Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Although pathologists typically focus their attention nearly exclusively on the neuropathologic changes of natural disease, therapy-associated neuropathology is increasingly playing a role, particularly now that post-therapy biopsies have become common. To avoid misdiagnosing a host of primary pathologic changes, such as tumor necrosis, vasculopathies, demyelination, cerebral infarction, inflammatory conditions, vasculitis, and others, it is critical to recognize iatrogenic changes induced by prior surgery, intravascular embolization (see Chapter 13 ), electrode placement (see Chapter 25 ), chemotherapy, and, especially, radiation therapy. The very therapies employed to enhance survival and quality of life can also be neurotoxic, and it is often difficult to predict which patients will be most vulnerable. Surgical complications are usually acute and consist mostly of hemorrhage, vascular damage, infarcts, coagulopathies, malignant cerebral edema with herniation, and postoperative infection. Similarly, preoperative tumor embolization has acute complications, such as infarct and hemorrhage. In neuropathology, post-embolization specimens are mainly encountered in the setting of large hypervascular meningioma resections, in which intratumoral necrosis and reactive changes are most commonly noted. Such acute manifestations are less problematic and will not be elaborated further. Similarly, the side effects of therapy on the peripheral nervous system are not covered.
The most serious side effects of radiation and chemotherapy, such as radiation necrosis, chemotherapy-associated leukoencephalopathy, and secondary neoplasms , are mostly chronic or remote in nature, often manifesting years after therapy. Some patients suffer to a much greater extent than others from therapy-induced neurotoxicity, although individual risks are difficult to predict. Nonetheless, the actively developing central nervous system appears to be particularly vulnerable, especially to radiation. Therefore, the management of infants and young children with malignant brain tumors can be particularly challenging. Other factors that alter risk of toxicity include the specific therapeutic modality and dosage, single therapy versus combined radiation and chemotherapy, genetic background, and idiosyncratic reactions. Furthermore, the assessment of new lesions after radiation is often challenging because radiation necrosis mimics tumor recurrence and superimposed radiation changes complicate tumor classification and grading.
Radiation necrosis or radionecrosis is most often encountered by pathologists on biopsy material as a late delayed manifestation, many months to years after therapy. A range of milder and sometimes reversible forms of damage are often managed by oncologists (i.e., rarely biopsied or autopsied) and referred to under the vague heading of radiation encephalopathy. Additional sources of therapeutic consternation (often during weekly tumor board discussions) are the radiologically detected examples of pseudoprogression (versus true progression), wherein magnetic resonance imaging (MRI) findings obtained several weeks to months after radiation initially suggest tumor progression, only to resolve on further neuroimaging follow-up (contrast this scenario with later discussion of pseudoresponse after use of vascular endothelial growth factor [VEGF] inhibitors). A variety of vascular changes due to radiation are lumped together under the heading of radiation vasculopathy, whereas a more diffuse form of white matter damage known as radiation leukoencephalopathy is usually encountered in the setting of large volume or whole brain therapy, although postradiosurgery examples have also been reported. Some of these are discussed in more detail under the Therapy-Induced Secondary Neoplasms section.
Injury to the nervous system is a common complication of radiation therapy, which was classically divided into acute, early delayed, and late delayed forms by Dr. Sheline in 1977. The acute and early delayed forms are thought to be mostly due to blood-brain barrier disruption and cerebral edema, with generally transient and reversible signs and symptoms. In contrast, late delayed effects typically occur years after therapy and are often irreversible injuries, including occasional fatal examples.
In adults, the incidence of radiation necrosis after conventional doses of CNS therapy ranges from 5% to 24% and is rarely seen with cumulative doses of standard fractionated radiation under 50 to 60 Gy to the brain or 45 Gy to the spinal cord. However, a non-necrotizing encephalopathy is even more common and may be encountered with lower dosages. Infants and young children are even more vulnerable and generally, the younger the patient, the higher the risk of radiation-induced damage.
Although all tissue types in the CNS are prone to radiotoxicity, the white matter is particularly vulnerable. Subcortical U fibers are often spared, although lesions can extend into the adjacent cortex or deep gray matter. Microscopically, small to midsize vessels show the most impressive pathologic changes, providing at least one potential explanation for the subcortical predilection. The susceptibility of endothelial cells to radiation damage is pivotal. Other proposed mechanisms of radiation damage include oligodendrocyte toxicity, coagulopathies, and immunologic disturbances such as autoimmune vasculitis. Large vessel disease also occurs, but much less frequently.
Radiation necrosis versus tumor recurrence is an extremely common differential diagnosis in post-therapy biopsy specimens ( Table 21.1 ). This distinction is critical, since the two are managed differently. Most commonly, there is a mixture of both residual/recurrent tumor and radiation necrosis, and some data suggest that the ratio of the two provides meaningful prognostic information. Specimens composed predominantly of radiation necrosis indicate a favorable response to therapy, whereas cellular tumor specimens with little necrosis suggest minimal cytotoxic effects (i.e., consider changing therapy). Also, the post-therapy tumor sometimes appears cytologically inert or “stunned” and it is difficult to know whether these cells are “coming or going”; in other words, it is not clear if tumor cells would have died given additional time or if they were in an early state of recovery. Further studies are needed to clarify these issues.
| Radiologic Feature | Therapy Effects | Glioma Recurrence/Progression |
|---|---|---|
| CT | Calcium in mineralizing microangiopathy | Rarely calcifies |
| Contrast-enhanced T1-weighted MRI | Rim-enhancing; “soap bubble” or “Swiss cheese” interior | Rim-enhancing; homogeneous hypointense center |
| FDG-PET | Decreased metabolism | Increased metabolism |
| Magnetic resonance spectroscopy (MRS) | All peaks decreased, except for lactate and/or lipid (i.e., necrosis) | Increased choline in regions of cellular tumor (Cho/Cr and Cho/NAA ratios > 1.8) |
| Diffusion weighted imaging (DWI) | Heterogenous, hypointense | Hyperintense (especially at margins) |
| Perfusion MRI | Decreased regional cerebral blood volume (rCBV) | Increased regional cerebral blood volume (rCBV) |
| Histologic Feature | ||
| Necrosis | Large foci of coagulative necrosis with hypocellular edges, dystrophic calcifications | Large or microscopic foci with hypercellular or palisading edges |
| Blood vessels | Telangiectatic Hyalinized Angionecrotic |
Microvascular proliferation (enlarged and multilayered “endothelial cells”) |
| Adjacent brain parenchyma | Rarefied or vacuolated, pale, and gliotic, with vascular changes listed above | Nearly normal or infiltrated by individual tumor cells |
| Cytologic atypia | Bizarre nuclei and abundant cytoplasm | High N/C ratio, except for classic gemistocytic and giant cells |
| Mitotic figures | Rare | Frequent (if tumor is high grade) |
| Ki-67 IHC | Low LI | Moderate to high LI b |
| MAP2 IHC | Glial cells negative | Glial cells positive |
| Mutant protein IHC a | Negative or rare positive | Positive |
a Only valid if mutant protein positive in pretherapy tumor specimen (e.g., IDH1 R132H positive glioma).
b Often the index is lower than in the original tumor, potentially because proliferating cells are more susceptible to radiation.
Although less severe than full-blown radiation necrosis and usually not life threatening, radiation encephalopathy tends to progress over many years and significantly diminishes quality of life. Neuroprotective agents, such as lithium, have been studied and may reduce the frequency of this complication. Individual risk factors for radiation damage are inadequately understood, but likely include superimposed medical disorders that also predispose to small vessel disease, such as diabetes, hypertension, and old age. In children, long-term survivors of irradiated brain tumors frequently develop moderate to marked cognitive deficits with losses in intelligence quotient (IQ), learning disabilities, hormonal deficits from involvement of the hypothalamic-pituitary axis, stunted growth, and psychomotor retardation. For instance, in an autopsy study of 22 pediatric glioma patients treated with radiation, there were foci of demyelination in 7, necrosis in 6, and cortical atrophy in 4 cases.
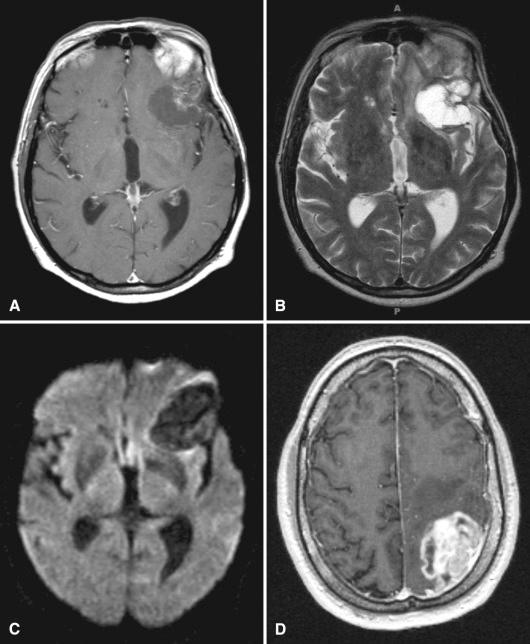
Radiation leukoencephalopathy and similar but less diffuse forms of damage often result in regions of increased T2-weighted and fluid-attenuated inversion recovery (FLAIR) MRI signals in the white matter. Over time, this may lead to tissue loss and hydrocephalus ex vacuo, a stark contrast to the increasing mass effect seen with tumor progression in diffuse gliomas. Foci of radiation necrosis, on the other hand, can be nearly impossible to distinguish from recurrent glioma in individual cases. Such cases or those with lesser degrees of injury such as blood-brain barrier disruption and inflammation are often referred to as “pseudoprogression” when the worrisome neuroimaging changes resolve partially or fully on follow-up imaging. Nonetheless, several useful imaging findings provide support for therapy effects versus true progression (see Table 21.1 ). For instance, on routine MRI, a “soap bubble” or “Swiss cheese–like” interior favors radiation necrosis, as does hypointensity on diffusion weighted imaging (DWI) ( Fig. 21.1A–C ). Unfortunately, these features are neither sufficiently sensitive nor specific. Other examples display heterogeneously or ring-enhancing masses that are indistinguishable from high-grade glioma ( Fig. 21.1D ). Newer radiologic modalities, such as perfusion MRI, positron emission tomography (PET), and magnetic resonance spectroscopy (MRS), have further enhanced the clinical distinction, although false positives and negatives remain fairly common and no single technique is entirely reliable. Therefore, tissue diagnosis remains the gold standard and a subset of such cases will undergo repeat surgery to resolve this essential question.
Depending on the relative proportions of necrosis, hemorrhage, edema, and gliosis, pathologic areas grossly appear as either firm, ill-defined, glioma-like masses or as soft, friable, infarct-like lesions with cystic degeneration ( Fig. 21.2A ). Gray-white matter junctions may be blurred. Necrotic foci appear yellow to tan-brown, with foci of dystrophic calcification being white and chalky. If there are superimposed foci of hemorrhage, they may have a variegated appearance, similar to that of glioblastoma.
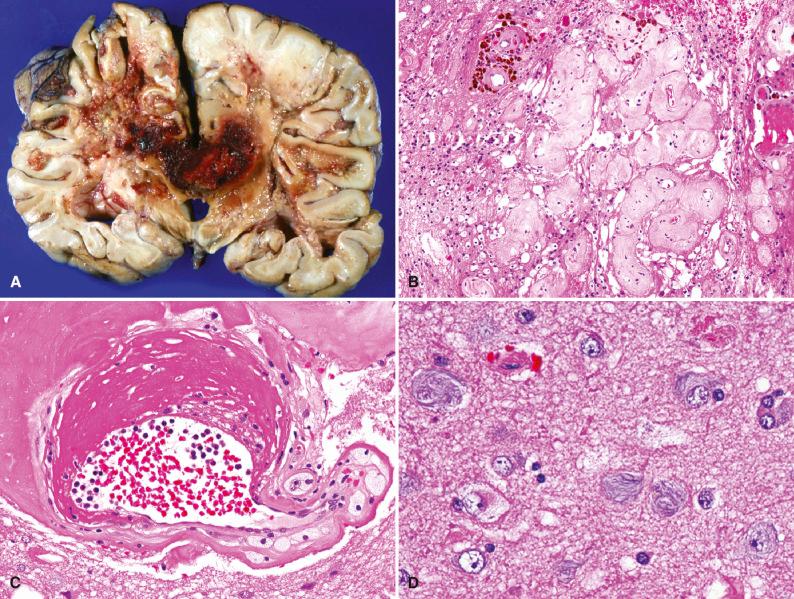
Although it is not always possible to distinguish radiation-induced from inherent tumor-associated pathology, there are several helpful histologic clues (see Table 21.1 ). Vascular changes are usually prominent and likely play an important pathogenic role in radiation damage. As one of the few actively proliferating cell types in the brain, the endothelial cell is particularly sensitive. Classic changes of late delayed radiation injury include coagulative and/or fibrinoid necrosis, thrombosis, hemorrhage, capillary telangiectasias, vascular hyalinization with luminal stenosis, and fibrinoid vascular necrosis ( Fig. 21.2B and C ), all of which facilitate hypoxic injury, myelin loss, and parenchymal necrosis. Dystrophic calcifications and histiocytic infiltrates are also common. Occasionally, neurodegenerative changes in the adjacent cortex ( Fig. 21.2D ) or vascular malformation-like aggregates of dilated blood vessels and endothelial proliferations are seen, which may be indistinguishable from sporadic cavernous angiomas and Masson tumors.
In general, the radiation effects from newer modalities (e.g., stereotactic radiosurgery) are essentially identical to those of conventional external beam therapy, although there is less experience with such post-therapy biopsies. The pathologic changes are often those of conventional radiotherapy, although the severity is exaggerated. Foci of almost amorphous, paucicellular, congealed fibrin-rich tissue are common (tissue looks “fried”). Viable tumor may be scant or totally absent and the peritumoral brain is often highly gliotic, sometimes with considerable radiation-induced atypia.
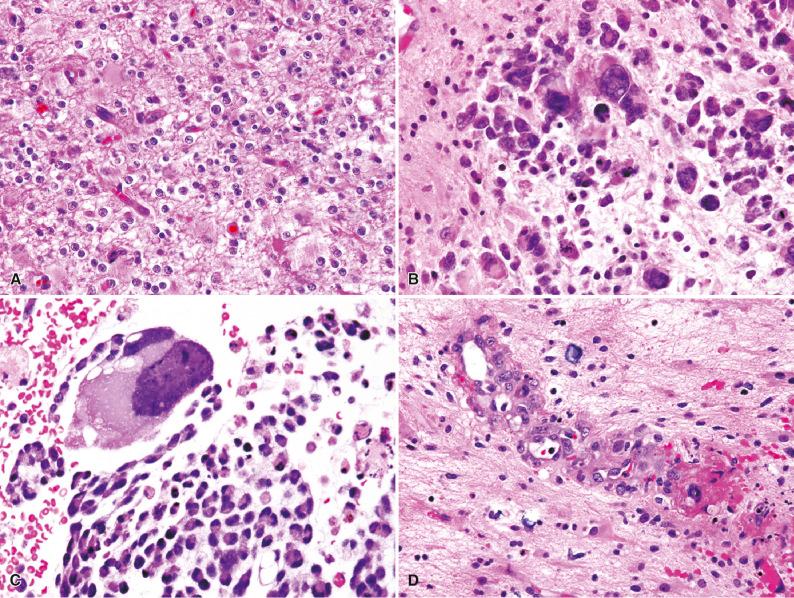
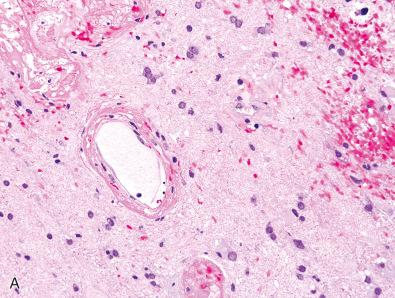
Radiation-induced cytologic atypia is common and similar to other parts of the body, involving tumor cells, endothelial cells, and adjacent brain parenchymal cells, such as reactive astrocytes and even neurons. Such changes pose substantial diagnostic challenges in terms of glioma classification and grading. For example, because radiation atypia is associated with both increased nuclear pleomorphism and the accumulation of eosinophilic cytoplasm, oligodendroglial neoplasms may artifactually appear more “astrocytoma-like” after therapy ( Fig. 21.3 ). In general, scattered cytomegalic cells with bizarre bubbly nuclei are more likely to be radiation induced, particularly if they are seen in hypocellular regions. Nevertheless, it is sometimes impossible to distinguish radiation-induced from innate tumor atypia. Furthermore, when this increased pleomorphism is coupled with radiation necrosis and telangiectatic vessels that may resemble the microvascular proliferation of glioblastoma, a previously radiated low-grade glioma can easily be overgraded, if the radiation changes are not discounted. Therefore, one should use strict diagnostic criteria for endothelial hyperplasia (multilayering of hypertrophic-appearing cells) and spontaneous tumor necrosis (e.g., palisading pattern) before increasing the grade of a previously irradiated glioma. In contrast, increased mitotic and proliferative indices are not typical of radiation damage and therefore represent more reliable features of tumor progression. As stated earlier, most post-therapy biopsies show some mixture of both viable tumor and radiation necrosis/radiation effects. In some examples where the latter predominates, one may be surprised by the presence of scattered viable tumor cells on special stains (e.g., IDH1 R132H), some of which may even resemble reactive astrocytes, in otherwise hypocellular “non-neoplastic-appearing” parenchyma ( Fig. 21.4 ). However, it remains unclear whether these scattered tumor cells are prognostic in the absence of a more cellular, mitotically active tumor.
Not applicable.
The most common differential diagnostic consideration involves recurrent glioma, as discussed earlier in the chapter in the sections “ Radiologic Features and Gross Pathology ” and “ Histopathology ” (see Table 21.1 as well). Rarely, vascular malformations or aneurysms enter into the differential diagnosis. Since these changes are also part of the spectrum of radiation vasculopathy, it may not be possible to distinguish the iatrogenic forms from their sporadic counterparts, although additional radiation changes in surrounding tissues favor a causal role for the radiation.
The vast majority of radiation-associated changes are recognizable on routine hematoxylin-eosin (H & E) sections. However, stains for Luxol fast blue–periodic acid–Schiff (LFB-PAS) and neurofilament protein may be useful for assessing the degree of white matter myelin loss and foci of necrosis, respectively. A Ki-67 stain can also be helpful for estimating the proliferative activity of residual/recurrent tumor cells, although it's important to note that labeling indices are often lower in recurrent glioblastomas than in the original tumor, likely reflecting the increased susceptibility of proliferating cells to radiation. Additionally, mutation-associated immunostains can be particularly useful for distinguishing neoplastic (e.g., IDH1 R132H, H3 K27M, or BRAF V600E positive) from non-neoplastic cells when a prior genetic alteration is already known (see Fig. 21.4 ). A slightly less specific, but sometimes useful immunostain is MAP2, since this neuronal marker is also expressed in most diffuse gliomas, but not in reactive astrocytes.
It is clear that some patients are more susceptible to radiation neurotoxicity than others, although in most cases, the predisposing factors are unknown or considered idiosyncratic. As mentioned previously, those who already suffer from small vessel disease are probably more susceptible to radiation damage, including disorders such as hypertension and diabetes, which have their own complex genetic factors. However, a few other familial conditions are also recognized. For example, NF1 patients are at greater risk for developing postirradiation strokes and other vasculopathies, such as moyamoya syndrome. Patients with ataxia-telangiectasia and other rare genomic repair syndromes are extremely sensitive to the damaging effects of ionizing radiation (and chemotherapy).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here