Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The field of tissue engineering embodies, like few other areas of scientific endeavor, the principles, aspirations, and the techniques of plastic surgery. However, the “boom time” promise of the early “frontier” tissue engineers and their pioneering endeavors have not been matched, to date, by real-world solutions that translated into useful outcomes for plastic surgery patients.
Unfortunately, the fortunes of the tissue engineering sector closely matched those of the turn of the century “tech bubble”; and in the wake of a historic stock market correction, suffered a similar collapse. What has been less well-described, however, have been some of the factors that created the “perfect storm” that made this course inevitable. Similarly neglected has been a discussion of the fact that this collapse led to a re-evaluation and a quiet re-birth of tissue engineering, which has now begun to take shape from the ashes of the collapse. Studying the factors that contributed to this collapse will reveal key lessons that may be used to ensure the success of tissue engineering Mark-II, and the longevity of plastic surgery in general.
From the early 2000s implosion of the tissue engineering sector have emerged a series of modified aims and realities that may loosely be termed as a “re-invention”. The dreams and hype of the 1980s and 90s were unable to be matched by the fundamental science capabilities of the time and the desired translation into solutions to supersede then-available conventional plastic surgical solutions did not eventuate. As a result, the role of today’s plastic surgeons at the cutting edge of the field was diminished and the leadership mantle was instead taken on by cellular and molecular biologists and translational science entrepreneurs.
As plastic surgeons, therefore, we must ask ourselves whether we are willing to now invest in our trainees and craft group, in order to in-build the fundamental science capability to remain conversant in the burgeoning scientific fields now required to engage in a “new wave” of tissue engineering. Are we willing to go back and learn from the problems that led to the crash of nearly 20 years ago and are we willing to apply the attitudinal changes required to make a real contribution and to drive the program? Or are we instead willing only to be passive consumers of products designed by biological engineers and biologists without our clinical input?
One truth that is certain is that given the cost, time, and expertise required to undertake meaningful science in the 2020s, a field that calls itself “tissue engineering” cannot exist independently of commercially viable products and imperatives; nor of the timelines and stringencies required to deliver them. Half a century on, we explore tissue engineering as a parable for plastic surgeons over-promising and under-delivering, and examine the lessons that must be incorporated into plastic surgery if we are to remain a viable and independent specialty into the next century. We examine some highs and lows of tissue engineering in plastic surgery and ask what might become of the field in future years. If we are to remain in the tissue engineering game for the next 50 years, there are two elements we must adopt overall:
Focus : Plastic surgeons must play to their clinical and personal strengths; and
Commitment : Plastic surgeons must invest in the skill sets and people to continue to evolve.
Plastic surgery seeks to restore or enhance the function and/or form of body tissues and organs of patients afflicted by congenital or developmental anomalies, or by a physical insult. The congenital or developmental anomaly may be either a sporadic or inherited genetic program mishap, or an acquired perturbation in utero. It may more recently be considered to include an ostensibly undisturbed genetic program that is inconsistent with the identity or perceptions of the patient’s self.
A physical insult that disrupts the patient’s tissues or organs may result either from the physical environment due to an external injurious force or agent; or due to a planned intervention by doctors seeking to combat a pathological condition such as malignancy, benign unwanted tissue growth or pathologic infective processes. From the dawn of surgery, the legendary figure of Sushruta who worked in India between the years 600–1000 BCE, anatomical examination and research have been a cornerstone of the surgical techniques applied to patients treated for punitive nasal amputations administered as punishment for adultery.
Sushruta identified the fact that a more thorough understanding of underlying science was required to inform application to patients:
[A]nyone wishing to acquire a thorough knowledge of anatomy must prepare a dead body and carefully observe and examine all its parts. Sushruta, 600-1000 BCE
Later, Italian surgeon Gaspare Tagliacozzi examined the anatomy of executed prisoners in order to learn the anatomy that would form the basis of his famous adaptations of the earlier version of reconstructive rhinoplasty that he performed to treat nasal injuries acquired in duels.
Tagliacozzi neatly summarized the aspirations of plastic surgery, which can also encompass the fundamental aims of tissue engineering:
We restore, rebuild and make whole those parts which nature hath given, but which fortune has taken away. Not so much that it may delight the eye but that it might buoy up the spirit, and help the mind of the afflicted. Gaspare Tagliacozzi
Since its inception, plastic surgery has necessarily required an inquisitive and pioneering mindset. Throughout the clinical practice of plastic surgery and more recently in the modern post-war era of plastic surgery, the practice has involved the surgeon experimenting through observation and trial and error with the limits of blood supply and tissue endurance.
From the first early-20th century theaters of war, through the 1940s, the founders of modern plastic surgery recognized the experimental nature of their revolutionary work. The “experimental” nature of the work of New Zealander Sir Harold Gillies ( Fig. 18.1 ) and his cousin Sir Archibald McIndoe, aided by Thomas Kilner, Rainsford Mowlem, and a host of other pioneers, earned their patients the moniker “The Guinea Pig Club”. The implication was that the prevailing treatments of debridement, amputation, and dressing wounds to heal by secondary intention could be extended to enhance the functional and aesthetic outcomes for wounded servicemen through observational experimentation and brinksmanship that would push the boundaries of what was then possible. The results were wound closure of defects that would once have necessitated amputation or crippling disfigurement of the face or limbs that would render a normal existence impossible. This represented a great evolution in surgical management that would spurn several generations of innovation and would eventually lead to the concept of tissue engineering.

Within three decades the limitations of random pattern and tubed pedicle flaps would become apparent and would stimulate the next great evolution in wound management. The pedicle flaps would necessitate 6 or more months of hospitalization and an extensive series of surgical procedures that were both resource-intensive and personally and physically taxing on the patient. Again, a generation of pioneering iconoclasts were called upon to move the specialty of plastic surgery into a new age.
In Boston, USA, Dr Joe Murray successfully transplanted a kidney between humans in 1954 and received the Nobel Prize in Physiology or Medicine in 1990. Also in the US, Dr Harry Bunke reported the first experimental replantation surgery of a rabbit ear in 1964, followed by the first primate digital replantation (toe to hand transfer) in 1966 and human tissue transfer of omentum to scalp in 1969. In Melbourne, Australia, Professor G. Ian Taylor mapped the blood vascular supply of the human body in the morgue of his local hospital, and in 1971 performed the first composite fasciocutaneous free tissue transfer when he transferred a groin free flap to an open ankle fracture ( Fig. 18.2 ) The “angiosome concept” described the entire body in vascular territories that could be utilized to transfer tissue from almost any donor site. In 1976, also in Melbourne, Taylor’s cross-town rival Dr Bernard O’Brien (see Fig. 18.2 ) described the use of microsurgery for the restoration of the physiology of the lymphatic system in the form of lymphatico-venous anastomosis surgery, and later, in the form of free vascularized lymph node transfer.
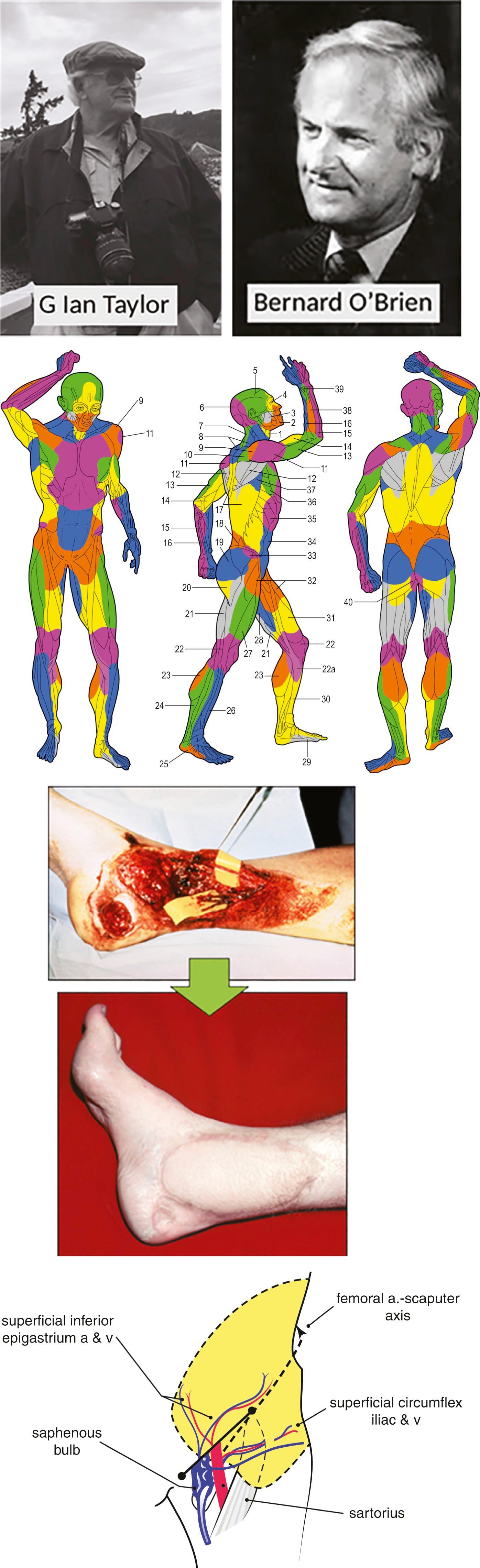
What had once been the stuff of science-fiction had now become clinical reality, and the coming decades would see the institution of the research performed by these and other visionary surgeons to create hundreds of variations of autologous free tissue transfer of 3D blocks of vascularized tissue for all manner of clinical applications. Microsurgery transformed the capability of plastic surgeons to treat congenital or developmental anomalies, and the physical insults acquired during the course of cancer treatment or in trauma in all areas of the human body. Finally, French surgeons performed the first composite tissue allotransplantation (CTA) of a hand in 1998, and in 2005, the first partial face transplantation to a living recipient. The first full face transplant was performed in 2010 in Spain, and since that time the technical exercise has been replaced by the more subjective art of clinical judgment of appropriate recipients. This has become the focus of CTA surgeons world-wide.
Open fractures and ungraftable soft-tissue defects in the limbs could be salvaged; and tissues could be transferred to cover critical exposures of underlying organs in abdominal, pelvic, cranial and chest wall defects, in which locoregional options were not available. Congenital hand deformities could be treated using free toe transfers, and genitalia, breasts, and the previously untreatable areas of the head and neck – such as jaws, laryngopharynx, and base of skull – could be reconstructed in ways that could previously only have been imagined. What had once been possible in many months, if at all, was now possible overnight ( Fig. 18.3 ). Plastic surgery had learned to transfer entire faces and hands from donor to recipient – two areas emblematic of human identity and function.
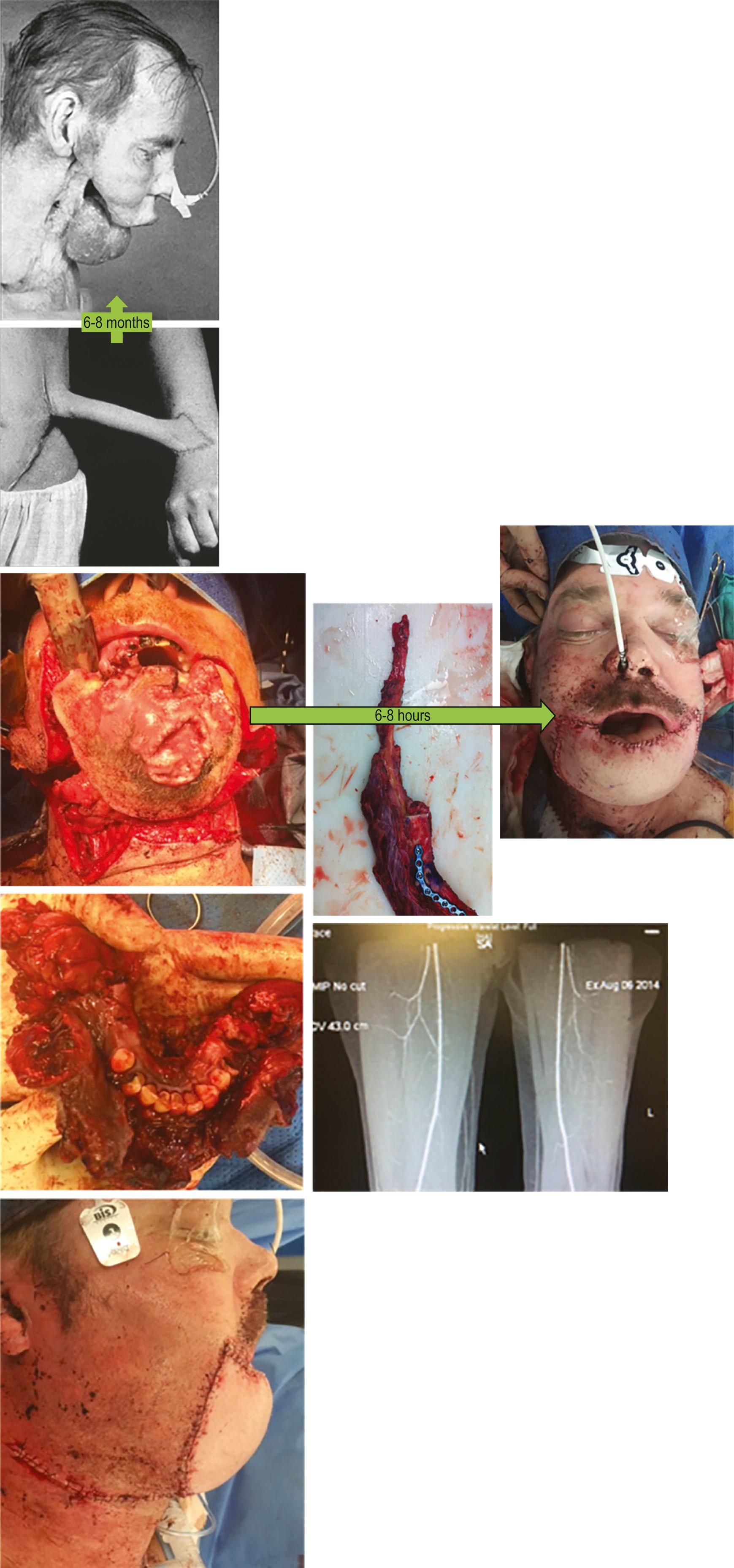
Microsurgery made it possible to achieve a superior outcome in a matter of hours. Here a free fibular flap is made into a neo-mandible and a second free flap is used to cover the external skin of the chin in a significant soft-tissue defect resulting from a p16-negative squamous cell carcinoma of the cutaneous chin.
Virtually overnight, an area of human endeavor had been created that would revolutionize the practice of reconstructive surgery, but which could be readily taught and learned to the extent that, today, microsurgery is a fundamental tool in the algorithm known as the “reconstructive ladder” of plastic surgery. Essentially, the success of microsurgery had been driven by a vast and worldwide unmet clinical need for the solution. It boasted the great attributes that modern companies seek; scalability and transferability of techniques. The requirements were core knowledge about the vascular anatomy of the body, training by experienced technicians, a relatively modest initial outlay to furnish a microsurgery unit with microscopes, a set of reusable instruments and micro-sutures. These giant steps had been accomplished by all of the pioneers and thanks to excellent international clinical fellowships and courses, microsurgery had been disseminated successfully to the four corners of the globe.
It was due in no small part to the amazing success of microsurgery that plastic surgery was able to take on the mantle as a significant contributor to human health. Microsurgery demonstrated the fact that plastic surgery could be a nimble and innovative specialty that could readily evolve and adapt to clinical needs. But where to from here?
Plastic surgery had managed to capture the public and, therefore, the funding bodies’ imagination for some years and to open a new frontier of human endeavor. Funding bodies such as the national hospital systems in some jurisdictions, and public research funding organizations and philanthropic donors in others, had funded research into microsurgery with good returns in human health metrics to the community.
If free flaps inherently involved “robbing Peter to pay Paul”, surely then, the final frontier for plastic surgeons was to achieve their goal of restoring form and function to tissues and organs that make up the organism and person as a whole – without paying the price of “robbing” the body of a donor tissue ( Fig. 18.4 ). For plastic surgery researchers taking this next step – particularly for patients in whom there was a paucity of donor sites or in whom the donor site trade-off was not acceptable – the next evolution, or at very least the next iteration, was needed.

In the 1990s, Langer and Vacanti encapsulated the discipline that had arisen from such noble and lofty aims as:
A new interdisciplinary field, tissue engineering, applies principles of biology and engineering to develop functional biological substitutes to restore, maintain or improve function in damaged tissue and diseased organs.
The dream of plastic surgeons was to create “off-the-shelf” body parts that could be utilized for patients in whom the donor sites were either non-existent or costly ( Fig. 18.5 ). In addition to “bulk filler” soft-tissue reconstruction, niche areas of unmet need also existed in more nuanced areas such as nerve and limb regeneration and inspired inquisitive minds to explore an exciting field of scientific endeavor.
The 1960s saw an emerging focus on biomaterials and engineering driven by the post-war challenges that drove a race toward global technological supremacy. A new substance that integrated the structural integrity of cross-linked polymer chains with an ability to absorb fluid, called hydrogels, had been developed at that time and was first applied to contact lenses and by the pharmaceutical industry for drug testing.
During the following decade the specialties of engineering and surgery combined when a like-minded engineer and surgeon described a coral-like structure that provided the structural integrity under greater force requirements that hydrogels lacked. In the late 1970s to early 1980s, burgeoning ink-jet technology was soon adapted for delivery of cultured cells in a 2D printed cell layer. In 1985 the term “tissue engineering” was introduced by Y.C. Fung, giving rise, between the years of 1987 and 1992, to a rapid expansion in biotech companies that could loosely be termed the “first biotech boom”. The epicentre of this boom was at the Massachusetts Institute of Technology (MIT) and Harvard University precincts in the US, and, to a lesser extent, other multidisciplinary institutions that sought to become the leading commercial translational institutions. At that time, the make-up of the fledgling tissue engineering sector was predominantly (90%) academic, with makeshift spin-outs and start-ups combining due to a strong investment appetite (90% private), without the governance and regulatory structures that are evident internationally today.
Europe and the UK were off to a slower start than was seen in the US, due to a more conservative investment risk appetite, a greater proportion of public funding that boasted higher funding hurdles, and a larger oversight role of more dominant government bodies on companies (e.g., the National Health Service in the UK) and on their products. In Japan much of the early cell work was only in the setting of state hospitals and academic centers, with no state scientists permitted to transition to also work in industry until 1998. This environment resulted in relatively slower approvals and a smaller number of large firms dominating the landscape and no start-ups to speak of; with a chief focus on exploiting already-approved products. Key regulatory changes in 2014 allowed cell cultivation outside hospitals and increased research speed and encouraged firms to develop their own products. This more supportive environment lead to a boom in nano-technology and the description of induced pluripotent stem cells (iPS)-related and stem cell products that would penetrate more deeply into the tissue engineering field, eventually giving rise to the cell-based therapeutics fields.
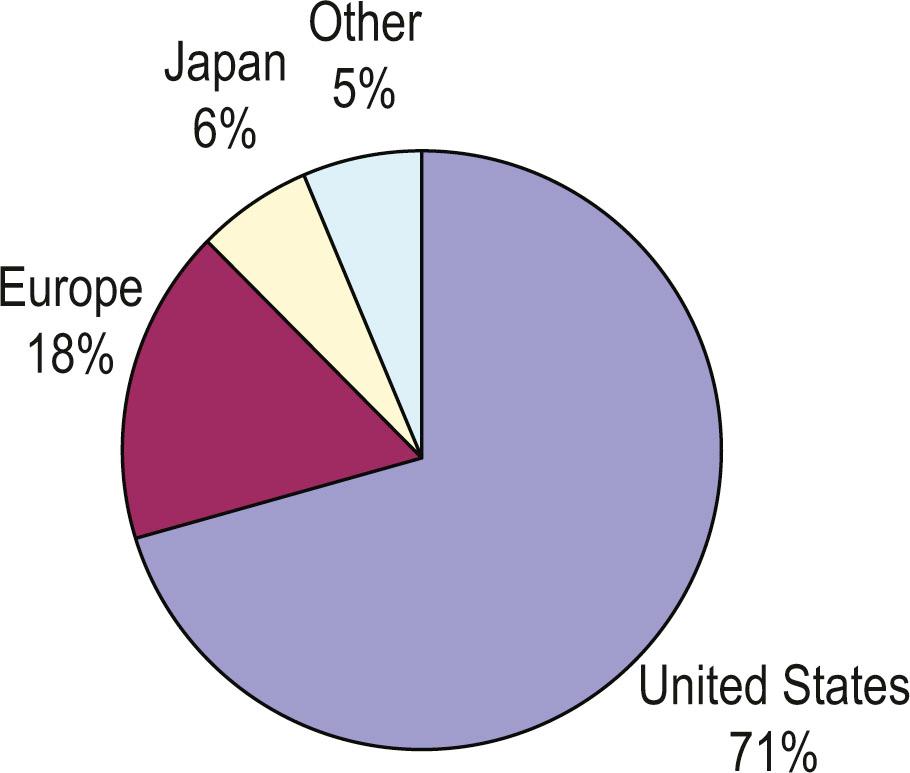
In Australia, the 1980s saw the O’Brien Institute transition from pursuits that had helped to play a key role in the development of microsurgery (particularly with regard to the lymphatic system ), toward a “vascular-loop” cell chamber-based tissue engineering focus. This transition would result in a key highlighting of the microenvironment and importance of tissue nutrition to the overall success of tissue engineering. Nevertheless, despite increasing interest globally, the majority of the intellectual property relating to tissue engineering continued to emanate from the US ( Fig. 18.6 ). A strong private investment appetite in the early 1990s continued to drive interest in the new field of tissue engineering, in which new opportunities to marry technology and reconstructive surgery fuelled the hope that the next generation of rebuilding human bodies was nigh.
During the latter part of the 1990s, the frenetic tissue engineering sector began to show signs of a “bubble market”. Issues started to appear in the translation of expanding media hype into rigorous experimental ideas, high-quality science, and eventually working solutions. A major sticking point in the tissue engineering model related to the inherent commercially unviable practice of using and handling living cells that were, by definition, hard to come by and to maintain; and which were marred in regulatory and ethical complexity. These factors led to poor commercial viability and inability of companies to achieve sufficient scale, and to a low rate of FDA and other regulatory approvals.
The consequence was low doctor and payer adoption of the proposed new technologies over conventional methods, reduced demand, higher costs, and diminished product recognition. US insurance providers were reluctant to pay for these unproven technologies, further eroding care-provider acceptance and inhibiting the efficacy of product marketing and broader integration into the health system.
Tissue engineered products fabricated by lab bench-scale processes remained prohibitively expensive and impractical with cost per product based on cost of cultivation, storage, transport in precise conditions of living cells far outstripping the budget available and the solutions that they had been intended to supersede. The lack of cost-effective production and distribution made tissue engineered solutions resource-intensive and of variable reproducibility and quality compared with more established treatments. Unfortunately, the result of poor competitiveness of tissue engineered solutions further contributed to the costs of production and many early firms became bankrupted, thus breaking continuity of any breakthroughs and developments that they might have made. Finally, investors lost patience with tissue engineering companies as they struggled to translate good ideas into real profits, eventually ceasing to fund the relatively speculative ventures behind the tech and biotech bubbles; and with them the relatively smaller tissue engineering industry. Whilst 1992 and 1998 saw minor corrections of the biotech sector, the major correction seen in 2000 would spell the end of many of the listed tissue engineering companies. The total capital value of tissue engineering companies dropped by a staggering 90%, from a total value of US$2.5 billion in 2000, to US$300 million two years later ( Fig. 18.7 ).
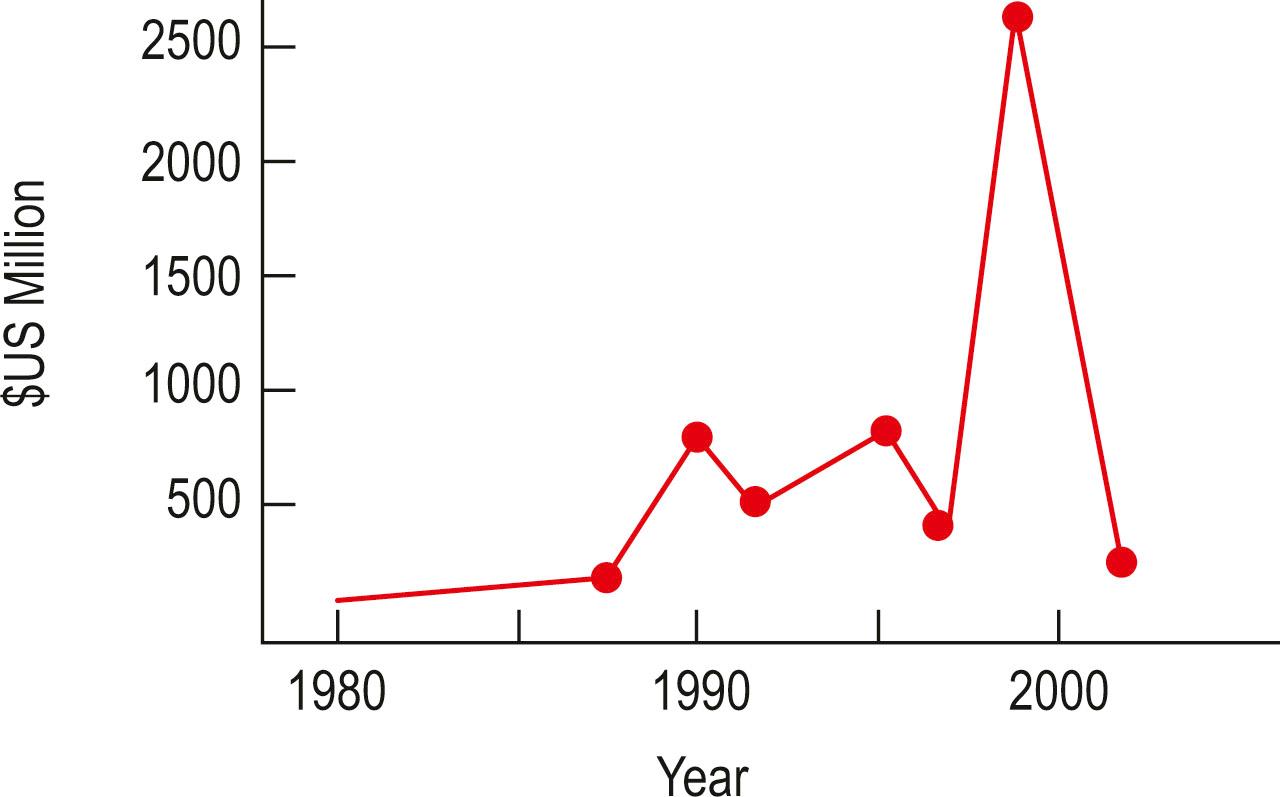
At the time of the collapse of the tissue engineering sector, 89 firms in 15 countries, employing 2600 full-time equivalent roles in research and development, were destroyed. Work in skin and cartilage structural biology had represented over 50% of tissue engineering and comprised over 800 FTE roles; however, at the time, the entire industry was yet to produce a single profitable product despite over US$4.5 billion research and development funds having been invested. Twenty products were in FDA trials; six of these were abandoned or failed. A total of four of these original 20 products were eventually approved, however, none of these managed to achieve commercial success, and no successful product portfolio was generated. As a result, by mid 2002, few of the early tissue engineering firms remained commercially viable and the industry had officially collapsed.
After having promised so much, the collapse of the tissue engineering market had left the sector in tatters. In order to understand the reasons behind the rise and fall, one must examine the global environment more broadly. Bouyed by unbridled optimism and unrealized expectation on behalf of both society and investors, the failure to create workable products led to disappointment and disaffection after a handful of years.
Unfortunately, the science capability at the time did not match the aspirations of the public and the increasingly expansive media outlets of the day. Insufficient thought had been given to a path to market and practical clinical application of the tissue engineering experimental models. There was insufficient business and industry expertise built into the largely academic ventures that in turn led to less emphasis on planning and governance; and slower and less direct passage through regulatory pathways.
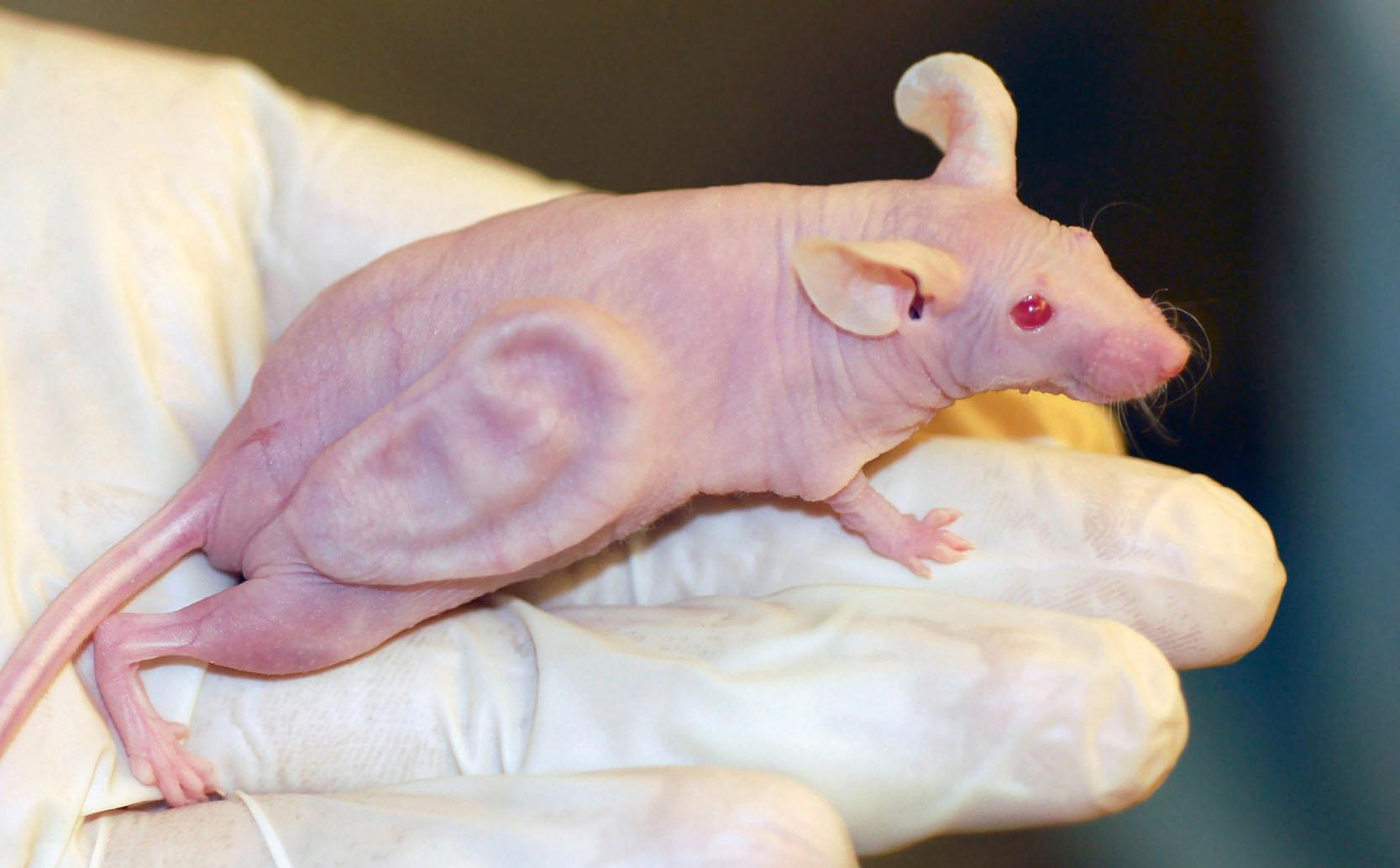
Insufficient forethought in the areas of target product profile and product differentiation from then gold-standard treatments; as well as poor process management and manufacturing quality assurance, meant that any progress made within the early funding rounds were not channelled into meaningful returns on investment. The public’s imagination was captured not only by block-buster science fiction movies and iconic television series, but it was also fanned by the headlines such as Dolly the sheep, cloned from adult stem cells in the UK, and the famous images of the Vacanti mouse, which boasted a bovine cartilage ear scaffold implanted beneath the back skin of an immunocompromised mouse ( Fig. 18.8 ). Unfortunately, much like the fate of the tissue engineering sector in the 1990s, the once bold ear scaffold of the Vacanti ear construct quietly involuted into an amorphous ball of scar tissue on the back of a nude mouse.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here