Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Studies of Mellanby, McCollum, Steenbock, Windaus, and others earlier in the 20th century resulted in the discovery and characterization of an important new bioactive substance termed vitamin D. Although this vitamin was found to be produced in the skin following exposure to sunlight, studies that rapidly followed indicated that the compound undergoes sequential hydroxylations in the liver to 25-hydroxyvitamin D 3 and then in the kidney to 1,25-dihydroxyvitamin D 3 (1,25(OH) 2 D 3 ), the biologically active form of the original vitamin. The discovery that its mode of action involves the regulation of gene expression led to the suggestion that 1,25(OH) 2 D 3 might represent a novel steroid hormone rather that a true vitamin. This hypothesis proved to be correct as a result of extensive mechanistic studies that were carried out during the ensuing decades.
It is now known that 1,25(OH) 2 D 3 operates in target tissues such as the intestinal tract, kidney, and bone through its capacity to activate a nuclear receptor/transcription factor termed the vitamin D receptor (VDR). This regulatory protein functions to modulate the expression of specific genes whose products are responsible for maintaining calcium and phosphorus homeostasis. This factor is also known to regulate many additional biological processes. Regulation is enabled through direct interaction of the 1,25(OH) 2 D 3 -bound VDR with specific DNA sequence elements (VDREs) located within individual, cell-specific genetic loci. The localization of the VDR at these sites provides a nucleation center for the recruitment of additional sets of coregulatory complexes that are essential for the diverse genetic and epigenetic processes that are ultimately required to modify gene expression. Common features of this mechanism are utilized by all the steroidal and lipophilic hormones.
1,25(OH) 2 D 3 functions to integrate the calcium and phosphorus regulating actions of intestine, kidney, and bone such that blood calcium and to a lesser extent blood phosphorus levels are maintained within tight limits. An elevation in circulating 1,25(OH) 2 D 3 levels accelerates the absorption of calcium and phosphorus by the intestine, the reabsorption of filtered calcium at the kidney, and under certain conditions mobilization of both calcium and phosphorus from the skeleton. 1,25(OH) 2 D 3 is produced in response to parathyroid hormone (PTH), a peptide whose elaboration from the parathyroid gland is exquisitely sensitive to blood calcium levels. Decreasing blood calcium triggers increased parathyroid gland production of PTH, which causes increased synthesis and elaboration of 1,25(OH) 2 D 3 and its subsequent actions on the three tissues responsible for elevating blood calcium and phosphorus levels. Importantly, elevated phosphate levels prompt the secretion of a second recently discovered hormone termed fibroblast growth factor 23 (FGF23), a bone cell-derived phosphaturic hormone, which acts in the kidney to increase phosphate diuresis. Other hormones are also involved in mineral metabolism, although to a lesser extent.
In this chapter, we provide an historical overview of the vitamin D endocrine system. We then provide a contemporary view of how the vitamin D hormone functions to modulate gene expression in the intestine, kidney, and bone. We also provide a focus on genes that are directly involved in intestinal calcium transport across the intestinal epithelium and describe the molecular mechanisms through which these genes are regulated by 1,25(OH) 2 D 3 . As will be evident, our understanding of these mechanisms has been greatly enhanced through the discovery and implementation of powerful new approaches for the study of gene expression which have also enabled an examination of epigenetic contributions linked to the expression of genes. In the final section, we discuss briefly newer discoveries of the actions of vitamin D and/or its receptor in the intestinal tract, including the novel activation of the VDR by lithocholic acid (LC) in the colon, the unexpected role of the VDR as an inducer of enzymes responsible for the detoxification of secondary fecal bile acids, and the potential impact of the vitamin D hormone on the microbiome.
Following the initial discovery of vitamin D, extensive work over the ensuing half century has revealed that vitamin D is produced, activated through hydroxylation, and then degraded by very specific metabolic processes. Indeed, vitamin D is synthesized in the skin following exposure to sunlight through a process that involves initial photolysis of cutaneous 7-dehydrocholesterol (provitamin D) to previtamin D followed by rapid isomerization to authentic vitamin D. Importantly, vitamin D was then discovered to undergo further metabolism, first in the liver to 25-hydroxyvitamin D 3 (25OHD 3 ) and then in the kidney to 1,25(OH) 2 D 3 . The enzymes responsible for these conversions are cytochrome p 450-containing mixed-function oxidases. Several hydroxylases have been identified that can carry out 25-hydroxylation of vitamin D, including mitochondrial CYP2R1, as well as microsomal CYP27A1, CYP2D11, CYP2D25, CYP2J2/3, and CYP3A4. CYP2R1, a 25-hydroxylase (25-OHase) recently discovered in the liver by Cheng et al. demonstrates highest affinity for vitamin D and is likely to be the most important of the 25-OHases. By far, the most critical hydroxylation of vitamin D that results in the synthesis of 1,25(OH) 2 D 3 , however, occurs in the kidney through the actions of mitochondrial CYP27B1 ( Fig. 51.1 ). First discovered by Fraser and Kodicek in 1970, this enzyme was cloned by St. Arnaud and coworkers as well as others and shown to be solely responsible for the synthesis of 1,25(OH) 2 D 3 . Importantly, genetic mutations in both CYP2R1 and CYP27B1 are associated with human disease, the former with a unique and perhaps rare form of hereditary rickets, the latter with the syndrome of vitamin D resistant rickets, type 1 (VDDR-1). The biochemical and skeletal phenotype of this latter syndrome has been recapitulated recently through genetic deletion of the Cyp27b1 gene in mice using homologous recombination. Recent studies of the deletion of Cyp2r1 in mice led to a significant reduction in circulating 25(OH)D 3 levels, but leave open the likelihood that other 25-hydroxylases may also contribute.
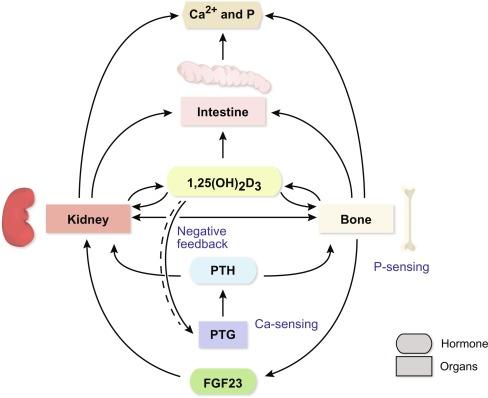
The activity of renal CYP27B1 is critical to the production and maintenance of physiologic levels of circulating 1,25(OH) 2 D 3 . As a consequence, the synthesis and activity of CYP27B1 are tightly regulated through factors that are elaborated in response to changes in blood calcium and/or phosphorus ( Fig. 51.1 ). Most notable is PTH, which is produced in the parathyroid glands in response to hypocalcemia, and which acts directly on the kidney to stimulate CYP27B1 gene expression. CYP27B1 is also modulated independently through phosphate signaling, although the mechanism through which this regulation is carried out has yet to be fully understood. Recent studies suggest that FGF23 also plays a key role in the modulation of CYP27B1 expression. FGF23 represents the long sought after hormone phosphatonin (or a member thereof) that is induced by 1,25(OH) 2 D 3 and is the major mediator of phosphorus homeostasis. Linkage of FGF23 to the regulation of phosphate was initially derived from phenotypes associated with tumor-induced osteomalacia (TIO), autosomal dominant hypophosphatemic rickets (ADHRs), and X-linked hypophosphatemic (XLH) syndromes. The FGF23 gene has been deleted and overexpressed and the emerging phenotypes strongly support this linkage. The molecular mechanisms and signaling pathways whereby PTH and FGF23 suppresses the expression of renal CYP27B1 also remain obscure, although new approaches are seeking to delineate details of this regulation. Importantly, completion of the vitamin D endocrine circuit involves a potent feedback mechanism through which 1,25(OH) 2 D 3 acts to suppress CYP27B1 expression in the kidney, to downregulate PTH expression and production by the parathyroid gland and, as stated earlier, to upregulate FGF23. A number of additional factors also regulate CYP27B1 including the sex and adrenal hormones, prolactin, and growth hormone.
With respect to catabolism, 1,25(OH) 2 D 3 is degraded through the primary actions of CYP24A1, a mitochondrial enzyme also found in the kidney and in virtually all vitamin D target tissues. As perhaps expected, its expression is strongly induced by 1,25(OH) 2 D 3 itself. The degradative pathway involves a third hydroxylation of 1,25(OH) 2 D 3 at carbon 24 to 1,24,25-trihydroxyvitamin D 3 (1,24,25(OH) 3 D 3 ) followed by multistep destruction of the vitamin D side chain to calcitroic acid. Hydroxylation at C-23 also occurs as well. 25OHD 3 is also hydroxylated by CYP24A1 leading to high circulating levels of 24,25-dihydroxyvitamin D 3 (24,25(OH) 2 D 3 ). Thus, CYP24A1 regulates not only 1,25(OH) 2 D 3 catabolism in all tissues but also controls the renal production of other metabolites including 25OHD 3 , 24,25(OH) 2 D 3 , and 1,25(OH) 2 D 3 , and their concentrations in the blood. A biological role for 24,25(OH) 2 D 3 has been hypothesized for many years, although little in vivo evidence exists to support this view. St. Arnaud and coworkers deleted the Cyp24a1 gene in the mouse, an action that resulted in hypercalcemia, hypercalciuria, renal calcification, and skeletal abnormalities; all of these effects were subsequently attributed to toxic circulating levels of 1,25(OH) 2 D 3 , which coexist as a result of Cyp24a1 deletion. As indicated above, one of the fundamental actions of 1,25(OH) 2 D 3 in all target cells is to stimulate the expression of CYP24A1 , thus initiating the means to its own self destruction. Interestingly, while the mechanism through which 1,25(OH) 2 D 3 induces CYP24A1 transcription was believed to be well understood at the molecular level, more recent studies using unbiased approaches have revealed a much more complex mode of activation by the vitamin D hormone through coregulatory regions located distal to the gene’s transcriptional start site (TSS). This regulatory mechanism represents a paradigm for the current view of how most genes are believed to be regulated by transcriptional activators.
Calcium and phosphorus homeostasis is maintained through the activity of the intestinal tract, kidney, and bone ( Fig. 51.1 ). These tissues serve to acquire mineral from the diet, to conserve mineral from glomerular filtrate, and to provide an immediately available source of skeletal mineral when the diet is deficient in calcium and/or phosphorus. Integrating the actions of these tissues so that serum calcium and phosphorus levels are maintained within tight limits and at supersaturating concentrations relative to mineralized bone is the central function of 1,25(OH) 2 D 3 . The actions of 1,25(OH) 2 D 3 in the intestine are therefore focused upon stimulating the production of proteins essential to the processes of dietary calcium and phosphorus absorption. 1,25(OH) 2 D 3 , particularly at aberrantly high concentrations, can also provoke calcium and phosphorus mobilization from the skeleton through a process involving both stimulation of bone-resorbing osteoclast activity as well as the induction of new osteoclast formation from cellular precursors. Interestingly, this mechanism involves the ability of 1,25(OH) 2 D 3 to induce expression of the autocrine TNF-like factor receptor activator of NF-κB Ligand (RANKL) from osteoblasts and osteocytes. This factor acts in turn on both osteoclast precursors and fully mature osteoclasts. The mechanism has a particularly profound consequence when dietary levels of calcium are insufficient. Accordingly, a homeostatic attempt in mice and in humans is made to maintain serum calcium and phosphorus levels at the expense of bone, which leads to bone demineralization, a weakening of the structure of the skeleton and increased risk of bone fracture. Interestingly, recent studies suggest that osteocytes, mature osteoblasts that have become fully encased in bone mineral, may also function to resorb bone and could represent critical targets of vitamin D action as well. Indeed, it is the osteocyte that elaborates FGF23 in response to a variety of stimulants including the vitamin D hormone. Finally, 1,25(OH) 2 D 3 also acts on the kidney to increase the resorption of calcium. Although this action is modest, the actual amount of calcium recovered through reabsorption can be highly significant due to the large daily load of calcium that is filtered by the kidney.
Central to the orchestration of intestinal, kidney, and bone actions by 1,25(OH) 2 D 3 are the additional systems that monitor the content of calcium and phosphorus in the blood. In the case of calcium, the level of this ion is continually monitored by the calcium-sensing receptor (CsR) in the parathyroid gland, which responds when calcium levels drop by increasing parathyroid gland secretion of PTH. As noted earlier, increased PTH levels enhance the synthesis of 1,25(OH) 2 D 3 , stimulating, in turn, both calcium uptake at the intestine and calcium reabsorption by the kidney. The actions of 1,25(OH) 2 D 3 in conjunction with increased PTH on bone serve to promote bone mobilization, which also leads to an increase in blood levels of mineral. While 1,25(OH) 2 D 3 also acts to increase phosphate levels in the blood via actions on the intestine, both PTH and FGF23 act collectively on the kidney to modulate phosphate reabsorption. With regard to FGF23, a novel mechanism is now known whereby FGF23 promotes a redistribution of the phosphate transporters NaPi2a ( SLC34A1 ) and NaPi2c ( SLC34A3 ) such that proximal tubular reabsorption of phosphate is reduced. FGF23’s ability to reduce circulating levels of 1,25(OH) 2 D 3 through downregulation of the renal CYP27B1 gene also results in a reduction in intestinal phosphate uptake. The molecular mechanisms whereby 1,25(OH) 2 D 3 induces the uptake of calcium as well as phosphorus across the intestinal epithelium as well as its molecular actions in kidney and bone will be considered in subsequent sections.
The VDR was discovered first in the chick intestine in the early 1970s, and rapidly determined to be expressed in other tissues such as kidney, bone, and parathyroid glands shortly thereafter. It is now clear that this factor is expressed in selected cell types in almost all vertebrate tissues, the majority of which do not play roles in calcium and phosphorus homeostasis. The VDR is a protein of approximately 50 kDa that is able to bind 1,25(OH) 2 D 3 with very high affinity and selectivity, an activation process that leads to a complex set of downstream events. This largely nuclear-localized protein was discovered subsequently to bind directly to DNA through a domain separate from that which binds ligand. These functional properties of the VDR provided further support for the hypothesis that the actions of 1,25(OH) 2 D 3 occur at the level of transcription. Details of the overall process of ligand-activated, VDR-mediated gene regulation are depicted in the model in Fig. 51.2 and will be considered in detail in subsequent sections. In this model, the VDR is activated by 1,25(OH) 2 D 3 , although the secondary bile acid LCA appears able to modulate receptor activity in the colon as well ( Section 51.7 ). While much was accomplished following the discovery of the VDR, it is clear that the most important advance following its identification was the cloning of the chicken VDR gene in 1987 and the subsequent cloning of the human and rat homologues as well. As the overall structural similarities of the VDR with that of several other steroid receptors cloned during that period of time were strikingly evident, the cloning of the VDR provided the final evidence that 1,25(OH) 2 D 3 operated in a manner mechanistically similar to that of other steroidal ligands. These latter achievements opened the door for a myriad of additional studies that have revealed not only the molecular domain structure of the protein but also precise details of its functional capabilities.
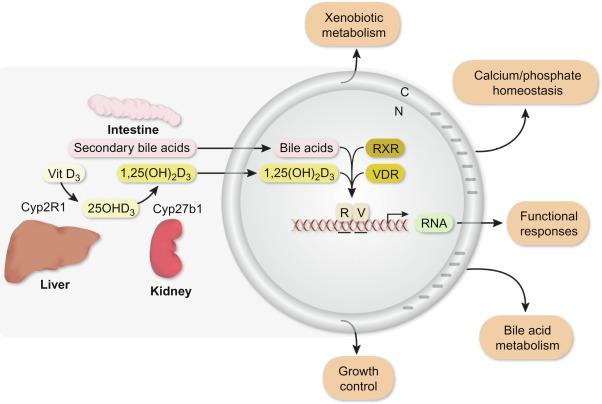
1,25(OH) 2 D 3 is known to regulate the expression of a number of genes, as summarized in the model in Fig. 51.2 , not only in intestine, kidney, and bone but also in other tissues. Perhaps its most ubiquitous regulatory target is CYP24A1 , which as indicated earlier functions negatively to downregulate cellular levels of 1,25(OH) 2 D 3 . In the intestine, 1,25(OH) 2 D 3 regulates the transcriptional output of the calcium-binding proteins (calbindins D9K and D28K), basolateral calcium-stimulated ATPases such as PMCA1b and TRPV6 (considered in more detail in a subsequent section). Proteins with analogous functions are also regulated by 1,25(OH) 2 D 3 in the kidney. In bone, however, 1,25(OH) 2 D 3 regulates a variety of additional genes whose products include the matrix proteins osteocalcin, osteopontin, and bone sialoprotein that are involved in bone osteoid formation and which control mineralization. 1,25(OH) 2 D 3 also regulates RANKL and OPG, two factors that as previously indicated participate in the bone resorbing activities of osteoclasts that are central to bone calcium and phosphorus mobilization. Investigation of each of these transcriptional targets at the molecular level has revealed the presence of specific DNA sequences or VDREs that represent high-affinity-binding sites for the VDR. In initial studies using traditional methodological approaches, many VDR-binding sites were found within several kilobases of the genes’ promoters. Further exploration of these VDREs has revealed adjacent binding sites for many additional regulatory proteins as well, suggesting that these regions are modular and likely mediate and integrate the actions of a variety of signaling pathways. VDREs are comprised largely, but not exclusively, of two directly repeated hexanucleotide half-sites of the consensus sequence AGGTCA that are separated by an identity-irrelevant three base pairs. Several additional configurations have been defined as well, although these sequences have yet to be fully validated via contemporary techniques. The response elements that mediate the actions of 1,25(OH) 2 D 3 in cis are similar, but not identical, to those for other nuclear receptors that utilize RXR as partner. Most striking is that while the arrangement of the half-sites and their sequences are retained, the degree of nucleotide spacing that exists between the two half-sites diverges. Thus, the diagnostic feature for a potential VDRE is not the sequence of the half-sites, but rather their three base pair separation. As will be seen in a subsequent section, evaluation of both the placement and structural organization of VDREs across thousands of VDR-binding sites identified through recent genome-wide analyses has confirmed the structural organization of these VDREs but not their most frequent locations relative to the genes they regulate.
1,25(OH) 2 D 3 is also known to suppress the expression of genes, among others, those that are targets of negative feedback regulation such as PTH and CYP27B1 . Early studies suggested that the sequences of the VDREs that mediate repression by 1,25(OH) 2 D 3 might be slightly different from those that mediate transactivation. Thus, small differences in the sequence of one or both of the half-sites might be capable of dictating suppression, suggesting that DNA sequence can act as a ligand and regulates VDR activity allosterically. A key example is that of the PTH gene. Additional studies, however, have revealed that repression likely occurs most frequently as a result of the ability of the VDR to displace or to prevent the binding of key transcription factors that are necessary for the maintenance of basal expression . Importantly, recent genome-wide studies suggest that the mechanisms of repression are not only cell type-specific but also highly diverse.
Accompanying the discovery of the first VDRE was the important finding that VDR binding to these specific DNA sequences was dependent upon an unknown nuclear factor. The identity of this protein was subsequently revealed when it was discovered that specific members of the steroid receptor family termed retinoid X receptors (RXRs) were capable of forming heterodimeric complexes with the VDR and other members of this class of steroid receptors. Indeed, one of the roles of 1,25(OH) 2 D 3 upon ligand activation of VDR is to increase this receptor’s affinity for its RXR partner such that cooperative, high-affinity DNA binding can be accomplished, as illustrated in Figs. 51.2 and 51.3 . The requirement of a VDR/RXR heterodimer for DNA binding differs from that of the true steroid receptors, which bind DNA as homodimers, but explains the structural need for two directly repeated DNA half-sites. Interestingly, it is known from both molecular as well as structural studies that the VDR binds to the upstream 5′ half-site of a typical VDRE, whereas RXR binds to the downstream 3′ half-site. This orientation is determined by unique domains within the two proteins that facilitate the formation of a dimer during the process of DNA binding. Whether this orientation is consistently maintained across many thousands of regulatory elements for the VDR/RXR heterodimer remains unknown, although protein-interaction studies of the complexes on DNA at the 3D level suggest that this structural orientation may be an essential requirement for high-affinity DNA binding.
Three different genes encode the RXRs, including RXRα, RXRβ, and RXRɤ. While all interact with the VDR in vitro, the precise hierarchy of interaction of the VDR with these related proteins that may occur in the same cell in vivo is not known. As indicated earlier, the RXRs also represent heterodimeric-binding partners for many other nuclear receptors, including those for retinoic acid, thyroid hormone, and peroxisome proliferators. Interestingly, RXR can be activated independently through several of its own ligands, which include 9- cis -retinoic acid and certain fatty acids. This feature of the RXRs both as an independent transactivator and as a common interacting component of heterologous nuclear receptors potentially links specific ligand-activated signaling systems together in cells where they are coexpressed depending upon the intracellular abundance of the RXRs. Early studies suggested that the role of RXR in VDR action was to enhance VDR-binding affinity at target VDREs. Indeed, this involvement has now been confirmed at hundreds of sites across both mouse and human genomes, as will be described in more detail in several sections to follow. These studies, however, have also revealed additional complexity in that while VDR DNA binding is largely dependent upon activation by 1,25(OH) 2 D 3 , DNA sites to which the VDR binds may already contain an RXR molecule, suggesting that the latter is capable of preoccupying VDR-binding sites for future activation. The molecular form of RXR (monomer, dimer, or heterodimer) bound to these sites is unknown. Finally, evidence also suggests that RXR may participate directly in the transcriptional activation process as well, although recent structural evidence suggests that only a single coregulatory factor is able to bind to the heterodimer when bound to DNA.
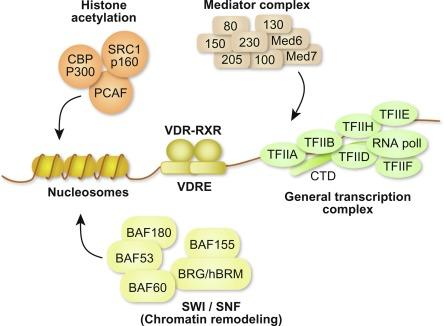
The association of the VDR/RXR heterodimer with specific DNA sequences, as with other nuclear receptor family members, initiates a series of processes essential to the modulation of transcriptional output at target genes. Although early studies suggested that the interaction of gene regulators such as the VDR with basal transcriptional machinery was sufficient to modulate gene output, it is now known that the process of transcriptional modulation is exceedingly complex and involves the receptor-dependent recruitment of multiple comodulatory complexes, each with unique function ( Fig. 51.3 ). Overcoming and/or restoring the inherent repressive state of chromatin requires the presence of regulatory machinery able to shift and/or displace nucleosomes (chromatin remodeling), alter the condensation state and thus the architecture of chromatin (histone modifications), and/or facilitate the entry of RNA polymerase II (RNA pol II) at appropriate sites. At least three complexes are believed to accomplish these specific actions. The first are the vertebrate ATPase-containing homologues of the yeast SWI/SNF complex that utilize the energy of ATP to remodel and reposition nucleosomes. This action increases the availability of binding sites for additional transactivators and facilitates the changes necessary for transcription to proceed. The second are complexes that contain either acetyl- or methyltransferases, deacetyltransferases (HDAC), or demethylases each of which function to modify the lysine or arginine containing tails of histone three and/or histone four. Certain methyltransferases place unique covalent methylation marks on specific histones highlighting TSSs (promoters) at potentially regulated genes across the genome; others are known to install marks that represent the covalent signature function of key regulatory regions of genes. Interestingly, these enzymatic activities link the genomic processes to cellular energy metabolism. Demethylases remove these marks, instilling a dynamic nature to these regulatory marks. These processes comprise part of complex, location-dependent epigenetic layers that are present at specific histones that functions to coordinate and mediate the expression of gene networks across the genome that are essential to the differentiation of cells and to the development and maintenance of their unique functional phenotypes. Alterations in the presence of these marks are able either to provoke or to emerge as a consequence of disease. Acetyltransferases and their corresponding HDACs covalently modify histones through acetylation marks as well. The marking of histones H3 and H4 through acetylation promotes chromatin decondensation, thus facilitating transcription factor access and the modulation of genes. HDACs, on the other hand, remove these marks thereby limiting activation potential. These activities are coordinated by coregulatory factors such as the p160 family SRC-1, SRC-2, and SRC-3 as well as CBP/p300, the corepressors SMRT and NCoR as well as one of several HDACs. A third complex is Mediator, believed to facilitate the entry of RNA pol II and perhaps to play a role in transcriptional reinitiation and other processes. Many additional complexes are known to interact with nuclear receptors as well; their precise roles in transcriptional modulation are currently being defined. Importantly, activation of the VDR by 1,25(OH) 2 D 3 enables the VDR/RXR heterodimer to interact with both the above coregulators and others.
The previous sections have defined a role for 1,25(OH) 2 D 3 in promoting both RXR heterodimer formation and the recruitment of cofactors at target genes. Indeed, it is now clear from molecular as well as structural studies that the potentiation of these interactions by 1,25(OH) 2 D 3 is due to the ability of the ligand to induce changes in VDR conformation. Conformational changes in the VDR are likely induced upon entry of 1,25(OH) 2 D 3 into the carboxy-terminal ligand-binding pocket, where it is stabilized by direct contacts with specific VDR amino acids. These conformational changes induced by 1,25(OH) 2 D 3 result in a rearrangement of several of the 12 alpha helices, which create both a contact heterodimer surface for RXR and a binding site for the coregulators, the latter arising from an interaction between helices 3, 4, and 12. A molecular view of this relationship has been acquired recently through cocrystallization of the rat VDR ligand-binding domain (LBD) with a peptide sequence that mimics the coactivator-interacting domain of MED1. This sequence conforms to the leucine-charged receptor interacting region of most coregulators and is comprised of a motif whose central core contains the amino acid sequence LXXLL. The motif LXXXIXXX I/L found in corepressors also binds to the same region of the VDR. Most importantly, however, recent studies using cryo-EM have now been successful in providing a three-dimensional view of the VDR bound to its cognate DNA sequence element while paired to its heterodimer partner RXR. This successful 3D image and its comparison to similar structures of RAR and PPAR provide new insight into the structural specificities of these three mechanistically related nuclear receptors.
Despite delineation of the mechanism of action described above, unequivocal evidence for the central role of the VDR as the comprehensive mediator of the actions of 1,25(OH) 2 D 3 was derived from numerous experiments of nature. Early clinical studies defined a rare human syndrome termed hereditary 1,25(OH) 2 D 3 -resistant rickets or HVDRR that is characterized by hypocalcemia, hypophosphatemia, severe skeletal and dentitial deformities and, in a portion of these cases, alopecia as well. Additional features include reduced circulating levels of 24,25(OH) 2 D 3 and perhaps most strikingly, extremely high blood levels of 1,25(OH) 2 D 3 . Patients are generally unresponsive to treatment with 1,25(OH) 2 D 3 although the infusion of calcium and phosphorus can lead to normalization of calcium and phosphorus levels in the blood and improve the skeletal physiology. Fibroblastic cells derived from these affected individuals appear to recapitulate the transcriptional regulating capabilities of 1,25(OH) 2 D 3 (or lack thereof). It was subsequently shown that this syndrome is derived from genetic mutations in the VDR gene, first in the exons encoding the DNA-binding domain that prevent association with VDREs at target genes and later in exons that encode domains affecting protein stability, RXR heterodimerization, coregulator complex formation, or that lead to specific alterations in the VDR protein itself. Interestingly, only mutations that lead to the deletion of specific regions of the receptor or of the VDR protein in its entirety result in the alopecic phenotype. This observation, coupled with additional research in mice, revealed that an intact VDR molecule was essential for maintenance of the hair cycle but that this activity did not require activation through 1,25(OH) 2 D 3 . Many of the features of HVDRR, including those at the skeleton, have now been recapitulated through the successful preparation of mice nullizygous for the VDR alleles. Not surprisingly, these mutant mice all retain alopecia. To address this issue, recent studies have explored the expression of wildtype and mutant forms of the human VDR from large, comprehensive transgenes that contain all of the regulatory regions of the human gene as well. Importantly, when placed in a VDR-null mouse background, the wildtype gene is able to fully rescue the diverse VDR-null phenotype. In contrast, however, a 1,25(OH) 2 D 3 -resistant mutant VDR unable to bind the hormone is capable of rescuing the alopecia but not the skeletal deformities that arise due to the receptor’s inability to mediate the pervasive and essential transcriptional activities that are dependent upon 1,25(OH) 2 D 3 . These genetic strains of mice have proven useful in clarifying both the skeletal actions of 1,25(OH) 2 D 3 as well as new physiologic possibilities not previously recognized.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here