Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A variety of benign and malignant lesions can develop in the trachea and lead to airway obstruction. The infrequent occurrence of tracheal pathology and its frequently insidious nature can lead to a delayed diagnosis for patients in whom these conditions exist. Advances in surgical and anesthetic techniques have enabled safe and effective resection with primary reconstruction for many of these lesions. For obstructive lesions whose extent precludes primary tracheal resection and reconstruction, there is still no universally definitive treatment, but several palliative measures exist to provide patients with a satisfactory airway. Most recently, several reports of tracheal replacements using tissue-engineered grafts have generated hope for these patients.
This chapter focuses on surgically correctable lesions of the trachea, including those that involve the subglottic larynx. Pathology and treatment of carinal lesions will be discussed in a separate chapter. Historical perspective, along with preoperative assessment, operative strategies, and postoperative care for patients with obstructive pathology of the trachea will be discussed in detail.
When the field of tracheal surgery was young, it was widely accepted that only four tracheal rings, or approximately 2 cm, of trachea could be resected safely with immediate reconstruction. It was the work of Dr. Hermes Grillo, widely considered the father of modern tracheal surgery, that led to the surgical practices used today. Grillo et al systematically investigated the limits of resection of the trachea to permit reconstruction without excessive anastomotic tension. Among other findings, these studies demonstrated that the tracheal blood supply entered in its lateral pedicles, and that safe mobilization of the trachea could be accomplished only with anterior and posterior dissection. Dedo and Fishman described laryngeal release maneuvers to minimize tension on lengthier tracheal resections. These contributions led to the realization that, in extreme cases, up to 50% of the human trachea could be resected and reconstructed without detrimental tension.
The trachea averages 11 cm in length from the lower border of the cricoid cartilage to the carinal spur, with an additional 1.5 to 2 cm of subglottic laryngeal airway. The structural support comes from 18 to 22 cartilaginous C-shaped rings, with about two rings per centimeter. The cricoid cartilage is the only complete cartilaginous ring in the normal airway. Most of the trachea lies within the thoracic inlet and the chest. Cervical hyperextension delivers up to half of the trachea into the neck, and flexion devolves most of the trachea into the mediastinum.
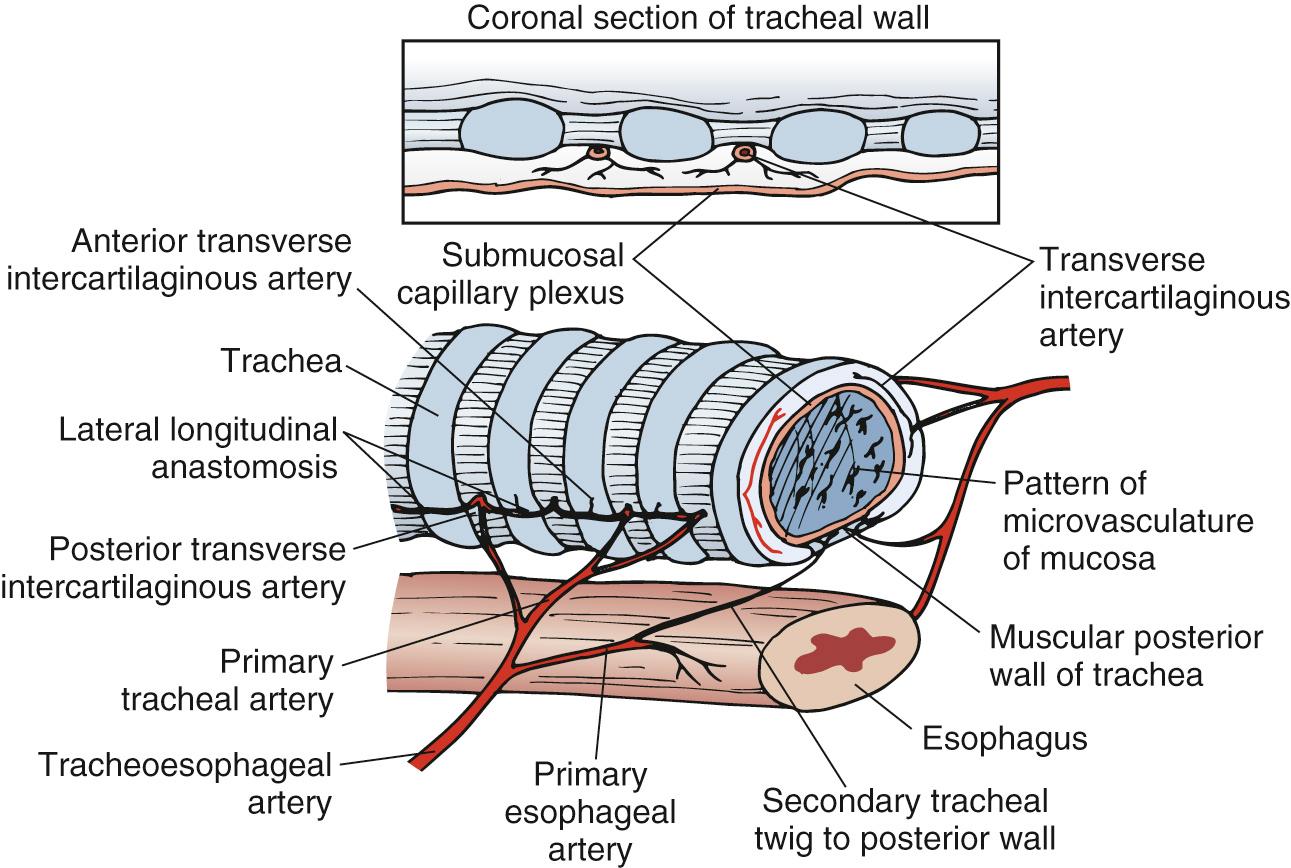
The blood supply to the trachea is provided by many segmental end arteries. The upper trachea is supplied principally by branches of the inferior thyroid artery, and the lower trachea by branches of the bronchial arteries. These vessels enter the trachea via very fine lateral pedicles and lack intersegmental collateralization ( Fig. 8-1 ). The pretracheal plane and the plane between the esophagus and the trachea are avascular. The recurrent laryngeal nerves ascend in the tracheoesophageal grooves bilaterally and pass medially to the inferior cornua of the thyroid cartilage to enter the larynx. The nerve on the left is present along the entire length of the trachea, whereas the right recurrent laryngeal nerve is present in the tracheoesophageal groove for only the proximal cervical trachea.
A wide spectrum of pathology can afflict the trachea, ranging from benign conditions to malignant ones. Most conditions lead to central airway obstruction with subsequent respiratory insufficiency. Others, such as fistulas between the trachea and innominate artery or esophagus, can also occur and be equally deleterious. Direct external trauma, blunt or penetrating, can result in a tear or complete disruption of the trachea at any level. Inhalational burn injuries are usually maximal in the proximal subglottic region and diminish in the more distal airway. In most cases, the tracheal rings are not destroyed. Idiopathic laryngotracheal stenosis appears to be a distinct disease entity, which leads to an inflammatory process in the subglottic region. This is an unusual problem almost exclusively occurring in white women most commonly in the third to fifth decade of life.
Iatrogenic injuries resulting from tracheal intubation have long been the most common lesion afflicting the airway. Postintubation injuries include granulomas, strictures, malacia, and tracheoesophageal and tracheoinnominate fistulas ( Fig. 8-2 ).
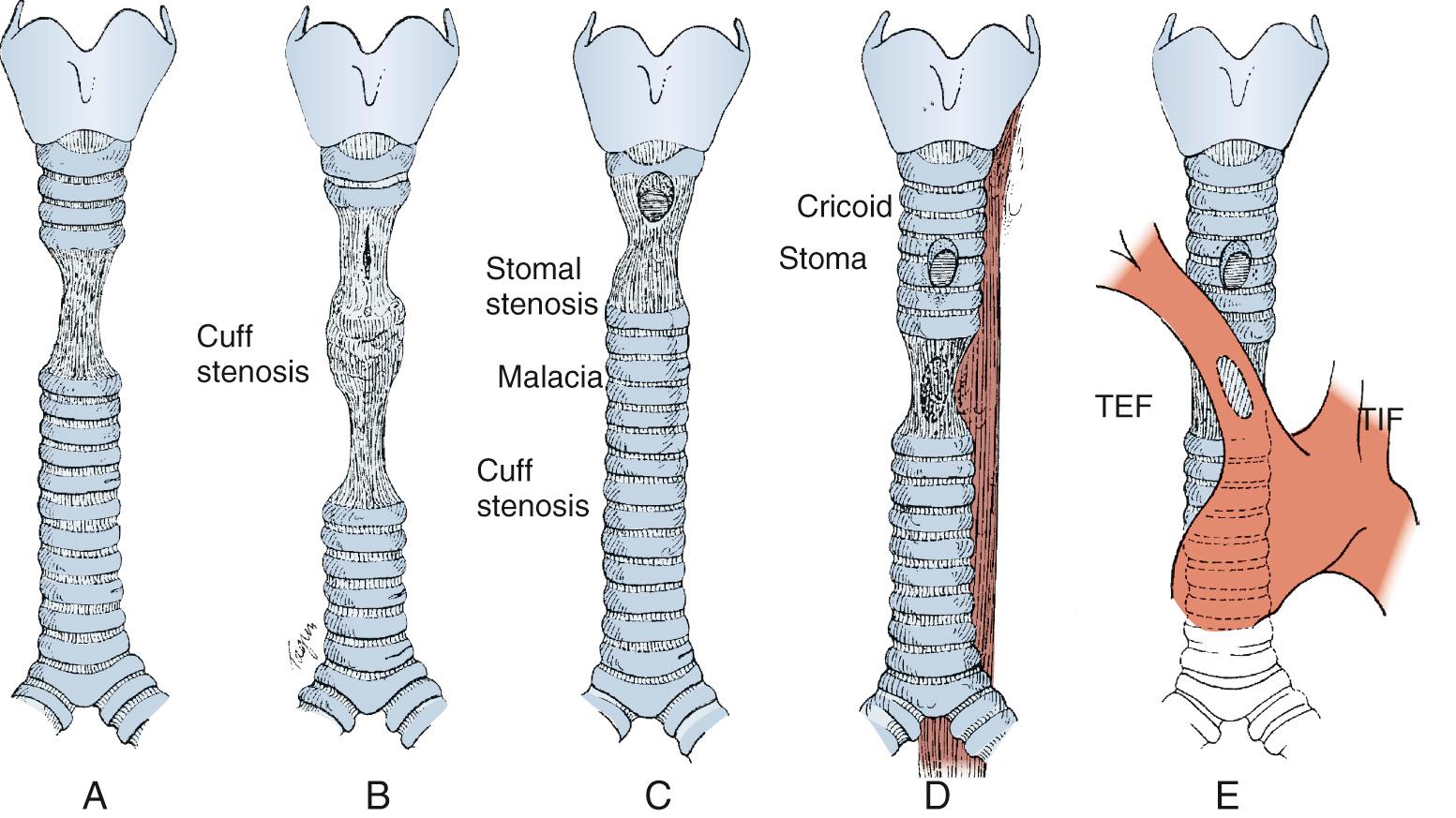
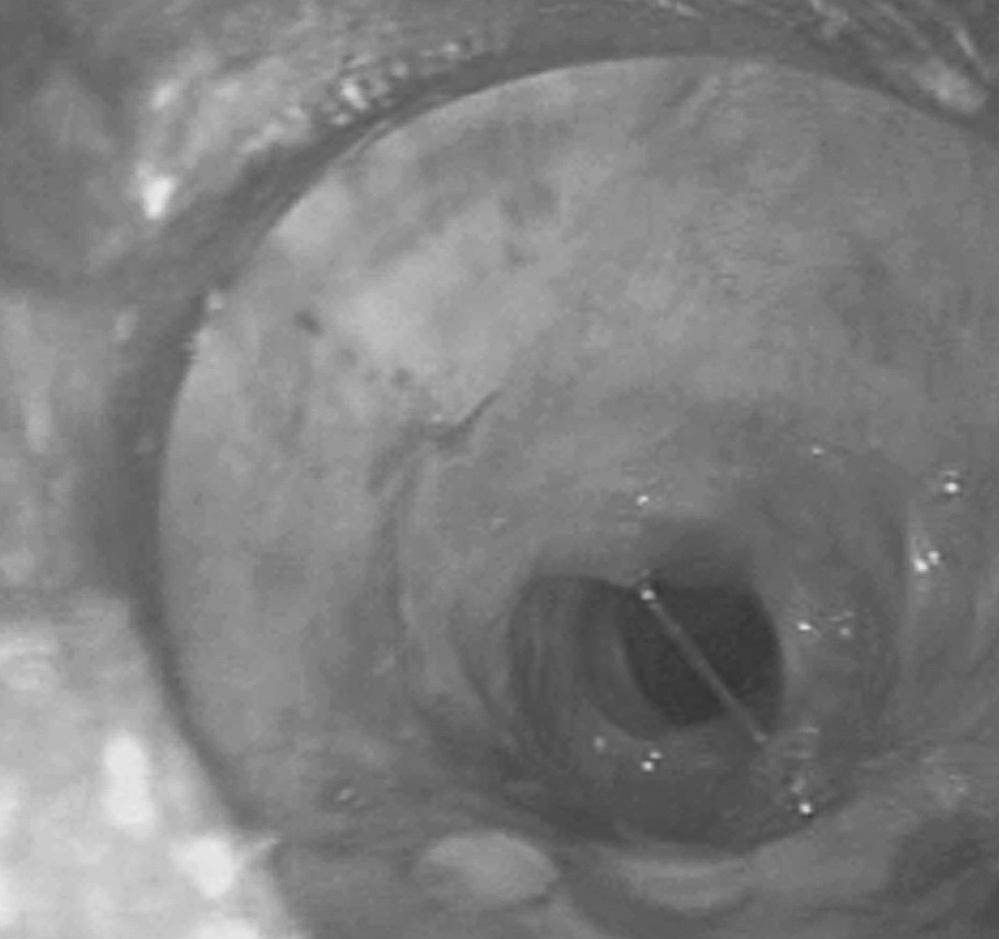
Postintubation tracheal stenosis represents the most common iatrogenic injury of the trachea. It occurs after intubation and develops principally at the level of the endotracheal tube (ETT) or tracheostomy tube cuffs. The radial pressure exerted from the cuff causes circumferential pressure necrosis, which results in cicatricial scaring stenosis ( Fig. 8-3 ). The route of entry of the ventilatory tube does not affect the occurrence of cuff injury, only its level. These lesions are seen in patients who have had only oral endotracheal intubation and in those with previous tracheostomy. The development of the large-volume, low-pressure cuff for tracheostomy and ETTs for ventilation has greatly lowered the incidence of cuff-related stenoses. However, overinflation of these cuffs can still result in local airway damage and subsequent scar formation, and it contributes to the continued incidence of these lesions. Stenosis in the subglottic region can occur because of prolonged intubation with ETTs, after cricothyroidotomy, or after high placement of a tracheostomy where the neck of the tube erodes through the cricoid cartilage.
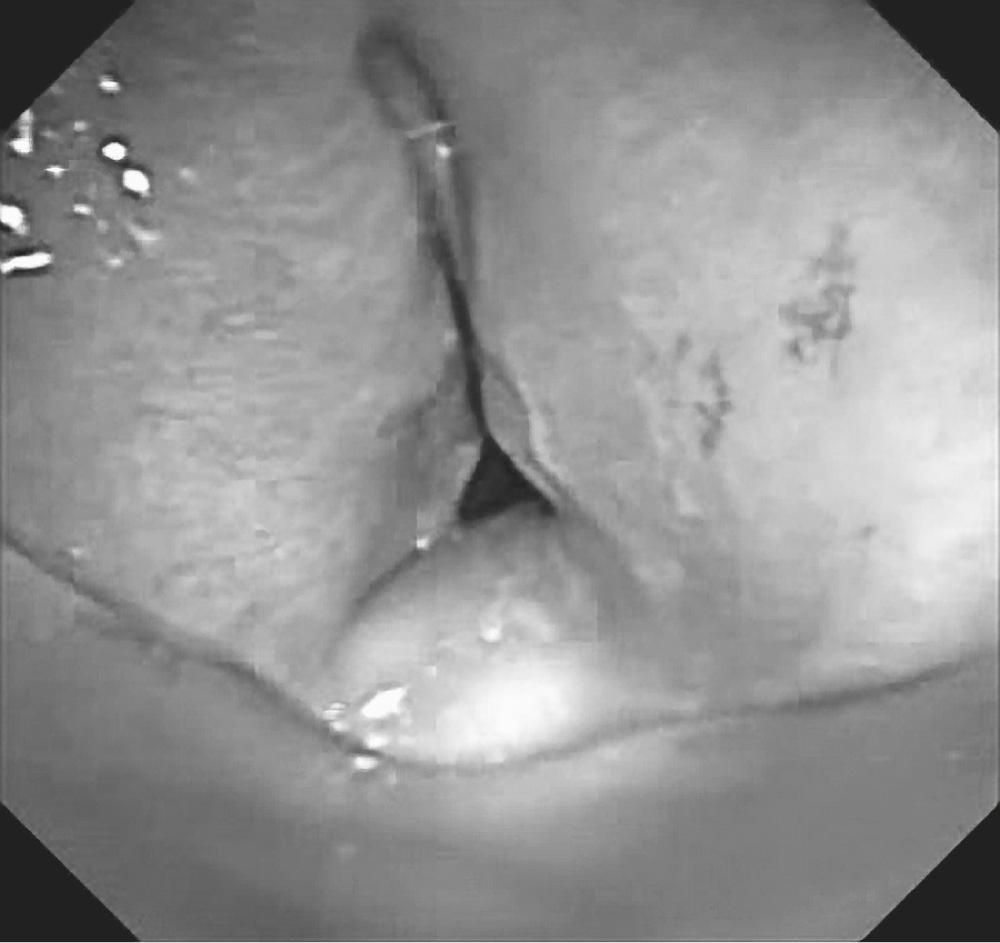
Stomal stenosis results from the gradual enlargement of a tracheal stoma and its eventual healing by contraction. This pulls the sides of the defect together, distorting the tracheal lumen to a triangular configuration, with the base of the triangle located posteriorly and consisting of the uninjured membranous wall ( Fig. 8-4 ). The large stoma can result from overzealous excision of anterior tracheal cartilage at the time of the initial tracheostomy, from erosive infection around the margins of the stoma, or, most commonly, from erosion resulting from leverage by equipment attached to the tracheostomy tube during ventilation. The stenotic process can extend into the subglottic larynx if the previous tracheostomy was placed inappropriately high in the trachea.
Prolonged endotracheal intubation combined with a nasogastric tube can lead to a tracheoesophageal fistula ( Fig. 8-5 ). This fistula results from pressure necrosis generated by a ventilating cuff in the trachea and a nasogastric feeding tube in the esophagus. Consequently, these fistulas occur in the shared location between the membranous tracheal wall and the anterior wall of the esophagus. Anterior injuries of the trachea can lead to the `development of a tracheoinnominate artery fistula. These most often result from direct erosion of the inner elbow of the tracheostomy tube in an inferiorly placed stoma, and they can arise either from a tracheostomy tube placed too low in the trachea or from an innominate artery lying high, near the sternal notch.
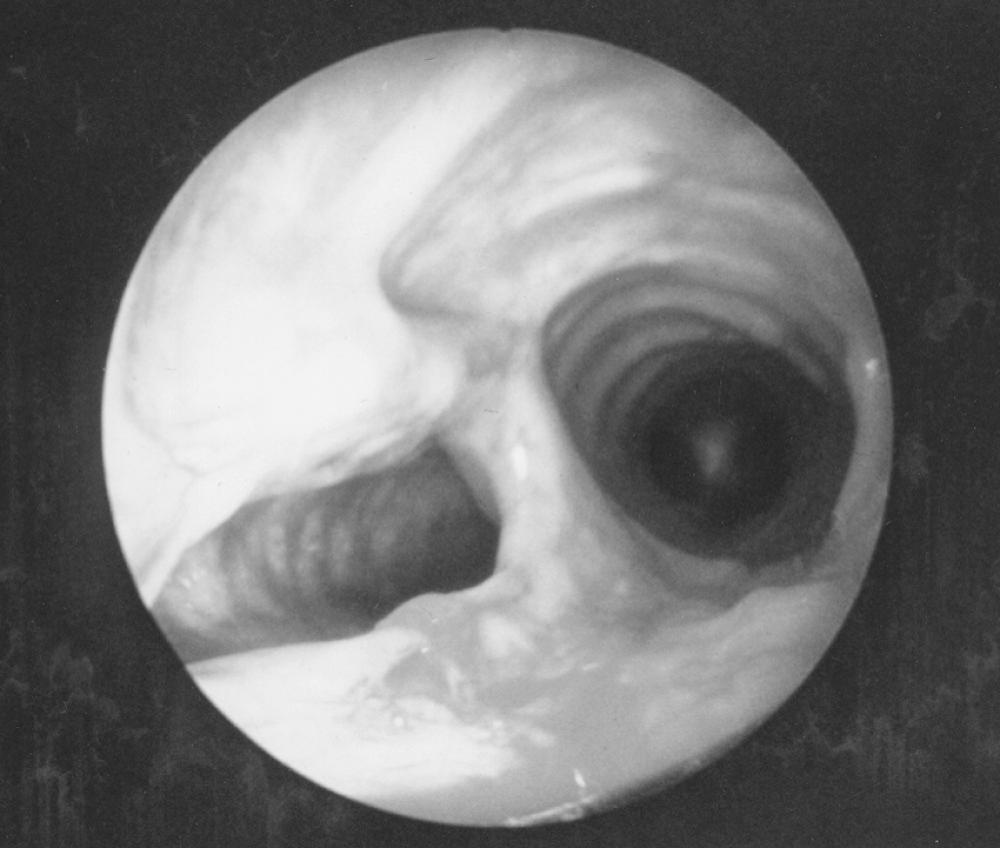
Rarely, a patient has stenosis of the airway without history of trauma, infection, inhalation injury, or airway intubation. Idiopathic laryngotracheal stenosis is a diagnosis of exclusion, characterized by an inflammatory cicatricial stenosis at the level of the subglottic larynx, cricoid, and upper trachea. Causes such as gastroesophageal reflux with silent aspiration, Wegener granulomatosis, and connective tissue disorders must be excluded. As part of the routine preoperative workup, all patients undergo serologic screening (antinuclear antibody [ANA], antineutrophil cytoplasmic antibody [ANCA]) for these conditions. Over 95% of patients with idiopathic laryngotracheal stenosis are female between the third and fifth decade of life, and they typically exhibit signs and symptoms of upper airway obstruction. Pharyngeal and laryngeal anatomy and vocal cord function are usually normal, whereas the inflammatory process leading to luminal narrowing is confined intrinsically to the wall of the airway and the immediately surrounding connective tissue of a short (<2.5 cm) segment at the level of the cricoid cartilage.
The biggest clinical challenge in the surgical management of idiopathic laryngotracheal stenosis lies in the unpredictability and variability of the future clinical course. When airway obstruction becomes severe and dilation does not provide sustained relief of symptoms, surgery may be entertained, with, however, the clear caveat that the disease may progress. Most patients are best treated with definitive laryngotracheal resection and primary reconstruction. Timing of resection and reconstruction is central and must be done when inflammation is minimal. Bridging the time to definitive operation may require repeated bronchoscopic dilation. We prefer dilation to be performed in the operating room with rigid bronchoscopes starting with pediatric sizes increasing to adult bronchoscopes.
Airway malacia may be a consequence of a postintubation injury; however, in patients with chronic obstructive pulmonary disease, malacia can develop in the lower trachea, main bronchi, and sometimes the more distal bronchi, even in the absence of prior intubation. When the patient attempts a deep expiration or a cough, the membranous wall approximates to the anterior, softened cartilaginous wall, causing nearly total obstruction. Subsequently, the posterior membranous wall elongates and becomes redundant. Afflicted patients may have dyspnea, cough, and secretion retention. It is possible to address the deformity surgically by pulling the ends of the cartilages toward one another posteriorly, restoring a more circular shape to the airway. The redundant membranous wall must be tacked to a posterior splinting material to prevent it from falling forward into the lumen. Various materials have been used for splinting, but none with complete success. These have included fascia lata, pericardium, lyophilized bone, polytetrafluoroethylene, and rigid plastic splints. Biomesh and cryopreserved aortic allografts can be useful biologic alternatives to synthetic tissues, especially when tissue quality or comorbidities are a factor, and airway erosion is of concern. To obtain maximum long-term relief, we currently use sheets of nonabsorbable polypropylene mesh to plicate the membranous wall as described by Rainer and colleagues.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here