Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Research from the author’s laboratory as cited here is the result of support from the National Institutes of Health. Figs. 61.2–61.4 were produced by AASarts.com .
The trace metals are roughly divided into the “trace” metals and the “ultratrace” metals. The latter term comprises a group of elements, which includes chromium, for which there are various degrees of evidence to support nutritional essentiality. Evidence may be limited to minimal effects such as growth reduction during a dietary restriction under controlled conditions of husbandry including ultraclean environments. In some cases, the ultratrace metals have no clearly established biochemical function. In contrast, the trace metals such as copper, manganese, selenium, and zinc have well-established, biochemically based, deficiency syndromes that define nutritional essentially. These metals have a well-characterized basis for essentiality derived from the animal nutrition studies and support essentiality for humans. Along with iron, copper and zinc are the metals upon which most of our knowledge about metal transport into and from the intestinal epithelial cell resides. Efficiency of utilization (bioavailability) includes factors such as diet composition which influences the extent of digestion and endogenous secretion as well as medical disorders leading to infection and inflammation.
Absorption of all trace metals from food and fluids consumed is influenced by their physical and biochemical properties. The extent to which these components are degraded into smaller components influences absorption of individual constituents. Solubility is a factor that influences trace metal absorption. Trace metals tend to be widely dispersed among foods. For example, zinc has important functions in cell replication and gene expression. Consequently in food ingredients, zinc is abundant where nucleic acids are concentrated, that is, grains. The high zinc content of wheat germ is an example. Zinc is abundant in muscle and provides a major food source. Selenium, manganese, and copper, having enzymatic roles, are concentrated in foods where metabolic activity is very high, that is, grains, and in organ meats and muscle. Animal- and plant-derived foods provide selenium as selenocysteine and selenomethionine, respectively. The trace metals are presented to the intestinal lumen initially as protein/peptide complexes.
The extent of digestion and transit times are determining factors for absorption ( Fig. 61.1 ). Trace metals tightly bound to high molecular mass compounds, for example, large peptides, may not interact with transporters responsible for uptake by enterocytes. Unless these dietary constituents are well digested these constitute an unavailable pool of a particular trace metal. The pool of a trace metal available for absorption most likely is composed of low-molecular mass compounds, for example, amino acid chelates, peptides, or “free” ions of the element. The binding affinities of various molecules for a specific metal dictate how much “free” ion exists.
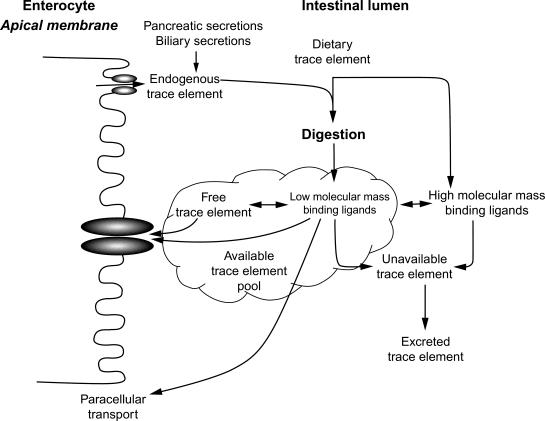
Unlike nutrients whose solubility is high, kinetic analysis of trace metal uptake is quite challenging. These micronutrients tend to be soluble in an acidic environment, but less so in a neutral milieu. Acidic gastric secretions tend to enhance solubility, but the stomach is not usually considered as a site of appreciable trace metal absorption. Recently, zinc uptake by the secretory compartment of the acid-secreting parietal cells of the stomach has been shown in vitro and may be coupled to acid secretion. Some zinc transporters (e.g., ZIP11) are highly expressed in the stomach and are downregulated with dietary zinc depletion. Such findings may cause further consideration of the stomach as a factor in trace metal absorption or may reflect functional roles in the gastrointestinal tract. How the acidic microenvironment of the intestinal apical surface influences trace metal uptake has not been studied. As the gastric contents enter the duodenum, neutralization is rapid and yields a concomitant reduction in solubility. Despite the neutral pH environment, the small intestine is the major site of trace metal absorption. Uptake for copper, manganese, and zinc follows saturable kinetics. Since the extent of absorption is a function of interaction of the metal ion with transporters at the apical surface of enterocytes, the more the interaction that exists with metal-binding dietary constituents of low affinity, the greater should be the uptake rate. Saturable kinetics (mediated component of uptake) implies that one or more transporter molecules are involved. As luminal concentrations of a trace metal increase, nonmediated uptake tends to be predominant. This is believed to represent paracellular transfer of metals and metal chelates, that is, those metals bound to small peptides and potentially trace metals presented in the form of nanoparticles. Transepithelial movement of trace metals occurs by poorly understood mechanisms. The demonstration that many transporters are localized to intracellular vesicles, endosomes, the ER, and Golgi of enterocytes suggests multiple transporters contribute to transcellular movement of metal ions. Mechanisms for transport across the basolateral membrane and the endothelium are also being defined. Efflux transporter genes for copper and zinc have been identified. The intracellular pools from which absorbed trace metals are drawn for cellular efflux are not well understood. These trace metals sequestered in endocytotic vesicles may be the substrates for efflux transporters at the basolateral membrane.
Kinetic analysis of trace metal absorption has employed many techniques, ranging from in vivo perfusion studies with humans to in vivo intestinal cell preparations and even membrane vesicles from experimental animals and immortalized cell lines. Unlike in vitro systems with relatively purified components, in vivo approaches designed to mimic the actual luminal environment can only approximate the true concentration of the metal in question. Nevertheless, measured kinetic parameters from these methods are frequently within the expected range, based on dietary consumption of these micronutrients and eventual concentrations in peripheral blood.
Evidence to support mediated transport for copper, manganese, selenium, and zinc absorption is substantial, but there is a fair degree of controversy regarding the components involved. For example, divalent metal ion transporter 1 (DMT1, SLC11A2) is believed to be the iron transporter responsible for Fe 2 + uptake by enterocytes. In vitro experiments have shown that when Xenopus oocytes are transfected with Dmt1 mRNA, a number of other trace elements, such as copper, zinc, or manganese are also transported. Based on that and other in vitro evidence, it has been suggested that this transporter is involved in the intestinal uptake of multiple elements. However, the transport of copper by DMT1 has been challenged in a more recent in vitro study. In addition, experiments with an intestine-specific Dmt1 knockout mouse model show that DMT1 is specific for iron absorption, but is not required for the absorption of copper, zinc, or manganese. Consequently, in vitro and in vivo experiments can yield different results and interpretations. Aside from a few in vitro competition experiments with transporters, the interactions of trace metals have not been thoroughly evaluated at the molecular level. Despite the lack of evidence, it does seem reasonable to suggest that transport activities for some trace metal transporter proteins are leaky and, thus, may transport both multiple essential trace metals. This situation may occur when the dietary availability of the natural metal substrate is low. Such circumstances may lead to the absorption of these metals with no known biological functions, for example, cadmium, which are considered toxic substances. Few studies have evaluated the influence of dysfunctional human transporter genes on trace metal absorption. A classic example is the malabsorption of 65 Zn in patients diagnosed with acrodermatitis enteropathica (AE) produced by mutated Zip4 (reviewed in Ref. ). Another example is mutated Zip14 that produces a form of ZIP14 which does not transport manganese properly and may prevent the normal hepatic transfer of absorbed manganese into the bile for fecal excretion.
Numerous metal transporter proteins documented to be expressed in various cell types may contribute to trace metal absorption or excretion, but have not yet been localized within cells of the gastrointestinal tract. Consequently, their individual roles in pathways of metal homeostasis have not been defined, but may influence interpretation of existing data. Further research, particularly studies using knockout rodents which do not express a specific transporter gene in the gastrointestinal tract may shed light on trace metal absorption mechanisms.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here