Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Thyroid disorders are among the most common endocrinopathies in women of childbearing age, yet diagnosis and management are often confusing during pregnancy. In large areas of the world, iodine deficiency is the predominant cause of these disorders affecting both maternal and fetal production of thyroid hormones. In the Western Hemisphere, these disorders are most often due to autoimmunity. The hormonal and immunologic perturbations during and after pregnancy and the dependence of the fetus on maternal iodine and thyroid hormone have profound influences on maternal thyroid testing and function, and consequentially pregnancy outcomes and fetal well-being.
The thyroid gland weighs approximately 20 to 25 g, and each lobe is divided into lobules, each containing 20 to 40 follicles that produce thyroid hormone (TH). A follicle consists of follicular cells that surround a lumen filled with a glycoprotein material called colloid , where thyroglobulin (TG; a tyrosine-rich glycoprotein) is synthesized and stored . Thyrotropin-releasing hormone (TRH; a tripeptide) is released from the paraventricular nucleus of the hypothalamus, traverses the pituitary stalk through the pituitary portal circulation, and is delivered to the anterior pituitary thyrotropes. TRH affects the production and release of thyrotropin (TSH; a glycoprotein). TSH is composed of α- and β-subunits, and the β-subunit confers specificity. The α-subunit is identical to follicular stimulating hormone (FSH), luteinizing hormone (LH), and human chorionic gonadotropin (hCG). TRH stimulation and negative feedback are mediated primarily from the inhibitory actions of circulating TH (both thyroxine [T 4 ] and triiodothyronine [T 3 ]), somatostatin, dopamine, glucocorticoids, and excess iodine that control TSH secretion. TSH binds to the TSH receptor on the thyroid follicular cells, which stimulates increased blood flow to the thyroid and induces thyroid growth, iodine metabolism, TG synthesis, thyroperoxidase enzyme (TPO) activity, and secretion of T 4 and T 3 .
Dietary iodine is reduced to iodide, which is actively trapped by the thyroid and is the rate-limiting step in TH biosynthesis by stimulating iodide organification. Iodide is oxidized back to iodine and binds to tyrosine residues on TG, which forms mono- (MIT) and diiodotyrosine (DIT). T 4 is formed with two DIT coupling, and T 3 is formed with an MIT and DIT coupling. Iodide oxidation, TG iodination, and MIT and DIT coupling requires TPO catalytic activity. When TH is required in circulation, TG is endocytosed, broken down by lysosomal enzymes to T 3 and T 4, and both are released from the gland into the circulation in a 14:1 (T 4 /T 3 ) ratio. T 4 is an inactive pro-hormone and the primary TH released from the gland. T 4 levels are genetically driven and remain in a tight window under normal conditions. More than 80% of circulating TH is T 4 , but T 3 is the active form with a 10-fold greater affinity for nuclear thyroid hormone receptors (THR). The majority of available T 3 results from deiodinase peripheral conversion of T 4 . Therefore, circulating T 4 is critical for peripheral T 3 availability. Moreover, T 3 levels are variable and highly influenced by conditions that impact deiodinase conversion of T 4 to T 3 such as illness and medications. In settings of iodine deficiency, T 3 is preferentially formed and negatively feeds back on TSH secretion, which may result in a low FT4 with a normal TSH (i.e., isolated hypothyroxinemia).
Circulating TH are highly bound to proteins (>99% of circulating T 4 and T 3 are bound; 0.03% are free), primarily thyroxine-binding globulin (TBG), prealbumin, transthyretin, and albumin. Free TH enters the cell and binds to nuclear THR, stimulating transcription and translation of new proteins in a concentration- and time-dependent manner. TH has diverse effects on cellular growth, development, and metabolism.
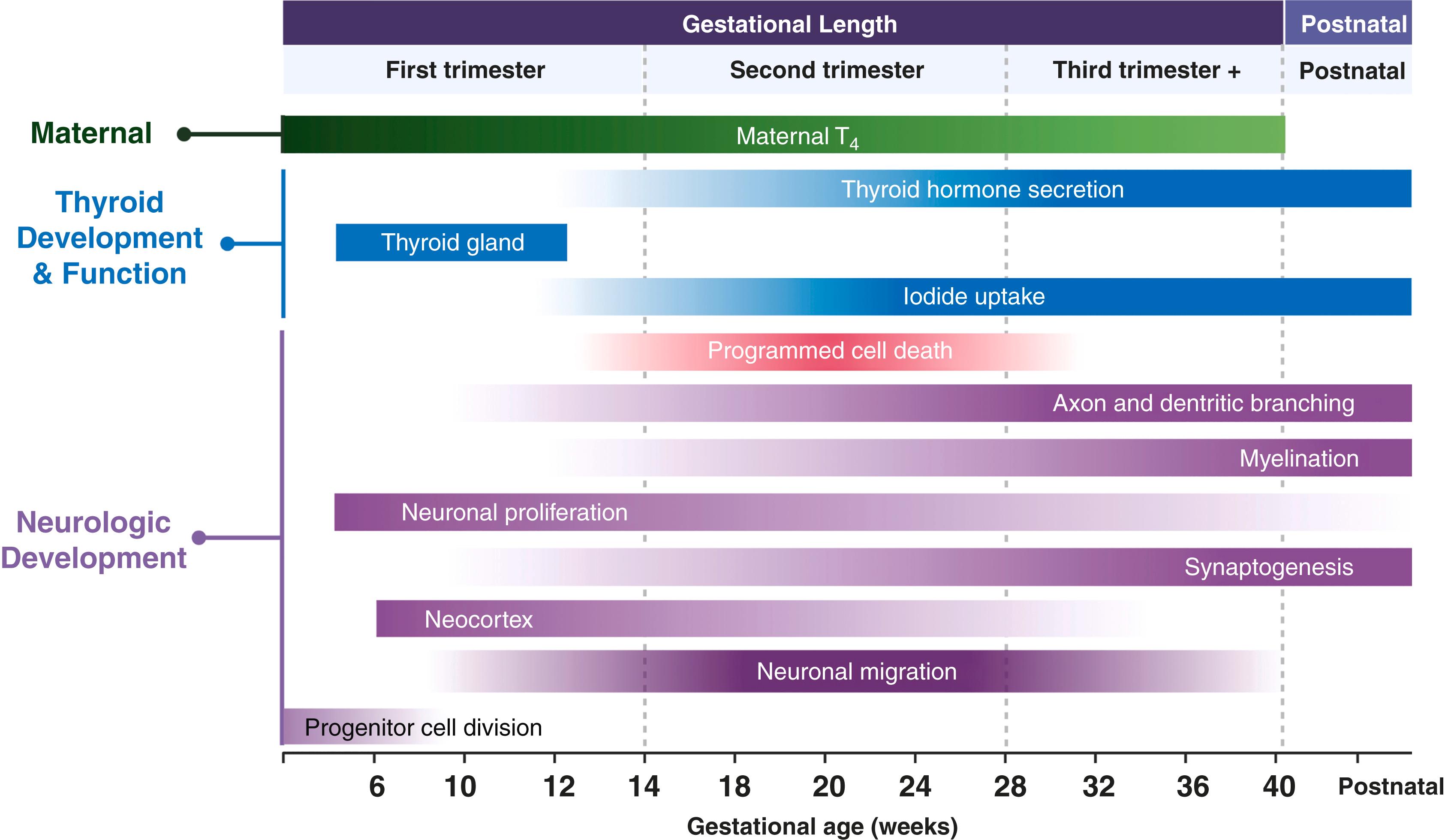
Pregnancy is a complex and dynamic metabolic state that is associated with alterations in thyroid economy. The major physiologic changes that contribute to changes in thyroid function include thyroid stimulation by hCG, estrogen-induced rise in TBG, changes in clearance of TH, increased iodine metabolism, and type 1 and type 2 deiodinase placental activity ( Fig. 61.1 ). The thyroid gland increases in size by 10%–15% in iodine-sufficient countries due to mild increases in vascularity and mild glandular hyperplasia, but is not generally notable on physical exam. In iodine-deficient areas, the gland may increase 20%–50% and may not be reversible, also known as the thyroid goiter of pregnancy. Pregnant persons with normal thyroid function can adapt appropriately to these alterations in thyroid and iodine metabolism. However, those with decreased thyroid reserve from Hashimoto thyroiditis or partial thyroidectomy may not be able to adequately increase TH production to meet the demands of pregnancy.
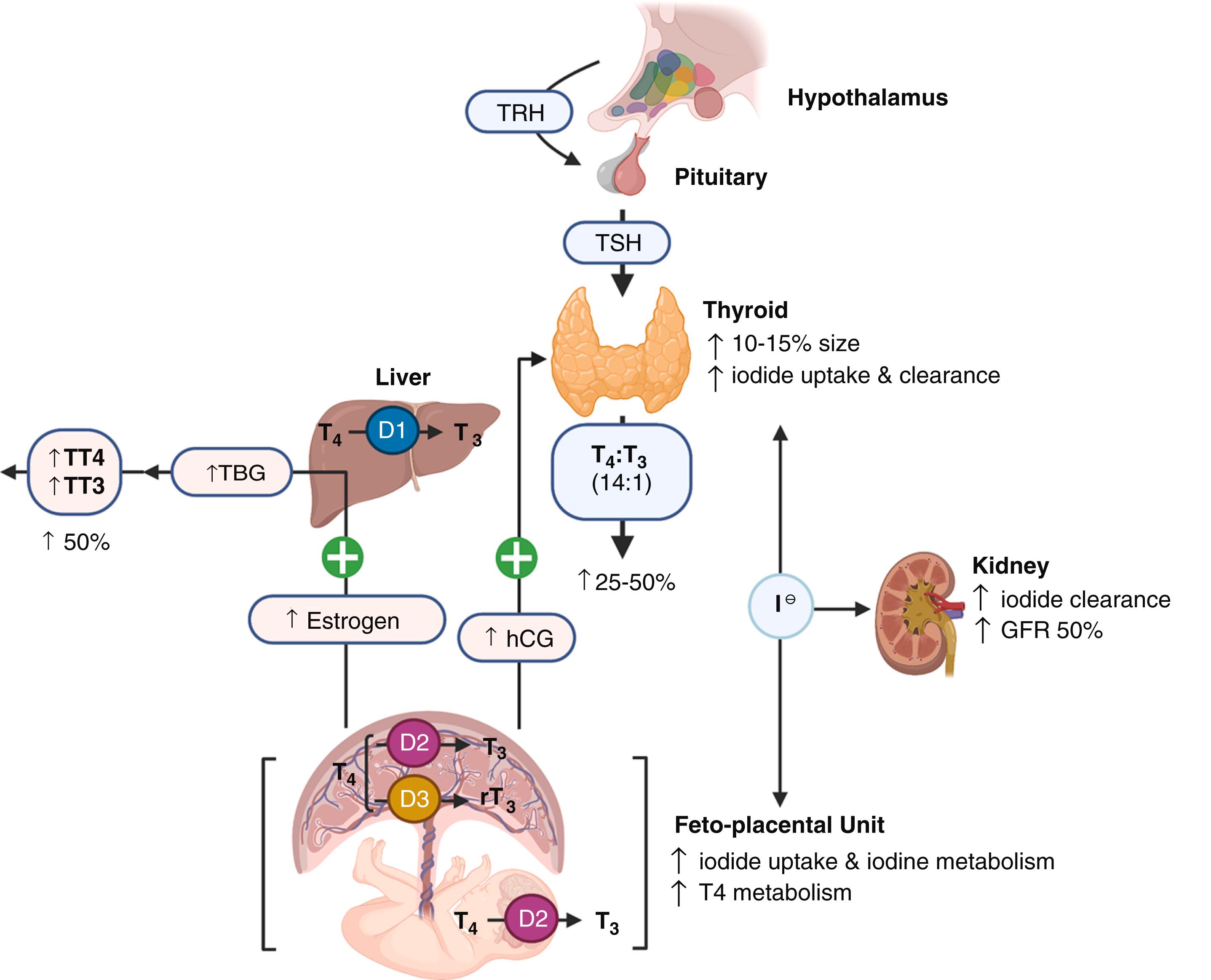
By 7 weeks’ gestation, the rising circulating estrogen concentrations increase TBG synthesis two- to threefold in the liver, TBG half-life is prolonged from 15 minutes to 3 days, and levels peak at 16 weeks, continuing until term. Under conditions of iodine sufficiency and the absence of any decreased thyroid reserve, the TBG concentration results in ∼50% increase in total T 4 and T 3 (TT4 and TT3) levels ( Fig. 61.2 ). With iodine insufficiency or a patient with decreased thyroid reserve, TSH levels may increase and FT4 and FT3 may fall. Measuring accurate free TH levels under high protein (TBG) levels in pregnancy is a challenge.
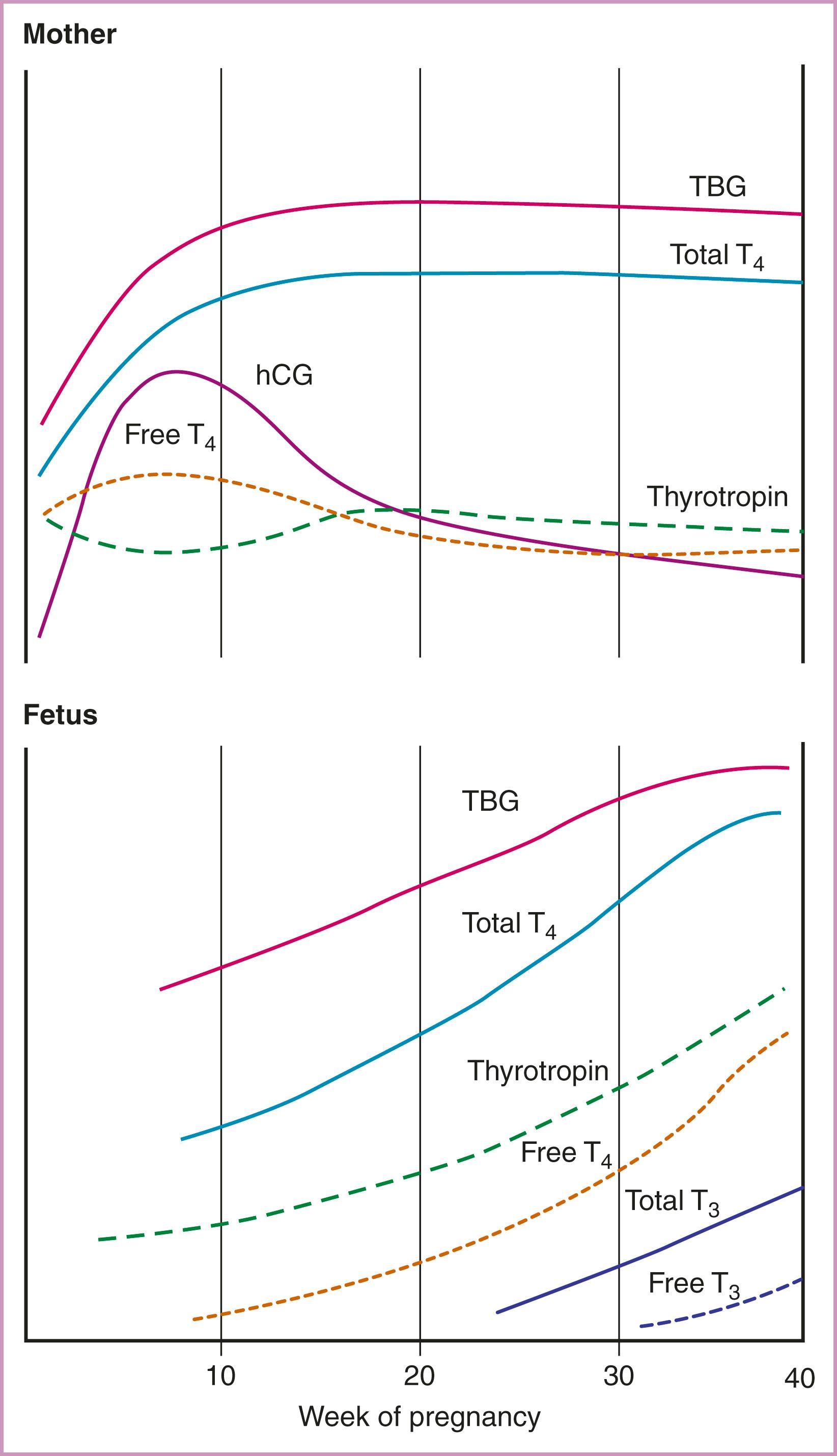
The thyroid gland is directly stimulated by hCG throughout the first trimester, peaking at approximately 8–12 weeks’ gestation with a subsequent decline and plateau thereafter. , It promotes TH production because of (1) the similar beta subunit structural homology (∼40%) of hCG and TSH, and (2) sialylation of hCG that modifies its half-life and affinity for the TSH receptor. TH production increases 25%–50% by the end of the first trimester. As hCG peaks late in the first trimester, the slight increase in T 4 negatively feeds back and transiently lowers TSH. Pregnant conditions associated with significantly higher hCG concentrations such as molar pregnancies, hyperemesis gravidarum, and multifetal gestations more frequently lead to suppressed TSH and less commonly, mild increases in T 4 levels. , Every incremental increase of 10,000 IU/L of hCG is associated with lowering basal TSH levels by 0.1 mIU/L. TSH suppression from the thyrotropic activity of hCG is not associated with adverse perinatal outcomes. TSH increases after the first trimester to term but may remain mildly suppressed through the third trimester.
The concentrations of T 3 and T 4 in circulation are controlled by TH secretion as well as peripheral tissue metabolism through primarily deiodinase enzyme activity but also sulfation and glucuronidation. As a result of the complicated interplay between local TH supply, transport, deiodinase activities, and degradation, serum T 4 and T 3 levels do not necessarily reflect tissue levels. T 4 is metabolized to active T 3 or bio-inactive reverse T 3 (rT 3 ) with three deiodinases that are found in both fetal and adult tissues: type 1 (D1), type 2 (D2), and type 3 (D3) iodothyronine deiodinases ( ![]() e-Fig. 61.3 ).
e-Fig. 61.3 ).
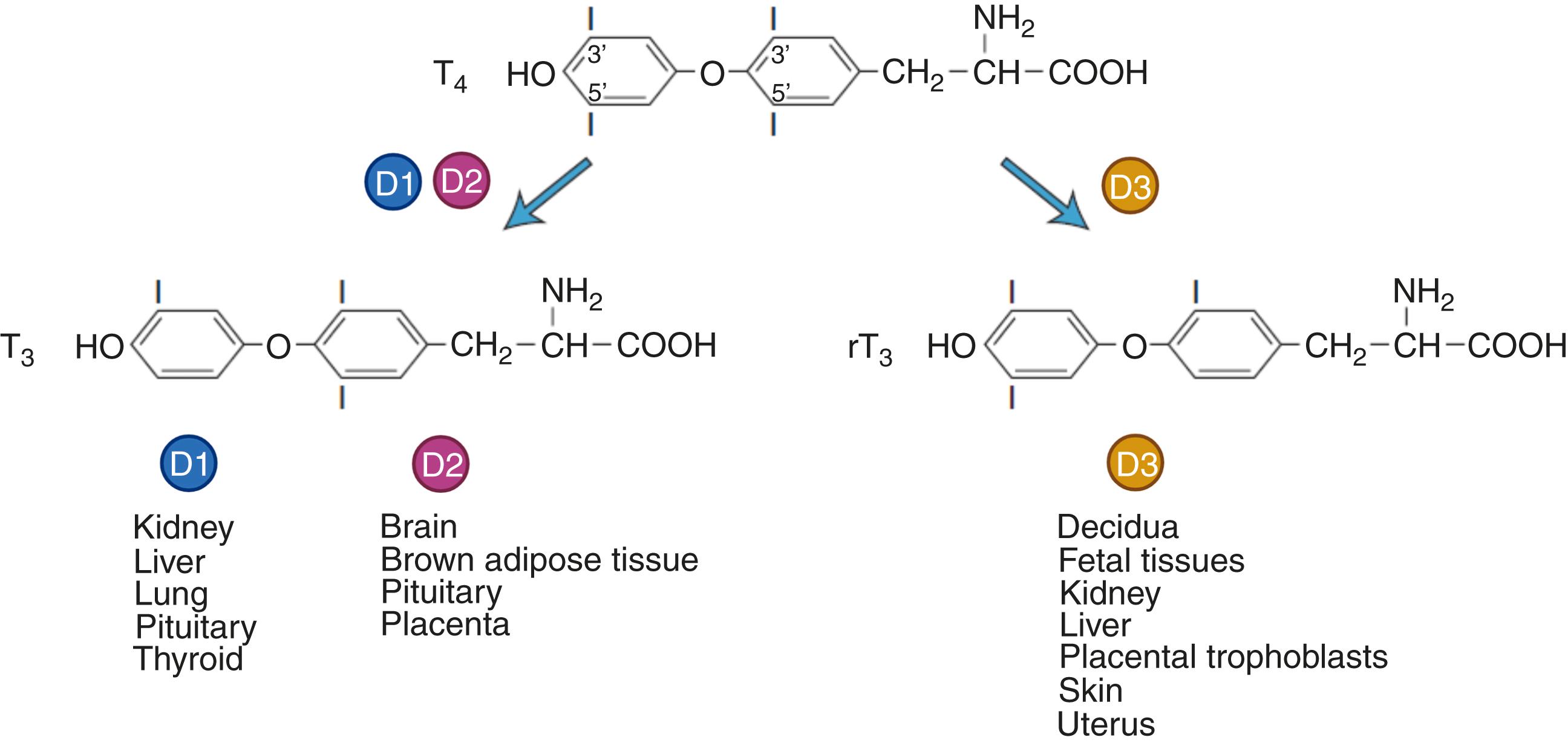
TH availability to the fetus is determined by plasma membrane thyroid hormone transporters, deiodinase activity, and binding proteins expressed in the placenta. Alterations in the peripheral metabolism of TH occur throughout pregnancy but are more prominent in the second half due to increased deiodinase activity in the placenta. The placenta expresses both D2 and D3 deiodinases, but predominantly expresses D3 (200-fold greater than D2) to protect the fetus from overexposure to maternal TH. Unlike D1 and D2 which increase formation of T3 in various tissues, D3 catalyzes the deiodination of T4 to reverse T3 (rT3) which is inactive. Placental D3 contributes to fetal iodide pool, which can be used for TH synthesis by the fetal thyroid gland. TH is important for trophoblast differentiation. The presence of D2, predominantly expressed in the cytotrophoblast in the first trimester, can maintain or increase T 3 production locally for fetal tissues, placental growth and development , and conditions when maternal T 4 concentrations are reduced.
In the nonpregnant state, the thyroid gland takes up to 80 to 100 μg of iodine daily. Iodide is absorbed and cleared by the kidneys (80%) and thyroid (20%). The maternal iodide pools are relatively lower in pregnancy due to increased maternal T 4 synthesis by the thyroid gland, the placental transfer of iodine for fetal iodine requirements, and renal iodide clearance that nearly doubles. Consequently, an increased consumption of iodine (50–150 μg/day) during pregnancy and lactation is required to maintain adequate T 4 production. Iodine readily crosses the placenta via the sodium/iodine symporter. The fetal thyroid develops by 10-12 weeks and is capable of iodine uptake and accumulation by 12 weeks’ gestation. A substantial amount of iodine is diverted toward the fetoplacental unit for fetal TH production.
Worldwide, iodine deficiency is still a common cause of severe neurodevelopmental dysfunction and impaired cognition. Iodine deficiency and hypothyroidism in pregnancy continue to be a worldwide problem. , In the United States, mild to moderate iodine deficiency is often present due to a lack of mandated iodine supplementation in salt and even more pronounced in other countries, including Europe. The National Academy of Medicine, formerly the Institute of Medicine, and the recently released United States Departments of Agriculture and Health and Human Services Dietary Guidelines for Americans recommend a total daily iodine intake of 150 μg/day prepregnancy, 220 μg/day for pregnant persons, and 290 μg/day during lactation. , The World Health Organization and the American Thyroid Association (ATA) recommend 250 μg/day of iodine during pregnancy.
Dietary iodine sources vary globally and across the United States. Salt iodization significantly decreased the rates of childhood goiters and has been the primary source of iodine fortification in the United States, but only 50% of table salts are iodinized. One teaspoon of iodized salt contains 272 μg of iodine. Other sources of iodine include dairy, commercially-baked breads, seafood, eggs, meat, and poultry. The American Academy of Pediatrics and ATA have recommended prenatal supplementation containing 150 μg potassium iodide as dietary adequacy is often difficult to determine. , As of 2017, only 40% of commonly sold prenatal vitamins contained iodine and 25% of those supplements used kelp-based sources. Kelp can have variable, but sometimes extremely high, iodine content and is not the recommended source in pregnancy.
Excess iodine can lead to transient inhibition of TH synthesis by decreasing peroxidase activity and organification to protect against overproduction of TH, a mechanism known as the Wolff-Chaikoff effect. Although this is usually followed by an “escape” of this effect by downregulation of the sodium-iodide transporter and decreased intrathyroidal iodine concentrations, pregnant persons with autoimmune disease or nodular goiters may not be able to escape from this effect and become hypothyroid in the setting of excessive iodine exposure. Because the fetal thyroid gland does not fully maturate until 36 weeks’ gestation, it may not be able to fully escape the acute Wolff-Chaikoff effect. As a result, the fetus is more susceptible to congenital or neonatal hypothyroidism and goiter with sustained excessive iodine exposure even when maternal euthyroidism is maintained.
Reduced iodine intake leads to impaired fetal and maternal TH synthesis, affecting T 4 more than T 3 synthesis. As noted, isolated hypothyroxinemia without an elevated TSH may be caused by iodine deficiency due to preferential synthesis of T 3 which requires less iodine to form than T4 and which negatively feeds back on TSH. More severe iodine deficiency increases TSH and TG levels and induces thyroid hypertrophy in an attempt to keep T 4 levels from falling, resulting in thyroid nodules and/or goiter. In a study of otherwise healthy pregnant women living under conditions of relative iodine restriction, thyroid volume, as assessed by ultrasonography, increased an average of 30% during pregnancy (goiter of pregnancy) with a higher risk for persistent goiter following delivery.
Importantly, preconception iodine intake may be more successful in maximizing thyroidal stores and is associated with higher FT4 and lower TSH levels. The beneficial effects of iodine on offspring development appear to be lost if supplementation is started after 10–20 weeks’ gestation, strongly supporting prepregnancy supplementation. Maternal preconception iodine to creatinine ratio is positively associated with child IQ at age 6–7 years after adjustment of maternal IQ and potential confounders. Neurodevelopmental outcomes were improved for children whose mothers were moderately deficient and received iodine supplementation early in pregnancy. Even with mild iodine deficiency, prolonged iodized salt intake improved maternal thyroid economy and reduced the risk of maternal thyroid insufficiency during gestation.
Iodine is an essential mineral in normal human breastmilk and intake should be increased after delivery. Iodine is taken up in the lactating mammary gland through the sodium/iodine symporter with the highest concentrations found in colostrum and levels decline with lactation duration. The sodium/iodine symporter is regulated by oxytocin and prolactin but no significant differences in iodine concentrations in milk of term or preterm lactating persons have been demonstrated. Full-term infants are recommended to have approximately 15 μg/kg of iodine, and 30 μg/kg in preterm infants for normal growth and development. Ultrasonography revealed that thyroid volume was 38% larger in neonates of untreated mothers compared with neonates of mothers treated with iodine supplementation. Antithyroid medications (ATD) are found in very low concentrations (<0.03%) in breastmilk and have not been demonstrated to significantly inhibit deiodination in breastmilk nor affect neonatal thyroid function.
Thyroid hormones are important for trophoblast proliferation, migration, and invasion. T 4 crosses the placenta more readily than T 3 , but the mechanisms and modulators of TH placental delivery to the fetus are not well known. TH enters and exits cells through six thyroid hormone transporters identified in the placenta: MCT8, MCT10, LAT1, LAT2, OATP1A2, and OATP4A but the relative contributions of each to placental TH transport are poorly understood. Once within the cell, TH cellular actions occur through nuclear THR binding.
Type 2 iodothyronine deiodinase (D2), which, like D1, increases T3 formation, is strongly expressed in the cytotrophoblast (CTB) in the first trimester, and the protein expression becomes attenuated with advancing gestation. Type 3 iodothyronine deiodinase (D3), which results in rT3, is strongly expressed in the syncytiotrophoblast (SCT) with activity ranging 100- to 400-fold greater than D2 in the placenta. The mRNA and protein expression for D3 is variable across gestation, suggesting an interplay and balance of D2 and D3 activity to fine tune TH levels in the placenta. TH is associated with alterations in trophoblast differentiation, invasion, and angiogenesis in a gestational age–dependent manner, potentially through regulation of cell adhesion molecules and inflammatory cytokines. T 3 exposure increases trophoblast secretion of hCG, progesterone, and human placental lactogen. Fetal growth restriction has been associated with changes in TH transporter expression as well as upregulation of THRs in the placental villi, which may contribute to the TH accumulation in CTB observed in growth-restricted placentas. However, the regulation of TH within the placenta, contributions to pathogenesis, and the relationship with adverse pregnancy outcomes remain unclear.
Transthyretin (TTR; a 56-kDa homotetrameric protein) is produced and secreted by placental trophoblasts from at least 6 weeks’ gestation and is proposed to play a role in transferring maternal T 4 to the fetal circulation. TTR is an important TH protein carrier in the blood that has been found to be synthesized and secreted in the placenta. Levels rise during the first trimester at a time when placental oxygen tensions are also rising. Levels peak at 13 weeks at which point TTR expression remains constant until term. It binds to extracellular T 4 and forms a complex that prevents inactivation by D3 in the placenta and may be partly responsible for the placental transfer of maternal T 4 over T 3 . The SCT internalizes the TTR-T 4 complex followed by an exocytosis into the fetal circulation. ,
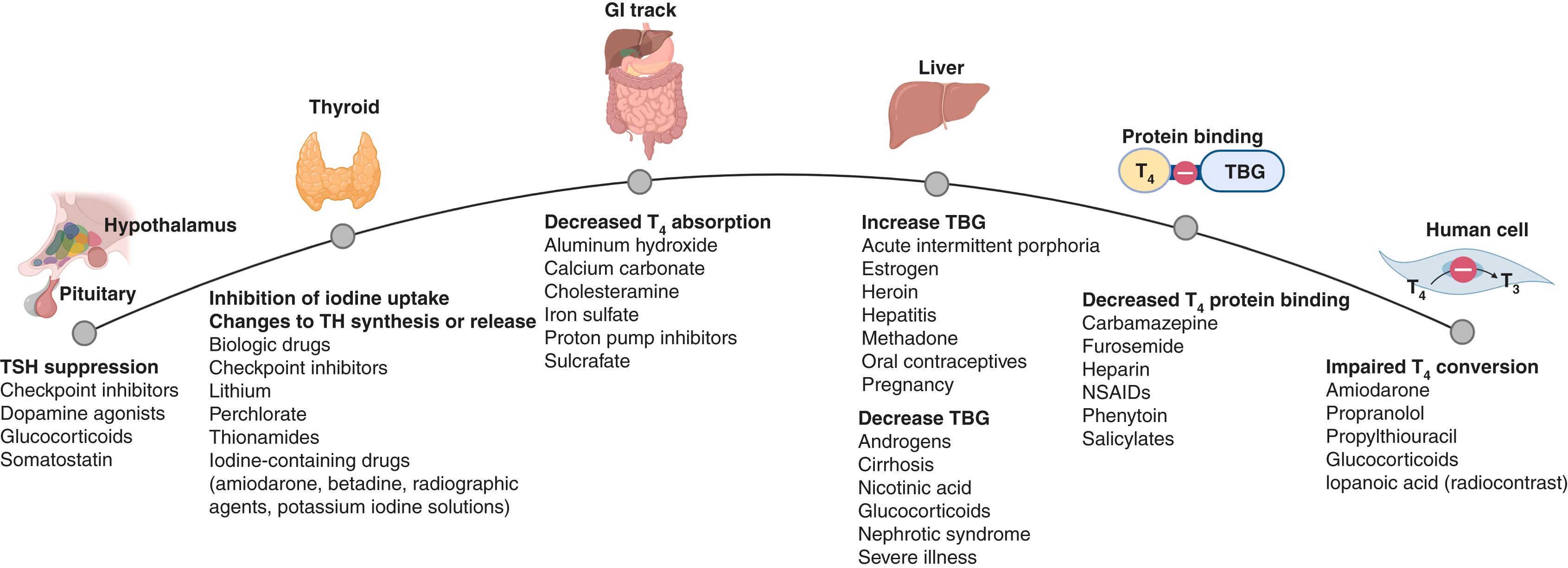
Many pharmacologic agents commonly used in pregnancy can be transported across the placenta to the fetus. The potential influence of the placenta on fetal thyroid and neurologic development is evident by the transfer of several agents that affect thyroid function and metabolism, absorption of thyroid hormones, and interpretation of thyroid function tests ( Fig. 61.4 ) . , Iodine-containing drugs have the potential to cause iodine overload in the fetus. Amiodarone is an antiarrhythmia drug that is used for digoxin and flecainide-resistant fetal tachyarrhythmias. It contains 37% iodine by weight and structurally resembles that of T 4 . Transient hypothyroidism is observed in ∼15% of adults and neonates exposed to intrauterine amiodarone. Therefore, caution for amiodarone use in pregnancy and appropriate neonatal and infant evaluation for continued use during lactation is advised.
TSH does not cross the placenta, and maternal TRH insignificantly impacts fetal thyroid function secondary to the low physiologic concentrations. Intraamniotic administration of T 4 preterm increases fetal maturation, reflected by an increase in the lecithin-to-sphingomyelin ratio and a decrease in neonatal respiratory distress syndrome (RDS). Furthermore, corticosteroid administered antenatally before 32 weeks’ gestation stimulates T 4 release with lower rates of RDS.
During the third week of gestation, the thyroid gland develops at the base of the tongue from a proliferation of endodermal cells between the first and second pharyngeal pouch. This thyroid diverticulum then descends inferiorly to the pretracheal position from 5 to 7 weeks’ gestation with division in the right and left lobes at approximately 5 weeks. During migration, the hollow thyroid diverticulum is filled with follicular cells of the gland. The parafollicular C-cells differentiate from the ultimobranchial bodies that arise from the 4th and 5th pharyngeal pouches, fuse with the superior dorsolateral aspect of the developing thyroid, and form the Zuckerkandl’s tubercle. The thyroglossal duct that connects the thyroid and the tongue during development degenerates by the 10th week of gestation. Incomplete degeneration of the duct can lead to abnormalities of the thyroid gland such as thyroglossal duct cysts and pyramidal lobe.
Hypothalamic and pituitary-vascular maturation occurs from 10–12 weeks with detectable TRH and continues through approximately 36 weeks’ gestation. Follicular growth is progressive from 12 weeks’ gestation when active trapping of iodide and TH synthesis can occur. Thyrocyte differentiation is complete with the primitive onset of TH synthesis at 12–14 weeks as progressive follicular growth occurs in the thyroid. TH synthesis parallels the increasing expression of thyroid-specific functional genes such as Tg , TPO , and NIS . Before the maturation of fetal thyroid function at ∼16 weeks’ gestation, fetal TRH, TSH, and T 4 concentrations remain low and the fetus is completely dependent on maternal TH supply.
At 18 to 20 weeks’ gestation, iodine uptake by the fetal thyroid gland, TH secretory activity, and serum TSH and T 4 concentrations begin to significantly increase (see Fig. 61.2 ). Concentrations of T 4 steadily increase from 2 μg/dL at 20 weeks to adult levels of 10 μg/dL at term, mediated by pituitary TSH secretion and stimulation of the thyroid gland. , The rising T 4 level has minimal negative feedback inhibition on fetal TSH secretion due to the immature hypothalamic-pituitary-thyroid (HPT) axis, which fully matures at 1–2 months after birth. A significant transfer of maternal TH persists through the late second and third trimester. Fetal dependence on maternal T 4 may continue through delivery based on evidence that 30%–50% of T 4 measured in cord blood is maternally derived and the fetal brain is partially protected in cases of congenital hypothyroidism due to genetic mutations, thyroid dysgenesis, and defects in iodide transport or organification.
Fetoplacental exposure to TH is regulated developmentally to prevent overexposure during critical embryologic stages in a tissue specific manner. T 4 is metabolized primarily to rT3 in the placenta and the fetus by D3 but also inactivated to a variety of sulfated thyroid hormones such as T 4 S, T 3 S, and rT 3 S. Because of the high D3:D1 ratio, fetal serum T 3 is negligible until 30 weeks. Near term, fetal T 3 increases in concentration to 50 ng/dL due to the developmental changes in tissue deiodinase activity. Fetal T 3 correlates with the progressive decline of serum rT 3 concentrations with advancing gestation. ,
Normal thyroid function is critical for neuronal proliferation, migration, synaptogenesis, myelination, and development of normal fetal brain structures ( Fig. 61.5 ). Given the fetal CNS is relatively impermeable to T 3 , T 4 is the primary TH that transfers across the placenta and enters the fetal brain, which is why maternal supplementation with T3 is not advised. Fetal T 4 levels are at least one-third of the maternal circulating levels in the first trimester and increase up to 50% by the second trimester. THs are present in the coelomic fluid by 5–6 weeks’ gestation. Nuclear THRs have been identified in the fetal brain by 5 weeks’ gestation and increase 10-fold by 16 weeks before significant fetal thyroid hormone production begins. T 4 binds to these receptors in the fetal brain and is converted to T 3 by D2, activating thyroid responsive genes that control brain development. The fetal brain has both D2 (astrocytes) and D3 (oligodendrocytes and neurons) that serve to provide the critical T 3 levels for brain development and spatially protect regions from excessive T 3 levels by conversion to rT 3 . D3 has a role during fetal development to regulate circulating T 4 levels, but the modulators of spatial and organ-specific regulation of T 4 are poorly understood.
The transport of T 4 and T 3 in and out of cells is controlled by several classes of transmembrane TH transporters including members of the organic anion transporting peptide family (OATPs), L-amino acid transporters (LATs), and monocarboxylate transporters (MCTs). Importantly, OATP-F is distributed widely in the human brain, preferentially transports T 4 , and facilitates T 4 uptake by astrocytes to be converted to T 3 by D2. Moreover, D2 has been observed in the fetal brain by 7 weeks’ gestation, underscoring the importance of maternal T 4 for conversion to T 3 for neuronal and astrocyte proliferation and migration in the fetus. Approximately 80% of T 3 identified in the cerebral cortex is secondary to local conversion from T 4 . Animal studies have clearly demonstrated that the developing brain is dependent on a supply of T 4 that is locally deiodinated to T 3 . Therefore, replacement with maternal T 3 does not readily cross the placenta nor the fetal brain, inadequately supporting the necessary brain T 3 levels required for normal brain development. Despite low circulating levels of T 3 , fetal brain levels of T 3 are 60%–80% of those of adults by 20–26 weeks’ gestation; D2 increases in the fetal cerebral cortex in parallel to the T 3 concentrations.
Because the embryonic/fetal period is when differentiation of major brain structures occurs in a relatively short amount of time, even small or subtle alterations in brain structure or function from low TH during fetal life can become progressively magnified over time. These CNS alterations produce long-lasting effects on brain anatomy, connectivity, and function. Immediate postnatal TH replacement appears to reverse alterations in TH-mediated gene expression. However, TH in early gestation is required to avoid neuronal migration defects, morphological alterations, or impaired motor coordination.
TH plays a role in the biochemistry of the brain, involving norepinephrine, epinephrine, dopamine, serotonin, and GABA neurotransmission in addition to changes in acetylcholinesterase. Maternal thyroid dysfunction is associated with affective mood disorders, autism spectrum disorders (ASD), and attention-deficit/hyperactivity disorders (ADHD) in the offspring. Glucocorticoids and TH have synergistic effects on neurodevelopment as well as fetal tissue maturation, which is reflected in the co-occurrence of T 3 and cortisol surges at term. Maternal stress or depression may alter fetal T 3 tissues levels and neurodevelopment. Tricyclic antidepressants (norepinephrine) and SSRIs (serotonin) enhance D2 activity (resulting in conversion of T 4 to active T 3 ), and cortisol decreases D2 activity in the brain.
Severe iodine deficiency is associated with increased rates of perinatal and infant mortality, pregnancy loss, maternal and fetal goiter, growth restriction, and offspring cognitive dysfunction. In areas with the most severe iodine deficiency (maternal iodine intake <25 μg/day), both maternal and fetal hypothyroidism is profound, leading to cretinism. It is characterized by extreme intellectual impairment with predominant neuromotor deficits including strabismus, deaf mutism, and disorders of gait and coordination. In fact, iodine deficiency is the leading cause of preventable severe cognitive deficits. , , Neurologic consequences of endemic cretinism result from insults to the developing brain beginning in the first trimester (i.e., deafness) through postnatal life (i.e., cerebellar abnormalities). The full syndrome of abnormalities can be prevented only when the iodine deficiency is corrected before the second trimester and optimally before conception. Neurologic abnormalities and intellectual deficiency depend ultimately on the timing and severity of the brain insult.
Observational trials suggest overall iodine insufficiency may result in differences in verbal IQ, reading comprehension, auditory processing, and fine motor skills more than performance IQ in iodine-insufficient pregnant women. Offspring also have a higher risk for ADHD. , The studies vary with respect to measures of iodine deficiency, the timing of the iodine exposures, and type and timing of neurodevelopment assessments. The vast majority of randomized trials supplementing pregnancies complicated by mild to moderate iodine deficiencies do not show an improvement in pregnancy or long-term child development, but these studies are challenged by the difficulties in quantitating true deficiencies at an individual level and the timing of iodine supplementation. , , Population studies have used urinary iodine concentrations (UIC) to determine sufficiency of iodine intake, with <150 μg/L defining deficiency in pregnancy. Although >90% dietary iodine is renally excreted, the UIC reflects recent iodine intake that is highly variable day to day and even hour to hour. The lack of a reproducible biomarker for chronic iodine insufficiency has made it difficult to identify which individuals would most benefit from iodine supplementation in randomized clinical trials (RCTs).
Immediately after birth, there is a surge of TRH and TSH with an increased T 4 conversion to T 3 and a moderate increase in T 4 levels. Within a few days, the increased TSH falls to adult levels through T 4 and T 3 negative-feedback inhibition. T 4 and T 3 concentrations return to normal adult levels within 4 to 6 weeks. Transient hyperthyroxinemia can be triggered by neonatal cooling and may represent an adaptation of thermogenesis in extrauterine life. ,
In premature neonates, the HPT axis is immature and the physiological TSH surge is dramatically lower or absent. FT4 and FT3 levels are low in the face of normal TSH levels and may take 3–8 weeks after birth to achieve similar levels as term infants. Transient hypothalamic hypothyroidism of prematurity has been observed in up to 50% of infants born under 28 weeks and it can be difficult to distinguish from central hypothyroidism. Transient hypothyroxinemia has been associated with cognitive and neurological delays; however, the target T 4 level needed to optimize brain development is unknown with no universal treatment consensus.
Newborn screening for congenital hypothyroidism is universal in the United States and ideally performed 2–4 days of age in term infants or within 7 days for preterm infants. TSH is often used for screening but some institutions use both TSH and FT4 to differentiate central hypothyroidism, hypothyroxinemia with delayed TSH elevation, thyroid hormone resistance, thyroid dysgenesis, and thyroid dyshormonogenesis.
Immunologic shifts in pregnancy, including the Th1-Th2 (T cell helper 1 to T cell helper 2) shift, are necessary to sustain pregnancy and maintain tolerance to a semiallogenic fetus. This immunologic shift is orchestrated primarily by placental tissues and passaged fetal cells that are able to modulate local and systemic maternal immune responses. , Some autoimmune diseases such as rheumatoid arthritis, multiple sclerosis, and Graves disease can demonstrate improvement of symptoms and disease severity. Alternatively, pregnancy may not impact maternal symptoms or disease severity, but the placental transfer of maternal autoantibodies may target the developing fetus, as often seen with Graves disease. The heterogeneity of the pregnancy effects on the course of autoimmune disease is suspected from contributions of immune shifts, dynamic reproductive hormonal changes, and potential contributions of fetal microchimerism.
Thyroid autoimmunity is described by the presence of antibodies to thyroglobulin (TG), thyroperoxidase (TPO), or thyroid-stimulating hormone receptor (TRAb) of either stimulatory (TSI) or blocking immunoglobulin (TBI). In iodine-replete areas, thyroid autoimmunity is the most common cause of thyroid disorders. Thyroid antibody positivity is more common in non-Hispanic White populations and increases with age. Antibodies to TG and TPO, as well as TRAb, similarly cross the placenta, but only antibodies to TRAb have been shown to affect fetal thyroid function and block (TBI), stimulate (TSI), or neutrally affect fetal thyroid hormonogenesis.
Although antibody titers generally decrease across gestation, baseline TSH concentrations with TPO antibody positivity are higher than TPO antibody–negative pregnant persons. Therefore, checking TPO antibody status among pregnant persons with TSH concentrations that are borderline (e.g., 3.0–3.9 mIU/L) may in some cases be considered to help direct TSH monitoring and potential levothyroxine (LT4) treatment. TPO and TG antibodies are found in 2%–20% of pregnant persons, but TPO antibodies compared to TG antibodies are associated more strongly with adverse pregnancy outcomes. Most euthyroid pregnant persons with positive TPO antibodies have normal pregnancy outcomes, but their presence is a marker of decreased thyroid reserve and associated with an increased risk for developing subclinical and overt hypothyroidism during pregnancy. Two studies have shown that 20% of women with TPO or TG antibodies developed subclinical hypothyroidism (SCH; TSH ≥4 mIU/L) during gestation despite early normal TSH values. , Therefore, euthyroid pregnant persons with TPO antibody positivity should be screened for developing abnormal TSH levels every 4–6 weeks through midgestation according to recent ATA guidelines. Over 90% of hypothyroid cases are attributable to Hashimoto thyroiditis, which is associated with >90% TPO antibody positivity. Given that the pathology of Hashimoto thyroiditis is identical to postpartum thyroiditis, TPO antibody positivity portends a 5- to 7-fold greater risk for postpartum thyroiditis. Therefore, TSH concentrations should be screened postpartum in euthyroid, TPO antibody–positive individuals.
Data overall support a modest association of miscarriage and preterm delivery with TPO antibody positivity compared to antibody-negative pregnant persons in euthyroid individuals. , , , Although earlier, smaller trials suggested an improvement in pregnancy outcomes in euthyroid, TPO-positive women, adequately powered RCTs have not demonstrated that LT4 or intravenous immunoglobulin treatment significantly increase live births rates. , The TABLET trial was a double-blind, placebo-controlled trial that randomized 952 euthyroid, TPO-positive women with a history of miscarriage or infertility to 50 μg LT4 or placebo during the preconception period to determine the influence of LT4 on live birth rates at ≥34 weeks’ gestation. The authors demonstrated no difference in the live birth, miscarriage, preterm birth, or neonatal outcomes. The T4-Life trial in the Netherlands is still ongoing and will examine the effect of LT4 among TPO antibody–positive euthyroid women and a normal TSH with a history of recurrent miscarriage on live birth rates. TPO antigens have been recently demonstrated in endometrium and the SCT. TPO antibodies have been speculated to interfere with implantation or trophoblast function, increase endometrial cytokines, and have cross-reactivity with hCG receptors, and they are associated with fetal resorption in murine models. However, further studies are needed to understand the biology of the association. ,
Prematurity is the leading cause of neonatal death worldwide and is complicated by the challenge of multiple etiologies. Although large individual prospective studies have failed to confirm TPO positivity and preterm birth, meta-analyses and cross-sectional studies have shown a significant association of positive TPO antibody status with spontaneous preterm birth compared to euthyroid or untreated controls. , , Currently, there is insufficient evidence to support treatment of LT4 in mothers with a normal TSH for the prevention of preterm birth or pregnancy loss. Conflicting reports have shown an association of TPO antibodies and placental abruption, gestational diabetes, preeclampsia, offspring impaired neurodevelopment, and peripartum depression. Additional studies are needed to address these inconsistences.
TRAbs are defined by any type of antibody that interacts with the TSH receptor. Receptor assays do not differentiate between stimulating (TSI) and blocking immunoglobulins (TBI), and bioassays are necessary to distinguish the functional properties. Commercially available TRAb bioassays demonstrate low interference with serum hCG, which is known to activate the TSH receptor and can be used reliably in pregnancy. TRAbs can persist for a variable amount of time following thyroidectomy and especially following radioiodine ablation (RAI) due to the tissue destruction and antigen release. Therefore, the antibodies may persist beyond TH level stability postsurgically or the 6-month interval recommended to avoid conception following radioiodine exposure. Although uncommon, TRAbs may switch from stimulating to blocking activity or vice versa, which many explain a variable disease course during pregnancy. Fetal and neonatal hyperthyroidism develops in 2%–5% of pregnant persons with Graves disease with stimulatory TRAb activity (TSI), which is much higher than antibody-induced neonatal hypothyroidism secondary to TRAb-blocking antibodies (TBI). The fetal thyroid becomes responsive to TRAbs at approximately 20 weeks’ gestation. The risk of fetal or neonatal Graves is even higher in women with extremely high levels of these antibodies. The half-life of immunoglobulins is 2–3 weeks but the antibody-induced hyperthyroidism can be observed for 3 months or longer, especially in individuals with Graves orbitopathy or in tobacco users. TRAb levels generally decrease across gestation but have rarely been observed to increase during pregnancy. ,
The physiologic changes of pregnancy influence TH metabolism and thyroid function tests. TSH is the most sensitive marker of primary thyroid dysfunction and should be used as the first-line test except in pituitary-hypothalamic disorders; nonthyroidal illnesses; clinical conditions with high levels of dopamine, glucocorticoids, or somatostatin analogues; and mild iodine deficiency (see Fig. 61.4 ).
Biotin can affect laboratory assessments of TH levels. Although the self-reported prevalence of biotin supplementation is low, the rates of biotin consumption have increased, particularly among women and older adults. High doses of biotin at 10 mg/day (often marketed to strengthen hair and nails) may interfere with immunoassays and when they do, typically cause elevations of T4 and T3 and falsely suppress TSH. Discontinuing the supplement for 1 week prior to drawing blood tests should be considered to avoid potential biotin interference.
TSH has a half-life of only ∼1 hour. TSH levels demonstrate a diurnal pattern, rising in the evening before the onset of sleep, reaching a peak between 11 p.m. and 5 a.m. and a nadir at ∼11 a.m. The magnitude of TSH signaling is primarily genetically driven and regulates T 4 and T 3 secretion. TSH is log-linearly related to T 4 such that individuals have significantly elevated TSH concentrations before any decrease in T 4 or T 3 levels out of the normal range. A reduction in the TSH reference range in the first trimester is notable in all pregnancies and usually persists through the second and third trimesters. A small percentage of pregnancies, particularly those complicated by multiple gestation or other conditions with higher levels of hCG, can have undetectable TSH (<0.01 mIU/L) with usually normal pregnancy outcomes.
Population-based studies demonstrate substantial differences in the TSH upper reference limit (2.5–5 mIU/L) that differ by trimester. These TSH variations are attributable to assay manufacturer differences, iodine intake, TPO antibody status, geography and ethnicity. , Data from studies prior to 2017 led to the historical definition for the upper limit of TSH as 2.5 mIU/L in the first trimester and 3.0 mIU/L in the second and third trimesters. , However in 2017, the ATA redefined TSH norms from data supported by a number of cohort studies including >60,000 subjects as well as the 15-center Maternal-Fetal Medicine Units Network (MFMU) RCT which screened over 97,000 US women. The MFMU trial used the 97.5th percentile of women screened to establish the upper limit of the TSH normal range, and women with positive TPOAbs were not excluded. , The ATA and the American College of Obstetricians and Gynecologists (ACOG) recommended TSH reference ranges based on local, population-derived, trimester-specific TSH reference ranges that exclude pregnant persons with a history of thyroid disease, suboptimal iodine intake, or thyroid autoimmunity as reflected by TPO antibody positivity. If these data are not available, ATA suggested a TSH reference range for pregnancy that is not trimester-specific and modified by reducing the lower reference range by 0.4 mIU/L and the upper range by 0.5 mIU/L, giving an approximate range of 0.1–3.9 mIU/L. A TSH ≥4.0 mIU/L but <10 mU/L was adopted to define subclinical hypothyroidism for pregnant persons if a pregnancy-specific TSH range was not available. The TPO antibody status of the women in the MFMU RCT was not described, but they were considered to be iodine sufficient by urinary iodine measures. Given that the prevalence of TPO antibody positivity in pregnancy is 9%–18%, exclusion of women who were TPO antibody positive to define the TSH ≥97.5th percentile might have resulted in lower TSH norms in the MFMU study, especially in the first and second trimesters. , TSH concentrations gradually rise in the second and third trimesters of pregnancy but generally remain lower than the nonpregnancy state secondary to hCG stimulation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here