Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Mitral Valve
Three-dimensional echocardiography (3DE) provides real-time, detailed, nonplanar images of the complex mitral valve (MV) apparatus, including the annulus, leaflets, chordae, and papillary muscles.
Quantitative analysis of MV anatomy, function, and motion using 3DE is significantly more accurate and reproducible than two-dimensional echocardiographic (2DE) planar imaging.
Assessment of degenerative MV disease with 3DE helps guide the optimal surgical strategy and improve postoperative outcome.
3DE provides mechanistic insight into the pathophysiology of ischemic mitral regurgitation (MR) and potential roles for surgical and transcatheter repair.
Multiplanar imaging provides significantly more accurate measurements of the severity of MR compared with 2DE.
3DE plays an important role before, during, and after multiple catheter-based interventions, including valvuloplasty for mitral stenosis, edge-to-edge repair for MR, and closure of perivalvular leaks.
Aortic Valve
The 3DE en face view improves the assessment of aortic root structures such as aortic valve leaflet number, aortic annular shape, and left ventricular outflow tract (LVOT) dimensions.
Assessment of aortic stenosis severity is improved by substituting the 3DE planimetered area of the LVOT area into the continuity equation, by replacing the numerator of the continuity equation by 3DE-derived stroke volume, or by direct aortic valve area planimetry.
Assessment of aortic regurgitation severity is improved with the use of 3DE planimetered vena contracta area.
With 3DE, good visualization of prosthetic aortic valve rings from the LVOT perspective and the aorta is possible; however, reliable visualization of prosthetic aortic valve leaflets remains challenging.
3DE improves characterization of valve masses.
3DE plays an important role before, during, and after percutaneous procedures such as transcatheter aortic valve replacement and closure of paravalvular regurgitation.
Advances in 3DE technology have ushered its use into mainstream clinical practice. It provides realistic images of the mitral and aortic valves and their spatial relationships with adjacent structures. It offers unique anatomic and functional insights that have furthered our understanding of the pathophysiology of valvular heart disease. In this chapter, we discuss the value of 3DE for the mitral and aortic valves in evaluating valve anatomy, volumetric quantification, presurgical planning, intraprocedural guidance, and postprocedural assessment.
The MV is a complicated 3D structure involving multiple, distinct anatomic components. Optimal interaction of the elements comprising the annulus, commissures, leaflets, chordae tendineae, papillary muscles, and left ventricle (LV) is crucial for its functional integrity.
The mitral annulus is a fibromuscular ring to which the anterior and posterior MV leaflets attach. The normal MV annulus has a 3D saddle shape with its lowest points at the level of the anterolateral and posteromedial commissures. This enables proper leaflet apposition during systole and minimizes leaflet stress. The annulus can be divided into the anterior and posterior annulus based on the insertion of the corresponding leaflets.
The MV has an anterior and posterior leaflet. The atrial, or smooth, surface is free of attachments, whereas the LV, or rough, surface connects to the papillary muscles by the chordae tendineae. The posterior leaflet, which has a quadrangular shape, is attached to approximately three-fifths of the annular circumference. The semicircular anterior leaflet is attached to approximately two-fifths of the annular circumference. Although the posterior leaflet attaches to a larger portion of the annular circumference, the leaflet is shorter than the anterior one.
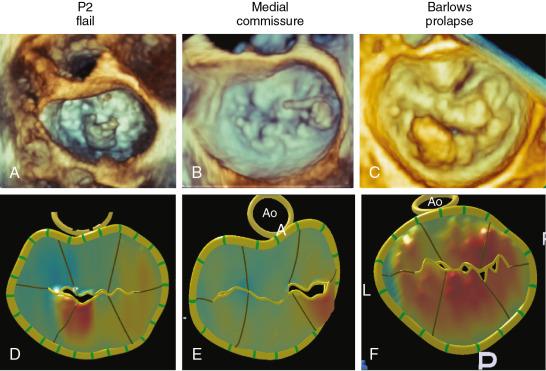
The leaflet segmentation proposed by Carpentier is the most widely classification used. This schema takes advantage that the posterior leaflet has two well-defined indentations dividing it into three separate sections or scallops . The anterolateral scallop is defined as P1, the middle scallop is defined as P2, and the posteromedial scallop is defined as P3. The anterior leaflet typically has a smoother surface and is devoid of indentations. The segment of the anterior leaflet opposing P1 is designated A1 (anterior segment), the segment opposite to P2 is A2 (middle segment), and the segment opposite to P3 is A3 (posterior segment) ( Fig. 2.1 ).
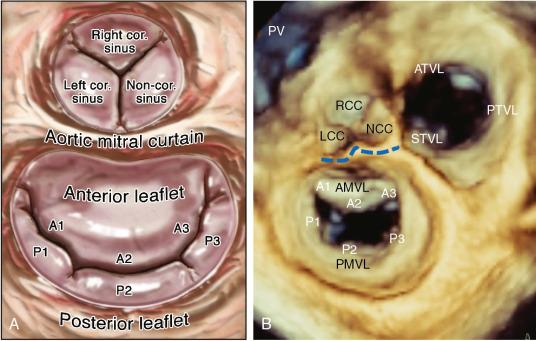
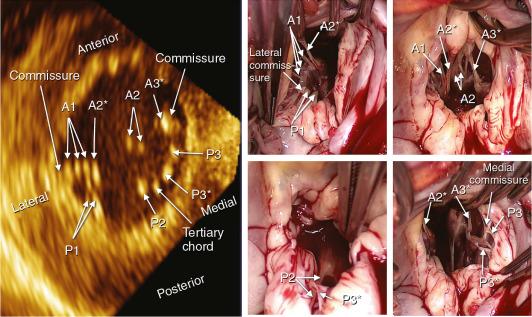
The commissures define a distinct area where the anterior and posterior leaflets appose each other during systole. Carpentier divides them into anterolateral and posteromedial commissures. The amount of tissue in the commissures varies from several millimeters of leaflet tissue to distinct leaflet segments.
The chordae tendineae are responsible for determining the position and tension on the anterior and posterior leaflets at LV end-systole. The chordae are fibrous extensions originating from the heads of the papillary muscles and infrequently from the inferolateral LV wall. They are named according to their insertion site on the mitral leaflets. Marginal or primary chordae insert on the free margin of the mitral leaflets and help prevent marginal prolapse. Intermediate or secondary strut chordae insert on the LV surface of the leaflets, preventing billowing while reducing tension on the leaflet tissues. , These chords may also play a role in determining dynamic LV shape and function due to their contribution to LV-valve continuity. , Basal or tertiary chordae insert on the posterior leaflet base and mitral annulus. Their specific function is unclear.
There are two papillary muscles—the anterolateral and the posteromedial—that originate from the area between the apical and middle thirds of the LV free wall. The anterolateral papillary muscle is composed of an anterior and posterior head, and the posteromedial papillary muscle is usually composed of anterior, intermediate, and posterior heads. Because the papillary muscles connect directly to the LV, any geometric change in LV shape can change the axial relationship of the chordae and leaflets, resulting in poor leaflet coaptation.
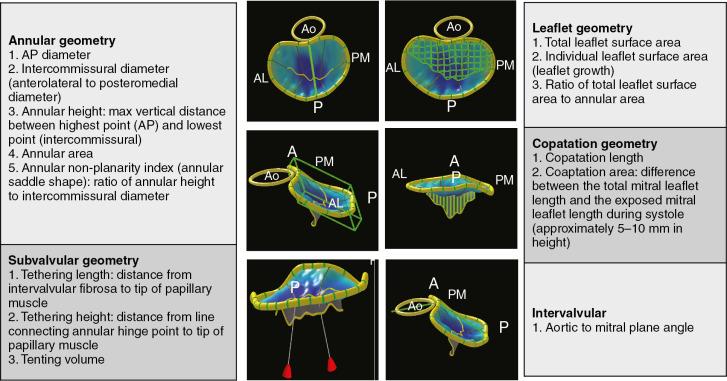
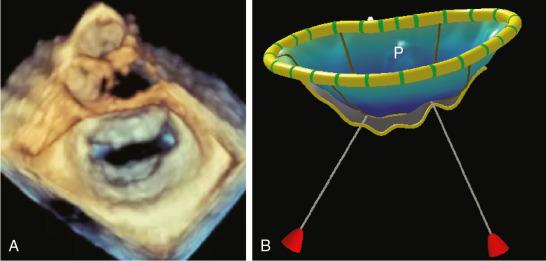
With the advent of 3DE imaging, new parameters quantifying annular, coaptation, leaflet, and subvalvular geometry were easily obtained. , These measurements provided insights into MV mechanics and have been instrumental in guiding MV repair because 3DE allowed classification of MV dysfunction ( Table 2.1 ). One study found a strong correlation ( r = 0.93, P < 0.0001) between chordal length assessed preoperatively by 3DE and intraoperative measurements, emphasizing its important role in preprocedural surgical planning ( Fig. 2.2 ). Fig. 2.3 shows the most commonly used parameters.
| Characteristic | Type 1 | Type 2 | Type IIIA | Type IIIB |
| Motion of leaflet margin | Normal | Prolapse or flail | Restricted leaflet opening | Restricted leaflet closure |
| Associated disease processes | Chronic atrial fibrillation Bacterial endocarditis |
Degenerative disease (Barlow disease, fibroelastic deficiency) | Rheumatic disease | Myocardial infarction Dilated cardiomyopathy |
| Associated lesions | Annular dilation Leaflet perforation |
Leaflet thickening Leaflet billowing Leaflet elongation Chordal thickening Chordal rupture |
Commissure fusion Leaflet thickening Chordae thickening |
Papillary displacement Chordae tethering Annular dilation |
The main views used to acquire 3D transthoracic echocardiographic (TTE) images of the MV are the parasternal long-axis and apical four-chamber views ( Table 2.2 ). Although the 2D TTE parasternal short-axis view provides an en face view of the MV, it displays only the MV leaflets en face from the LV perspective.
| Imaging | View | Mitral Valve | Aortic Valve |
| Acquisition | Transthoracic | Parasternal long-axis view Apical 4-chamber view |
Parasternal long-axis view Parasternal short-axis view Apical 3-chamber view |
| Transesophageal | Mid-esophageal ≈0° 4-chamber view Mid-esophageal ≈120° long-axis view |
Mid-esophageal ≈60° aortic valve short-axis view Mid-esophageal ≈120° long-axis view |
|
| Display | Orient the aortic valve at the 12-o’clock position whether the valve is viewed from the LA or the LV | Orient the right coronary cusp at the 6-o’clock position whether viewed from the aorta or the LVOT |
When using 3D transesophageal echocardiography (TEE) for imaging, the 60-degree MV bi-commissural and 120-degree long-axis mid-esophageal views are best for visualizing the entire MV structure and its associated components. The transgastric long-axis view of the MV is best used with multiplanar mode to assess subvalvular and valvular structures. 3D TEE provides significantly better anatomic detail compared with TTE due to its higher spatial resolution.
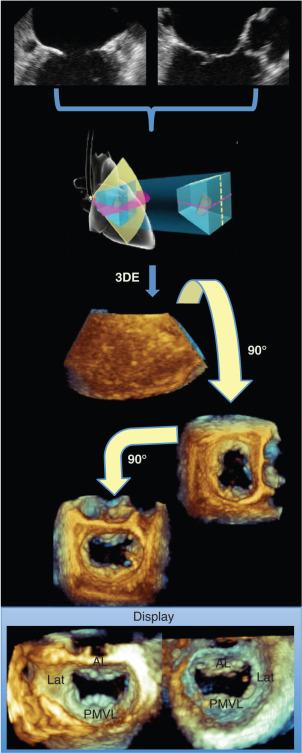
After the 3D data set is acquired, plane cropping and rotation of the pyramidal volume can be performed to present a dynamic en face 3D rendering of the MV. 3DE facilitates communication between the imager and the cardiovascular surgeon because it can display the MV in a manner similar to the way the surgeon visualizes the valve in the operating room when approaching the valve from the left atrium (LA). This surgical view is obtained by viewing the MV from the LA and rotating the valve such that the aorta is directly above it at the 12-o’clock position ( Fig. 2.4 ).
MV prolapse is the most common cause of MR in developed countries. 3DE technology has considerably improved the ability of physicians to diagnose and surgically treat MV prolapse, which primarily results from two distinctive types of degenerative diseases: Barlow disease and fibroelastic deficiency ( Table 2.3 ). Barlow disease results from an excess of myxomatous tissue, which is an abnormal accumulation of mucopolysaccharides in one or both of the leaflets and many or only a few of the chordae. In contrast, fibroelastic deficiency results from acute loss of mechanical integrity due to abnormalities of connective tissue structure and/or function. It usually results in a localized or unisegmental prolapse due to elongated chordae or flail leaflet due to ruptured chordae ( Fig. 2.5 ).
| Differentiating Characteristics | Barlow Disease | Fibroelastic Deficiency |
| Pathology | Excess of myxomatous tissue | Impaired production of connective tissue |
| Typical age of diagnosis | Younger (<40 years) | Older (>60 years) |
| Duration of disease | Years to decades | Days to months |
| Physical examination | Mid-systolic click and late systolic murmur | Holosystolic murmur |
| Leaflet involvement | Multisegmental | Unisegmental |
| Leaflet lesions | Leaflet billowing and thickening | Thin leaflets with thickened involved segment |
| Chordae lesions | Chordal thickening and elongation | Chordal elongation and chordal rupture |
| Carpentier classification | Type II | Type II |
| Type of dysfunction | Bileaflet prolapse | Prolapse and/or flail |
| Complexity of valve repair | More complex | Less complex |

Many studies have shown that 3DE is superior to 2DE in accurately diagnosing the location of degenerative disease. 3DE is less operator-dependent, and more reproducible than 2DE at any level of expertise. The diagnostic accuracy of 3D and 2D TEE was compared for a large number of patients undergoing MV repair due to prolapse, and echocardiographic findings were compared with surgical ones. 3D TEE correctly identified the prolapsing scallop in 92% of patients versus 78% of patients using 2D TEE. The use of parametric maps, which are 3D images of the MV transformed into color-encoded topographic displays of MV anatomy in which the color gradations indicate the distance of the leaflet from the mitral annular plane toward the LA, have also improved diagnostic accuracy for novice readers ( Fig. 2.6 ). One study found that 3D color-coded parametric maps of the MV allowed easy differentiation of MV prolapse versus MV billowing without the need to inspect multiple planes as needed with 2DE.
In addition to its superior accuracy in diagnosing degenerative disease, 3DE also has the ability to differentiate Barlow disease from fibroelastic deficiency. When 3D quantitative parameters are used to differentiate patients with or without degenerative MV disease, billowing height and volume were the strongest predictors of degenerative MV disease. 3D billowing height with a cutoff value of 1.0 mm differentiated normal from degenerative disease without overlap, whereas 3D billowing volume with a cutoff value of 1.15 mL differentiated Barlow disease from fibroelastic deficiency. These measurements were found to be highly reproducible.
3DE studies have increased our understanding of the pathophysiologic differences between fibroelastic deficiency and Barlow disease. These two entities have different alterations in annular dynamics and leaflet tissues. , In addition to significant leaflet prolapse, patients with Barlow disease have blunted annular dynamics during the cardiac cycle that exceed the extent of LV and atrial remodeling. This suggests that there may be a primary abnormality of the mitral annulus that contributes to MR severity. These annular changes may also explain the development of mitral annular disjunction that leads to a functional decoupling between the ventricle and the mitral annulus. Despite greater prolapse severity and annular enlargement, patients with Barlow disease can have quantitatively similar MR severity compared with patients with fibroelastic deficiency due to compensation by increased mitral tissue reserve/distensibility during mid-to-late systole. In contrast, patients with fibroelastic deficiency have relatively preserved annular function but little tissue reserve with reduced systolic leaflet area changes. They develop severe MR with fewer morphologic changes. Overall, these differences suggest that different surgical approaches may be required for repair.
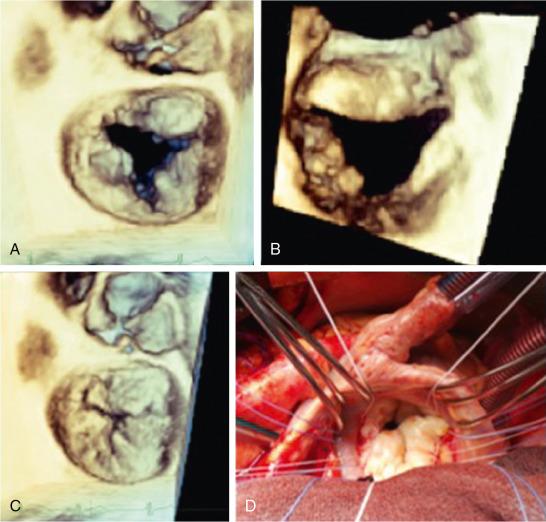
With 3DE en face visualization of the MV, there has been greater recognition of the presence of deep cleft-like indentations in patients with degenerative valve disease ( Fig. 2.7 ) and the importance of differentiating them from true clefts due to atrioventricular septal defects with or without an intact septum. Understanding the role of these clefts in contributing to the MR is needed to determine feasibility for transcatheter edge-to-edge repair procedures and as a possible source of residual MR after device placement.
Ischemic MR is a pathophysiologic outcome of LV remodeling arising from ischemic heart disease. Classically, it was thought to primarily result from posteromedial papillary muscle dysfunction due to this muscle’s dependence on a single blood supply. In the past decade, however, multiple 3DE studies have shown that papillary muscle dysfunction is not responsible for ischemic MR. A wide spectrum of geometric distortions that result from LV remodeling cause this type of valve dysfunction. The observations provided by 3DE have helped reshape our understanding of ischemic MR.
The MV is dynamic and changes from a saddle shape (i.e., hyperbolic paraboloid) during systole to a flatter configuration during diastole. During systole, competing forces act on the MV leaflets. Increased LV pressure acts to push the leaflets toward the LA, and tethering forces from the chordae act to pull the leaflets in the direction of the LV. The saddle-shape morphology is thought to balance these forces by optimizing leaflet curvature and minimizing mitral leaflet stress. In the setting of a myocardial infarction and resultant LV remodeling, an outward and apical displacement of the posteromedial papillary muscle occurs, which tethers the MV leaflets into the LV, restricting their ability to coapt effectively at the level of the mitral annulus. The mitral annulus also dilates, making leaflet coaptation even more difficult.
Although some have used the terms ischemic MR and functional MR interchangeably, they have different meanings. Unlike ischemic MR, in which displacement of the posteromedial papillary muscle predominates, functional MR is a result of bilateral papillary muscle displacement (i.e., symmetric tethering) typically caused by dilated cardiomyopathy. The direction of the MR jet can help differentiate the two types of valvular dysfunction. The ischemic MR jet is usually eccentric and directed toward the posterior “restricted” leaflet, whereas the functional MR jet is commonly centrally directed toward the roof of the LA ( Fig. 2.8 ).
3DE has provided insights into the pathophysiology of functional and ischemic MR. For example, several investigators using 3DE showed that increased sphericity of the LV, rather than contractile dysfunction, contributes significantly to bilateral papillary displacement, resulting in functional MR. , 3DE showed that anterior-apical myocardial infarctions that extend to the inferior apex can also cause ischemic MR. This suggests that inferior myocardial infarctions are not solely responsible for ischemic MR but that this entity can develop even when the myocardium immediately underlying the posteromedial papillary muscle is not directly involved.
Mitral leaflet tethering is a major factor contributing to development of ischemic MR. 2DE has been extensively used to calculate MV tenting area and tenting length, but studies have shown that the asymmetry of these single-plane measurements is commonly inaccurate compared with intraoperative findings. 3DE overcomes this limitation by providing more accurate and reproducible measurements. In one of the first studies to examine leaflet tethering with 3DE, patients with severe MR were shown to have significantly larger tenting lengths and volumes compared with control patients. This study found that the leaflet site where peak tenting occurred was different in each individual, suggesting that different chordae are involved in the disease process. Although MR severity is affected by the degree of tenting, tenting asymmetry is associated with greater degrees of regurgitation.
Conformational changes of the MV annulus contributes to the development of ischemic MR. Multiple studies have shown that the annulus dilates and flattens, becoming essentially adynamic throughout the cardiac cycle. , 3DE imaging has revealed more subtle anatomic changes such as greater dilation in the anteroposterior dimension and greater overall dilation and flattening in anterior compared with inferior infarcts. , 3DE has been used to evaluate the dynamic changes in MV annular surface area and annular longitudinal displacement throughout the cardiac cycle. It has been demonstrated that the mitral annular surface area is larger and the annular pulsatility and displacement lower in patients with ischemic MR. As the mitral annulus enlarges, it loses its motility, becoming progressively unable to modify its shape throughout the cardiac cycle.
One of the most intriguing findings of 3DE is that while leaflet tethering and annular geometric changes drive the development of ischemic MR, leaflet growth occurs in an attempt to compensate for the decrease in leaflet coaptation. In one of the earliest studies to examine this phenomenon, Chaput et al found that leaflet area increased by 35% in patients with LV dysfunction. Two months after a myocardial infarction, tethered leaflet area and thickness were shown to be significantly increased compared with nontethered leaflets. Studies using molecular histopathology showed that this leaflet growth was due to an increase in smooth muscle α-actin in tethered leaflets, indicating endothelial-mesenchymal transdifferentiation.
A 3DE study examined the interactions among leaflet tethering, annular dilation and flattening, and leaflet elongation. The study authors measured multiple variables, including tenting length and volume, total leaflet area, total annular area, and coaptation length and area. They demonstrated that mitral leaflet coaptation decreases proportionally with the increased displacement of the papillary muscles, despite the presence of compensatory increased total leaflet area. The ratio of total leaflet area to total annular area required to ensure proper coaptation in mid-systole was decreased in patients with severe MR compared with patients with only mild MR. Coaptation area was the strongest determinant of MR severity. The reason why some patients develop sufficient compensatory leaflet growth while others do not remains unknown.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here