Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Since the US Food and Drug Administration (FDA) approved the first thoracic endovascular aortic endograft repair (TEVAR) in 2005, its use has rapidly increased. , In a relatively short period of time, TEVAR for descending thoracic aortic aneurysms (DTAA) and various other thoracic aortic pathology has not only become the preferred treatment method but also the standard of care. Recently, the Society for Vascular Surgery (SVS) Clinical Practice Guidelines for TEVAR were updated and will be referenced throughout the chapter. The level of recommendation will be supported by a standardized grade and level of supporting evidence.
Thoracic aortic disease remains one of the leading causes of death in the United States and accounts for more than 11,000 deaths annually, with an estimated incidence of 5.6 to 10.4 cases per 100,000 person-years. Based on recent epidemiologic data, the incidence and prevalence of DTAA appears to be increasing and is attributed to an aging population, more frequent thoracic imaging, and improved educational awareness of patients and healthcare providers. , The average age of diagnosing a DTAA patient is 65 years, with a male–female ratio of 1.7:1. An underlying genetic component is often present; up to 20% of first generation relatives will be affected by aneurysmal disease. Risk factors associated with DTAA development include hypertension, tobacco use, atherosclerosis, family history and prior aortic dissection. Repair is recommended for all symptomatic and ruptured aneurysms regardless of the diameter. When comparing open surgical repair to TEVAR, similar criteria should be rigorously applied: diameter ≥5.5 cm, and rapid aneurysm expansion (≥5 mm per year). The 5-year risk of rupture is negligible for small aneurysms <4 cm, 16%–31% for aneurysms 40–59 mm in diameter and if >60 mm the risk of rupture increases to 15.6% annually. , This is supported by the SVS Guidelines (grade I [strong] with a moderate quality of evidence [B]). Patients determined to be high surgical risk should be considered for repair at a larger diameter (>6 cm) according to the SVS Guidelines recommendations (grade 2 (weak) and a low quality of evidence [C]). , , There are select situations in which repair at a smaller diameter should be considered, including non-saccular aneurysms, symptomatic, and those associated with connective tissue disorders. Inflammatory diseases (giant cell arteritis, Behçet disease, Takayasu arteritis) are infrequently associated with thoracic aortic aneurysms but, if present, DTAA repair should be delayed until the acute inflammatory component becomes quiescent. Connective tissue disorders associated with thoracic aortic aneurysms include Marfan syndrome, Loeys–Dietz syndrome, Ehlers–Danlos syndrome, Turner syndrome, and bicuspid aortic valve. In addition, gene mutations SMAD3, TGFB2, TGFB3, MYH11, ACTA2, and MYCK are associated with aortic dissections and aneurysms. Repair should be considered at a diameter of 45–50 mm due to the risk of rupture. It is recommended that Marfan patients undergo elective repair at a diameter of 50 mm and Loeys–Dietz syndrome has an even smaller recommended diameter threshold (40–50 mm) due to its rapidly progressive course. In addition to DTAA diameter and the presence of a connective tissue disorder, the aneurysm location has been noted to affect rate of growth. Aneurysms located in the mid-descending thoracic aorta tend to grow at a quicker rate compared to those located elsewhere. Typically, degenerative aneurysms enlarge at an average rate of 1–2 mm/year, in comparison with isolated DTAA, which expand at a rate of 3 mm/year.
Cardiovascular risk reduction and associated medical therapy is indicated in those patients with asymptomatic descending thoracic aortic aneurysms that do not meet objective criteria for repair as recommended by the SVS Guidelines (high quality of evidence [A]).
The many considerations supporting TEVAR over open surgical repair are based on physician experience, patient anatomy, and underlying patient comorbidities. Anatomically, if considering TEVAR suitable, proximal and distal landing zones (>20 mm) are required to provide adequate seal, along with appropriately sized arterial access to deliver the endograft. However, anatomy is only one parameter to consider when planning treatment: age, quality of life and medical risk assessment play significant roles in selecting the appropriate treatment option. , A 2016 Cochrane review concurs that clinical equipoise still exists between TEVAR and open repair of thoracic aortic aneurysms (TAAs) due the paucity of supporting data.
According to the 2020 SVS Guidelines, among patients who are suitable candidates for either open surgical repair or TEVAR (within the criteria of the device’s IFU), TEVAR is the preferred approach for elective repair. The level of recommendation is grade I (strong) with a high quality of evidence (A). However, this enthusiasm for TEVAR should be tempered by the paucity of long-term outcomes data and obligation for lifelong endograft surveillance. Strong consideration for open surgical repair in young patients is needed due to the lower need for reintervention associated with open repair. For DTAA in higher surgical risk individuals, the risk–benefit analysis favors an endovascular approach in most. Longer-term data is accumulating and supports a more liberal use of TEVAR in lower-risk individuals, due to the demonstratable early perioperative risk reduction with TEVAR. Despite the compelling evidence supporting TEVAR, physicians should continue to advocate for TEVAR based on the same criteria used for conventional open repair.
In patients without favorable aortoiliac anatomy for TEVAR, the risk–benefit assessment becomes more challenging. The incremental risks of planned branch vessel occlusion should be carefully assessed when considering TEVAR; presumably this option would be advantageous in select situations (i.e., cardiac or pulmonary comorbidities that preclude safe open repair or “high-risk” anatomic conditions). In these situations, the safety of sacrificing a branch vessel should be balanced against continued observation and/or open surgical repair. If sacrificing a branch is unacceptable, alternative options include snorkel/chimney endovascular technique, fenestrated/branched endografts, and hybrid approach with surgical debranching and TEVAR.
The durability of TEVAR is compromised by treating aortic pathology outside of the device-specific instructions for use (IFU), including large diameter necks, angulated aortic segments, use of chimneys/snorkels and circumferential thrombus lining the seal zones. Significant angulation of the aortic arch (>60 degrees) adjacent to the aneurysm not only increases the difficulty of device deployment but also may compromise seal. TEVAR is contraindicated in patients who fail to meet IFU-defined anatomic criteria and should be performed with caution in patients that have life-threatening allergies to nickel, titanium or stainless steel. In addition, anyone unable to comply with follow-up surveillance imaging should be excluded from TEVAR. In a few select situations TEVAR may be used as a temporizing life-saving measure, such as patients with active hemorrhage from an aorto-esophageal fistulae or mycotic aneurysm. The SVS Guidelines support endograft use for symptomatic mycotic/infected TAA only as a temporizing measure (grade 2 [weak] and low quality of evidence [C]).
Recent endograft advancements have included lower delivery profiles, improved fixation, better conformability, and techniques that provide improved precise deployment. Seven thoracic endograft devices were approved for use in the United States, although due to device advancements three have been phased out and are no longer available for commercial use in the US. These devices include the Cook Medical Zenith TX2 Thoracic Stent Graft, Medtronic Valiant Thoracic Stent Graft with Captivia Delivery System, and the Gore Conformable TAG Thoracic Endoprosthesis ( Table 80.1 ).
| Device | Enrolled | SCI | 30-day Mortality | 1-year Endoleak Rate | Technical Success | Freedom ARM |
|---|---|---|---|---|---|---|
| Gore TAG a | 140 | 2.8% | 2.1% | 3.9% | 98% | 97%, 3 years |
| Cook TX2 Starz Trial b | 160 | 5.6% | 1.9% | 3.9% | 98.8% | 94.1%, 5 years |
| Medtronic Talent Valor Trial c | 195 | 8.7% | 2.1% | 12.2% | 99.5% | 96.9%,1 year |
| Gore TAG Conformable with active control d | 127 | 3.2% | 2.4% | 3.9% | 97.6% | 96.1%, 1 year |
| Medtronic Navion e | 87 | 1.1% | 2.2% | 2.5% | 100% | 97.7%, 30 days |
| Cook Zenith Alpha f | 110 | 0% | 0% | 8.4% | 98% | 99%, 1 year |
| Terumo Relay Plus g | 133 | 3% | 5.3% | 4.5% | 97% | 91.3%, 5 years |
b Cook TX2 Starz – Pivotal Trial
c Medtronic Talent – Pivotal Trial
d Gore TAG Conformable – SURPASS Observational Registry
e Medtronic Navion – Valiant EVO global clinical trial
f Cook Zenith Alpha – International multicenter trial; Pivotal Trial
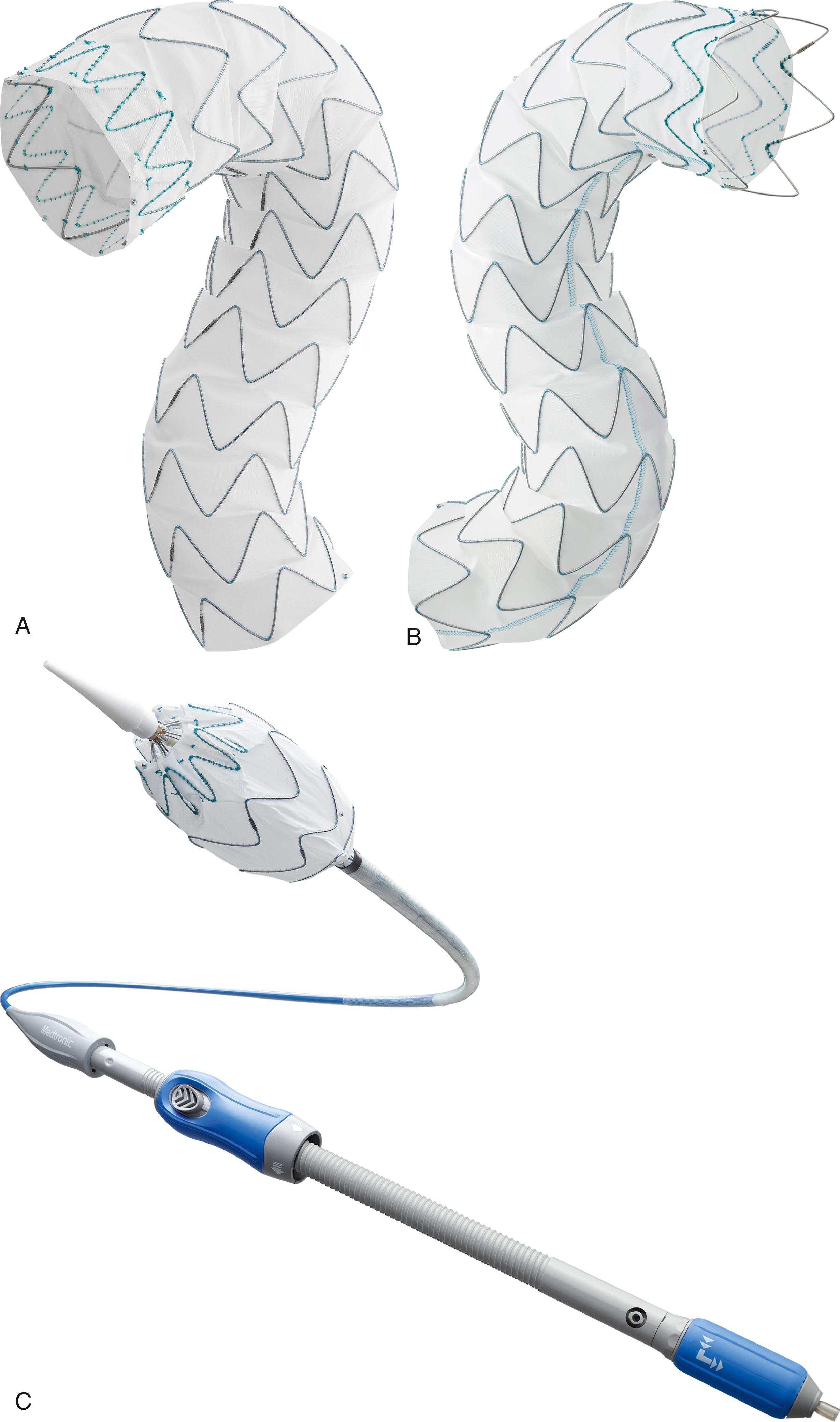
In 2005, the Gore Thoracic Aortic Graft (TAG) Endoprosthesis (W.L. Gore & Associates, Flagstaff, AZ) was the first thoracic endograft approved in the United States. The Conformable TAG (C-TAG) Endoprosthesis was approved January 2012 and created to better conform to the aortic wall and minimize the risk of type I endoleak using a one-step deployment. The advancement of the delivery and deployment system is represented by the most recent generation of Gore thoracic endoprosthesis. The Gore TAG Conformable Thoracic Stent Graft with Active Control System (W.L. Gore & Associates, Flagstaff, AZ) received FDA approval in 2019. Similar to prior Gore TAG devices it is constructed with an expanded polytetrafluoroethylene (ePTFE) graft reinforced with ePTFE/fluorinated ethylene propylene film. A proprietary sutureless graft attachment process and an external nickel–titanium (nitinol) self-expanding stent are affixed to the graft ( Fig. 80.1A ). The staged delivery system has a lower profile and is distinctly different from prior generations. It features a nested handle that requires a two-step graft deployment which improves control and accuracy ( Fig 80.1B ). In situ device angulation of the leading end is possible throughout the deployment process. In comparison to the Gore Conformable TAG Endoprosthesis, the new generation has two separate constraining sleeves rather than one ( Fig. 80.1C ). Each step in the graft deployment requires actuation of a separate handle component . The entire endograft deployment requires four separate steps and has two optional device angulation steps. Removal and actuation of the first deployment handle expands the endograft to its intermediate diameter (50% nominal size) and is designed to allow continuous blood flow through and around the graft lumen. The absence of a windsock effect minimizes hemodynamic instability and ensures precise positioning of the stent graft. Unlike prior devices, graft expansion is initiated from the leading to the trailing end. The angulation control dial can now be rotated to adjust angulation and orthogonal placement of the proximal endograft. Removal of the secondary deployment handle expands the endograft to its maximum diameter and does so by initiating deployment from the trailing end to the leading end ( Fig. 80.1D ). Even after complete endograft expansion, the angulation control remains accessible for improved orthogonal placement. During the deployment, the endograft remains secured to the delivery system with a locking wire. After device expansion, the locking wire is removed from the handle, and the angulation assembly handle is removed with the delivery system. The device lengths, diameters, and delivery profile are documented in Table 80.2 . Based on the IFU, the current Gore device is designed to be oversized 6%–33% and has specific oversizing windows that enable optimal radial fit with various device diameters. The benefits of a lower profile device were noted in the SURPASS European registry as 45.7% of 127 patients underwent percutaneous access.

| Device | Diameters (mm) | Lengths (mm) | Delivery Profile (F) | Tapered Transition | FDA |
|---|---|---|---|---|---|
| Gore TAG Conformable with active control | 21, 26, 28, 31, 34, 37, 40, 45 |
100, 150, 200 | 18–24 ID | 4 mm | 2019 |
| Medtronic Navion | 20, 22, 25, 28, 31, 34, 37, 40, 43, 46 |
52–223 CoveredSeal 59–229 FreeFlo |
18–22 OD | 5–6 mm | 2018 |
| Cook Zenith Alpha | 24, 26, 28, 30, 32, 34, 36, 38, 40, 42, 44, 46 |
105–233 proximal 142–211 distal |
16–20 ID | 4 mm | 2015 |
| Terumo Relay Plus | 22, 24, 26, 28, 30, 32, 34, 36, 38, 40, 42, 44, 46 |
100, 150, 200, 250 | 22–26 OD | 4mm | 2012 |
In 2018 the Medtronic Valiant Navion Stent Graft System received FDA approval. It is the next-generation device based on a modified Valiant thoracic stent graft with Captivia Delivery System (Medtronic, Santa Rosa, CA). Navion has a lower delivery profile and represents a 4-F reduction compared to prior Valiant devices. The Navion device is composed of a multi-filament woven polyester graft attached to sinusoidal nitinol self-expanding stents placed on the outside of the graft material. There are two different proximal graft configurations: a CoveredSeal with a covered leading end and FreeFlo design with eight-peaked bare metal open cell stents on the leading end that evenly distribute radial force across the proximal neck ( Fig. 80.2A,B ). In direct comparison with Valiant, Navion has a device with a longer length (225 mm) and across the board smaller graft diameters. The tapered endografts have been refined and the proximal to distal graft diameter reduction is 5 to 6 mm. To aid in the treatment of aneurysms located in the arch, the tapered tip has been shortened to 1 cm and tip capture feature added. In addition to the lower profile, a hydrophilic sheath coating facilitates device insertion in challenging anatomy ( Fig. 80.2C ) (see Table 80.2 ). Oversizing 10% to 20% in relation to the native aorta is recommended. The pivotal study results were reported in the Valiant EVO global clinical trial. There were no deployment failures, two secondary procedures were required in the first 30 days, few endoleaks, two deaths and the freedom from all-cause mortality was 97.7%. Percutaneous access was used in >50% of patients in the pivotal trial. The Valiant Navion Thoracic Stent Graft was voluntarily recalled by Medtronic in February 2021. The company initiated this action in response to information obtained from the Valiant EVO Global Clinical Trial indicating concerns of stent fractures and endoleaks. Instructions were sent to physicians to cease use of the device in new patients. The Medtronic Valiant Captivia Stent Graft remains available for use.
In September 2015 the Cook Zenith Alpha (Cook Medical, Bloomington, IN) received FDA premarket approval. This lower profile devices (16–20 F), and hydrophilic sheath improve access and device delivery. The two-piece modular Alpha graft consists of external nitinol self-expanding stents and a woven polyester fabric sewn together ( Fig. 80.3A ). To improve aortic wall apposition, the graft material externally lines both the proximal and distal ends of the graft. Like the earlier generation TX2 device, Alpha uses active fixation barbs at both ends of the graft. The Pro-form proximal suture modification has improved device conformability in the angled aortic arch and has minimized endograft infolding ( Fig. 80.3B ).The device is constrained by several trigger wires which are released after device placement; removing the wires releases the partially constrained leading graft edge. Gold radiopaque markers at both ends of the device improve visualization ( Fig. 80.3C ). Similar to the TX2 device, a wide range of Alpha diameters and lengths are available ( Table 80.2 ). Straight and tapered configurations are available with a 4-mm decrease in graft size proximal to distal. Torsello et al. reported on device durability after treating 70 aneurysms and penetrating aortic ulcers. At 1 year, there were 3 type Ia endoleaks, 2 type Ib endoleaks and a mortality of 7.1%. Only one case required reintervention for an endoleak and there were no open conversions. , The lower profile Alpha device permitted percutaneous access in 36% of patients in the pivotal trial with only one case requiring the use of a conduit.

This third-generation device was formerly known as the Bolton Relay Thoracic Stent-Graft with Plus Delivery System (Bolton Medical, Sunrise, FL). It is designed as a one-piece thoracic endograft made of woven polyester fabric sewn to self-expanding polished nitinol stents ( Fig. 80.4A ). Longitudinal support is provided by a pre-curved nitinol support strut that aids aortic conformability. The device received FDA approval in September 2012. The transport delivery system consists of a hydrophilic braided outer coaxial sheath, flexible secondary sheath with a through lumen that facilitates device advancement and provides precise endograft placement ( Fig. 80.4B ). Active proximal fixation is provided by bare stents on the Relay Plus device; in addition, a non-bare stent (NBS) configuration is available ( Fig. 80.4C ). The device is available in straight and tapered designs. Oversizing of 10% to 15% is recommended in the IFU. After endograft deployment, the leading edge remains constrained to avoid a windsock phenomenon ( Fig. 80.4D ). A wide range of diameters and lengths are available ( Table 80.2 ). The Relay Plus device offers the longest TEVAR lengths (250 mm). The RESTORE registry evaluated 150 European patients that underwent aortic repair; nearly 65% were treated for thoracic aneurysms. The 30-day mortality was 10% and 2-year reintervention rate 9%. No open conversions were required.

The Terumo Relay Pro is the next generation low-profile stent graft designed to improve access in patients with smaller vessels. The Relay Pro has a 3- to 4-F size reduction compared to the Relay Plus. Both bare stent and non-bare stent configurations were included in the US Pivotal trial which completed enrollment in 2019. CE Mark approval was obtained for relay Pro in 2018. The Regeneration Study enrolled 31 European patients with thoracic aortic disease. The technical success was 100%, 30-day freedom from aneurysm-related mortality was 100% and freedom from device-related major adverse events was 94%. Twenty-six of 133 patients required conduits for device insertion; despite the lower profile, no percutaneous procedures were performed.
Careful anatomic assessment of and meticulous preoperative planning are essential for successful TEVAR, including confirmation of adequate arterial access and suitable landing zones. Preoperative fine-cut computed tomographic angiography (CTA) from the neck to the femoral access vessels is essential and provides all necessary anatomic information (see Ch. 29 , Computed Tomography). Three-dimensional centerline reconstructions assess aortic anatomy, landing zones, optimal access sites and endograft sizing.
Many TEVAR procedures require an open surgical exposure for arterial access due to the large sheath size. However, a recent trend for percutaneous access is driven by physician experience, lower profile devices and advancement of percutaneous closure devices. For safe device insertion, suitable iliac and femoral access vessels are required. , Small diameters (<7 mm), existing calcification and vessel tortuosity have a profound impact on the procedure especially if present in combination. The right femoral artery is typically favored for device insertion, whereas the contralateral femoral artery is reserved for diagnostic imaging. An ideal access vessel should be ≥7 mm in diameter to accommodate a 22-F sheath and ≥8 mm for a 24-F sheath. The proximal external iliac typically has the smallest diameter and is prone to calcified plaque which may restrict device insertion. This is a particular problem in women, whose iliac and femoral arteries tend to be slightly smaller than men. Prior TEVAR studies documented the need for an iliac conduit in 15% of patients. If the narrowest segment of the external iliac is smaller than the expected diameter of the planned TEVAR device, consider shifting access to the common iliac artery. Rarely, the entire iliac system is hostile and consideration should be given to alternative access techniques or direct aortic access. An alternative approach for dealing with unfavorable iliac anatomy is the use of an internal endoconduit. A 10- or 12-mm covered stent is deployed across the diseased iliac segment and aggressively inflated, which creates a controlled vessel rupture. The dilated 10-mm stent graft will allow sheath sizes up to 22 F, and the 12-mm stent graft up to 24 F. Planned internal iliac artery embolization may be needed if the occlusive disease is adjacent to the internal iliac artery. Presently, the use of lower profile hydrophilic sheaths (Cook Alpha, Medtronic Navion) and stiff guide wires appear to have minimized the need for adjunctive techniques.
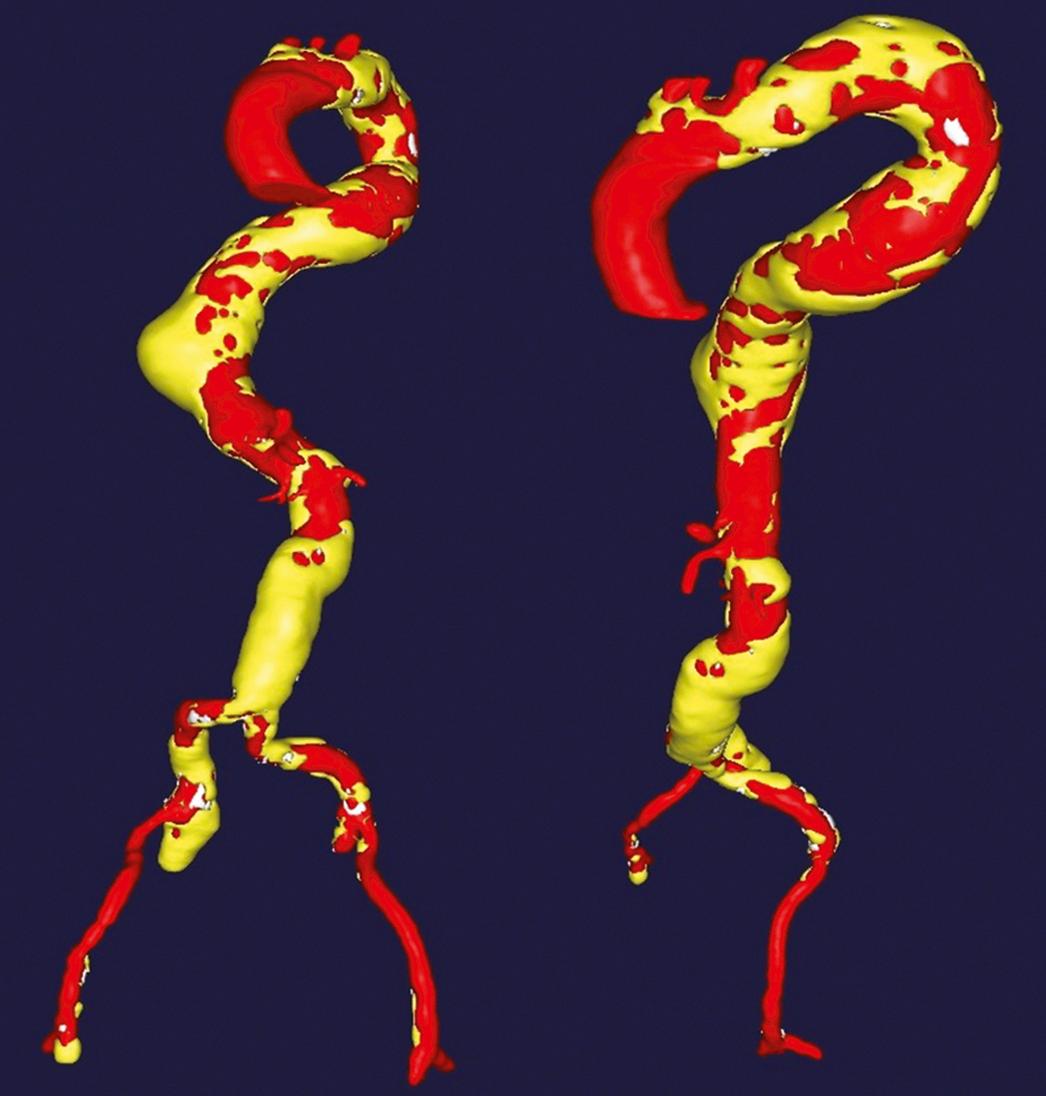
The abdominal aorta rarely presents an anatomic hindrance to device insertion, but tortuosity of the thoracic aorta can be challenging in the presence of aneurysmal disease because it frequently elongates and exaggerates normal thoracic aortic curves. As illustrated in Figure 80.5 , this is particularly problematic in a large descending thoracic aneurysm where cephalocaudal elongation causes exaggeration of the posterior–anterior course of the aorta as it enters the aortic hiatus. This tortuosity, if combined with increased angulation at the transition from the arch to the proximal descending aorta, may cause difficulty with device tracking due to bending of the delivery sheath at the multiple flexion points as it negotiates the angulated aorta.
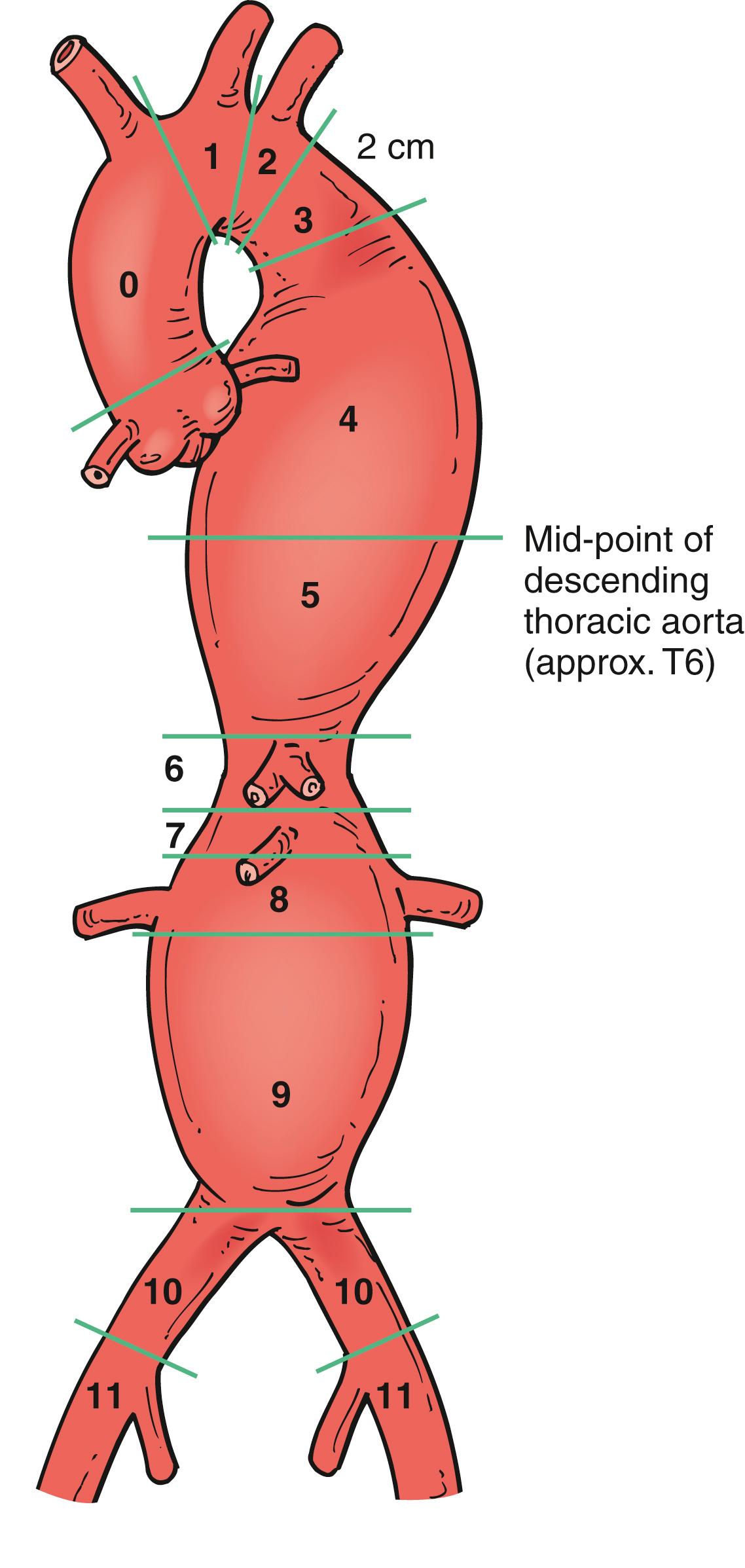
Adequate TEVAR fixation in the proximal and distal landing zones is essential. As a general rule, ≥20 mm of normal-appearing aorta is required for an adequate seal zone but when landing in angulated aortic segments one should consider a minimum distance of 25–30 mm. The TEVAR reporting standards published by the Society for Vascular Surgery (SVS) describe 11 landing zones for aortic disease ( Fig. 80.6 ). Several publications have documented the frequency of neurologic events correlates with location of the proximal landing zone. , The proximal attachment zones define the proximal edge of the endograft in relation to branch vessels: zone 0 – proximal to the innominate artery; zone 1 – distal to the innominate and proximal to left common carotid artery (CCA); zone 2 – distal to left common carotid artery and proximal to the left subclavian artery (SCA); zone 3 – <2 cm from left SCA without covering it; zone 4 – >2 cm distal to left SCA extending to the proximal half of the descending thoracic aorta (above T6 vertebral body); zone 5 – distal half of the descending thoracic aorta and proximal to the celiac artery; zone 6 – celiac origin to top of superior mesenteric artery (SMA); zone 7 – SMA origin to suprarenal aorta; zone 8 – covers at least one renal artery (pararenal aorta); zone 9 – infrarenal aorta; zone 10 – common iliac artery; and zone 11 – external iliac artery.
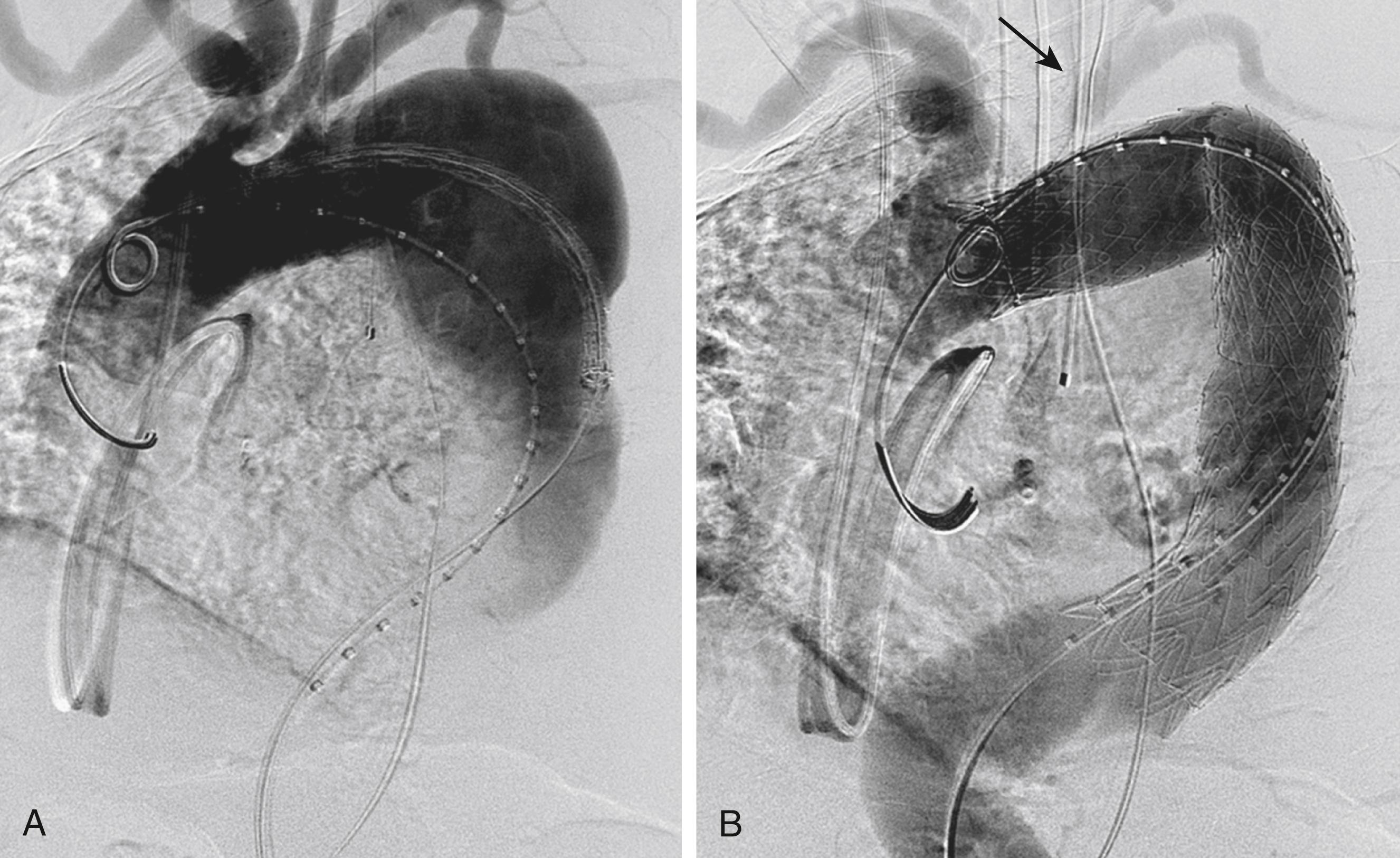
In atherosclerotic aneurysms, the degenerative process usually involves normal-appearing adjacent aorta. Although ≥2 cm is considered adequate for most cases, longer seal zones are preferable in angulated aortic segments to decrease the occurrence of type I endoleaks and device migration. However, the desire to cover longer aortic segments should be tempered by the associated risk of spinal cord ischemia. If anatomically feasible, the landing zones should be parallel in configuration, distal to the left SCA, and proximal to the celiac artery ( Fig. 80.7 ). Device placement in curved aortic segments, generally near the left SCA, presents added complexity with seal. The longitudinal stiffness of the endograft can limit device apposition along the inner aortic curve. If the endograft is angled cephalad and away from the inner curve, bird beaking can occur which increases the risk of device failure. In these regions, the sealing length should focus on the inner aortic curve rather than to the outer aortic wall. The proximity of the seal zones to major branches should carefully be measured with centerline measurements and three-dimensional imaging to avoid inadvertent branch vessel coverage. If indicated, an aortic arch debranching procedure should be considered, which extends the proximal landing zone while maintaining branch vessel patency. Alternative less invasive approaches to maintain branch vessel patency include branched endografts, in situ graft fenestration and chimney/snorkel techniques (see Ch. 82 , Fenestrated and Branched Endograft Treatment of Juxtarenal, Paravisceral, Thoracoabdominal, and Aortic Arch Aneurysms: Device Selection and Technical Considerations).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here