Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The prognosis for patients with symptoms due to severe valvular aortic stenosis treated medically is poor, with death rates as high as 51% at 1 year and 68% at 2 years for a series of patients at very high risk for surgical invention.
Balloon-expandable and self-expanding bioprosthetic valves mounted in a mesh frame are the most commonly used transcatheter heart valves. Many new valve technologies are in development and undergoing clinical investigation.
Data from multiple randomized trials indicate that transcatheter aortic valve replacement (TAVR) results in higher 1- and 2-year event-free survival rates than medical therapy, regardless of estimated surgical risk.
Evaluation of potential TAVR candidates requires a collaborative, multidisciplinary approach by a heart valve team that includes interventional cardiologists with expertise in structural heart disease, cardiac and vascular surgeons, anesthesiologists, imaging specialists, and specialized nurses.
Evaluation of patients should identify those for whom significant improvement in survival and quality of life is likely. Comprehensive evaluation, including neurocognitive assessment, frailty, functional status, and social support, is important.
Transthoracic and transesophageal echocardiography, cardiac computed tomography, and invasive angiography are used to perform anatomic evaluations necessary for procedural planning before TAVR.
Randomized trials and large registries of TAVR indicate procedural success rates of more than 95%, 30-day survival rates of more than 95%, meaningful improvement in quality of life, and acceptable complication rates (e.g., procedure-related stroke < 2%, vascular access site complications < 5%, permanent pacemaker rates ≈ 5%). TAVR has been validated as an equal or superior alternative to surgical aortic valve replacement across the spectrum of surgical risk.
Expanding uses for TAVR in patients with low-flow, low-gradient aortic stenosis, bicuspid aortic valves, moderate aortic stenosis with left ventricular systolic dysfunction, and asymptomatic severe aortic stenosis are areas of active investigation.
More than 300,000 TAVR procedures have been performed. Alternatives to TAVR include surgical aortic valve replacement and balloon aortic valvuloplasty.
Patients with symptomatic valvular aortic stenosis (AS) have a poor prognosis without intervention. In one series, these individuals, when treated with medical therapy alone (including balloon aortic valvuloplasty [BAV]), had death rates of 51% at 1 year and 68% at 2 years. Treatment entails valve replacement by open surgical aortic valve replacement (SAVR) or transcatheter aortic valve replacement (TAVR). SAVR for severe AS has been the gold standard for decades and offers excellent long-term results. However, during the past decade, TAVR has emerged as an effective and minimally invasive treatment for aortic valve disease. Although percutaneous TAVR was initially reserved for patients at excessive risk for traditional surgery, , it is now commonly used for patients at low, intermediate, and high risk for surgery and has outcomes that are similar or superior to those of the traditional surgery. ,
BAV can be used as a temporizing measure for the treatment of severe AS, but it does not provide a definitive solution. Initial results were disappointing, with neither hemodynamic nor clinical improvement over the long term. BAV is recommended as a palliative therapy or as a bridge to SAVR or TAVR in patients with severe symptomatic AS.
TAVR started with the work of Andersen and colleagues in pigs in 1992 and was followed by the work of a number of other groups. Cribier and associates reported the first implantation of a transcatheter aortic valve in humans in 2002. In 2006, Webb and coworkers first described delivery of a transcatheter aortic valve by a retrograde approach through the femoral artery, a technique that has become the predominant approach today. From these initial advances, the field rapidly evolved as a result of groundbreaking technologic advances and robust clinical research.
TAVR has become a safe and common procedure used widely across the developed world. Since its U.S. Food and Drug Administration (FDA) approval in the United States in 2011, more than 150,000 TAVR procedures (commercial and research) have been performed. , TAVR is approved for the treatment of native calcific AS, and its use as a treatment for expanding indications, including native aortic regurgitation (AR), bicuspid aortic valve disease, and degenerated bioprosthetic valves, continues to provide areas of active clinical research.
This chapter presents available valve designs, summarizes outcome data from TAVR randomized trials and registries, and discusses issues related to patient selection in adults with AS, and describes future directions of the field.
Two types of aortic valves for percutaneous implantation are most commonly used and commercially available: balloon-expandable valves and self-expanding valves ( Fig. 12.1 ).
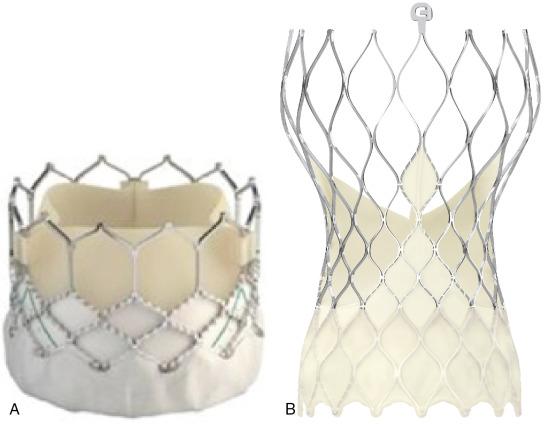
Balloon-expandable prostheses for human implantation include the first-generation Cribier-Edwards valve, the modified second-generation Sapien (i.e., Sapien and Sapien XT) series of valves, and the third-generation Sapien 3 valve (all from Edwards Lifesciences Corp., Irvine, CA).
The Edwards Sapien 3 transcatheter heart valve comprises a balloon-expandable cobalt-chromium alloy tubular frame, within which are sewn bovine pericardium leaflets. It has a low delivery profile (14 Fr for 20-, 23-, and 26-mm valves and 16 Fr for the 29-mm valve) and an outer skirt that is designed to minimize paravalvular leaks (PVLs) (see Fig. 12.1 ). For transarterial implantation, the transcatheter valve is crimped onto a Commander delivery catheter (Edwards Lifesciences) and introduced through a sheath placed in the femoral artery. When transfemoral access is not feasible, multiple alternative access sites have been described, including the subclavian artery, carotid artery, transcaval access using the inferior vena cava to obtain access to the abdominal aorta, suprasternal access, direct aortic access, and transapical access (through the left ventricular [LV] apex).
The Sapien 3 valve is balloon expanded within the diseased native valve under rapid ventricular pacing, displacing the diseased native leaflets and anchoring in the calcium of the native aortic annulus. Whereas the Sapien 3 valve is delivered by a 14- or 16-Fr sheath, the older-generation Sapien XT/NovaFlex transfemoral system used a 16- or 19-Fr sheath. The earliest-generation devices used in the Placement of Aortic Transcatheter Valve (PARTNER) trial (discussed later) required the use of larger-diameter (22–24 Fr) sheaths.
The self-expanding valve with the longest history and the most published data is the CoreValve system (Medtronic, Minneapolis, MN). The iteration of the CoreValve with the most contemporary data is the Evolut R (see Fig. 12.1 ), a supra-annular valve made from porcine pericardium that features a self-expanding nitinol frame and an extended scalloped sealing skirt that conforms and seals to the native aortic annulus to minimize paravalvular regurgitation. The Evolut-R system can be recaptured to optimize positioning before final valve deployment and does not require rapid ventricular pacing at the time of deployment.
The CoreValve Evolut R is compressed within an EnVeo R delivery system catheter (Medtronic) and introduced into the femoral or subclavian artery through a 14-Fr–equivalent system for the 23-, 26-, and 29-mm valves or a 16-Fr–equivalent system for the 34-mm valves. After the valve is positioned correctly within the diseased native valve, the delivery catheter is withdrawn, releasing the valve. The multistage frame is anchored within the aortic annulus, but because of its length, it also extends superiorly to anchor in the aorta above the coronaries. The latest iteration of this valve is the Evolut Pro valve, which has an external pericardial wrap designed to reduce paravalvular regurgitation. This system requires adequate vascular access to allow for a delivery system that is 2-Fr larger than the comparable Evolut R valve.
Other well-studied valve systems are displayed in Fig. 12.2 . The Lotus valve (Boston Scientific, Natick, MA) ( Fig. 12.2 D) has a braided nitinol frame with bovine pericardial leaflets that is deployed by controlled mechanical expansion within the annulus. It is fully repositionable before release and has an adaptive seal on the outside of the valve to improve sealing and reduce paravalvular regurgitation. The latest iteration, Lotus Edge, features technology that aims to minimize the depth of deployment and interaction in the left ventricular outflow tract, potentially lowering the need for permanent pacemaker placement. It has been approved for commercial use in patients at high surgical risk and is under active investigation for those at intermediate surgical risk.
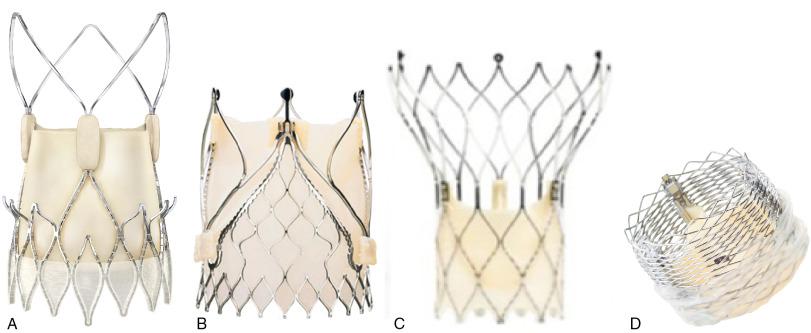
The Portico transcatheter aortic heart valve (St. Jude Medical, St. Paul, MN) (see Fig. 12.2 C) is a self-expanding, nitinol-based valve that is made of bovine pericardium, delivered transfemorally, and fully repositionable and retrievable. Similar to the CoreValve, it extends from the annulus to the supracoronary aorta to assist in coaxial alignment and fixation. In contrast to the CoreValve leaflets, which are supra-annular, the leaflets with the Portico device are intra-annular.
The Acurate neo valve (Symetis/Boston, Ecublens, Switzerland) (see Fig. 12.2 A) is a transfemoral aortic bioprosthesis composed of a porcine pericardial tissue valve sutured within a self-expanding nitinol stent covered by a pericardial skirt on the inner and outer surface of the stent body to improve sealing and reduce PVL. The valve is supra-annular but is designed to have a smaller footprint in the ascending aorta and therefore provide easier access to the coronaries. Transfemoral and transapical delivery systems are available.
The JenaValve (JenaValve Technology, Irvine, CA) (see Fig. 12.2 B) is an aortic porcine root valve mounted on a nitinol, self-expanding stent that can be delivered by a transfemoral or transapical approach. The frame design is unique in that it anchors by clipping onto the native leaflets rather than by radial force in the left ventricular outflow tract. It is currently the only transcatheter aortic valve that has been approved for the treatment of AS and AR. The mechanism of anchoring may also help protect against important complications such as coronary obstruction by holding the native leaflets away from the coronary ostium.
The Edwards Sapien, Medtronic CoreValve, and Boston Scientific Lotus systems are approved for commercial use by the FDA in the United States. The other platforms are commercially available in Europe. Clinical trials are ongoing in the United States for evaluation of the Lotus and Portico valve systems.
The primary results of the PARTNER trial at 1 and 5 years are presented in Table 12.1 . , , , This trial used early-generation TAVR systems (i.e., Sapien 23-mm and 26-mm valves with 22-Fr and 24-Fr femoral delivery catheters) in centers with limited operator experience with TAVR.
| TRIAL | Risk Category | Mean Age (yr) | % Male | Mean STS Score (%) | No. of Patients | Device Studied and Comparator | Primary End Point | Rate of Primary End Point (%) |
|---|---|---|---|---|---|---|---|---|
| PARTNER | High | 83 | 46 | 11.8 | 699 | Sapien vs. SAVR | Death at 1 yr |
|
| CoreValve U.S. Pivotal | High | 83 | 53 | 7.4 | 747 | CoreValve vs. SAVR | Death at 1 yr |
|
| PARTNER 2 | Intermediate | 82 | 54 | 5.8 | 2032 | Sapien XT vs. SAVR | Death or disabling stroke at 2 yr |
|
| SURTAVI | Intermediate | 80 | 56 | 4.5 | 1660 | CoreValve/Evolut R vs. SAVR | Death or disabling stroke at 2 yr |
|
| PARTNER 3 | Low | 73 | 69 | 1.9 | 1000 | Sapien 3 vs. SAVR | Death, stroke, rehospitalization at 1 yr |
|
| Evolut Low Risk Trial | Low | 74 | 65 | 1.9 | 1468 | CoreValve/Evolut R/Evolut Pro vs. SAVR | Death or disabling stroke at 2 yr |
|
| CHOICE | High | 81 | 36 | 5.9 | 121 | CoreValve vs. Sapien XT | Device success |
|
| REPRISE III | High | 83 | 49 | 6.8 | 912 | Lotus vs. CoreValve/Evolut-R | Death, disabling stroke, and moderate or great PVL at 1 yr |
|
| NOTION | Low | 79 | 53 | 3.0 | 280 | CoreValve vs. SAVR | Death at 1 yr |
|
Cohort B of the PARTNER trial compared transfemoral TAVR with standard therapy (including BAV). The mean operative mortality risk identified by the Society of Thoracic Surgeons (STS) score of patients enrolled in this trial was 11.6%. At 1-year follow-up, the rates of death were 50.7% for the standard therapy group and 30.7% for the TAVR group. Based on these results, only five patients needed to be treated with TAVR to prevent one death at 1 year (i.e., number needed to treat = 5) ( Fig. 12.3 ). TAVR was associated with a significant reduction in symptoms at 1 year as assessed by New York Heart Association (NYHA) functional class (74.8% of surviving patients who had undergone TAVR were in NYHA class II or lower vs. 42.0% of those treated with standard therapy) and by Kansas City Cardiomyopathy Questionnaire (KCCQ) scores. The rate of major stroke was numerically higher with TAVR but not statistically significant, whereas the rates of vascular complications and major bleeding were significantly higher (see Table 12.1 ).
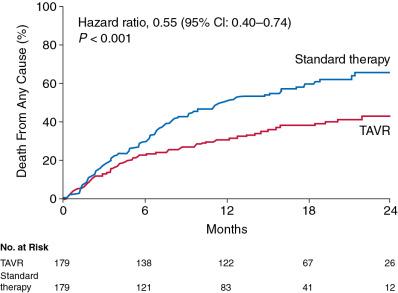
The 5-year follow-up of PARTNER Cohort B showed that the mortality rate remained lower for the TAVR group compared with that for the standard therapy group (71.8% vs. 93.6%, hazard ratio [HR]= 0.50, 95% confidence interval [CI]: 0.39–0.65, P < 0.0001).
Echocardiography after TAVR showed durable hemodynamic benefit (aortic valve area of 1.52 cm 2 and mean gradient of 10.6 mmHg at 5 years), with no evidence of structural valve deterioration. TAVR did not improve the mortality rate for patients with an STS risk score higher than 14.9% on entry into the trial.
Cohort A of the PARTNER trial compared transfemoral and transapical TAVR with SAVR. One-year data showed the noninferiority of TAVR (i.e., death from all causes of 24.2% with TAVR vs. 26.8% with standard surgery) ( Fig. 12.4 ). Rates of major strokes were numerically but not significantly higher in the TAVR group compared with the surgical group (3.8% vs. 2.1%, respectively, at 30 days; P = 0.20). Major vascular complications were significantly more frequent with transcatheter replacement (11.0% vs. 3.2%, P < 0.01), whereas adverse events that were more frequent after SAVR included major bleeding and new-onset atrial fibrillation.
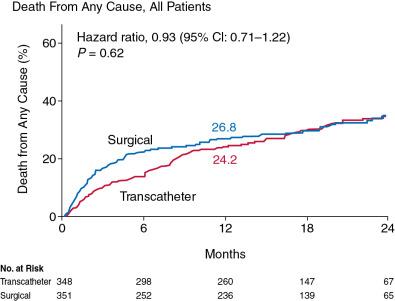
The 5-year follow-up of PARTNER Cohort A showed similar rates of death for the TAVR group and the surgical group (67.8% vs. 62.4%, respectively, HR = 1.04, 95% CI: 0.86–1.24, P = 0.76). There was no structural valve deterioration requiring SAVR in either group. Moderate or severe AR occurred more often in the TAVR group (14% vs. 1%, P < 0.01) and was associated with an increased 5-year risk of death (72.4% for moderate or severe AR vs. 56.6% for mild AR or less, P = 0.003).
Self-expanding valves have been associated with higher rates of survival at 1 year compared with surgical valve replacement in patients at high operative risk. In the CoreValve U.S. pivotal trial, patients with severe symptomatic AS at high operative risk were randomized to TAVR with the CoreValve self-expanding bioprosthesis or to SAVR. The results are summarized in Table 12.1 . The mean STS score was 7.4%. The rate of the primary end point of death from any cause at 1 year was lower for the TAVR group compared with the surgical group (14.2% vs. 19.1%, P < 0.01 for noninferiority). The rate of major adverse cardiovascular and cerebrovascular events at 1 year was significantly lower for the TAVR group than the surgical group (20.4% vs. 27.3%, P = 0.03). The rates of any stroke were lower in the TAVR group compared with the surgical group (4.9% vs. 6.2% and P = 0.46 at 30 days; 8.8% vs. 12.6% and P = 0.10 at 1 year). Major vascular complications and permanent pacemaker implantations were significantly more frequent in the TAVR group than in the surgical group, whereas bleeding, acute kidney injury, and new-onset or worsening atrial fibrillation were significantly more common in the surgical group. The rates of paravalvular regurgitation were higher in the TAVR group than in the surgical group at all time points after the procedure.
The PARTNER 2 trial randomized intermediate-risk patients to TAVR with a Sapien XT valve or to SAVR. The results are summarized in Table 12.1 . The mean STS score for each group was 5.8%. The trial showed that TAVR was noninferior to SAVR with respect to the primary end point of death (16.7% after TAVR and 18.0% after surgery, P = 0.001) or disabling stroke (6.2% after TAVR and 6.4% after surgery, P = 0.001) at 2 years. In the transfemoral-access cohort, TAVR resulted in a lower rate of death or disabling stroke than surgery (HR = 0.79, 95% CI: 0.62–1.00, P = 0.05), whereas in the transthoracic-access cohort, outcomes were similar between the two groups. TAVR also resulted in lower rates of acute kidney injury, severe bleeding, and new-onset atrial fibrillation, whereas surgery resulted in fewer major vascular complications and less paravalvular aortic regurgitation.
Additional data from observational studies demonstrated that TAVR may be superior to SAVR in intermediate-risk patients. In the Sapien 3 intermediate-risk registry, 1077 patients with a mean STS score of 5.2% and an average age of 81 years were assigned to receive the Sapien 3 valve (88% by transfemoral access through 14-Fr and 16-Fr delivery systems) at more than 50 sites in the United States and Canada. , One-year outcomes of these patients, including all-cause mortality and incidence of strokes, reintervention, and aortic valve regurgitation were then compared with outcomes of intermediate-risk patients treated with SAVR in the PARTNER 2A trial using propensity-matching analysis (see Table 12.1 ). This study was conducted in the same centers as the randomized PARTNER 2A study. The clinical events committee and the echocardiography and CT core laboratories were the same for this registry as for the PARTNER 2A study. At 30 days, the mortality rate was 1.1% after TAVR compared with 4.0% for the surgical arm of PARTNER 2A. Stroke rates were also significantly lower after TAVR compared with SAVR in this intermediate-risk population (disabling strokes: 1.0% vs. 4.4%). Although rates of paravalvular regurgitation were higher with TAVR than with SAVR, other important complications, including bleeding, atrial fibrillation, and acute kidney injury, were lower with TAVR.
The reduction in early complications translated to improved late outcomes. For the primary composite end point of death, stroke, and moderate or severe AR at 1 year, TAVR was superior to SAVR (−9.2%, 95% CI: −13.0 to −5.4, P < 0.0001). Based on this analysis, the study authors concluded that TAVR should be a treatment alternative to SAVR for intermediate-risk patients.
The Surgical Replacement and Transcatheter Aortic Valve Implantation (SURTAVI) trial randomized 1746 intermediate-risk patients with severe symptomatic AS to TAVR using of a self-expanding prosthesis or to SAVR. Clinical outcomes of these patients at 30 days, 12 months, and 24 months are shown in Table 12.1 . The mean STS score was 4.5%. At 24 months, the estimated incidence of the primary end point of death from any cause or disabling stroke was 12.6% for the TAVR group and 14.0% for the surgery group (95% CI: −5.2% to 2.3%; posterior probability of noninferiority > 0.999).
As in the PARTNER 2 trial, surgery was associated with higher rates of acute kidney injury, atrial fibrillation, and transfusion requirements, whereas TAVR led to higher rates of residual AR and need for pacemaker implantation. TAVR resulted in lower mean gradients and larger aortic valve areas than SAVR. Structural valve deterioration at 24 months did not occur in either group. Based on these data, TAVR with a self-expanding valve was considered a noninferior alternative to surgery in patients with severe AS at intermediate surgical risk.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here