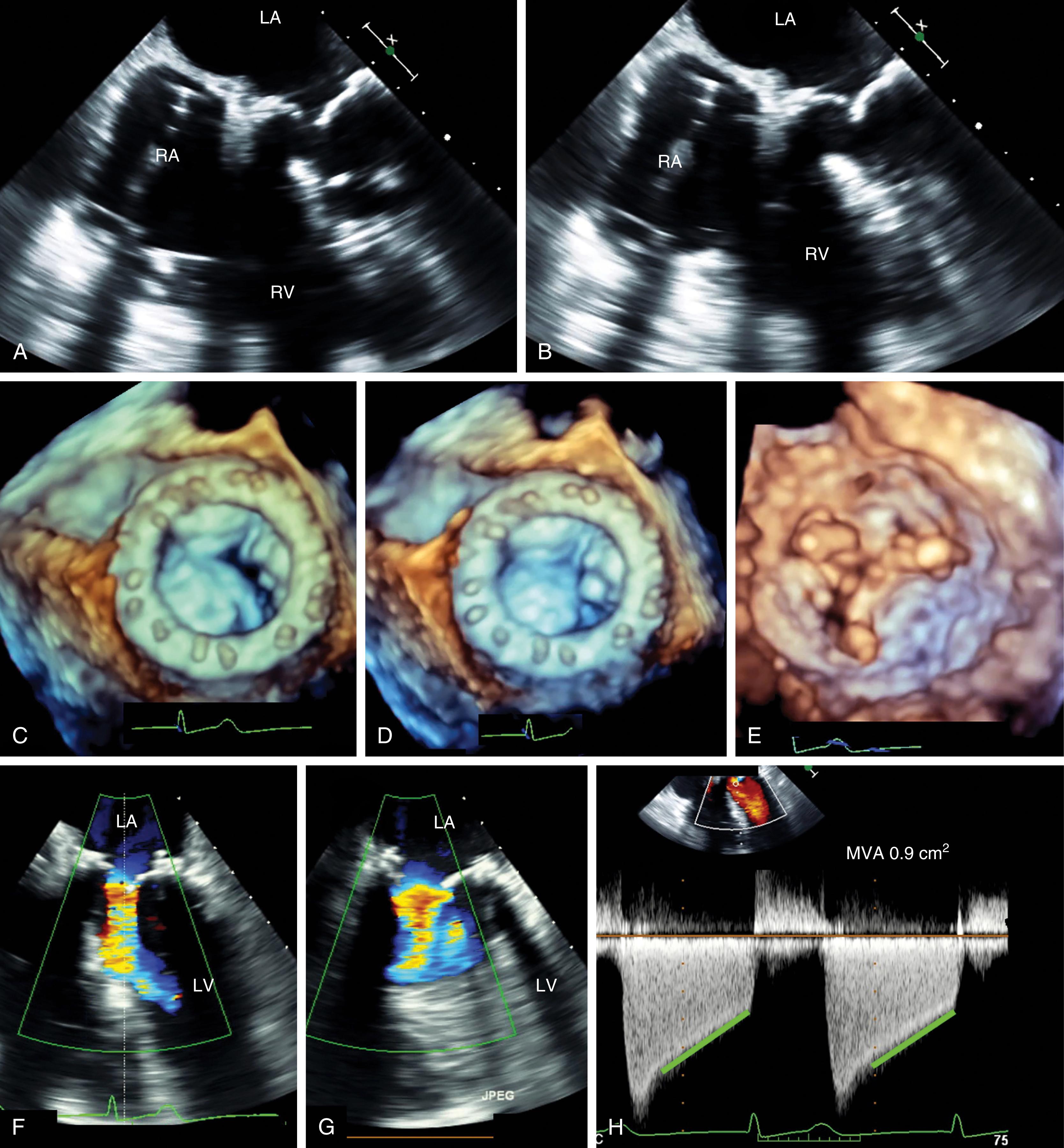
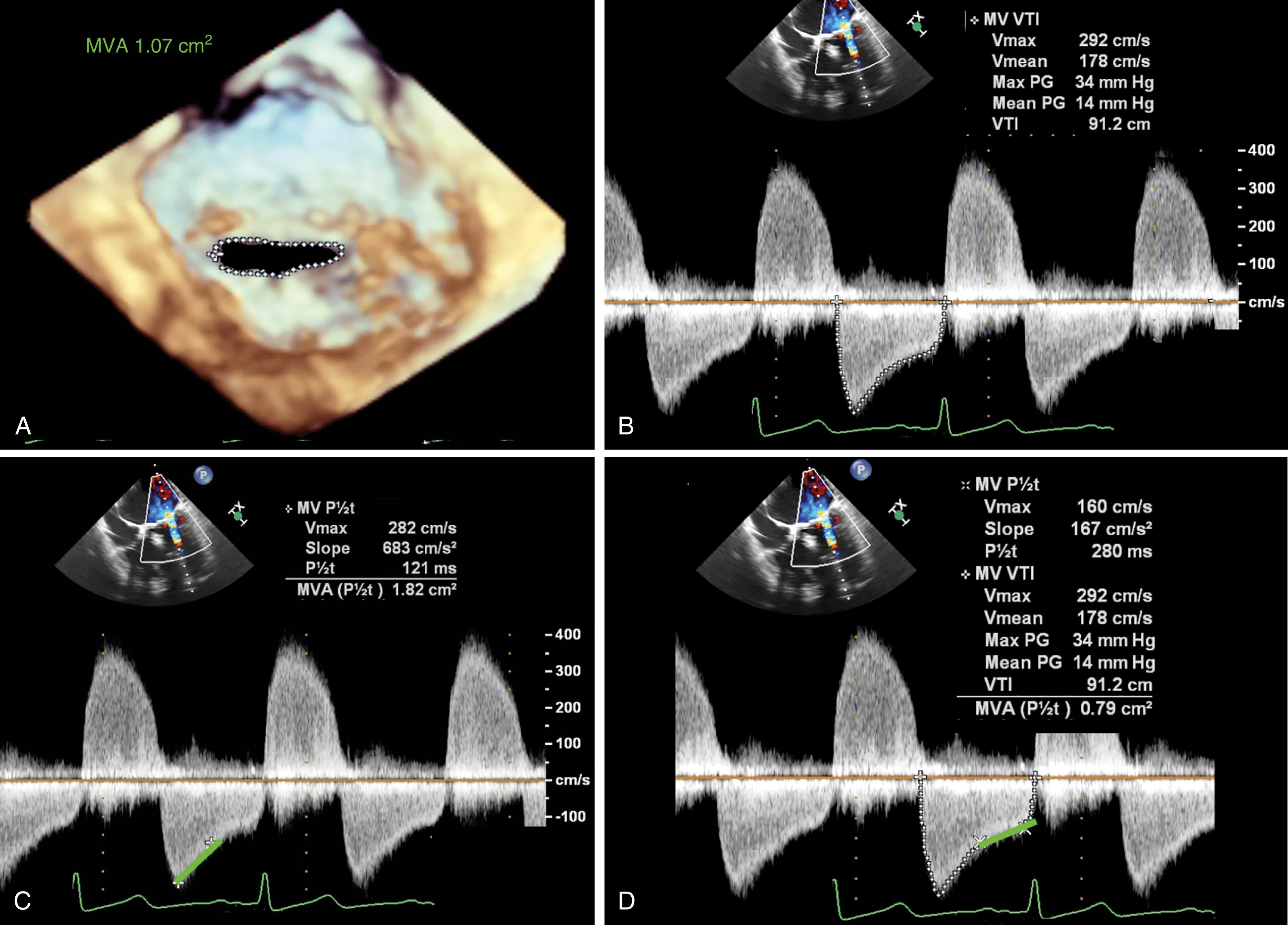
The American Society of Echocardiography level 1 recommendations for assessing MV patency include peak transvalvular jet velocity, pressure gradients, and MVA calculated from the pressure half-time (PHT) or measured by planimetry (see Figs. 11.54 and 11.55 ). , , The most direct measurement for tissue valves is planimetry made possible by 2D or, more recently, 3D imaging, the latter by using a multiplanar reconstruction (MPR) analysis. Whereas 2D planimetry is performed from TG MV short-axis imaging, 3D planimetry is obtained using volumetric data from ME windows.
Peak pressure gradients are calculated from the peak transvalvular jet velocity using the simplified Bernoulli equation. Mean pressure gradients are obtained by tracing the TVI across the MV to integrate the various jet velocities allowing calculation of the mean gradient. Since the peak gradient is influenced more by the net compliance between the left atrium and LV, the mean gradient is of greater importance.
The Doppler data are affected by changes in cardiac loading conditions, cardiac output (CO), and net compliances, coexisting valvular or congenital lesions, specifically including MR, AV dysfunction, and an atrial septal defect (ASD). The calculation of the MVA from the PHT is done by applying an empirically derived value (see Fig. 10.73 ).
Variables that increase the LA pressure or reduce LV pressure increase gradient and may overestimate MS severity. Doppler measures of MVA, including the PHT, are affected by these variables. , Variables that reduce LA pressure or increase LV pressure do the opposite. For example, an ASD with left to right flow, moderate or greater AI, or net positive change in the LV-LA compliance causes an overestimation of MVA by shortening the PHT.
The deceleration time (DT) is an extension of the PHT and is the time from the peak velocity to zero velocity across the orifice. Using the DT to calculate MVA requires knowing that PHT is 29% of the DT.
The transmitral TVI may be bimodal with a more rapid initial deceleration followed by a slower decline in gradient. It is recommended to measure the PHT from the slower decline as it better reflects the contribution of the MVA.
Degrees of MS follow similar charts for native and prosthetic valves. Significant (more than mild) MS is considered with a peak velocity greater than 1.8 m/s, a mean transvalvular gradient greater than 5 mm Hg, and a peak gradient greater than 12 mm Hg. An MVA less than 1.5 to 2.0 cm 2 is considered stenotic. PPM has been described for prosthetic MVs and considered for an indexed effective orifice area (EOAi; cm 2 /BSA [m 2 ]) less than 1.25 cm 2 /m 2 .
Different methods are used to assess the EOA, including the PHT, the continuity equation, and the flow convergence method. The continuity equation can be simply described by the following equation ( Fig. 11.58 ):
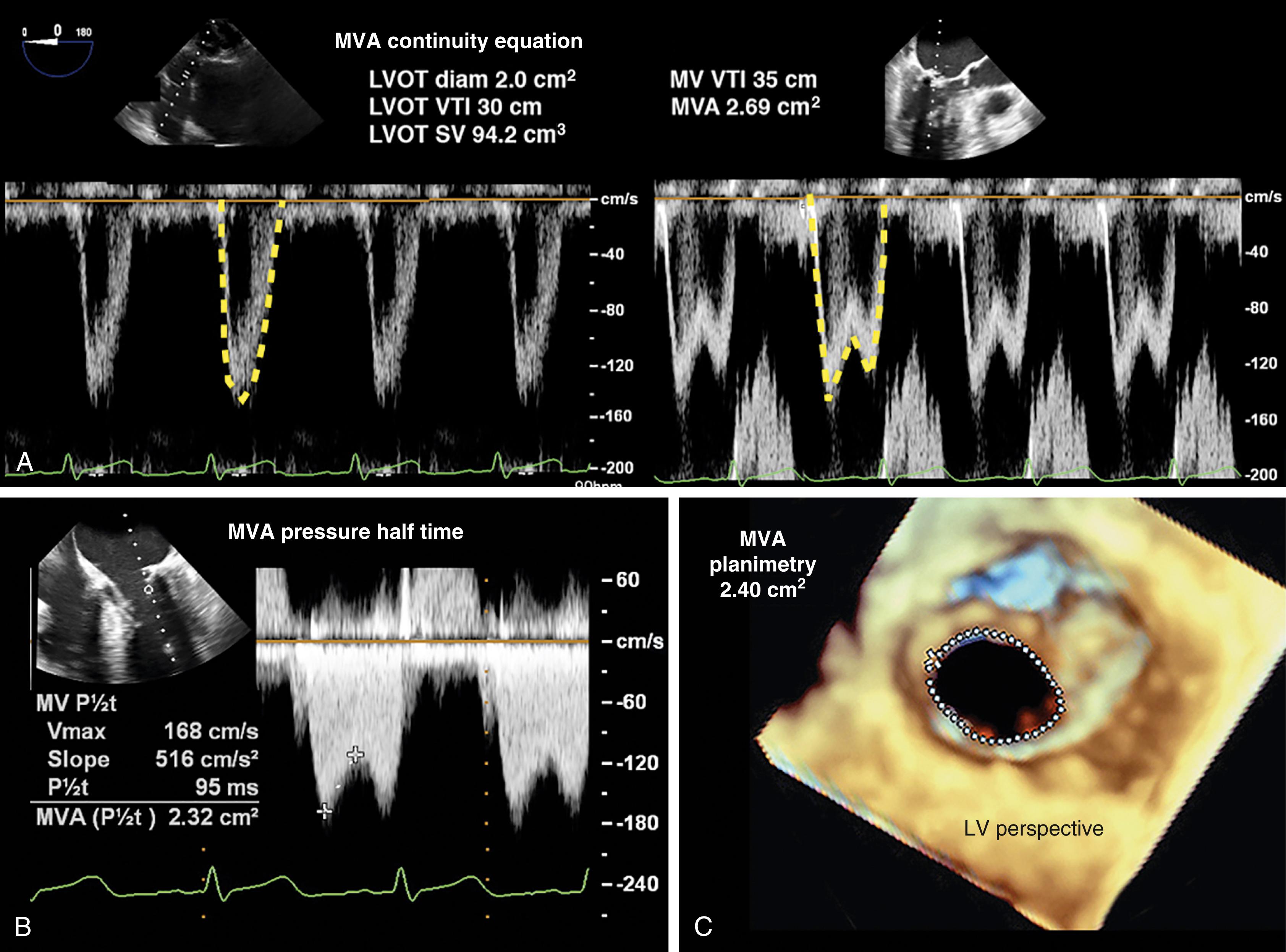
The SV can be measured from a reference site using Doppler echocardiography (e.g., pulmonary artery [PA] or LVOT), a noninvasive method (e.g., arterial waveform), or using thermodilution CO if a PA catheter were present.
PISA or flow convergence method has been described to measure MVA and assumes geometric assumptions that variably exist for native valves and less so for prosthetic valves (see Fig. 11.55 ). Qualitatively, the presence of a flow convergence area supports the diagnosis of mitral stenosis.
To avoid geometric assumptions and other variables listed, a Doppler velocity index (DVI) also may be used to assess prosthetic MV patency.
For cases in which imaging is difficult, a modified Gorlin equation can be employed. In this equation, the CO is divided by the square root of the peak transmitral pressure.
Invasive therapy is indicated for symptomatic patients with severe MS (MVA <1.5 cm 2 ) (see Fig. 11.59 ). Percutaneous mitral commissuroplasty (PMC) is preferred to open surgery, and surgery is only performed when PMC is contraindicated, which includes an MVA greater than 1.5 cm 2 , LA thrombus, significant MR (>2+), unfavorable anatomy for PMC, and/or when patients are scheduled to undergo other cardiac surgery procedures.
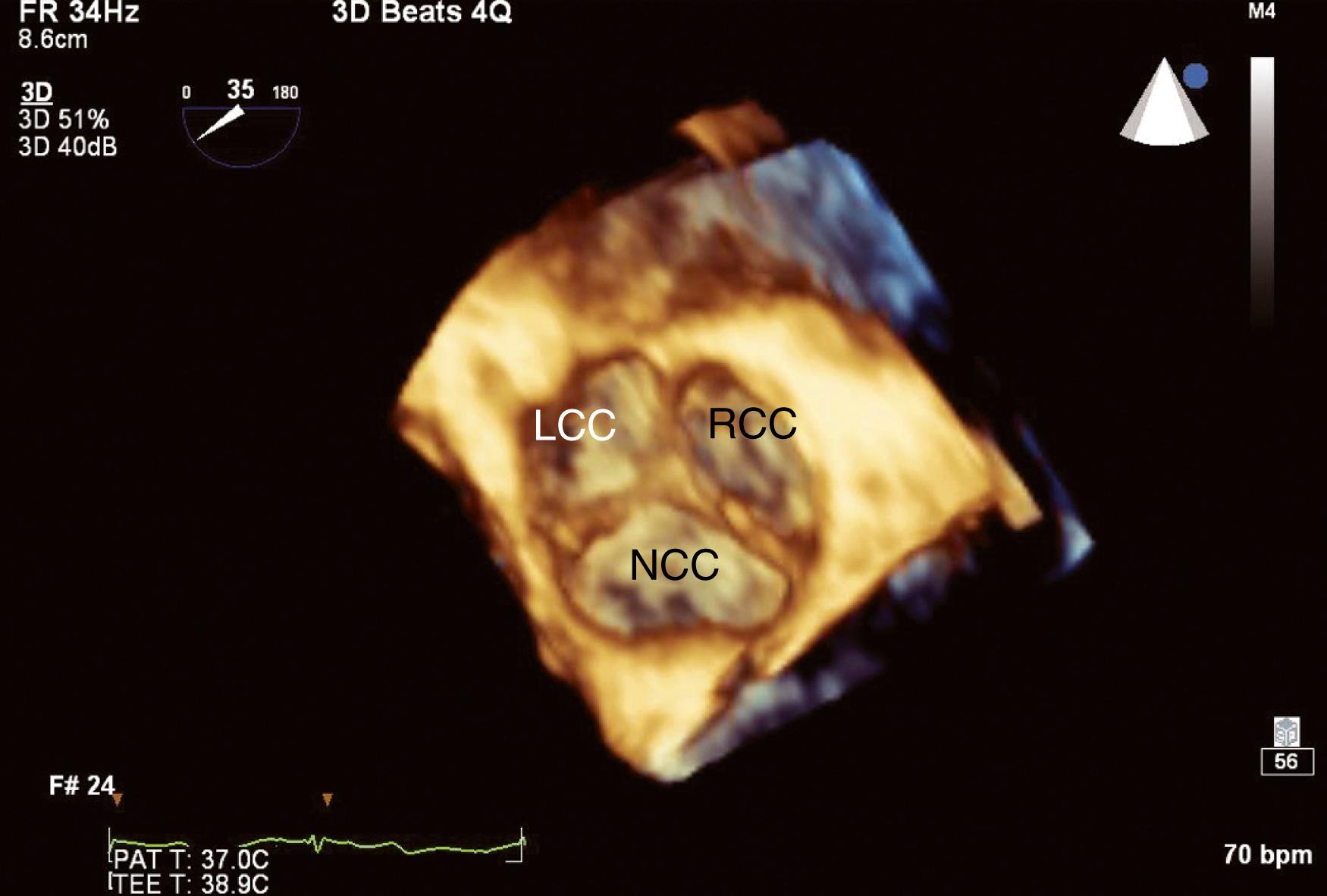
The normal AV is a three-leaflet semilunar valve with thin leaflets (<2 mm) and an EOA ranging from 2 to 4 cm 2 . The three leaflets, or cusps, are equal in size and symmetric and labeled as left, right, and noncoronary cusps, depending on their association with a coronary artery. The leaflets cover an area that is 40% greater than the area of the open orifice, indicating significant overlap. The coapting length between any two leaflets is generally greater than 6 mm. The three leaflets coapt centrally along a thickened nodular area (Nodules of Arantius) above the annular plane (ie, no prolapse).
The AV is a part of the LVOT that extends from the subvalvular to supravalvular area, being defined as the region between the anterior mitral leaflet and the septum immediately below and extending to the sinotubular junction (STJ) within which the AV is attached ( Fig. 11.60 ). The supravalvular tissues include the sinuses of Valsalva and the STJ, which then extends into the ascending aorta. There are three sinuses of Valsalva: left, right, and non. Each is defined by the presence of the respective coronary ostia (right and left) or its absence (non). The sinuses appear as outpouches and serve an important role, one of which is to provide a reservoir of blood during diastole to optimize coronary artery filling and flow. The sinuses have a diameter ranging from 29 to 45 mm (19 mm/m 2 ).
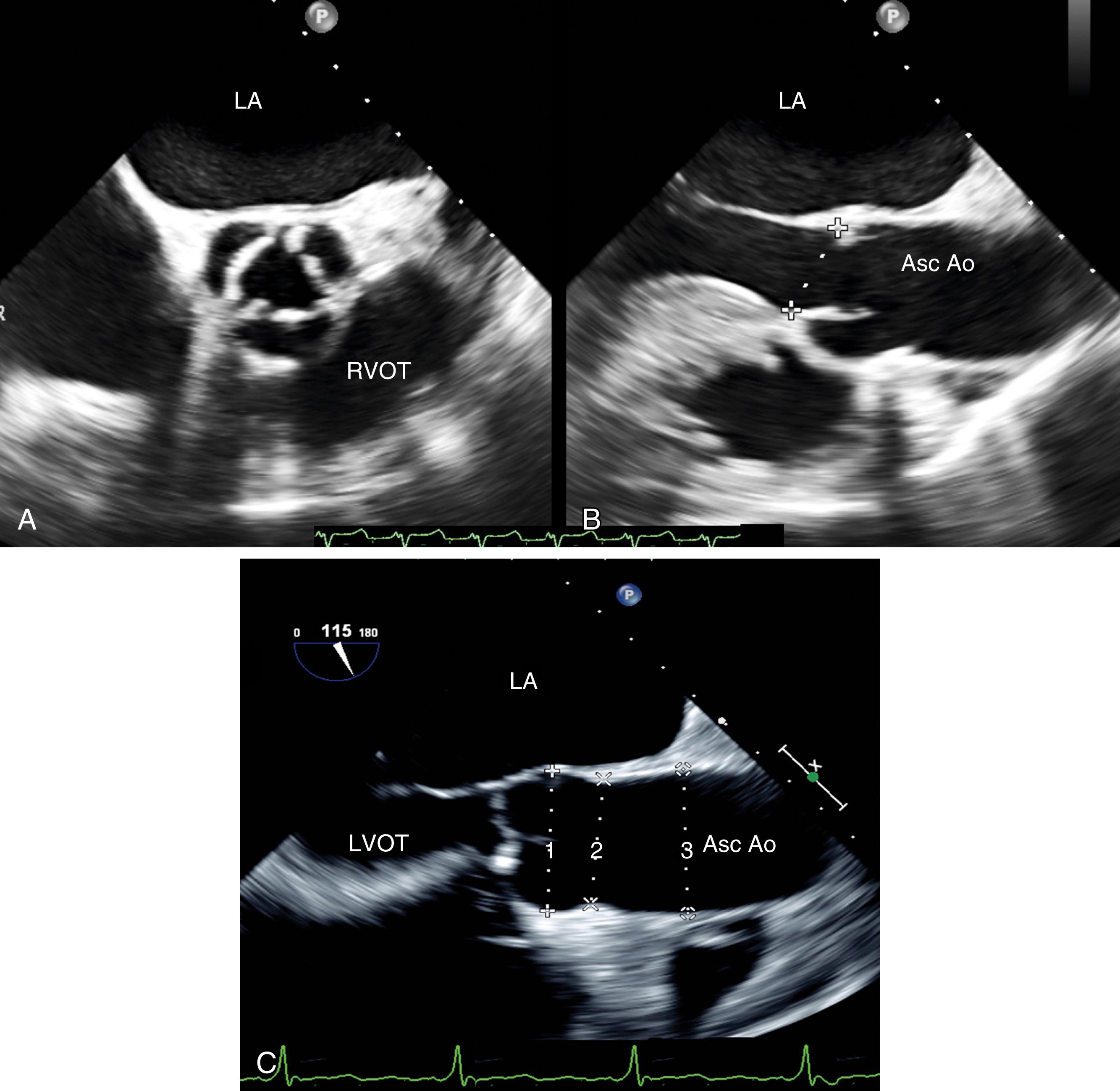
The leaflets open (ventricular systole) and close (ventricular diastole) in accordance with the pressure gradient between the aorta above and the LV below. Although this is the main driving force guiding leaflet motion, the anatomic position and connection between the AV and surrounding tissues also contributes to the opening and closing of the valve. The interplay between the valve leaflets and surrounding tissues or muscle reduces resistance and tension on the leaflets to facilitate both opening and closing. Prior to ventricular ejection, the ascending aorta begins to enlarge and accounts for the initial leaflet separation. Prior to diastole, blood begins to pool in the coronary sinuses creating vortical forces and pressure, which initiate leaflet closure.
Prior to the evaluation of the prosthetic or repaired AV, it is important to know the details of the surgical procedure and the normal hemodynamic functions that are expected for the replaced or repaired valve. , , Surgically aortic valve replacement (SAVR) involves prosthetic valves with annular sizes 19, 21, 23, and 25 mm being most commonly placed. In general, 19, 21, and 23 mm valves result in EOAs of 1.1, 1.4, and 1.6 cm 2 , respectively (see Table 11.3 ). , , , While stentless bioprosthetic valves are anticipated to have respectively larger EOA, initially the hemodynamic profile may appear inferior due to geometric changes in the presence of perivalvular aortic edema and hematoma, which resolve within 6 months of surgery.
Transcatheter aortic valve implantation (TAVI) includes prosthetic valves sized 20 to 31 mm in diameter with estimated aortic valve area (AVA) ranging from 2.4 to 6.6 cm 2 . While the SAVR bioprosthetic valve has three defined struts, the TAVI valve has a curtain that surrounds the leaflets.
While AV repairs are mostly performed for regurgitant valves, repair of congenital AV stenosis has been reported. The appearance of the repaired valve depends on the repair, but by and large results in a singular orifice with a flow profile similar to a normal native valve. While delineation of the pre-repair dysfunction helps guide the repair, the postrepair evaluation predicts long-term function. This includes assessment of length (>4 mm) and level (above annulus) of leaflet coaptation, presence of residual AI, and the size of the annulus.
The 2D examination of the AV includes assessments of the supravalvular and subvalvular tissues. Although a comprehensive examination is necessary, particular echocardiographic windows of importance include ME short-axis and ME long-axis windows to image the AV and the tissues above and below in short- and long-axis views. TG windows are useful for quantitative Doppler as the Doppler beam can be best aligned with blood flow across the aortic root.
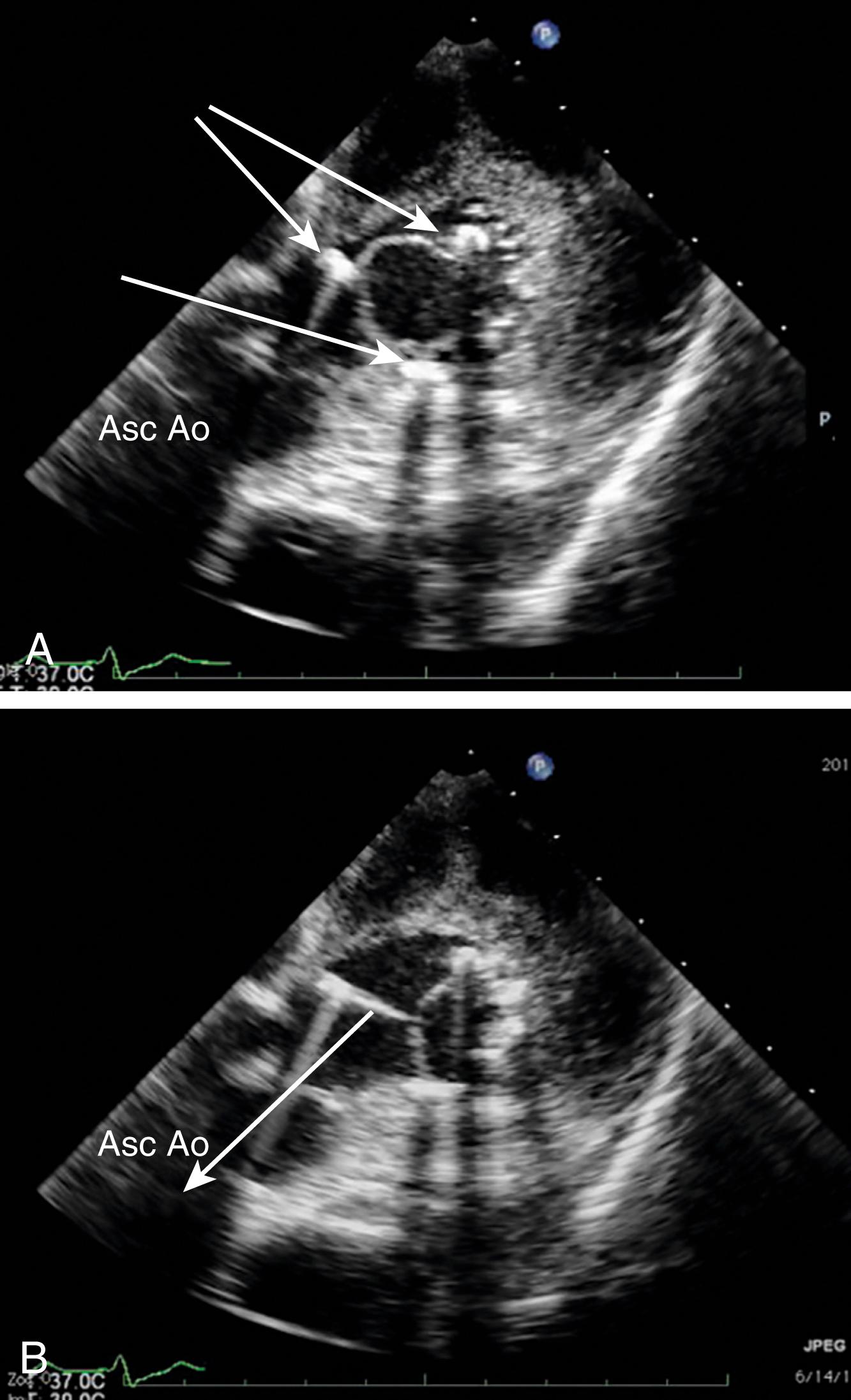
Leaflet number, thickness, and mobility, and the presence of masses, the latter of which may represent fibrinous strands, endocarditis, tumor, or fractured calcium deposits, are key to determining the etiology of dysfunction and determining whether or not the valve can be repaired or should be replaced. Any evidence of instability and/or hypoechoic planes within the aortic wall or between the valve and the aortic wall suggest valve dehiscence and/or root infection or abscess ( Figs. 11.61 and 11.62 ). , The comprehensive examination defines whether or not AI is due to a primary valve dysfunction or secondary to aortic root pathology (e.g., aortic dissection or root dilation). Secondary cardiac effects of AS or AI, including LVH, left heart dilation, and dysfunction, allude to the severity of dysfunction and timing of invasive treatment.
The Doppler (color, PW, CW) examination allows both qualitative and quantitative evaluations of patency and competence, including presence, direction, and width of normal and abnormal blood flow in and around the native, the repaired, and prosthetic valves. ,
The post cardiopulmonary bypass examination is similar to the pre-examination ( Fig. 11.63 ). The stented and stentless bioprosthetic valves can be differentiated by the presence or absence of both the stent and the supportive struts with the stent ( Fig. 11.64 ). Defined normal forward and regurgitant flows are described for prosthetic valves. It is important to recognize the presence of periprosthetic thickening due to edema or hematoma which begins to resolve in the early postoperative period. For patients with stentless or repaired valves, this may result in a smaller EOA and elevated transvalvular gradients in the immediate postoperative period followed by a significant increase in EOA over the first 3 to 6 months associated with resolution of the perivalvular swelling (see Fig. 11.64 ).
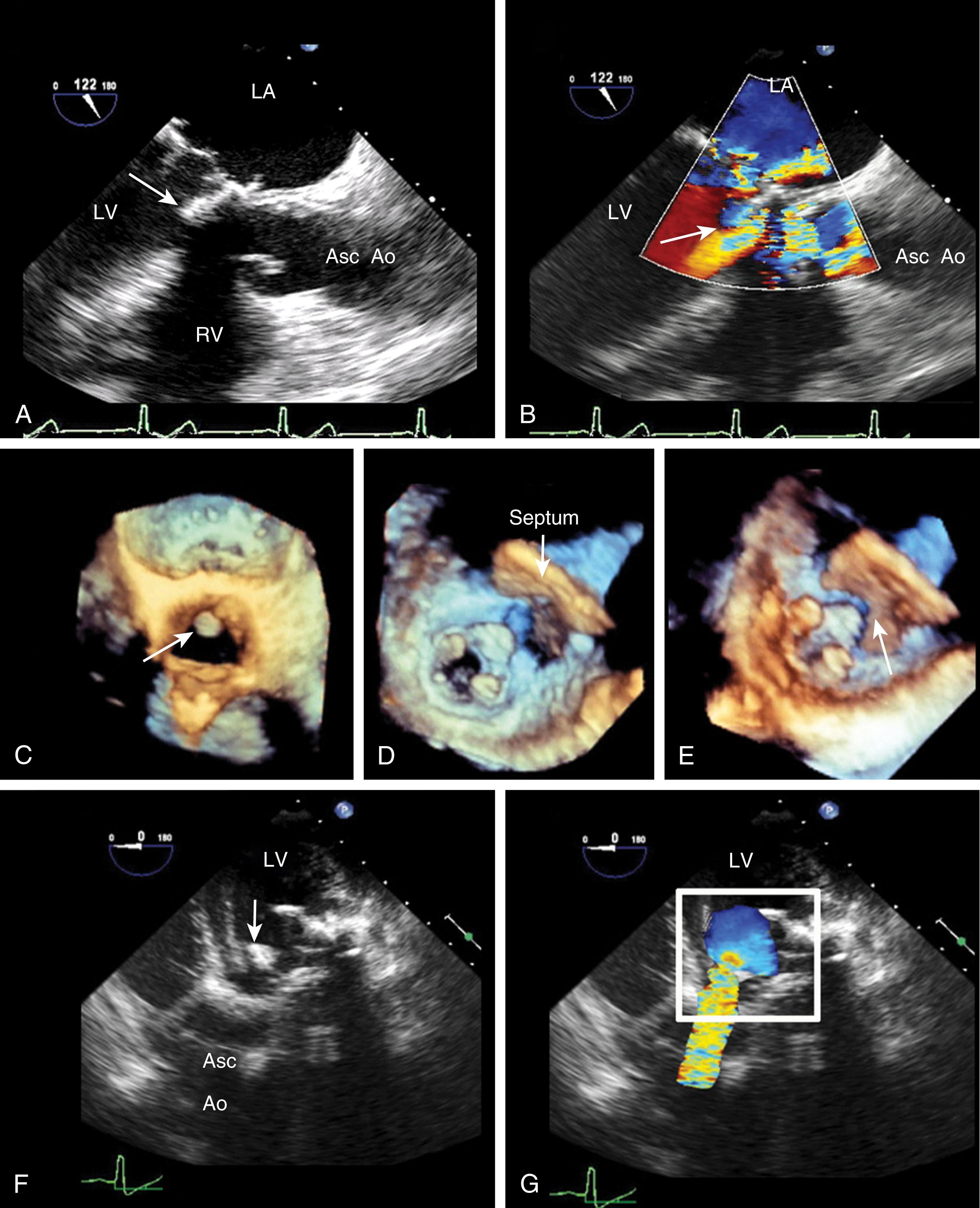
AV regurgitation results from either primary aortic leaflet or valve abnormalities or secondarily from ascending aortic pathology. , , , Trace or mild AI may be seen in the absence of significant pathology; however, this occurs in the minority of cases (<20%). Similar to other valves, regurgitation can be divided based either on mobility and/or leaflet pathology. Type I dysfunction includes AI due to aortic root dilation ( Fig. 11.65 ). Root dilation includes those tissues extending to the STJ, but not beyond (ie, the ascending aorta). Type II dysfunction reflects excess leaflet mobility noted by either leaflet prolapse or flail ( Figs. 11.66 and 11.67 ). The presence of fenestrations, seen with fibroelastic deficiency, is suggested by multiple areas of regurgitation and may hinder successful repair. Type III dysfunction also reflects restricting leaflet motion ( Fig. 11.68 ). Repair should be considered for type I and type II dysfunctions.
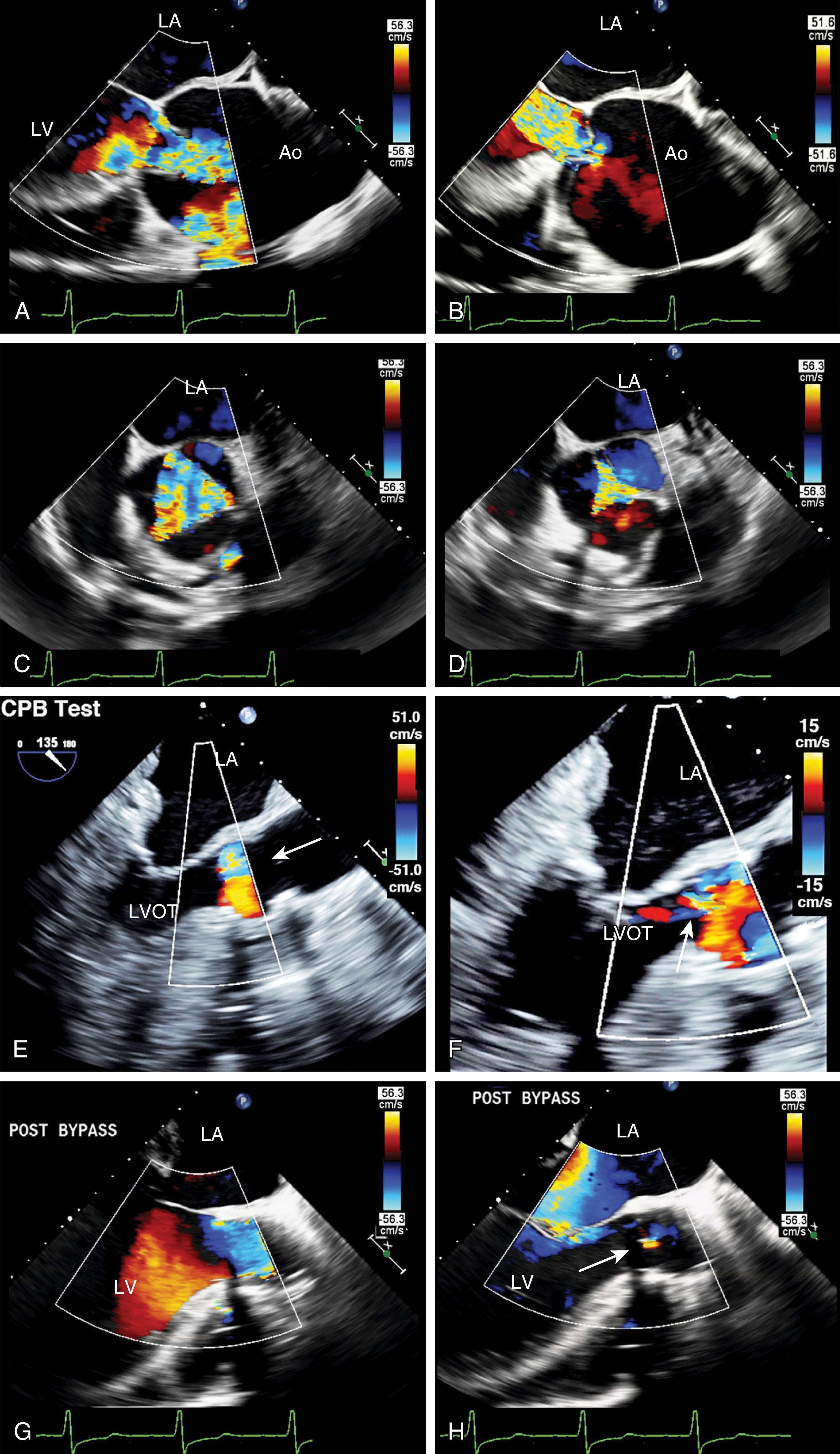
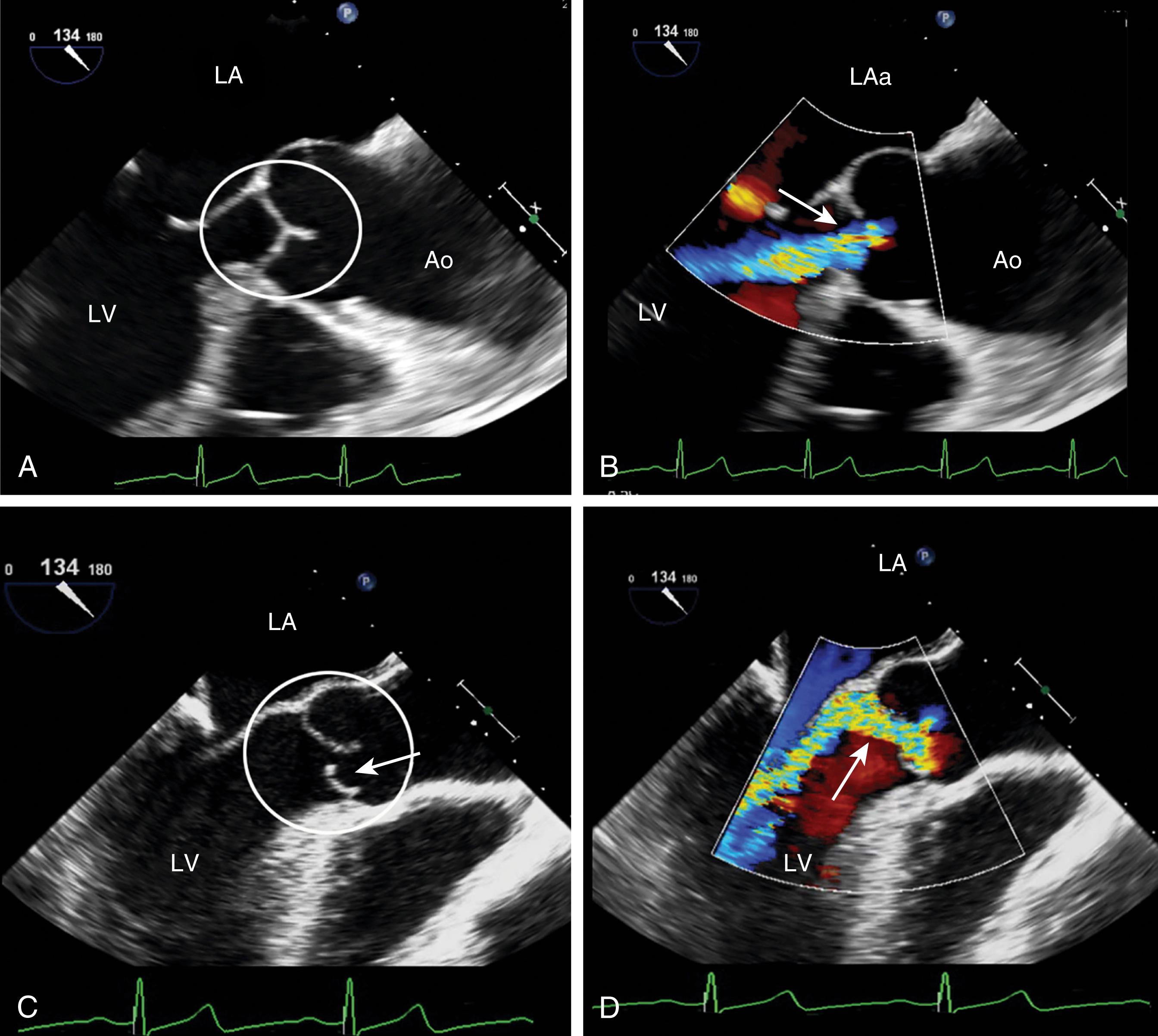
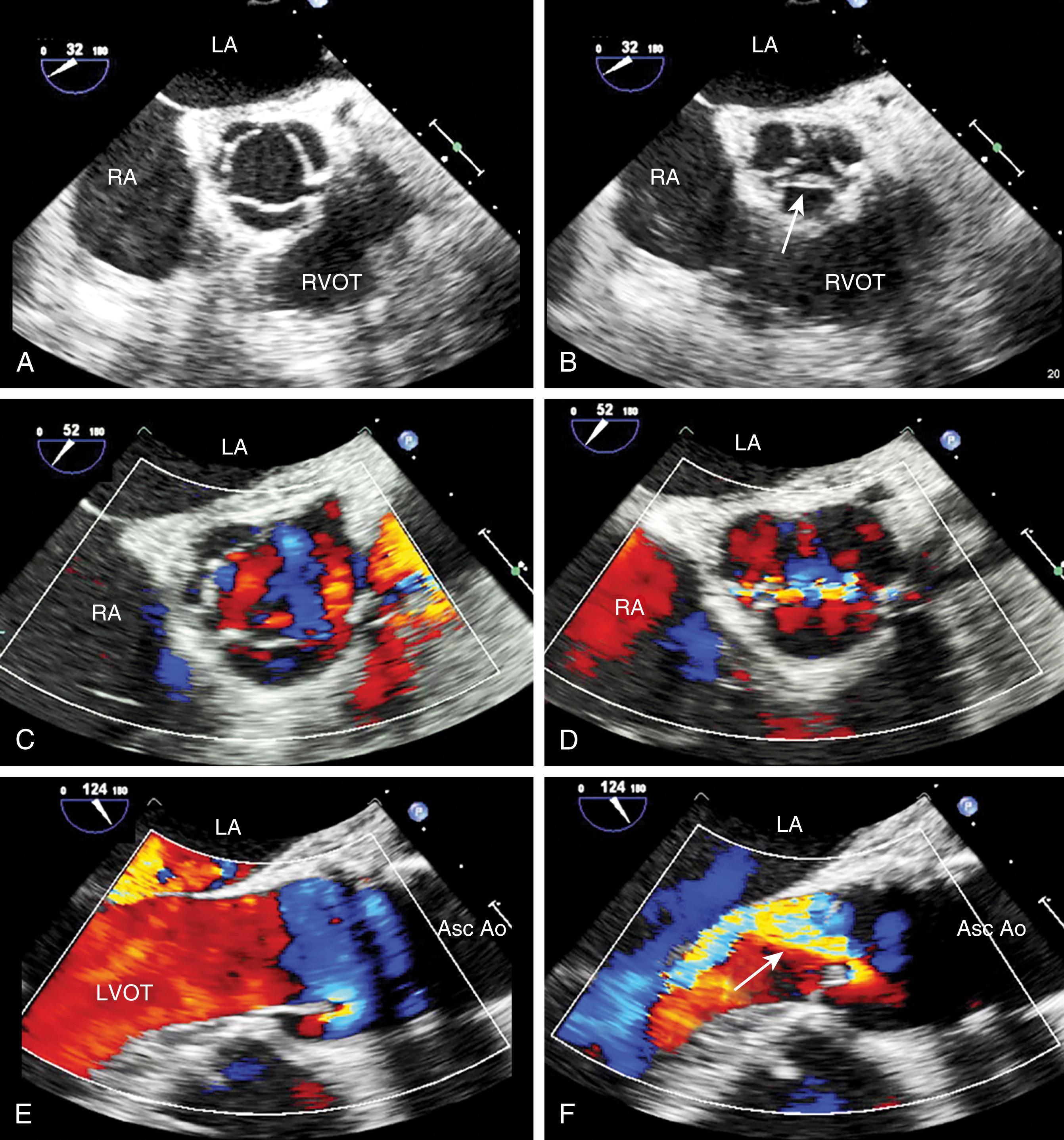
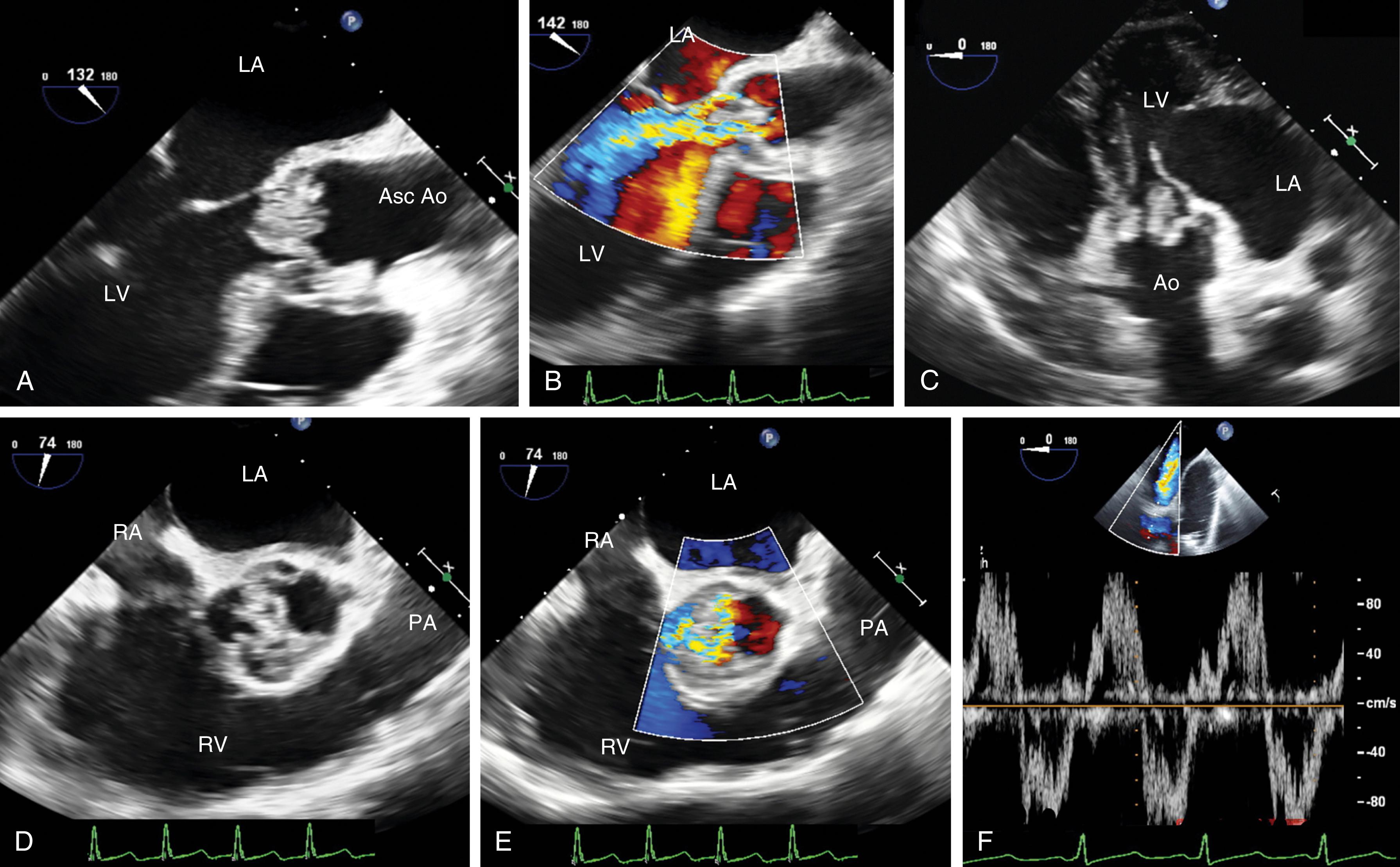
Primary valve pathologies include bicuspid AV, quadricuspid AV, myxomatous degeneration (fibroelastic disease), fenestrations, endocarditis, calcification (often associated with AS), radiation therapy, rheumatic disease, and trauma ( Figs. 11.66–11.69 and Table 11.6 ). The AV also can be involved in association with a subvalvular membrane or a ventricular septal defect (VSD) ( Fig. 11.70 ). Type II dysfunction includes prolapsing or flail leaflets. Multiple color Doppler regurgitant jets are consistent with leaflet fenestrations. Masses, calcification, rheumatic disease, or radiation therapy reduces leaflet mobility. Secondary causes include aortic root pathology such as dilation, dissection, and infection in which the aortic leaflets may be involved or appear normal (see Figs. 11.61 and 11.65 ). Determining the cause of AI is important to determine the best mode and timing of therapy, and whether the valve can be repaired or requires replacement.
| Mild | Moderate | Severe | |
|---|---|---|---|
| Angiographic grade | 1+ | 2 + | 3–4+ |
| Jet density/appearance | Faint/incomplete | — | Dense/complete |
| Color Doppler jet width/LVOT width | <0.25 | 0.25–0.65 | >0.65 |
| Doppler vena contracta (cm) | <0.3 | 0.3–0.6 | >0.6 |
| Pressure half-time (ms) | >450 | 250–450 | <250 |
| Pulsed-wave Doppler ascending/descending aorta | — | — | Reversal |
| Regurgitant volume (mL/beat) | <30 | 30–60 | >60 |
| Regurgitant fraction (%) | <30 | 30–60 | >60 |
| Regurgitant orifice area (cm 2 ) | <0.3 | — | >0.3 |
| Secondary findings | LAE, LVE, LVSD, PHTN | ||
Prosthetic valves have a “normal” signature or expected regurgitant jets and should be differentiated from “abnormal” flows. Normal or nonproblematic regurgitant jets have a VC less 3 mm in width and do not extend far from the valve. , , , , Bioprosthetic valves typically display a narrow central regurgitant jet, homografts should have no AI, while bileaflet mechanical leaflets display a variety of regurgitant jets depending on the valve type. Imaging parallel to the mechanical valve leaflets will allow better visualization of the normal two regurgitant jets directed centrally. A single-leaflet valve (or tilting disk valve) has one larger and one smaller jet, while the ball-in-cage valve will have a surrounding or circular series of small jets. Abnormal paravalvular regurgitant jets are greater than 3 mm in width and/or are eccentrically directed away from the middle. Smaller jets may also be considered abnormal, but in the absence of obvious pathology (mobile materials; valve instability), these do not tend to progress over time. All paravalvular jets are abnormal; however, if the VC is less than 3 mm, then they are not likely to progress.
Etiology of prosthetic valve incompetence is similar to that of native valves and includes endocarditis, leaflet deterioration or degeneration, restricted leaflet mobility, or valvular dehiscence/abscess. As with all prosthetic valves, the defect may occur within the aortic prosthesis or around it (paravalvular).
Repair of the native AV is generally applied to regurgitant valves. , , , Although the goal is to eliminate regurgitation, mild or less AI is acceptable (see Fig. 11.65 ). The location and direction of the regurgitant jet depends on the prerepair pathology and the repair technique. The repair can be assessed before separating from cardiopulmonary bypass by filling up the proximal aorta and generating pressure on the valve (see Fig. 11.65 ). Either by noting spontaneous echo contrast or with the color gains adjusted lower, it is possible to assess residual regurgitation prior to separating from cardiopulmonary bypass. Long-term outcome (absence of recurrent AI and/or need for reoperation) is predicted by the immediate postrepair absence of leaflet prolapse, a coaptation length >6 mm, and no residual AI ( Figs. 11.65 and 11.71 ). , ,
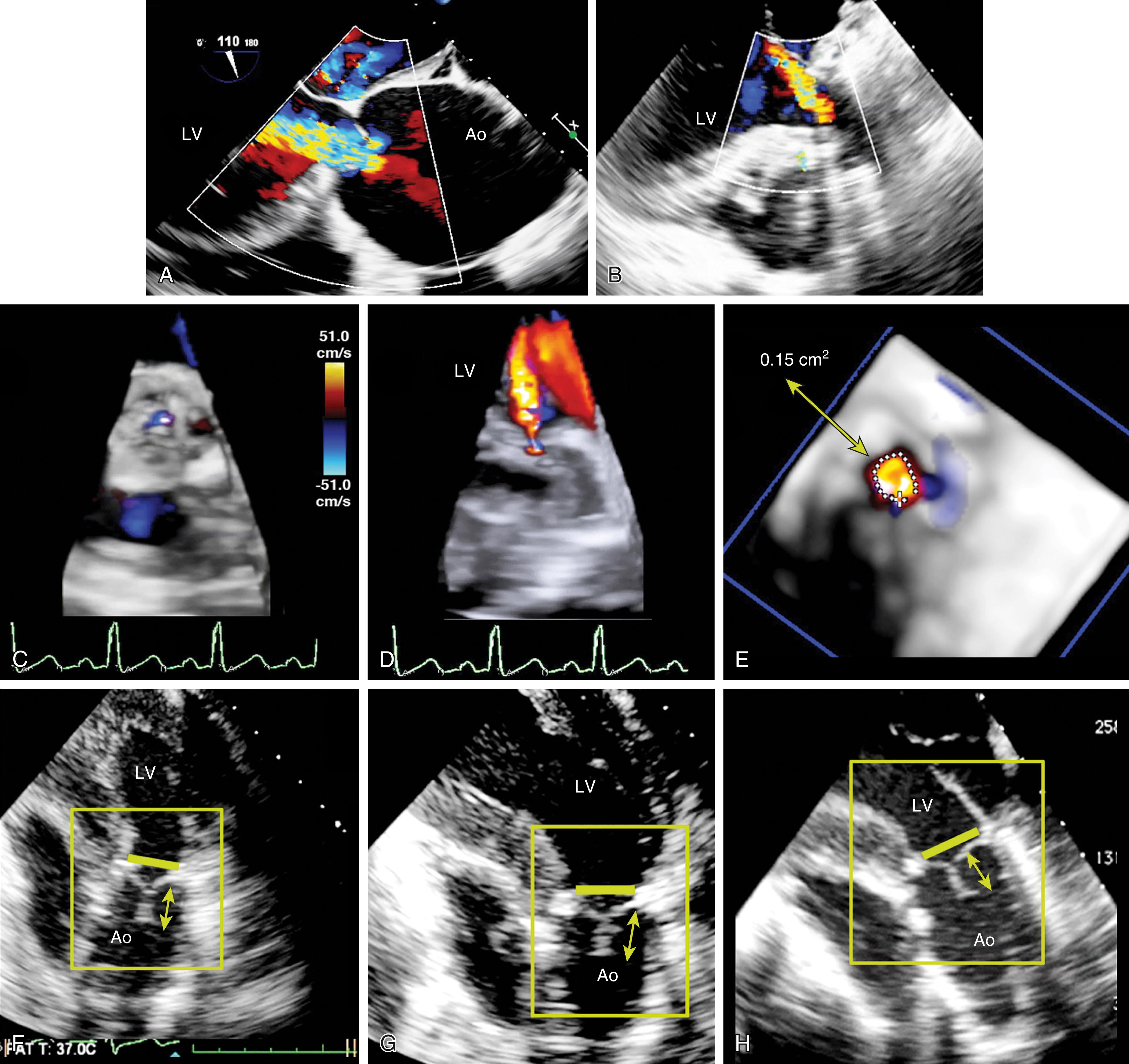
Assessing regurgitant flow of the AV is similar for both native and prosthetic valve disease (see Table 11.6 ). It is necessary to know the range of normal native valve function and signature flows for prosthetic valves. Two similar prosthetic valves from different companies may have different signature jets. Assessing AI severity begins with a qualitative assessment using CFD to identify the presence of a regurgitant jet direction. Color Doppler jet area is less accurate to quantify AI severity; therefore other indirect and direct methods are employed. Measurement of the VC (narrowest jet width just beyond or downstream to the aortic regurgitation [AR] orifice) and the ratio of the proximal jet width to the width of the LVOT attempt to assess the actual defect or as close to the regurgitant orifice as possible (see Fig. 10.68 ). The limitations to the VC and jet width ratio occur when either the LVOT cannot be seen clearly due to imaging artifact from prosthetic materials or when the AI jet is either eccentric and/or complex. The PHT or DT is used to assess the pressure changes across the regurgitant orifice as a reflection of the ROA. A small PHT (<450 ms) reflects more than mild AI ( Fig. 11.72 ). However, the PHT is affected by net chamber compliance. The PHT is shortened with increasing LV end-diastolic pressure, vasodilator therapy, and in patients with a dilated compliant aorta. It is lengthened in chronic AI.
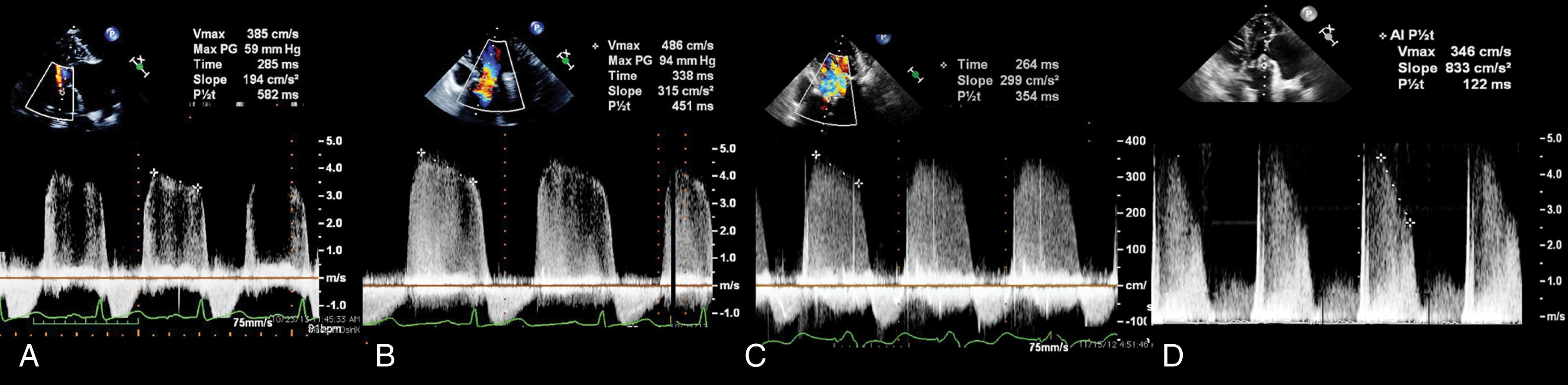
The PISA method has been utilized to measure ROA and regurgitant volume ( Fig. 11.73 ). If the ROA were multiplied by the TVI (CW Doppler), the result would be the volume flowing across the orifice in each cardiac cycle. Qualitatively, the presence of a flow convergence area suggests that more than mild AI is present.
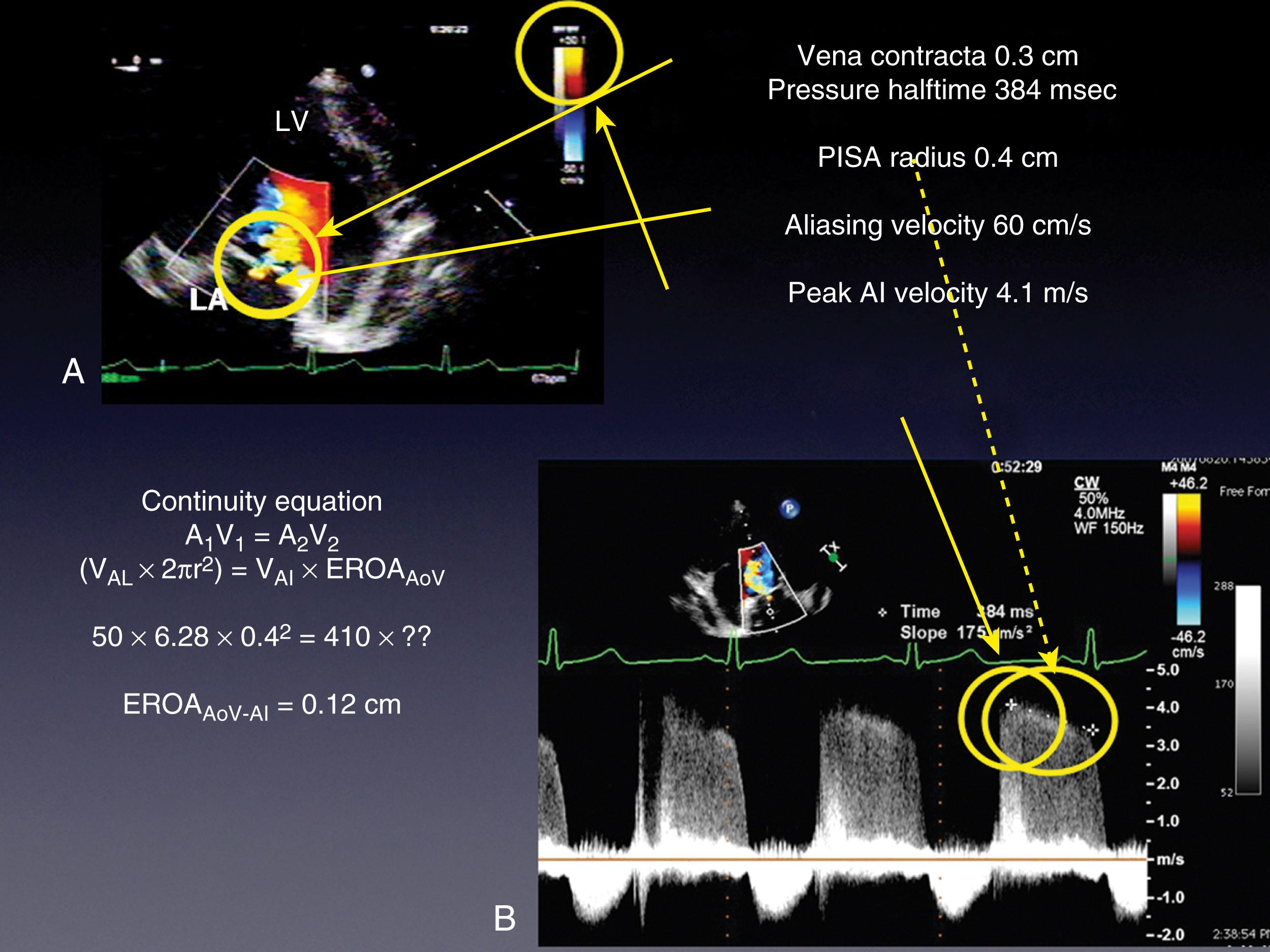
Other Doppler methods include documentation of diastolic flow reversal (severe AI) in the ascending or descending aorta, the former being more sensitive, while the latter is more specific. The continuity equation may be used to determine the regurgitant volume as the difference between the SV across the PA and the AV.
When feasible, combining color Doppler with 2D or 3D imaging, the ROA can be measured by planimetry. A full-volume 3D color data set allows MPR analysis of the narrowest portion of the regurgitant jet. Visualization of the three levels of the regurgitant jet is useful to better define the anatomy and severity of AI. While qualitative imaging includes both ME and TG windows, most of the quantitative assessment is best achieved from TG windows where blood flow and the Doppler beam can be best aligned to allow measurement of the maximum flow velocities (see Figs. 11.63 and 11.71 ).
Assessing severity of regurgitant flow , is similar for native and prosthetic AVs; however, artifacts may hinder specific or direct measures of the regurgitant orifice (see Table 11.6 ). Measurement of proximal regurgitant jet widths is simple but less accurate than MPR analysis. Regurgitant jet widths less than 3 mm are either consistent with normal regurgitant flows or, if abnormal, is not likely to progress. CW Doppler profile of the AI jet can qualify severity. A dense and more complete profile supports more than mild AI. Quantitative measures supporting more than mild AI include VC greater than 3 mm, regurgitant jet width/LVOT diameter ratio higher than 25%, PHT less than 400 ms, ROA greater than 0.3 cm 2 , regurgitant volume greater than 30 mL/beat, and a regurgitant fraction greater than 30%. Severe AR is suspected with a VC greater than 0.6 cm and an ROA greater than 0.6 cm 2 . Diastolic flow reversal in the aorta is consistent with more than moderate AR. Qualitatively, the presence of flow convergence suggests the presence of more than mild AI. Using the flow convergence method, mild, moderate, and severe AR are defined as ROA of less than 15 mm 2 , 15 to 30 mm 2 , and greater than 30 mm 2 , respectively. These ROAs are associated with regurgitant volumes of 30, 30 to 60, and greater than 60 mL/beat.
Secondary changes in cardiopulmonary function depend on the acuity and severity of AI and how the system is able to compensate. Severe acute AI causes a volume overload without time to compensate, causing increased left heart pressures, pulmonary hypertension, pulmonary edema, and right heart failure. By contrast, chronic AI allows time to compensate for the increased load on the left heart. Initially, ventricular hypertrophy and increased contractility maintain hemodynamic functions; however, over time, ventricular dilation and contractile dysfunction follow. Left heart compliance remains stable, and LV and PA pressure remain low to mildly elevated until late in the course.
With the goal of preventing long-term dysfunction, surgical treatment of AI should be performed ( Fig. 11.74 ). Patients with symptomatic severe AI should undergo replacement or repair if feasible. Surgery is also indicated for asymptomatic patients with severe AI and LV systolic dysfunction defined as an LVEF less than 55%, and/or a dilated LV described as an LV systolic diameter larger than 50 mm or LV end-diastolic diameter greater than 65 mm. Surgery is also recommended for asymptomatic severe AI in patients undergoing other cardiac surgical and/or ascending aortic procedures.
Changes in the native AV architecture occur with age including leaflet thickening found in more than 20% of patients older than 65 years. There is a 4% incidence of AS found in patients older than 85 years. The vast majority of fixed native AS is due to elderly (or senile) calcific trileaflet pathology. , This is followed by bicuspid AS and rheumatic AS (see Figs. 10.56 and 10.57 ; ; Table 11.7 ). Less common outflow obstructions are due to unicuspid valve, supravalvular stenosis (e.g., Williams syndrome), and subvalvular obstruction, such as a subvalvular membrane (see Fig. 11.70 ). Hypertrophic obstructive cardiomyopathy is a dynamic form of obstruction that should be distinguished from fixed forms of obstruction as management is different (see Chapter 18 ). There can be coexistence of obstruction at different levels.
| Mild | Moderate | Severe | |
|---|---|---|---|
| Jet velocity (m/s) | <3.0 | 3.0–4.0 | >4.0 |
| Acceleration time (ms) | <100 | >100 | |
| Mean pressure gradient (mm Hg) | <25 | 25–40 | >40 |
| AV area (cm 2 ) | >1.5 | 1.0–1.5 | <1.0 |
| AV area index (cm 2 /m 2 ) | >0.85 | 0.85–0.65 | <0.65 |
| Velocity ratio (TVI lvot /TVI A v ) | 0.35–0.30 | 0.30–0.25 | <0.25 |
| Secondary findings | LAE, LVH, PHTN, RAE, RVE, TR | ||
Trileaflet calcific AS is characterized by calcium collection on the leaflet mostly at the leaflet tips and proximal. Rheumatic stenosis involves commissural fusion and thickening of the trileaflet valve with a central triangular orifice. Rheumatic AV disease most often occurs in conjunction with MV disease. Bicuspid AS results from fusion of the commissures with or without calcification. The most common configuration of a bicuspid AV is fusion of the right and left commissures with or without a raphe seen. Fusion of the left and noncoronary cusps is least common. A subvalvular membrane may be a simple protrusion of tissue or a circumferential narrowing of the subvalvular space. Supravalvular stenosis appears as an hourglass-shaped deformity in the supravalvular space with or without irregularities of the coronary arteries. Delineating where the obstruction occurs is extremely important to therapy decision-making.
Mild prosthetic AS is not uncommon with the majority of prosthetic valves placed. , Prosthetic valve areas are less than a normal native AV in the same annular space. Although AVA data vary among different prosthetic valves, in general, a 19-, 21-, or 23-mm prosthetic AV yields an EOA of approximately 1.1, 1.3, and 1.5 cm 2 , respectively (see Table 11.3 ). Abnormal prosthetic valve stenosis is suspected based on symptoms of heart failure and fatigue, as well as recorded high gradients and/or lower than expected AVA. Causes of prosthetic AS include thickened and/or restricted leaflet motion or mass (pannus, thrombus, infection) obstruction ( Fig. 11.75 ). , Causes of an elevated gradient in the presence of a normally functioning valve include subvalvular obstruction (hypertrophic obstructive cardiomyopathy or a subvalve membrane), supravalvular obstruction, or PPM.
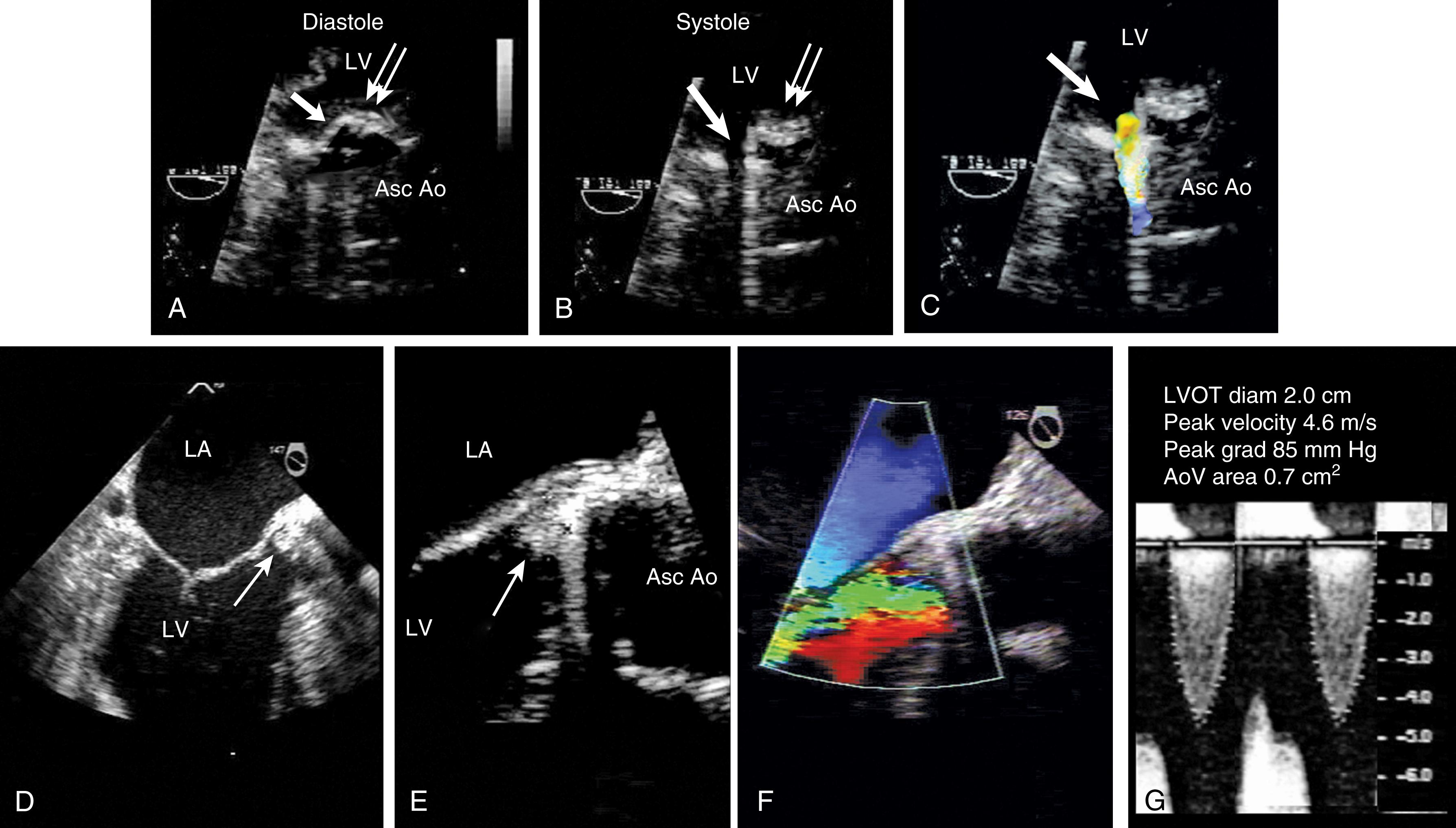
Outflow obstruction causes an increase in LV stress and pressure. To compensate (i.e., maintain forward flow), left ventricular hypertrophy develops and the ventricular contractility increases to overcome the resistance. Although the left ventricular hypertrophy is an important compensation, it also results in diastolic dysfunction and a smaller internal chamber volume. With further progression, hemodynamic function changes and stability becomes more dependent on a regular heart rate with atrial contraction, adequate diastolic filling time, and maintaining ventricular preload. Continued increase in wall stress, left ventricular hypertrophy, and diastolic dysfunction is accompanied by increased myocardial oxygen consumption (MVO 2 ), with reduced coronary flow reserve increasing the risk of ischemia, contractile dysfunction, and hemodynamic instability. Myocardial fibrosis develops replacing more normal myocardium, hemodynamic and clinical decompensation occurs, and regression of left ventricular hypertrophy is less likely to occur even after surgical relief of the obstruction. This is further complicated by secondary changes such as increased LA size and dysfunction with arrhythmias, pulmonary hypertension, and pulmonary edema, and ultimately right-sided heart dysfunction.
Assessing forward flow across the native and prosthetic AV is similar and defines valvular hemodynamics and severity of dysfunction (see Table 11.7 ). A normal contour of the AV flow velocity profile is a low-velocity (<1.5 m/s), laminar, centrally directed, early peaking (peaking after one-third of the ejection period) asymmetric triangular shape ( Fig. 11.76 ). Prosthetic valves project forward flow in different ways depending on their configuration. While bioprosthetic valves have a single forward flow similar to native valves, mechanical valves can have two (single-leaflet) or three (bileaflet) forward projecting jets. Knowledge of expected normal flows helps differentiate them from abnormal valvular flows.
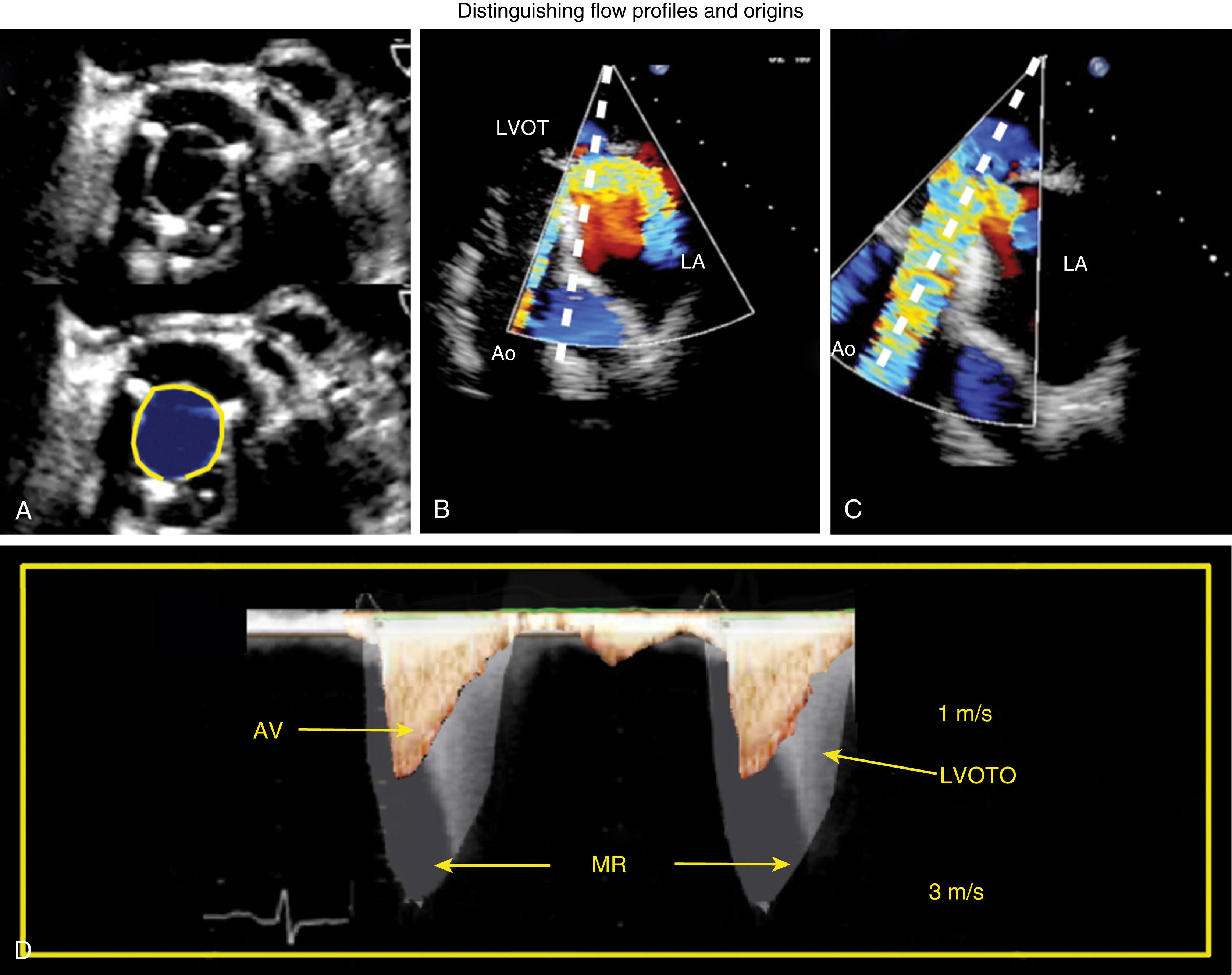
Qualitative assessment of forward flow mainly employs color and PW Doppler imaging to identify and locate turbulent or high-velocity flows (aliasing or exceeding the Nyquist limit) directing the location for further quantitative analyses. Defining the site (s) and severity of obstruction as either subvalvular (subvalvular membrane or hypertrophic obstructive cardiomyopathy), valvular, and/or supravalvular assists in defining a therapeutic plan. Since coexistent AI increases forward flow, velocities, and gradients, assessing AI severity will contribute toward a comprehensive assessment of obstruction severity.
For high-flow states associated with left ventricular outflow obstruction, CW Doppler is used to quantify velocities and gradients. The CW Doppler profile of AS appearing as relatively symmetric crescendo-decrescendo, with a delayed rounder peak compared to the normal native valve, can be considered (see Figs. 10.59 and 10.60 ; ). , The acceleration time is longer or delayed (>100 ms) compared to the relatively short period to the peak velocity ( < 80 ms) seen with a normal AV. , Given the potential overlap and proximity to other high-velocity systolic flows, the profile for AS should be distinguished from those of subvalvular LVOT obstruction and MR ( Figs. 11.76 and 11.77 ).
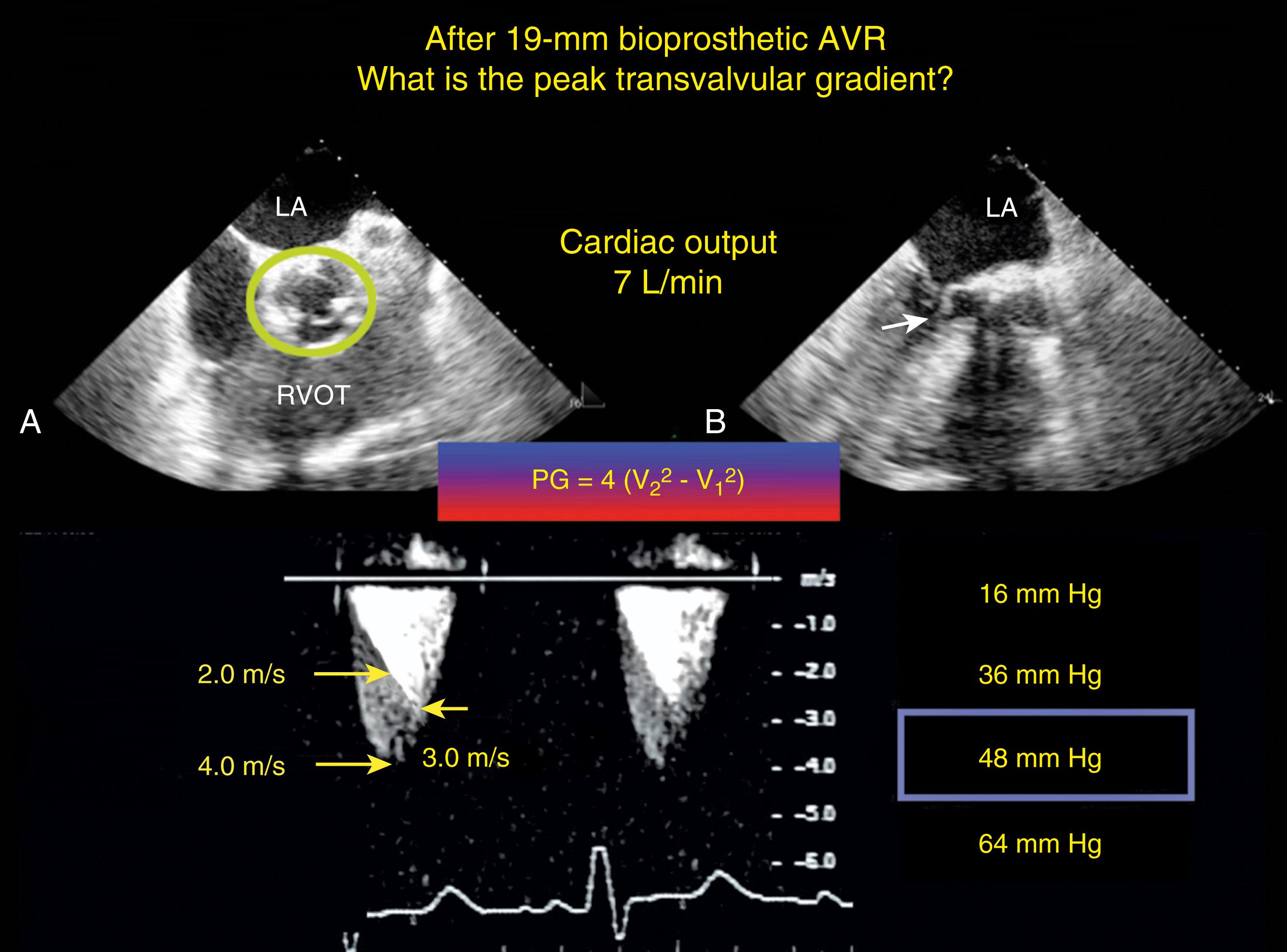
The American Society of Echocardiography level 1 recommendations for quantitative AS assessment include measurement of transvalvular jet velocity, calculation of the mean transvalvular gradient, and assessment of the AVA. The former two are affected by chamber pressures and flows. , The latter can be directly measured using planimetry or calculated using the continuity equation.
Outcome data and treatment algorithms are based on transvalvular jet velocities and valve area. A velocity greater than 4 m/s is considered severe and aortic valve replacement (AVR) is recommended. For intermediate velocities, other considerations are included in the decision algorithm such as the AVA, coexisting AI, and evaluation for conditions that might reduce forward flow velocities (see below). Jet velocity, and therefore gradient, is dependent on blood flow or CO, as well as the ability to align the ultrasound beam with blood flow. Since peak and mean gradients are calculated from velocities using the Bernoulli equation, caution must be considered when the subvalvular (LVOT) velocity is elevated (>1.4 m/s) at which point the subvalve velocity should be included in the modified Bernoulli equation in the calculation of tansvalvular gradients. As many as 10% to 15% of AS cases may be associated with subvalvular outflow narrowing or obstruction (see Fig. 11.77 ).
Although a mean gradient less than 40 mm Hg does not suggest severe AS, it does not rule it out either. Measuring the AVA by planimetry or calculating it by using the continuity equation may define more accurately the severity of AS by providing an anatomic area or EOA, respectively. An AVA <1.0 cm 2 is considered severe AS. Planimetry is more commonly performed from the ME AV short-axis window. Accurate planimetry may not be possible owing to degree of calcification, imaging artifacts, and the inability to visualize the inner edges of the AV in a planar fashion. The continuity equation (conservation of mass) allows calculation of the AVA from Doppler data and a measurement of the LVOT diameter (subvalvular). Clinical data show that peak velocities (V LVOT and V AV ) can be substituted for the TVI of the respective sites. ,
Here r is the blood density. Since the density of blood does not change, r can be removed.
A potential source of error in the calculation of AVA by continuity equation is the need to measure the diameter of the LVOT, which is assumed to be circular, which, alone, is a potential error. Two-dimensional measurements have a 5% to 10% error. When the LVOT diameter is halved (radius) and then squared, the total error may be as great as 15.20%. An AVA of less than 1.0 cm 2 is considered severe AS. There is ample three-dimensional data reporting that 85% to 90% of LVOT is elliptical and that that LVOT diameter measured during 2D imaging represents the short-axis diameter of the LVOT. ,
To avoid errors of the 2D LVOT measurement, a simplified dimensionless index (DVI or velocity ratio [VR]) inherent in the earlier continuity equation (V 1 /V 2 or TVI 1 /TVI 2 ) compares LVOT and AV flows using either TVIs or peak velocities.
A value less than 0.25 is consistent with severe AS. For the great majority of patients, the LVOT diameter is less than 2.2 cm. If inserted into the continuity equation, a VR of 0.25 would yield an AVA less than 1.0 cm 2 if applying a diameter of <2.2 cm to the area calculation. A VR of 0.2 would be consistent with severe AS even for an LVOT diameter as large as 2.5 cm.
Parameters consistent with abnormal (more than mild) prosthetic AV obstruction are similar to that of the native AV. A transvalvular jet velocities greater than 3 m/s, mean gradient greater than 25 mm Hg, and an acceleration time greater than 100 ms are consistent with prosthetic valve stenosis. , As with native valves, valve function should be quantified by calculation of the AVA. For all assessments, hemodynamic data should be recorded to help define the meaning of Doppler data.
A DVI or VR less than 0.35 suggests prosthetic valve stenosis and should prompt further evaluation. While a VR greater than 0.35 for smaller prosthetic valves (19 and 21 mm) suggests normal opening, larger valves are associated with higher VR (>0.40). ,
The valve area should be considered in the context of each individual case and indexed per BSA. An AVA of 1.0 has a different impact on a patient with a BSA of 1.5 as compared to one with a BSA of 2.0. An indexed AVA (AVAi) less than 0.65 cm 2 /m 2 is consistent with severe AS, and an AVAi greater than 0.85 cm 2 /m 2 is consistent with mild AS. PPM describes the mismatch between the area of the prosthetic valve selection (AVA) and the patient’s size (BSA).
Data suggest that short- and long-term outcomes are improved with a properly sized valve. Adverse outcomes are seen with severe PPM (i.e., <0.65 cm 2 /m 2 ). PPM may also have less clinical impact on older adults (older than 75 years) and/or smaller patients (BSA <1.75 m 2 ).
Analyses should be performed at a time when patient hemodynamics (CO/SV) are optimized or at least known. This is especially important for the condition known as low-gradient AV stenosis:
Low-gradient AV stenosis is seen in patients with a reduced SV across the valve, which occurs as a result of reduced LVEF (<40%), CO or SV, arrhythmias, and significant MR. For such cases, calculation of AVA is necessary. If the AVA is less than 1.0 cm 2 , a stress echocardiogram (either exercise or dobutamine) should be considered to differentiate between true AS and pseudo-AV stenosis, the latter being the result of reduced opening forces. Hemodynamic goals of the stress test include an increase in the heart rate of either 10 to 20 beats per minute, an increase of more than 100 beats per minute, and/or an increase in SV or EF of 15% to 20%. True AS is characterized by a rise in the mean gradient greater than 40 mm Hg with a persistent AVA less than 1.0 cm 2 . An increase in the AVA greater than 1.0 cm 2 supports pseudo-AV stenosis. An absence of a hemodynamic response to stress (ie, no change in LVEF) suggests a lack of contractile reserve, which is associated with increased morbidity and mortality with surgery.
Other conditions that affect the echocardiographic-derived gradient include poor alignment of the ultrasound beam with blood flow, which may underestimate the true flow velocity and subsequently calculated gradient.
Increased transvalvular velocities can be seen in the presence of AI and/or a high-flow state. A higher jet velocity may be recorded and overestimate the severity of AV obstruction. Given the proximity of the MV during TG Doppler interrogation of the LVOT and AV, erroneous measurement of the mitral regurgitant jet will cause overestimation of the true transaortic valve velocity. The two flow profiles can be differentiated in that MR is a holosystolic jet compared to a decrescendo-crescendo flow profile of AS (see Fig. 11.76 ). Finally, sequential narrowings (subvalvular or supravalvular stenoses or obstructions) affect detection of the gradient across the AV (see Fig. 11.77 ).
Another issue regarding Doppler assessment of transvalvular gradients is poor alignment between the Doppler ultrasound beam and blood flow, which causes an underestimation of flow velocity and calculation of pressure gradients.
The peak gradient during echocardiography is an instantaneous peak gradient and is larger than that measured during catheterization, which is the gradient between the peak transvalvular pressure and the peak LV pressure. During catheterization, the two peaks occur at different times.
A phenomenon called pressure recovery results in differences between gradients measured during Doppler echocardiography and cardiac catheterization. When flow crosses the stenotic AV, potential energy is converted to kinetic energy and a high pressure gradient is recorded. , However, downstream from the stenotic valve, energy is lost to turbulence and viscous forces. Energy may be converted back to potential energy, causing a rise in pressure or pressure recovery. The amount of pressure recovery is related to the differences in the cross-sectional area (CSA) of the ascending aorta.
Pressure recovery is related to the ratio of the EOA/AoA or the EOA of the AV and the CSA of the ascending aorta. Pressure recovery is most significant for patients with an ascending aortic diameter less than 3.0 cm.
Although not often discussed for SAVR, the location of the coronary ostia is a critical part of the evaluation prior to TAVI. During SAVR, the surgeon can identify the coronary ostia and position the prosthetic valve and struts to avoid any obstruction. This is not the case during the insertion of the TAVI valve for which a circumferential supporting curtain, the height of which ranges from 5 to 10 mm, can cause immediate or delayed coronary ostial obstruction. Approximately 90% to 94% of the time, the left and right coronary ostia take off below the STJ, and 10% of the time (L > R), the ostia take off within 10 mm of the AV annulus, increasing the risk of ostial obstruction. Additional risk of coronary ostial occlusion includes a small sinus of Valsalva (diameter <30 mm). Identifying the location of the coronary ostia and the root size are important to define coronary risk for TAVI, but are also important to define the same risk during SAVR.
Surgical or transcutaneous AVR is indicated for symptomatic severe AS ( Fig. 11.78 ). Surgical AVR also should be considered for asymptomatic patients with severe AS, or patients with moderate AS with a likely rapid progression (>0.1 cm 2 /year), while undergoing other cardiac or aortic procedures. Elective AVR for asymptomatic patients with severe AS is considered to prevent irreversible myocardial changes. AVR for low-gradient (mean <40 mm Hg) severe AS may require assessment of reserve function, ie, viability studies. The decision to perform SAVR or TAVI is an evolving discussion that balances age risk and longevity of the patient, risk of coronary obstruction and future need for coronary intervention, and the durability of the prosthetic valve ( Fig. 11.79 ). ,
The risk of coronary artery obstruction is significant for both SAVR and TAVI, perhaps more for TAVI given the circumferential curtain. Nevertheless, assessment of coronary height (annulus to ostia) is important to predict and prepare prior to AVR.
The normal TV area is 4 to 6 cm 2 and the normal annular diameter is less than 28 mm. The TV has three unequal-sized leaflets (septal, anterior, and posterior) surrounded by a 3D saddle-shaped ellipsoid annulus that contracts and changes its shape and area during the cardiac cycle. ,
The septal leaflet is attached to the medial or septal wall, which is in continuity with the fibrous trigone of the heart. The posterior leaflet is the smallest of the three leaflets. The anterior leaflet is the largest. The leaflets are attached via chordae to three respective papillary muscles and each papillary muscle gives off chords to at least two leaflets. Congenital variations range from two to nine papillary muscles and as many as six leaflets.
The function, shape, size, and planarity of the annulus are affected by changes in right atrial (RA) and RV size, shape, and function. As the annulus dilates, it becomes more planar, circular, and less contractile. The septal region is attached to the fibrous trigone, it stays relatively stable, however, while the antero-lateral annular regions dilate away from the fibrous skeleton, and is associated with the development of TR.
The importance of TV function and its impact on right-sided heart function and patient outcome is increasingly appreciated. As a result, TV surgery has increased either alone or in association with surgical procedures on the left side of the heart. The vast majority of TV surgery is for regurgitant valves; however, both severely regurgitant and stenotic valves result in clinically relevant right heart failure and warrant surgical consideration. , Moderate TV dysfunction should receive consideration for surgery when patients are undergoing cardiac surgical procedures for the left side of the heart.
The majority of cases are due to functional or secondary TR, and more than 80% are repaired. Valve replacement may be considered for cases with high risk of repair failure. Valve replacement is performed for TV stenosis, primary valve disorders (endocarditis, rheumatic valvitis, carcinoid syndrome, traumatic rupture), nonrepairable regurgitant valves, or for clinically relevant recurrent regurgitation after repair. , Details of the surgical procedure complement the postcardiopulmonary bypass echocardiographic evaluation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here