Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Since its introduction into clinical practice, transcatheter aortic valve implantation (TAVI) has proven to be a transformational technology, where the implantation of the new valve is done without the need for complex surgery, usually via the femoral artery under local anesthesia, which results in a quicker patient recovery time and lower hospital costs. Management of severe aortic stenosis is moving quickly toward TAVI for lower-risk patients, resulting in a higher potential patient pool.
In a recent meta-analysis of periprocedural complications, compared with surgical aortic valve replacement (SAVR), TAVI was associated with a reduced risk for stroke, bleeding, and renal failure; however, it was associated with an increased incidence of vascular complications and pacemaker implantation.
Despite the high success rate of TAVI (>95% in the contemporary era), a significant effort toward a better understanding of the common complications that might occur during the procedure or after this valve implantation technique is needed.
Reducing the incidence of periprocedural complications is especially important in lower-risk patients, a population who can expect good results with SAVR, and for whom the rate of periprocedural complications after TAVI needs to be very low for TAVI to be cost effective compared with SAVR.
In this chapter we review the incidence, predictive factors, and clinical implications of the major related procedural and device complications of TAVI.
Vascular complications (VCs) are among the most frequent and serious complications of TAVI.
Despite improvements in operators’ experience, patient selection, and lower-profile devices, VCs remain frequent.
According to the Valve Academic Research Consortium-2 (VARC-2) criteria, major VCs are still reported in approximately 12% of the cases that receive first-generation valves.
A significant reduction in VCs has been shown in recent clinical trials of newer devices, with an incidence of 5% to 8% (resulting from smaller sheath sizes, flexible delivery systems, and other aspects).
Many of the factors that predict VCs are nonmodifiable, so risk reduction with respect to these can only be accomplished through a careful selection of patients. Patient-related risk factors that are associated with an increased threat of VCs include the following:
Female gender
Renal failure
Peripheral vascular disease with significant atheromatous calcification (especially when circumferential)
Concomitant peripheral vascular disease
Procedural-related risk factors for VCs include the following:
Increased sheath to femoral artery diameter ratio (>1.05)
Systems with >18-French sheaths
Operator inexperience
On the other hand, the Medtronic CoreValve self-expandable device (Medtronic [Dublin, Ireland]) has been associated with a lower risk of VCs. Similarly, the newer-generation lower-profile SAPIEN XT valves (18-/19-French delivery systems; Edwards Lifesciences [Irvine, CA]) and especially the Edwards SAPIEN 3 valve systems (with even smaller delivery structures) achieved lower rates of major VCs compared with the first-generation valves. New-generation TAVI delivery systems with smaller and expandable sheaths, such as the Evolut R/Evolut PRO, are expected to further reduce the VC rate.
The selection of vascular access requires detailed and accurate assessment of vascular anatomy, primarily using multidetector computed tomography (MDCT) or invasive angiography. MDCT provides a clear and complete three-dimensional assessment of the iliofemoral system, including vessel tortuosity, extent of vessel calcification, and identification of atheroma/dissection, if present ( Fig. 13.1 ).
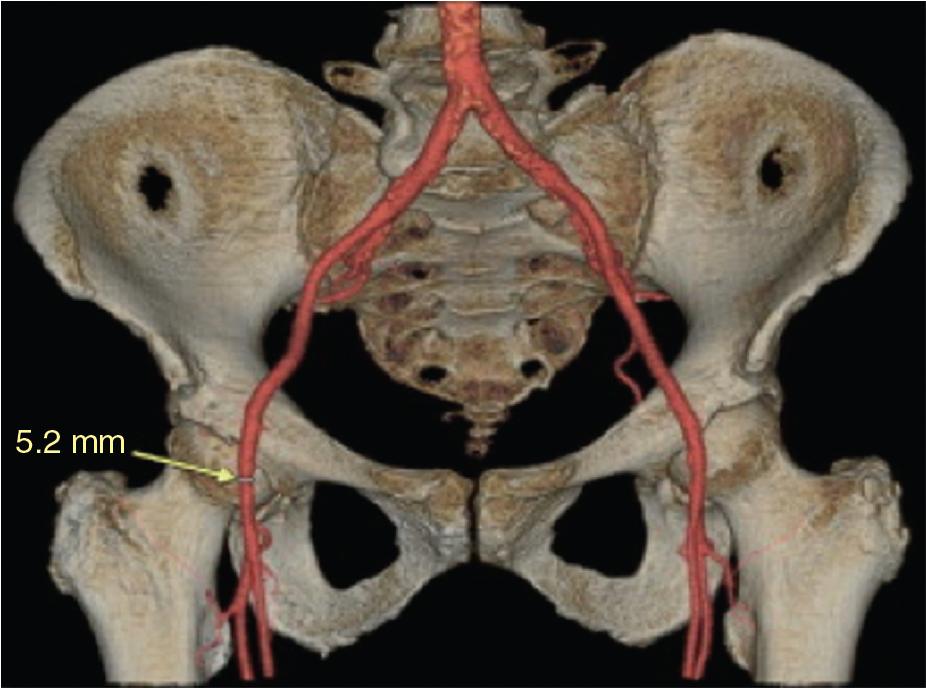
For patients in whom TAVI is being planned, a consensus statement by the Society of Cardiovascular Computed Tomography outlines the recommendations for assessment of the access site and the aorta.
For the femoral route, the image acquisition is typically gated and extends from the arch of the aorta to below the femoral head.
Fluoroscopy can be used to facilitate femoral puncture, using a radiopaque marker to “label” the position of the femoral head, or digital subtraction angiography to puncture the vessel in real time.
The use of real-time two-dimensional (2D) ultrasound has been shown to do the following:
Improve first-pass success rate
Reduce the number of attempts and time required for vascular access
Reduce unintentional venous punctures
Most important, result in a lower rate for overall VCs in patients undergoing coronary procedures through the femoral access, with some data suggesting its potential for reducing VCs in the TAVI population as well
Other procedural strategies to reduce VCs for transfemoral TAVI such as the use of radial artery for secondary access and the use of unilateral access have also shown promising results, but data are still limited.
The minimal size required for the use of an 18-French delivery system is 5.5 to 6.5 mm, with the 14- to 16-French systems allowing for an even greater margin of safety.
Smaller sheath sizes with newer-generation devices makes the transfemoral access feasible in a larger fraction of the TAVI population.
Early experiences showed that around 70% of TAVI procedures used the femoral access point. More recent data have shown that the transfemoral approach has been used in over 90% of contemporary cases.
This shift in practice has major prognostic implications, because transfemoral TAVI is associated with a 20% relative reduction in mortality compared with alternative (nonfemoral) approaches and also versus SAVR.
Still, when the femoral calibers are not compatible with the transfemoral approach, and especially when calcifications are severe, concentric, or located anywhere from the aorto-iliac bifurcation to the femoral bifurcation, this could represent a potential contraindication for transfemoral access, and an alternative route should be considered (see Chapter 7 ).
The subclavian approach can be used, which, in terms of VCs, has rates comparable with the femoral route.
With regard to transcaval access, the most well-known complication is cavo-aortic fistula, although the data are sparse because this approach is relatively new and not frequently used.
Transapical and transaortic approaches that were abundant in the early days of TAVI are quite rare in the current period, but some centers still use it more frequently than others based on the local surgical expertise.
Common VCs include the following:
Aortic dissection
Aortic rupture
Pelvic (iliac-femoral) vessel dissection
Pelvic vessel rupture
Access site hematoma
Pseudoaneurysm formation
Aortic dissection is a relatively rare (≈0.2% of cases) but potentially deadly complication of TAVI.
The standard of care for type A aortic dissection is open surgical repair because of the high early mortality without prompt intervention.
Type B aortic dissections are usually managed medically (except in cases of serious complications such as visceral malperfusion). It is essential to carefully weigh the risks and benefits of a complex open procedure versus medical management.
All dissection patients should have intensive medical therapy, with strict heart rate and blood pressure control using intravenous β-blockers (or, if β-blockade is contraindicated, a dihydropyridine calcium channel blocker or sodium nitroprusside).
Interestingly, there have been reports of successful use of thoracic endografts in the ascending aorta to treat type A aortic dissection; however, the use of this technology is dependent on the specificities of each case (e.g., patient’s anatomy, size of the aorta/sinotubular junction, involvement of the coronary arterial origin). It is expected that in the near future, as the ascending aortic endograft technology develops, and this might become an ideal solution in cases of TAVI-induced type A aortic dissection.
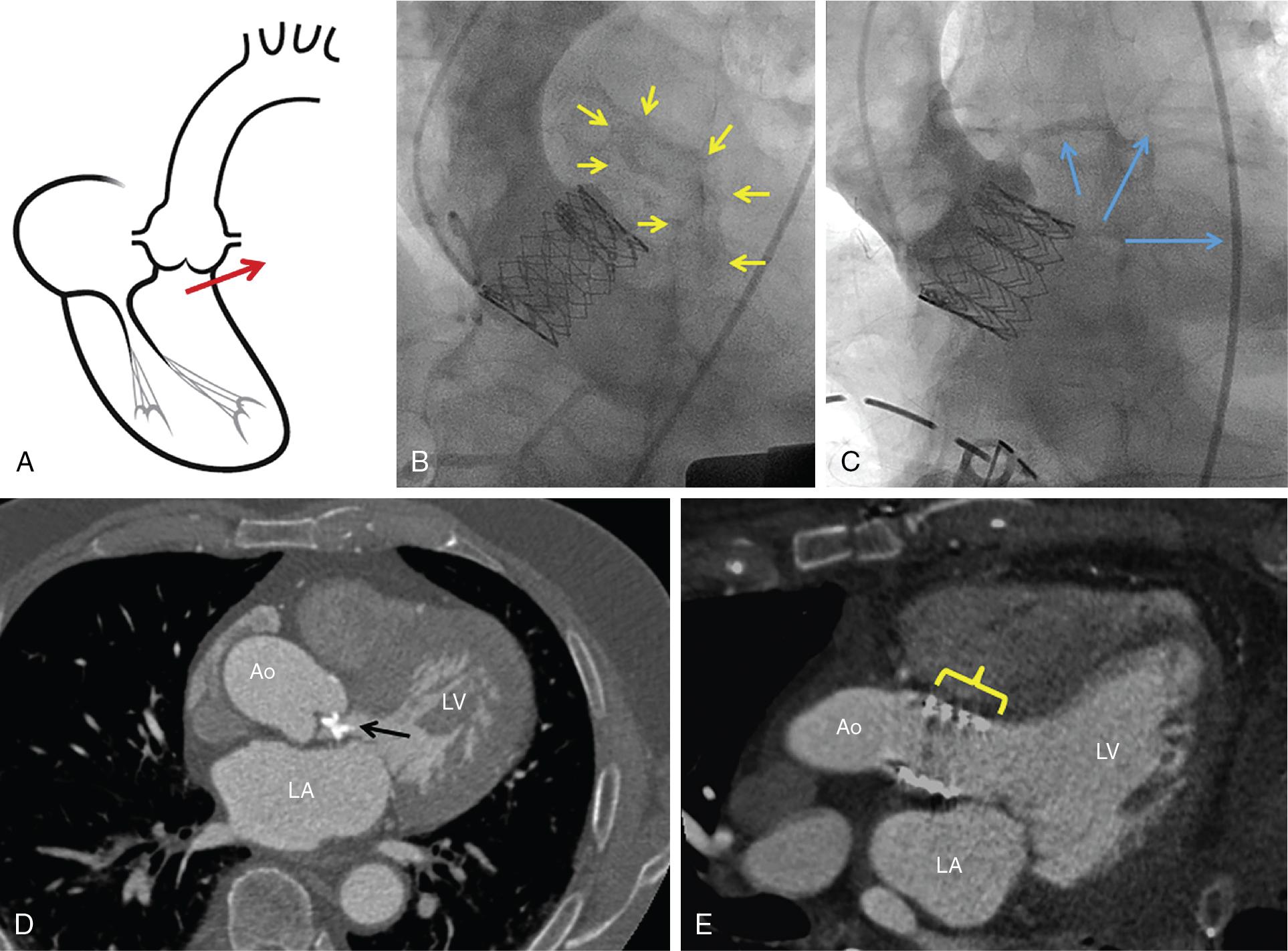
Ascending and descending thoracic aorta dissection and perforation are generally caused by catheter/wire trauma or vascular sheath insertion, or occur after aortic valvuloplasty. Aortic rupture is a rare occurrence during TAVI (≈0.1% of cases), but with possible catastrophic consequences ( Fig. 13.2 ). The most frequent anatomic site of rupture is the aortic annulus, although left ventricular outflow tract (LVOT, 10%), sinus of Valsalva (16%), and sinotubular junction (6%) ruptures have also been described ( Fig. 13.3 ). The management options include urgent surgical versus endovascular repair with covered stent grafts, depending on the location of the perforation.
Despite appropriate interventions, mortality with aortic rupture remains high (up to 48%, and can be as high as 75% in cases of uncontained rupture).
Particular care is needed when placing sheaths and advancing catheters in markedly ectatic vessels.
A careful exploration of the aorta should always be performed, in order to identify potential anatomic features associated with an increased risk of vascular rupture/dissection. These relative contraindications include tortuosity, presence of aneurysms, thrombotic appositions, or aortic arch calcifications.
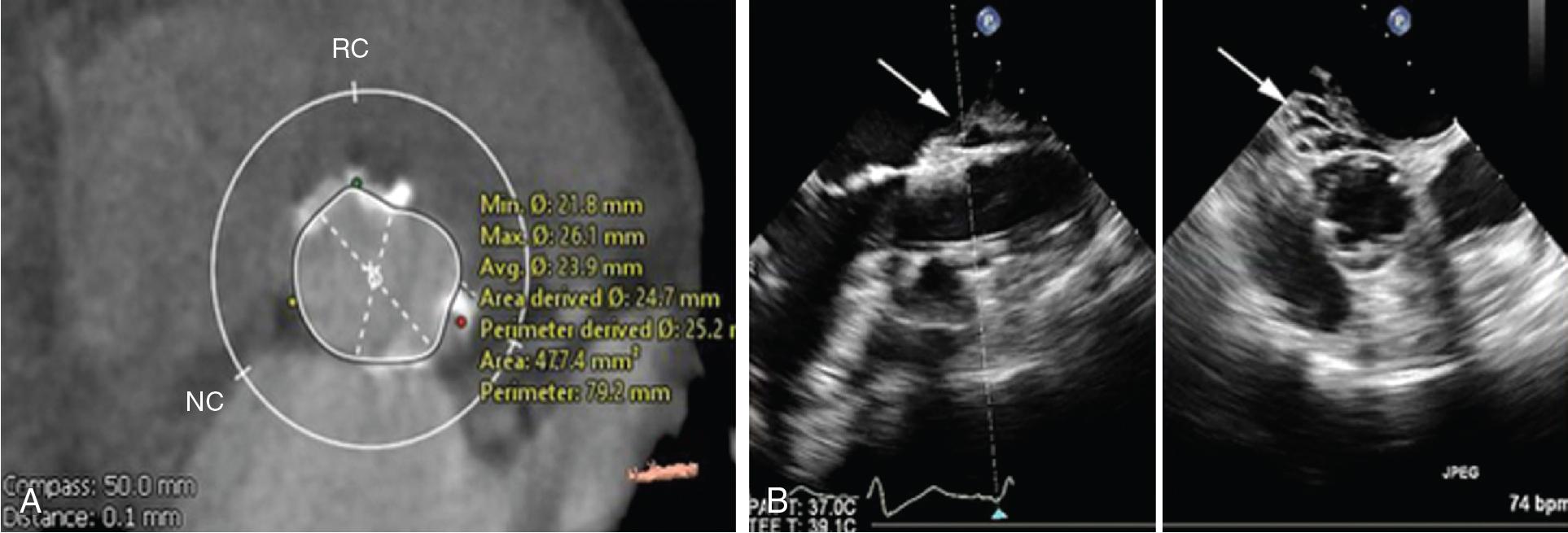
In a large multicenter TAVI cohort, the calcium score was significantly higher in patients who experienced landing zone rupture compared with other patients ; thus a high burden of LVOT/subannular calcification is recognized as the most important predictor of device landing zone rupture. Perhaps more important than the calcific burden is the distribution of calcium, because a higher calcium volume in the upper LVOT has been associated with the risk of rupture ( Fig. 13.4 ).
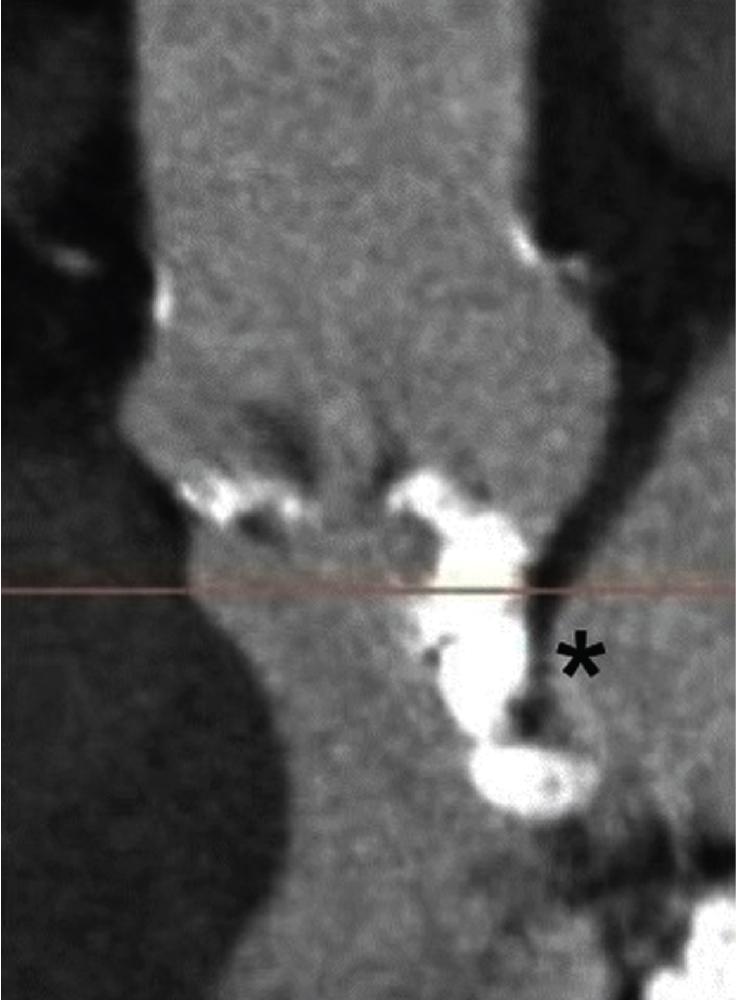
The choice of valve prosthesis is also critical:
Self-expandable valve is preferable in case of patients with high-risk LVOT calcification patterns and shallow sinuses of Valsalva.
Additionally, it must be noted that postdilation significantly increases the risk of landing zone rupture (especially with >20% area of oversizing).
Close surveillance and a repeated MDCT assessment are crucial in ruptured patients, because an adverse evolution is possible up to several hours or days after the rupture event.
The most common VC when using the transfemoral route is pelvic vessel dissection (iliac and femoral arteries).
Pelvic vessel dissection occurs in approximately 6.5% of TAVI patients, even in high-volume centers with experienced operators.
The most typical involved vessel is the external iliac artery.
The dissection can occur during the initial progression of the delivery system or during sheath withdrawal.
Extensive dissections can lead to lower limb ischemia, which, depending on the spread of the dissection and the vessel territory that is involved, can be critical, if there is predisposition to thrombus formation and vessel occlusion, or subcritical, if there are compression symptoms with blood extravasation ( Fig. 13.5 ).
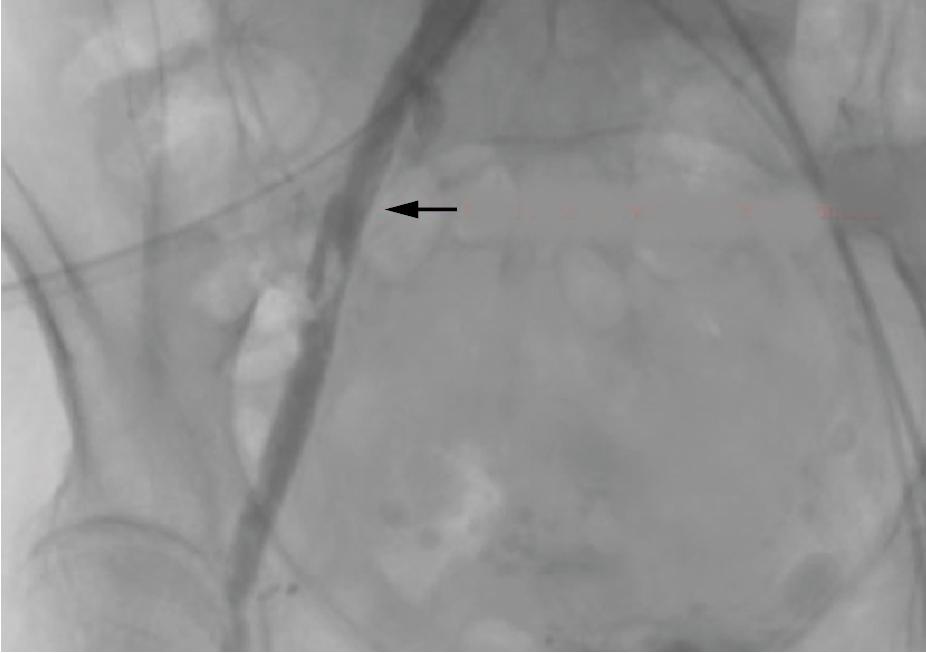
Invasive angiography, computed tomography angiography (CTA), or vascular Doppler can confirm the diagnosis.
In emergency situations, a balloon inflation can seal the dissection and/or tamponade the bleeding site.
It is essential to select the correct balloon size needed for transient occlusion; this should be based on the preprocedural MDCT sizing.
Some cases will require the use of endovascular stenting, covered stenting, or open surgical intervention, to reconstruct the damaged vessel and restore the flow to the affected lower limb.
Iliofemoral rupture is another feared complication of TAVI, but the incidence has diminished significantly with the use of smaller and more compact delivery systems (3%–5%).
Pelvic vessel rupture is mostly evident after the sheath is withdrawn, because while the sheath is in place it frequently acts as a seal for the tear.
As soon as the pressure seal is taken off, a vast pelvic bleed can occur with a rapid clinical deterioration ( Fig. 13.6 ).
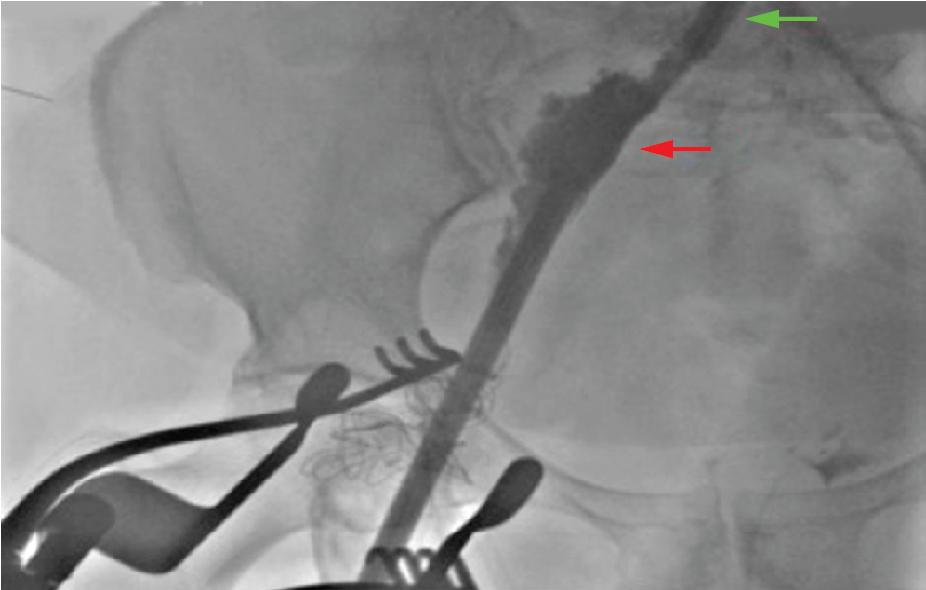
Careful attention should be given to the patient’s hemodynamics at the time of the sheath removal, immediately after vascular closure, because major defects will be evident by then.
Usually, angiography is performed immediately after complete sheath removal and vascular closure, in order to assess any focal dissection or to detect any extraluminal contrast extravasation.
The management of rupture could be a quick sheath reintroduction to seal the site or, more commonly, a proximal balloon tamponade while a contralateral (or even ipsilateral using a lower vascular stick) balloon delivery and inflation is employed.
Usually, massive fluid repletion, quick anticoagulation reversal, and covered stent placement are required; although, in cases of complex dissections, surgical intervention might be needed.
Covered stenting is usually preferred to surgical repair when the injury is below the inguinal ligament and above the femoral bifurcation ( Fig. 13.7 ).
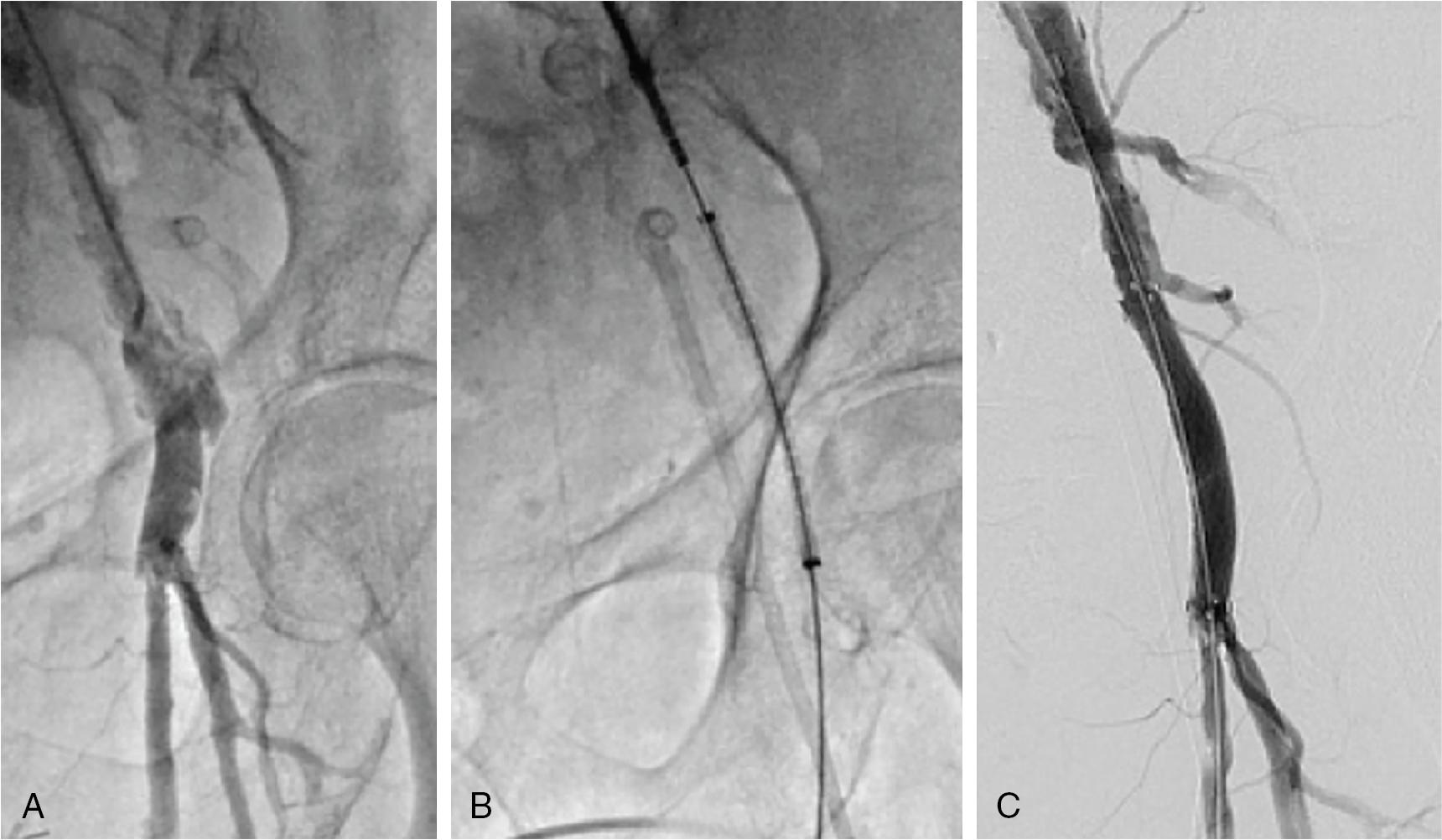
Small tears can extend and cause clinical instability only a few hours postprocedure, so close follow-up of the patients is essential.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here