Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The anatomic and functional characteristics of the aorta, which may at first glance appear relatively straightforward, are now recognized to be complex. Recent insights from both modern imaging technology and better understanding of the hydraulic principles associated with the variety of aortic diseases have helped the medical community to realize the multiple facets of in vivo aortic pathology, as well as its varied clinical presentation.
Diagnostic modalities such as transesophageal echocardiography (TEE), cardiovascular magnetic resonance (CMR), and spiral computed tomography (CT) have all been shown to be useful to interrogate the aorta, both in chronic disease and in acute aortic syndromes. X-ray contrast angiography, the former gold standard in acute and chronic aortic syndromes, has been relegated to a secondary role after the emergence of the noninvasive techniques, most importantly CMR, with their high sensitivity, specificity, and practical advantages. However, none of the diagnostic modalities listed above is ideal for all patients, and for a given individual, knowledge of both accuracy and limitations in the presenting clinical scenario are required. Although the information content of CMR may greatly overlap with established methods such as echocardiography, CT, or angiography, the technique is more comprehensive and offers more options including four-dimensional (4D) functional imaging.
Besides images with high soft tissue contrast without any radiation, CMR can demonstrate and quantify functional parameters beyond anatomic depiction. Combining anatomical and functional information in a single acquisition means that CMR can potentially provide a more comprehensive evaluation of thoracic aortic disease, including aortic valve morphology and function. CMR is an ideal imaging modality for surveillance in a relatively young patient population requiring long-term or even lifelong follow-up care. Although the cost-effectiveness of CMR has not been proven in all areas, CMR is the preferred modality in both aortic disease, including aneurysm and dissection, and its precursors, and in congenital and inherited heart diseases. This chapter focuses on emerging advantages of CMR with respect to a spectrum of aortic pathologies.
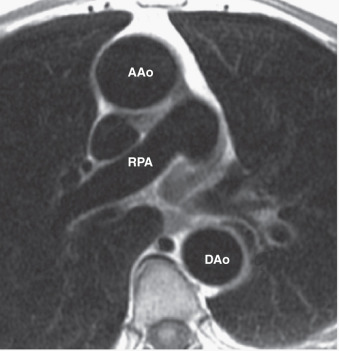
Spin echo T1-weighted imaging provides the anatomic detail of the aortic wall and pathologic conditions such as atheromatous plaques, intimal flaps, or intramural hemorrhage and is still the basis of any aortic study, whereas T2-weighted images (repetition time: 2/3 RR; echo time: 80 to 100 ms) can be used in tissue characterization of the aortic wall or blood components. Electrocardiogram (ECG) triggering is essential in minimizing motion and pulsatility artifacts. Slice thickness of 3 to 8 mm and an echo time (TE) of 20 to 30 ms are standard, whereas repetition time (TR) is determined from the RR interval of the ECG. A shorter acquisition time can be achieved with fast spin echo pulse sequences whereby a long train of echoes is acquired by using a series of 180-degree radiofrequency (RF) pulses; washout effects are even more substantial than in conventional spin echo techniques. A superior black-blood effect is achieved by using preparatory pulses (such as presaturation, dephasing gradients, and preinversion) with one or more additional RF pulses outside the plane to suppress the signal intensity of in-flowing blood and nullify the blood signal ( Fig. 43.1 ). Therefore “black-blood” fast T1-weighted and T2-weighted spin echo sequences have improved image quality, and constitute the method of choice for morphologic assessment of the thoracic aorta. Images are acquired in axial and additional planes, depending on the anatomy and diagnostic problems, to define the extent of the disease in three-dimensional (3D) space.
Gradient echo techniques provide dynamic and functional information, although with fewer details of the vessel wall. The bright signal of the blood pool on gradient echo images results from flow-related enhancement obtained by applying RF pulses to saturate a volume of tissue. With a short TR (4 to 8 ms) and low flip angle (20 to 30 degrees), maximal signal is emitted by blood flowing in the voxel and ECG-gated acquisition provides a high degree of temporal resolution throughout the cardiac cycle (up to 20 to 25 frames) to be displayed in cine format. Flow-related enhancement is produced by inflow of unsaturated blood exposed to only one RF pulse. As result, the laminar moving blood displays a bright signal in contrast to stationary tissues. The signal can be reduced if the flow is low, as in aortic aneurysms. Mural thrombi can be identified by persistent low-signal intensity in different phases of the cardiac cycle. Turbulent flow produces rapid spin dephasing and results in a signal void, providing additional information in many pathologic conditions such as coarctation, aortic valve insufficiency, aortic aneurysm, and dissection. Particularly in aortic dissection, the detection of entry and reentry sites is a special capability of functional CMR that can be helpful in planning both surgical and endovascular therapy. Accurate quantitative information on blood flow is obtained from modified gradient echo sequences with parameter reconstruction from the phase rather than the amplitude of the magnetic resonance (MR) signal; this is also known as flow mapping or phase contrast or velocity-encoded cine CMR ( Fig. 43.2 ). In each pixel of velocity images, the phase of the signal is related to the velocity component in the direction of a bipolar velocity phase-encoding gradient. In the phase image, the velocity of blood flow can be determined for any site of the vascular system. Flow velocity is calculated by using a formula in which velocity is proportional to change in the phase angle of protons in motion. MR maps of flow velocity are obtained two-dimensionally, which is particularly important in profiles of nonuniform flow, such as in the great vessels. Quantitative data on flow velocity and flow volume are obtained from the velocity maps through a region of interest. The mean blood flow is estimated by multiplying the spatial mean velocity and the cross-sectional area of the vessel. Vector mapping has been used to describe flow patterns in different aortic diseases (e.g., hypertension, aneurysms, dissection, Marfan syndrome, coarctation).
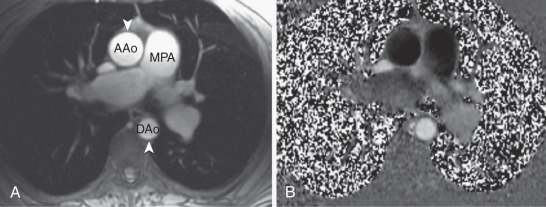
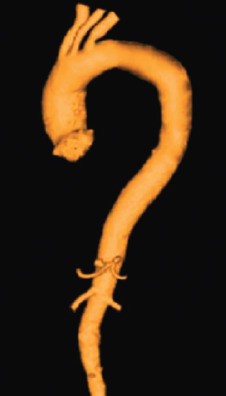
A variety of magnetic resonance angiography (MRA) techniques, including various pulse sequences, methods of data acquisition, and postprocessing, have been developed, but first-pass 3D contrast-enhanced MRA constitutes the method of choice for the evaluation of the aorta. The technique relies on the contrast-induced T1-shortening effects of the gadolinium (Gd) contrast agent, whereby saturation problems with slow flow or turbulence-induced signal voids are avoided. During the short intravascular phase, the paramagnetic contrast agent provides a precise signal in the arterial or venous system, enhancing the vessel-to-background contrast-to-noise ratio, irrespective of flow patterns and velocity and not only delineates the dissection lamella, but also filling of critical side branches. Intravenous bolus timing is necessary to ensure peak enhancement during the middle of CMR acquisition and not exceeding the acquisition time. Improved gradient systems allow a considerable reduction of the minimum TRs and TEs and the acquisition of complex 3D datasets within a breath-hold interval of under 30 seconds. With the support of maximum intensity projection (MIP) images and the 3D multiplanar reformation, this technique delineates all the morphologic details of the aorta and its side branches in any plane in a 3D format ( Fig. 43.3 ). Some caution has to be exercised in patients with poor renal function because this agent has been associated with the development of nephrogenic systemic fibrosis. From a technical point of view, blood pool imaging can be acquired without the use of a contrast agent using an ECG-gated and respiratory-navigated balanced steady-state free precession (SSFP) acquisition. After an intervention, first-pass contrast-enhanced angiography is instrumental in assessing the success of a procedure and in quantifying false lumen thrombosis, a well-established prognostic indicator. The image acquisition is timed according to the arrival of the contrast bolus in the proximal unaffected aorta and thrombosis is assumed to be present when there is no contrast agent in the false lumen. However, recent studies have shown that the flow rates in the false lumen are highly variable and often very slow. The use of a new CMR technique with gadofosveset trisodium blood pool agent in equilibrium state is encouraging.
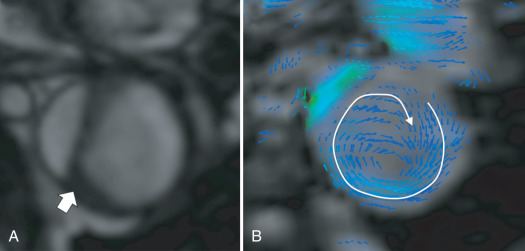
Finally, phase contrast sequences may be used to assess blood flow and velocity in both lumens of a dissection. This imaging technique does not play a major role in the context of diagnostic imaging; however, it appears to be an interesting research tool and potentially provides parameters for a prognostic assessment of a given patient. These sequences can also be used to acquire 3D velocity information (Vx, Vy, Vz) for each voxel, within a 3D volume, over time (frequently called 4D phase contrast-CMR) ( Fig. 43.4 ). This acquisition offers the potential to study aortic hemodynamics, flow patterns, and derived vessel wall parameters, such as wall shear stress. This technique can help to identify entry tears between the true and false lumens and to stratify patients according to their risk of aneurysm formation.
Four-dimensional time-resolved angiography with keyhole (TRAK) is a contrast-enhanced MR angiography technique that can acquire multiple time-resolved 3D volumes over time using image acceleration techniques. It can be used to acquire several phases of contrast distribution, including, but not limited to, the arterial and venous phases. This technique can be used clinically to characterize flow-related phenomena, such as false lumen thrombus distribution and endoleak.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here