Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Prior to the advent of robotics, a consensus panel of expert urologic surgeons judged laparoscopic radical prostatectomy to be “extremely difficult,” and the highest level of urologic surgical complexity in their ranking system. The robotic approach has certainly facilitated the performance and dissemination of laparoscopic radical prostatectomy throughout the United States. Once the transperitoneal approach to the prostate was perfected laparoscopically, some of the initial pioneers developed a totally extraperitoneal approach to minimally invasive radical prostatectomy. It was just a matter of time until robot-assisted radical prostatectomy was performed extraperitoneally, a development facilitated by the smaller, second-generation robotic platform, the Da Vinci S™. The more concise arms of robotic devices marketed allow less clashing in relatively more confining space of Retzius, while sitting at the remote console decreases the physical demands of the laparoscopic approach on the surgeon.
The concept is to mimic open radical retropubic prostatectomy as far as possible, and to use the advantages of robotic instrumentation and pneumoperitoneum to minimize blood loss and facilitate careful dissection. Compared to open radical retropubic prostatectomy, extraperitoneal robotic radical prostatectomy utilizes essentially the same steps, albeit in a slightly different order. After all, both procedures are performed in the retropubic or extraperitoneal space of Retzius and with the same goal: complete prostate extirpation, nerve sparing as indicated, and in many cases, bilateral pelvic lymphadenectomy.
The patient is placed supine on a butterfly beanbag specifically made for robotic pelvic surgery that is secured to the operating table. Legs are placed in Allen stirrups and spread apart and then lowered slightly with flexion at the hip joints. Arms are carefully tucked and secured at the sides and held in place by the beanbag, as are the shoulders. After prepping and draping, a Foley catheter is placed sterilely in order to decompress the bladder.
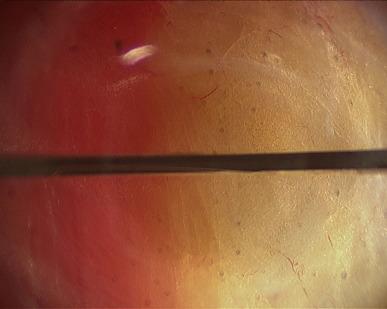
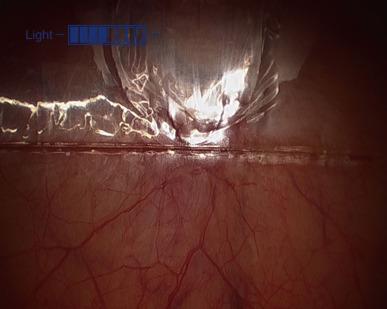
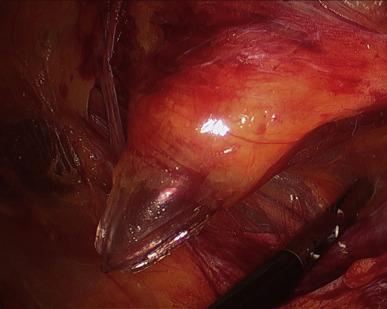
Rather than using Veress needle insufflations, the retropubic space is best entered through a 12 mm infraumbilical skin incision, in similar fashion as for total extraperitoneal (TEP) inguinal hernia repair. Through this incision the anterior rectus fascia is identified and incised. Our preference is to incise directly with a Visiport angled 60–80° to the vertical, aiming slightly caudally, though others prefer the open cut down Hasson technique (note that the Visiport does not accommodate the standard 12 mm robotic camera, but only a laparoscopic 10 mm or robotic 8 mm camera). At this point the Visiport is gently advanced between the rectus muscle bellies, generally to the left of the linea alba ( Figure 38.1 ) and is then slid over the posterior sheath toward the arcuate line at a more acute angle (30° to the skin) aiming caudally and into the space of Retzius. The arcuate line is passed over as the horizontal fibers of the posterior sheath give way to transversalis fascia, and then the Visiport is pushed left and right in a fan-shaped manner to gently open up the retropubic potential space for subsequent balloon dilatation. The Visiport is removed and a kidney-shaped laparoscopic balloon (PDBS2 AutoSuture™, Covidien, Mansfield, MA) is blindly inserted into the infraumbilical incision just past the incised anterior rectus fascia and angled southward into the space of Retzius in front of the bladder and behind the pubis. At this point the balloon is manually insufflated and the expanding balloon is visualized through the port with the laparoscopic zero degree lens ( Figure 38.2 ). The inferior epigastric artery and veins are easily identified superolaterally through the clear developing balloon as it pushes the parietal peritoneum away superiorly. Finally, the balloon is deflated and removed, and the Visiport trocar is reinserted into the space and insufflation is started. The whole case can be performed with pressures ranging from 12 mmHg to 20 mmHg; it is always safest to use the lowest possible insufflation pressure to maintain the space. If the space is intact, it should appear concave, football shaped, and have flimsy areolar tissue within it overlying the structures of the deep pelvis.
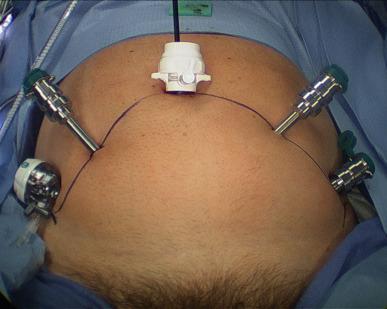
At this point the remaining ports are placed, all with blunt trocars, and essentially in a fan-shaped position around the pubic midline. Reusable metal robotic ports (3 × 8 mm) are used, as well as a 12 mm disposable assistant port (Ethicon Xcel for example). The configuration is as in Figure 38.3 , with ports angled caudally about 45 – 70° from the vertical and generally placed along the line from the anterior superior iliac spine (ASIS) to the umbilical incision, with a minimum of 8 cm between robotic ports. The first port is 8–9 cm left lateral to the umbilical port along the line to the left ASIS; then that is used to insert a blunt alligator grasper, which can facilitate dissection of the peritoneum cranially and posterior for right lateralmost port placement 2 cm superior and 2 cm medial to the right ASIS ( Figure 38.4 ). This port gets tunneled subcutaneously until it reaches the line between the right ASIS and the umbilical port. It then gets pushed in deeper and into the true pelvis gliding over the spermatic cord and lateral to the peritoneal reflection, which is often noted here ( Figure 38.5 ). Finally, the right medial 8 mm robotic trocar is placed 8–9 cm to the right of the umbilical port along the line toward the right ASIS, and then the left-most 8 mm robotic port for the fourth robotic arm is placed near the left ASIS but again some 2 cm superior and medial to it, then tunneled subcutaneously toward the left ASIS–umbilical port line, before passing into the pelvis and over the spermatic cord. It is crucial to angle all of the ports approximately 45–70° caudally on insertion (e.g., toward the legs) in order to avoid the peritoneal space, which is often visible at the time of port placement, and to watch the trocars go in under direct vision.
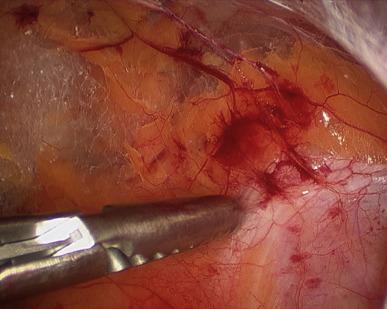
The patient is then placed in modest Trendelenburg (full Trendelenburg is rarely needed) and the space is analyzed. A peritoneotomy would be noticed at this point as the space would appear small and its superoposterior wall (peritoneum) would be billowing into the field. This relatively rare occurrence (about 1/20 cases in experienced hands) need not change one’s operative plan. A small/microscopic peritoneotomy, which lets gas into the peritoneal cavity, can be managed by inserting a Veress needle into the abdomen for continuous venting of this space during the operation. Alternately, since the bladder has been “taken down” already, a small inadvertent peritoneotomy can purposely be enlarged on one or both sides to render the extra- and transperitoneal spaces contiguous, but still without losing some of the advantages of extraperitoneal surgery, such as the fact that the bladder is already taken down, and the veil of tissue composed of the urachus and medial umbilical ligaments maintains separation between the patient’s bowels and the pelvic space.
The Da Vinci robot is then moved into place between the legs. If the legs need to be spread a bit more or lowered to avoid clashing with the robot, this is easily accomplished by adjusting the Allen stirrups. Docking is done carefully and remembering to keep the ports angled even while docking to avoid inadvertent peritoneotomy. Robotic arm number three comes in left lateral-most and must be docked such that it does not clash with the stirrup, left knee, left foot, or pelvis. Then, under direct vision, the robotic instruments should be advanced into the retropubic space. Our preference is for a ProGrasp forcep in the port at far left (arm #3), a bipolar Maryland grasper in the more medial left robotic port (arm #2), a monopolar scissor in the right robotic port (arm #1), and a zero degree lens in the camera port. The 12 mm assistant port is used for a right-sided assistant and allows for retraction, suction, clip placement, and also specimen (lymph node packet(s)) extraction.
The extraperitoneal space at this point only needs to be expanded a bit in order to appear identical to the standard open retropubic space to which surgeons are accustomed. It is worthwhile to spend several minutes dissecting through areolar tissue until the entire pubis and midline symphysis are clearly identified. It then helps to de-fat the area as well until the endopelvic fasciae are visible ( Figures 38.6 and 38.7 ). The superficial dorsal vein is usually apparent as fat gets dissected off of the anterior prostate and it should be taken with electrocautery. The fat overlying it generally ramifies along with its proximal branching centrally and to the right and left, over the prostate and toward the bladder; with bipolar diathermy these branches can easily be taken and the fat and superficial dorsal venous complex removed to better expose the prostate. In recent years, we have been sending this fatty tissue for pathological analysis because a minority of patients (5–15%) will have periprostatic lymph node(s) contained within.
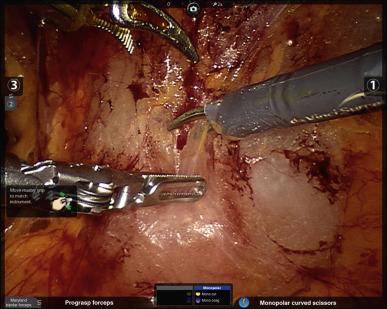
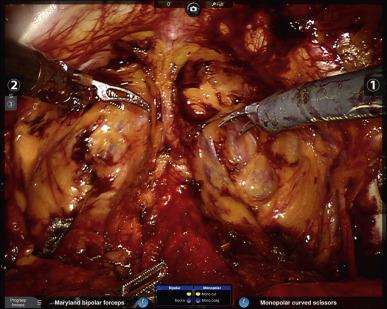
Flimsy tissues overlying the external iliac vessels and spermatic cords should then be incised to facilitate easy access to the pelvis for the assistant and fourth robotic arm. Any inguinal hernias or herniated fat that are in the way should be reduced. Finally, identification of important landmarks ensues: the puboprostatic ligaments and endopelvic fasciae for the prostatectomy, and the external iliac veins (EIV) and obturator nerves for men who will be receiving pelvic lymph node dissection (PLND). It is particularly important to make sure to assess the position of the right EIV in comparison to the right assistant port, and ideally to either advance that port past the vein or keep it quite superolateral to the vein such that instruments that are placed through the port do not risk traumatizing the vein.
We prefer to perform PLND prior to prostatectomy, though it certainly can be accomplished subsequent to the mobilization of the prostate. In either case, preparation for PLND involves identifying the EIV and obturator nerves. The former can usually be found, even if there is much fat overlying them, by looking for venous waveforms just above the pelvic brim. Once found, the fascia overlying the EIVs must be incised and the sidewall accessed under the veins. The assistant’s suction device can be placed in this space while the operator dissects the nodes off the EIV and looks for the obturator nerve. If not obviously under the EIV it can often be found by looking for the obturator foramen, which often has a fat plug in it. The nerve courses through the foramen and out of the pelvis by hugging the foramen superolaterally; once it is found the nodal packet under the EIV and above the obturator nerve should be clipped distally and handled with the ProGrasp for dissection as proximally as indicated. A standard PLND can certainly be performed in this space, taking all level I and II nodes, as the bifurcation of the iliac vessels is reachable (as in open radical prostatectomy, which as mentioned, is performed in the same space and with the same limitations). With the ProGrasp holding the nodes anteriorly, two arms remain for dissection, cautery, and retraction. In addition, the wrist of the arm on the side of the PLND can be used to hold back preperitoneal fat if it gets in the way, while still allowing the instrument to be used as a grasper/dissector. Extended PLND depending on the definition may or may not be able to be performed, as presacral nodes are not reachable without mobilizing the bladder more than can be done extraperitoneally; a 30° lens may be used to facilitate deeper and more medial dissections at the internal iliac artery(ies) and vein(s). In any case, we favor using Hem-0-Lok™ clips (Weck, Teleflex, Limerick, and PA) to secure lymphatic pedicles, rather than bipolar cautery or suction whenever possible. A harmonic scalpel is also a great tool for PLND and for sealing small lymphatics, but is certainly not necessary. The nodes are typically extracted after the dissection, through the 12 mm assistant port, though they can also be left in their location for later extraction with the prostate specimen. A drain should be placed at the conclusion of the case whenever PLND is performed, particularly when confined to the extraperitoneal space.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here