Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Platelets, once regarded simply as ”blood dust,” are now recognized to play essential roles in hemostasis. Not only do they form a hemostatic plug and initiate thrombus formation in the event of vascular injury, but they also repair minute vascular damage that occurs on a daily basis. Platelets also participate in wound healing and angiogenesis via the delivery of key growth factors to sites of vascular injury and interact with the innate immune system. Disorders associated with platelet production carry significant morbidity and mortality in humans because of hemorrhage, thrombosis, bone marrow (BM) fibrosis, BM failure, and/or hematologic malignancy. Thrombocytopoiesis is a complex process in which platelets are generated from their precursor cells, megakaryocytes, via a complex process. For a long time, the extreme rarity of megakaryocytes significantly hampered studies aimed at understanding the molecular mechanisms underlying platelet biogenesis. However, the purification and cloning (in 1994) of thrombopoietin (TPO), the major megakaryocyte cytokine, has enabled considerable progress to be made. Recent application of whole exome and genome DNA sequencing has further stimulated the discovery of new disease-associated genes involved in human thrombocytopoiesis. These new insights provide an important foundation for improved diagnosis and treatment of disorders of thrombocytopoiesis. This chapter reviews the current understanding of megakaryocyte biology and platelet production, highlighting connections with human disease.
Although platelets were described as early as the 1840s, it was not until 1906, in a seminal study by James Homer Wright, that their origin from megakaryocytes was first recognized. Megakaryocytes are large polyploid cells that reside predominantly within the BM during postnatal life. They are rare cells, constituting only about 0.05% to 0.1% of nucleated BM cells under normal steady-state conditions. They develop from hematopoietic stem cells (HSCs) through a process known as megakaryocytopoiesis. According to the classical model of megakaryocytopoiesis, HSCs give rise to multipotent progenitor cells (MPPs) which sequentially transition to common myeloid progenitor (CMP) cells, bipotential megakaryocyte-erythroid progenitor (MEP) cells, and Mk progenitor (MkP) cells which will ultimately generate platelet-forming mature megakaryocytes ( Fig. 29.1 ). In the past decade, single-cell transplantation and lineage tracing studies have demonstrated the existence of lineage-biased HSCs and underscored the heterogeneity of traditionally defined HSC and MPP cell compartments. Thus, the canonical model of hematopoietic hierarchy has been challenged by the emerging concept that hematopoiesis is a dynamic continuous process in which lineage priming and multilineage potential coexist. Notably, megakaryocytopoiesis has been at the center of this paradigm shift. The first functional evidence that megakaryocytes derive from HSCs bypassing the MPP stage was suggested by Adolfsson and colleagues in 2005. Subsequently, several groups have shown that megakaryocyte-biased HSCs exist in both murine and human hematopoiesis supporting the concept that megakaryocytic lineage branches out earlier than previously appreciated. In vitro culture and transplantation assays have established that a common MEP capable of Mk-erythroid differentiation represents an intermediate step between MMP and MkP. Recent evidence employing lineage tracing in situ has challenged the existence of MEPs during unperturbed hematopoiesis thus underscoring the potential limitations of stress hematopoiesis such as cell culture and transplantation models. Furthermore, the concept that megakaryocytic lineage undergoes a ”fast-tracked” differentiation process has been also documented in response to acute inflammation in murine models and in patients with myelofibrosis (MF) which is a condition characterized by Mk lineage hyperplasia ( Chapter 72 ). According to the revised model of megakaryocytopoiesis (see Fig. 29.1 ), megakaryocyte differentiation is initiated earlier during hematopoiesis from a pool of lineage primed HSCs and/or MMPs the fate of which is differentially modulated in conditions such as steady-state, stress hematopoiesis or inflammation. In recognizing the complexity and plasticity unveiled by this emerging research, it is conceivable that the new and the canonical pathways of megakaryocytic differentiation exist in the context of different physiological or experimental conditions.
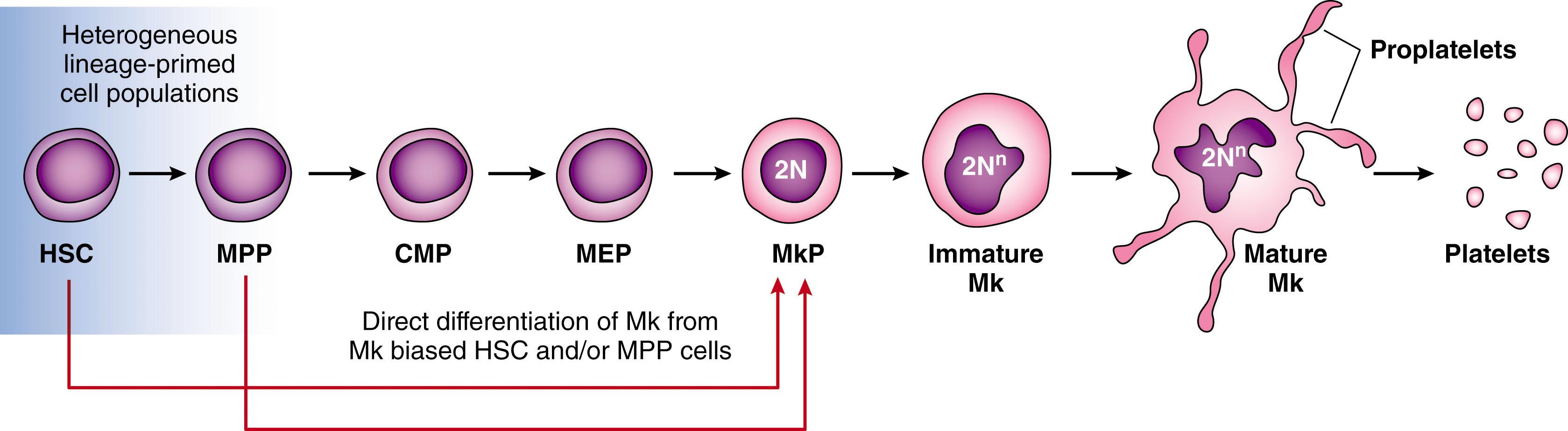
Like other hematopoietic progenitor cells, once committed to the megakaryocytic lineage, MkPs undergo a series of dramatic maturational steps ultimately tailored to their final task of platelet production. These include changes in proliferative capacity, cell size, nuclear content, organelle biogenesis, membrane development, and cytoskeletal rearrangement.
This process can be conceptually divided into three broad stages: proliferating MkPs, which contain normal DNA content (2 N/4 N), nonproliferating immature megakaryocytes (4 N to 8 N DNA content), and nonproliferating mature megakaryocytes (DNA content 8 N to 128 N). In vitro culture using semisolid media and mitogenic cytokine cocktails revealed that within the MkP compartment reside cells with high-proliferative-potential colony-forming cell (Mk-HPP-CFC), burst-forming unit-megakaryocyte (BFU-Mk) cells and more mature MkPs with very limited proliferative potential, the colony-forming cell-megakaryocyte (CFU-Mk). The proliferative capacity of the cells within the MkPs compartment can be demonstrated in vitro by the size of the colonies and the number of Mk-HPP-CFC, BFU-Mk, and CFU-Mk.
The immature megakaryocytes have an intermediate DNA content and are transitional cells intermediate between proliferating progenitor cells and postmitotic, mature megakaryocytes. They cease to proliferate and switch to endomitosis , also known as abortive mitosis, a process in which cells replicate their DNA but fail to undergo cytokinesis. This is accompanied by an increase in cell size and DNA content (up to 64 to 128 N), organelle biogenesis, membrane development, and cytoskeletal rearrangement. This culminates with the generation of mature, polyploid megakaryocytes that reach diameters of 50 to 100 μm and DNA content as high as 64 to 128 N. On BM specimens, morphologically recognizable megakaryocytes can be defined as megakaryoblasts (stage I), promegakaryocytes (stage II) and granular or “platelet shedding” megakaryocytes (stages II and IV) ( Fig. 29.2 ).
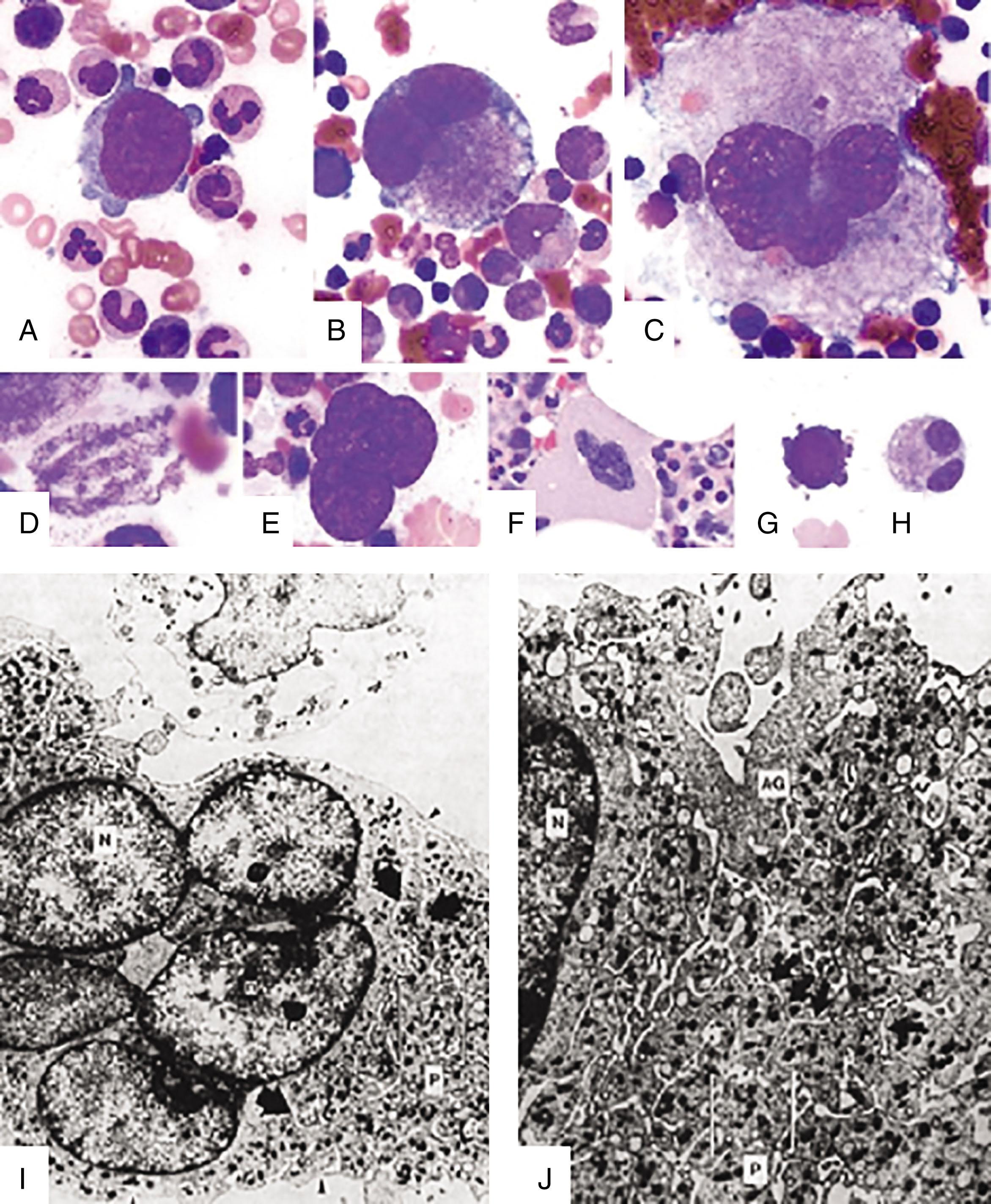
Mature megakaryocytes contain a large multilobulated polyploid nucleus and have abundant cytoplasm, which contains platelet-specific secretory granules, alpha (α-) granules, and dense granules (see Fig. 29.2 ). The biogenesis of α-granules and dense granules begins in immature megakaryocytes, and both granule types develop concomitantly. α-Granules are 200 to 500 nm in diameter and have a dense center and fine granular matrix. Megakaryocytes synthesize many of the constituents of α-granules and target them to the granules. These include vWF, fibronectin, P-selectin, fibrinogen receptors, PF4, coagulation factor V, and plasminogen activator inhibitor-1, among others. In addition, some constituents, such as fibrinogen, are taken up by megakaryocytes via endocytosis and/or pinocytosis and stored in α-granules. It was once thought that α-granules were a homogeneous population of vesicles. However, it has become clear that there are distinct populations of α-granules containing different constituents, and that these can be differentially released during platelet activation. Dense granules are 200 to 300 nm in diameter and consist of a halo encircling an electron opaque core. They contain many soluble hemostatic factors such as serotonin, catecholamines, adenosine, adenosine 5′-diphosphate, adenosine 5′-triphosphate, and calcium. Their limiting membranes contain glycoproteins such as αIIbβ3, glycoprotein Ib (GPIb), and P-selectin, which are also present in α-granules, as well as unique membrane proteins such as granulophysin. Multivesicular bodies serve as intermediates in the biogenesis of both α-granules and dense granules. It has been proposed that they constitute a sorting compartment between α-granule and dense granule components.
Mutations in the NBEAL2 gene have recently been linked to gray platelet syndrome (GPS) (OMIM 139090), a disorder of impaired platelet α-granule synthesis. This gene encodes a large BEACH domain containing protein that shares homology with the LYST gene product. LYST is involved in vesicular trafficking and is mutated in Chediak–Higashi syndrome (OMIM 214500), a disorder that includes impaired platelet dense granule biogenesis ( Chapter 123 ).
The megakaryocyte cytoplasm contains at least two complex membranous systems: the demarcation membrane system (DMS) and the dense tubular network (DTS) (see Fig. 29.2 ). The DMS consists of an extensive network of tubular and flattened membranous structures that interconnect with one another and communicate with the extracellular space. Whole-cell patch-clamp studies in living rat megakaryocytes show that they are electrophysiologically contiguous with the plasma membrane. The open canalicular system of platelets share many features of the megakaryocyte DMS and may represent a remnant of this structure. The DMS serves as a vast membrane reservoir for proplatelet and platelet formation. The DTS of megakaryocytes is distinct from the DMS. Unlike the DMS, it fails to stain with surface membrane tracer dyes, indicating a lack of communication with the plasma membrane. The DTS is thought to be a site of platelet prostaglandin synthesis.
The recent work uncovering that megakaryocytes are hierarchically closer to HSCs than previously appreciated underscores the similarities between the two cell entities. HSCs and megakaryocytes have common signaling pathways (TPO signaling), surface and cytoplasmic molecules expression (CD41, CD150, CXCR4, TPO receptor, von Willebrand factor), and transcription factors (RUNX1, GATA2, TAL1, ETV6, FLI1, and MEIS1). TPO signaling is not only important for megakaryocyte proliferation and development but also plays a role in HSCs survival, self-renewal, and expansion. Lastly, HSCs and megakaryocytes occupy the same niche at the BM vascular sinusoids, where they physically contact one another. One recent study also suggests that megakaryocytes are necessary to maintain HSC quiescence. The teleologic explanation for such a close relationship between HSCs and megakaryocytes remains to be further explored.
Megakaryocytes derive their name from their large and complex nuclei. This arises from an atypical cell cycle, termed the endomitotic cell cycle ( Fig. 29.3 ) (see comprehensive review by Mazzi et al. Like normal diploid cells, the cycle begins with a G1 phase, followed by S phase (DNA replication), and a G2 phase. The cells then enter the M phase, but unlike normal diploid mitotic cells, fail to complete late cytokinesis and karyokinesis. The defective abscission leads to the accumulation of the replicated DNA in the same cell. The process is repeated in subsequent rounds of DNA replication leading to a polyploid cell with a multilobulated nucleus encapsulated by a single nuclear membrane. Mature human megakaryocytes have been observed to reach ploidy levels as high as 128 N. The term endoreduplication has at times been used erroneously to describe megakaryocyte polyploidization. Endoreduplication correctly refers to a cell cycle that involves DNA replication but no entry into the M phase enabling polyploidization in Drosophila , plants and trophoblasts.
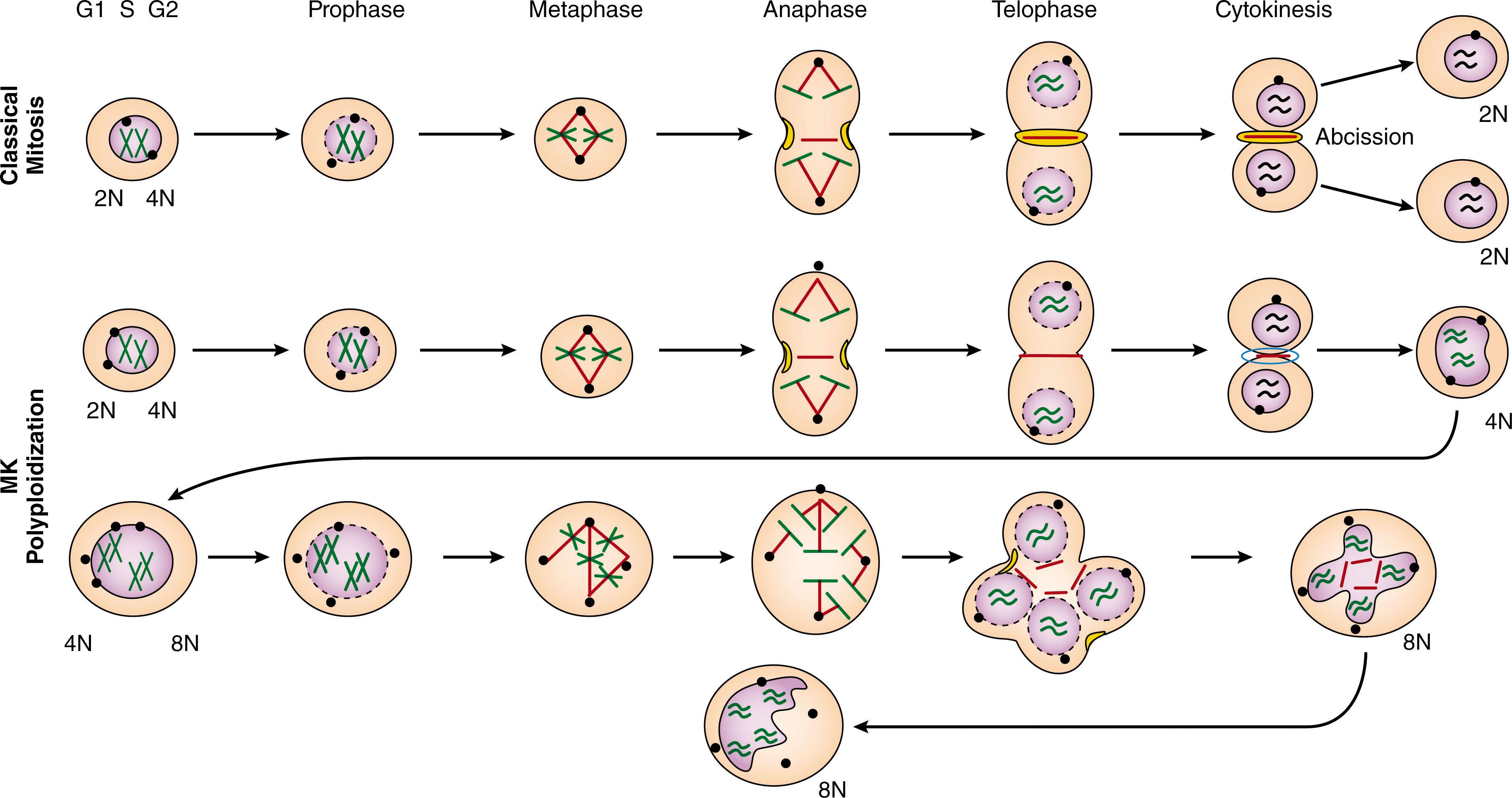
The reason that megakaryocytes undergo endomitosis is not known. It has been speculated that it provides a means for generating the abundant membrane, protein, biosynthetic cargo, and energy required for the dramatic final stages of proplatelet elaboration and platelet release. Several circumstantial pieces of evidence support this model. First, it is known that megakaryocyte DNA content correlates with megakaryocyte cell size, mRNA content, protein production, and eventual numbers of platelets released. Second, an increased DNA content of megakaryocytes precedes increases in platelet count during recovery from acute thrombocytopenia. Third, increases in cytoplasmic volume and maturation occur predominantly, if not completely, in stage II and III megakaryocytes, which do not synthesize DNA. Fourth, in polyploid megakaryocytes (4 N to 32 N), all alleles of the genes studied (i.e., ITGA2B [GPIIb], VWF, ACTB [β-actin], HSPA1 [HSP70], MPL, FLI1, and ZFPM1 [FOG-1]) have been found to be transcriptionally active.
The molecular mechanisms mediating endomitosis in megakaryocytes are incompletely understood. Studies investigating endomitosis have been hampered by the rarity of megakaryocytes, difficulty separating direct effects from general perturbations of cell maturation, complications associated with synchronizing the cell cycle, use of transformed cell lines, and potential differences between rodent and human megakaryocytes.
Two classes of proteins control the cell cycle in mammalian cells. These are the cyclins, so named for their cyclical synthesis and degradation during the cell cycle, and cell division kinases (Cdks, also known as cyclin-dependent kinases ). Together, these two families of proteins form a protein-kinase complex in which the regulatory unit is the cyclin and the catalytic unit is the Cdk. The role of these kinase complexes in cell cycle control is complex. At least seven members of the cyclin gene family and seven distinct Cdk genes have been identified.
Given the importance of cyclins and Cdks in controlling the cell cycle, they have been the focus of considerable attention in investigations of the mechanisms underlying megakaryocyte endomitosis. The most compelling evidence probably exists for the role of the D-type cyclins in megakaryocyte endomitosis. The D-type cyclins are unique in that their activity can be modulated by extracellular mitogens. Megakaryocytes express cyclin D3 and, to a lesser extent, cyclin D1. Levels of both of these factors increase after treatment with TPO. Overexpression of cyclin D3 results in increased megakaryocyte ploidy in transgenic mouse models. Complexes of cyclin D3 and its major kinase subunit, Cdk2, show high kinase activity in polyploid cells. Antisense knockdown of cyclin D3 levels suppresses endomitosis and abrogates normal development of primary mouse megakaryocytes.
Cyclin D1 is a direct target gene of GATA1, a transcription factor required for megakaryocyte polyploidization and maturation. Overexpression of cyclin D1 in transgenic mice increases megakaryocyte modal ploidy compared with nontransgenic littermates, and the combination of cyclin D1 and Cdk4 kinase activity restores polyploidization of GATA1-deficient murine megakaryocytes. Conversely, enforced expression of p16 ink4a , a cell cycle inhibitor of Cdk4/6, blocks polyploidization in murine megakaryocytes. p16 ink4a is also potently repressed by GATA1.
Cyclin E −/− mice have impaired megakaryocytopoiesis with reduced modal ploidy. These mice also have defective trophoblast development, another tissue characterized by atypical mitosis. Cyclin B1/CDC2 is a mitotic cyclin complex. Yeast strains deficient in cyclin B1 or CDC2 undergo an additional round of DNA replication without cytokinesis. Several studies have shown that low levels of cyclin B1/CDC2 are required for the progression of endomitosis in megakaryocytic cell lines. However, studies of primary megakaryocytes have shown normal cyclin B1 and CDC2 levels and functional mitotic activity during endomitosis.
Aurora-B kinase (also called AIM-1 kinase ) is involved in late anaphase and cytokinesis, and mRNA transcript levels of Aurora-B kinase have been reported to decrease during polyploidization of primary megakaryocytes and megakaryocytic cell lines. This suggests that Aurora-B kinase may play a mechanistic role in megakaryocyte endomitosis. However, functional activity of Aurora-B kinase appears normal in late anaphase of endomitotic primary megakaryocytes, indicating that a simple deficiency of Aurora-B kinase activation is an unlikely mechanism to explain endomitosis. A polo-like kinase (PLK-1) is a serine-threonine kinase required for assembly of the mitotic spindle, separation of chromosomes during anaphase, and exit from mitosis. PLK-1 mRNA and protein levels decrease during polyploidization of murine megakaryocytes, and enforced expression of PLK-1 in primary murine megakaryocytes impairs endomitosis. However, the effects of overexpression are modest, preferentially affect lower-ploidy megakaryocytes, and are complicated by alterations in cell cycle kinetics.
Microtubules play key roles in mitosis by enabling the formation of the mitotic spindle. Therefore, factors that regulate their assembly have also been investigated as candidates involved in megakaryocyte endomitosis. The protein regulator of cytokinesis 1 (PRC-1) is involved in mitotic spindle elongation and cytokinesis. However, no differences in PRC-1 levels were detected in primary murine megakaryocytes undergoing polyploidization compared with nonendomitotic precursors. Stathmin is a microtubule-depolymerizing factor that plays an important role in the regulation of the mitotic spindle. Levels of stathmin are inversely related to the level of ploidy of megakaryocytic cell lines and primary megakaryocytes. Inhibition of stathmin in K562 cells increases their propensity to undergo endomitosis when induced to differentiate into megakaryocytes, and overexpression of stathmin prevents the transition from mitotic to endomitotic cell cycles. Conversely, lentiviral-mediated overexpression of stathmin in primary megakaryocytes delayed cytoplasmic maturation and impaired their ability to achieve high levels of ploidy. Together, these findings support a possible role of stathmin in modulating endomitosis.
During mitosis of normal diploid cells, a spindle assembly checkpoint prevents the progression of anaphase until all of the chromosomes are aligned with the mitotic spindle and each sister chromatid is properly attached to spindle microtubules originating from the opposing spindle pole. This ensures that each daughter cell receives the proper complement of chromosomes. The anaphase-promoting complex (APC) is a multisubunit protein complex with ubiquitin ligase activity that regulates chromosome segregation and anaphase progression by targeting key factors for degradation. Since some chromosomal missegregation occurs during megakaryocyte endomitosis, several groups have examined the expression levels and/or activity of certain APC components and associated factors. These studies have shown no significant difference in protein levels of the core APC protein CDC27 or the kinetochore-associated signaling protein hsMAD2 in primary murine megakaryocytes undergoing polyploidization compared with nonendomitotic precursors. Haploinsufficiency of BUBR1, a key component of the spindle checkpoint, perturbs megakaryocyte development and polyploidization in mice but does not cause alterations in circulating platelet counts.
Cytokinesis requires the assembly and activity of an actin-based contractile ring which enables cell separation. This process is regulated by RhoA involving actin polymerization, myosin II accumulation, and activation which provide the contractile forces for abscission. The failure to complete cytokinesis during endomitotic cell cycles in megakaryocytes involves functional and quantitative defects in the RhoA, Rock, F actin, and myosin II. Silencing of nonmuscle myosin heavy chain IIB (Myh10) by the transcription factor RUNX1 is also required for efficient megakaryocyte polyploidization. Further studies will be required to fully dissect the molecular pathways involved in megakaryocyte endomitosis.
It has been estimated that each megakaryocyte produces between a few hundred to several thousand platelets. The exact mechanism by which this occurs has been controversial, with several competing models proposed in the past. It was initially suggested that the DMS established platelet fields, which defined territories of prepackaged platelet contents. These fields would generate platelets directly upon the breakdown of the megakaryocyte cytoplasm. However, prevailing evidence supports an alternate model in which platelets are released from dynamic megakaryocyte pseudopod extensions called proplatelets . This model was first proposed by Becker and De Bruyn in 1976 and supported by ultrastructural studies later in the 1980s. Italiano et al., extended these earlier studies on proplatelet formation and platelet biogenesis using the videomicroscopy of cultured murine megakaryocytes (see Chapter 123 ). Briefly, platelet biogenesis begins with a reorganization of unique cortical microtubules within the megakaryocyte to produce large pseudopodia structures from one pole of the megakaryocyte. This spreads across the megakaryocyte, generating extensions that elongate into complex branching tubular proplatelet processes. During this time, organelles travel along microtubules within the shafts of the proplatelets and are loaded into the proplatelet tips where they are captured. This phenomenon likely accounts for the ability of each megakaryocyte to generate such a large number of platelets. The DMS serves as an extensive membrane reservoir for these processes. Proplatelet formation is regulated by a pathway involving Rho GTPase proteins, Rho-associated kinase (ROCK), the MYH9 gene product myosin IIA, and myosin light-chain kinase.
Hematopoiesis develops in distinct waves during embryonic development. In mammals, the first hematopoietic progenitors are found inside blood islands of the yolk sac. These give rise to a distinct population of large erythrocytes, termed primitive erythrocytes , which express unique globin genes and retain their nucleus longer than adult-type or ”definitive” erythrocytes. ”Definitive” hematopoiesis arises later during embryogenesis from HSCs that develop de novo from the ventral aspect of the dorsal aorta in the aorto-gonad-mesonephros (AGM) region. These then seed the fetal liver, which serves as a major site of hematopoiesis during gestation. Eventually, hematopoiesis shifts to the BM (and spleen in mice), where it is sustained postnatally.
MkPs have been detected in the yolk sac as early as embryonic day 7.5 (e7.5) of mouse development. They are capable of generating proplatelets and platelets after in vitro culture. Circulating platelets have been detected in the mouse embryo as early as e10.5. Megakaryocytes cultured from early yolk sac have features somewhat distinct from those cultured from adult BM, such as lower modal ploidy, smaller size, different cytokine requirements, and faster kinetics of platelet generation. These unique progenitors disappear by e13.5. In addition, mixed erythroid-megakaryocyte colonies derived from the early yolk sac give rise to primitive erythrocytes. It has therefore been suggested that a separate wave of ”primitive megakaryocytes,” akin to ”primitive erythrocytes,” exists during the early yolk sac stages of hematopoiesis. These rapidly maturing megakaryocytes may prevent hemorrhages from the developing vasculature until definitive hematopoiesis is available to provide a steady supply of platelets.
Several pieces of evidence suggest that fetal megakaryocytes also have unique features compared with adult BM-derived megakaryocytes. They are smaller, have a greater proliferative capacity in vitro, have lower ploidy and form fewer platelets. This could be caused by either cell-intrinsic differences or possibly their interactions with a distinct microenvironment. The developmental differences between fetal and adult megakaryocytes are reflected by disease conditions that affect neonates. In response to increased platelet demand, adult megakaryocytes increase their number, size, and ploidy which replenish the platelet counts. By contrast, neonatal megakaryocytes are not “equipped” to respond to increased platelet demand in the same manner which is believed to contribute, at least in part, to neonatal thrombocytopenia. Two congenital disorders of megakaryocytopoiesis in humans, Down syndrome-associated transient myeloproliferative disorder (DS-TMD) and thrombocytopenia with absent radii (TAR) resolve spontaneously after the newborn period, suggesting specific effects on fetal megakaryocytopoiesis. Congenital amegakaryocytic thrombocytopenia (CAMT) is a disorder in which megakaryocytes are absent or present in very low numbers due to mutations in the gene encoding for c-MPL which is the receptor for TPO, the master regulator of megakaryocytopoiesis. In adults, this defect is consistently associated with severe thrombocytopenia, while in infants it coexists in some cases with relatively normal platelet counts or in other cases with the appropriate number of megakaryocytes in the BM. These observations and recent findings in murine models indicate that developmental differences exist in the response to abnormal c-MPL/TPO signaling in neonatal and adult megakaryocytes.
The features of fetal megakaryocytes have additional clinical implications including neonatal thrombocytopenia and the delayed platelet engraftment often observed when umbilical cord blood is used as a graft source for human stem cell transplantation. Conversely, due to their proliferative potential, neonatal megakaryocytes represent a valuable cell source for expansion and manipulation towards ex vivo manufacturing of megakaryocyte or platelet-based cellular therapies.
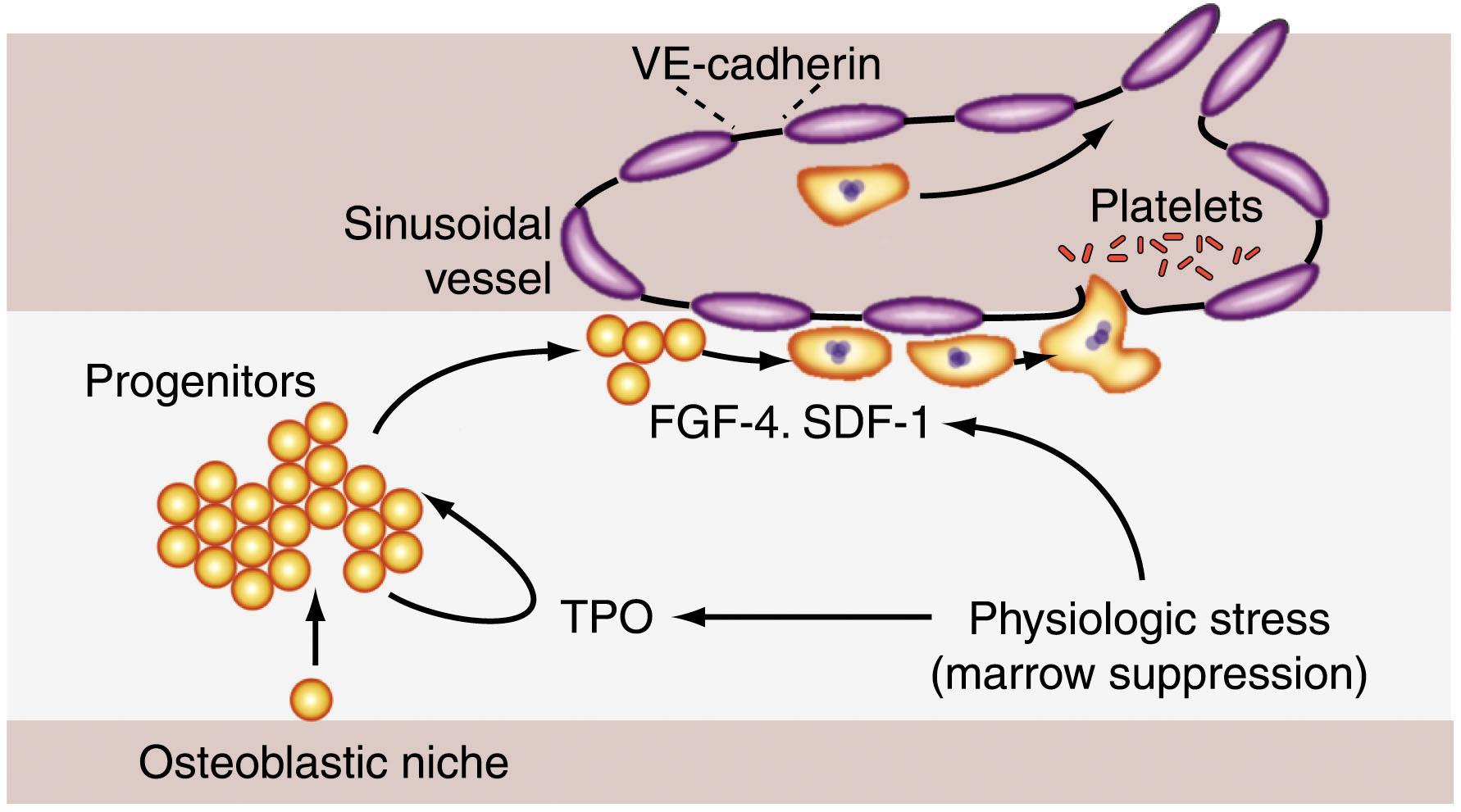
There is mounting evidence that the proliferation and terminal maturation of MkPs occur in distinct spatial compartments within the BM (see Chapter 14 ). In a simplified model, the BM space can be conceptually divided into distinct regions, a space adjacent to the cortical bone (an “osteoblastic niche”), an intermediate zone, and a “vascular niche” containing sinusoidal vessels lined with specialized BM endothelial cells. HSCs are thought to reside in a quiescent state adjacent to the bone. Under appropriate conditions, they are recruited to generate hematopoietic multipotent progenitor cells which are then subject to expansion and lineage commitment under the influence of various cytokines and likely other signaling molecules. This is where TPO is postulated to affect MkP proliferation and survival. Rafii and colleagues have shown that the chemokines stromal-derived factor-1 (SDF-1; also called CXCL12) and fibroblast growth factor-4 (FGF-4) promote migration and attachment of murine MkP cells (which express the receptor for SDF-1, CXCR4) to the vascular endothelium, where they physically attach, mature, and produce intercalating pseudopod structures. In fact, exogenous SDF-1 and FGF-4 restores thrombocytopoiesis in TPO −/− or TPO receptor (c-Mpl) −/− mice to near wild-type (WT) levels. This occurs in the absence of enhanced MkP proliferation and requires direct physical interaction with BMECs. Based on these findings, Avecilla et al., have proposed a model in which MkPs proliferate in an immature developmental state (in response to TPO) in a nonvascular niche ( Fig. 29.4 ). However, once the progenitors reach and adhere to the sinusoidal vessels in the vascular niche in response to chemokines, proliferation ceases, and terminal maturation and platelet release ensue. Work from other investigators supports this model. Multiple electron microscopic studies have captured megakaryocytes extending proplatelet processes through vascular endothelium and into BM sinusoids, and in vivo imaging studies have documented this process in living mice.
In addition to the BM, the lung and the spleen represent important sites of residence for megakaryocytes. Historically, the presence of platelet-forming megakaryocytes in the lung have been documented in humans as early as 1893, described in detail by Howell and Donahue in 1936 and later confirmed by several reports. The significance of such observations has been controversial but recent evidence confirms that the lung is not only a tissue of residence for megakaryocytes but a site conducive to platelet biogenesis. It has been postulated that two types of megakaryocytes exist in the lung; those residing in the pulmonary interstitium and those originating in the BM which are present in the lung vasculature where they release platelets. It remains debatable whether lung-based thrombocytopoiesis is a physiological process, driven by increased platelet demand or both. Furthermore, it is not clear to what degree lung-generated platelets contribute to the total pool of circulating platelets.
Megakaryocytes are normally present in the spleen in rodents where the spleen remains a site of hematopoiesis throughout adult life. In humans, the presence of megakaryocytes in the spleen is a consequence of pathological conditions such as extramedullary hematopoiesis (EMH). Prototypical EMH occurs in primary MF which is also characterized by abnormal and hyperplasic megakaryocytes ( Chapter 72 ).
The increased understanding of the biological events underlying the generation of platelets from megakaryocytes led to the development of novel strategies that might eventually supplement or replace donor-derived platelet transfusions. Ex vivo manufacturing of platelets from primary HSCs or induced pluripotent stem cells (iPSCs) has been pursued and near-clinical doses of platelets have been achieved by utilizing highly optimized ex vivo cultures and/or state-of-the-art bioreactors. In addition, megakaryocyte-biased cellular products that have the ability to naturally release platelets in the patient’s own body after infusion has been proposed to mitigate repeated platelet transfusions. Clinical implementation of these approaches has the potential to overcome the limitations associated with donor-dependent platelet transfusions and mitigate the increasing demand for donor platelet transfusions which is compounded by dependency on volunteer donors, short storage time, and lifespan after infusion as well as shortages in supply due to inclement weather and holidays.
Furthermore, gene therapy of megakaryocytes derived ex vivo from CD34 + HSCs or iPSCs has also been explored with the goal of correcting inherited bleeding disorders such as Glanzmann thrombasthenia, hemophilia or Bernard-Soulier syndrome.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here