Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
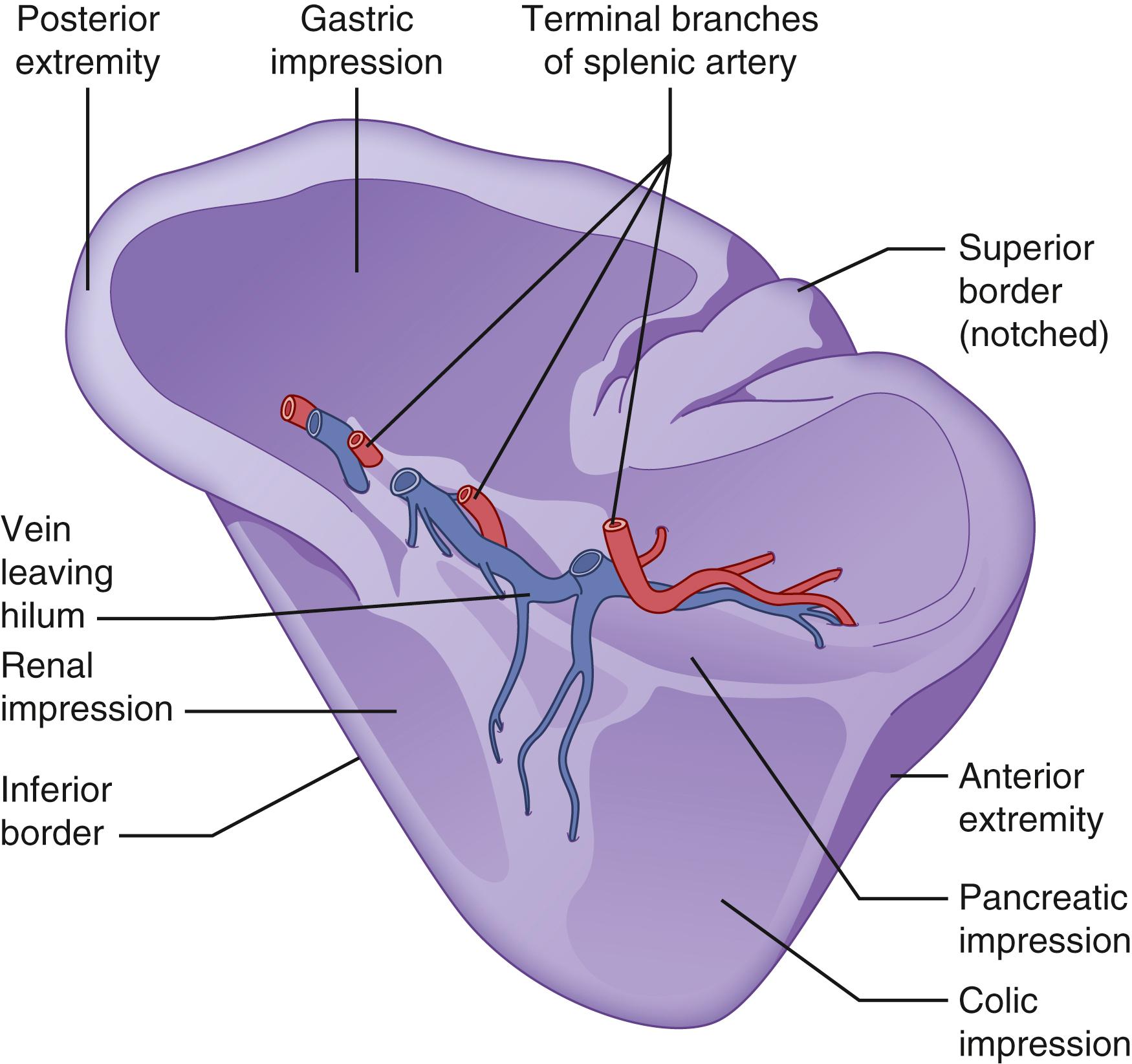
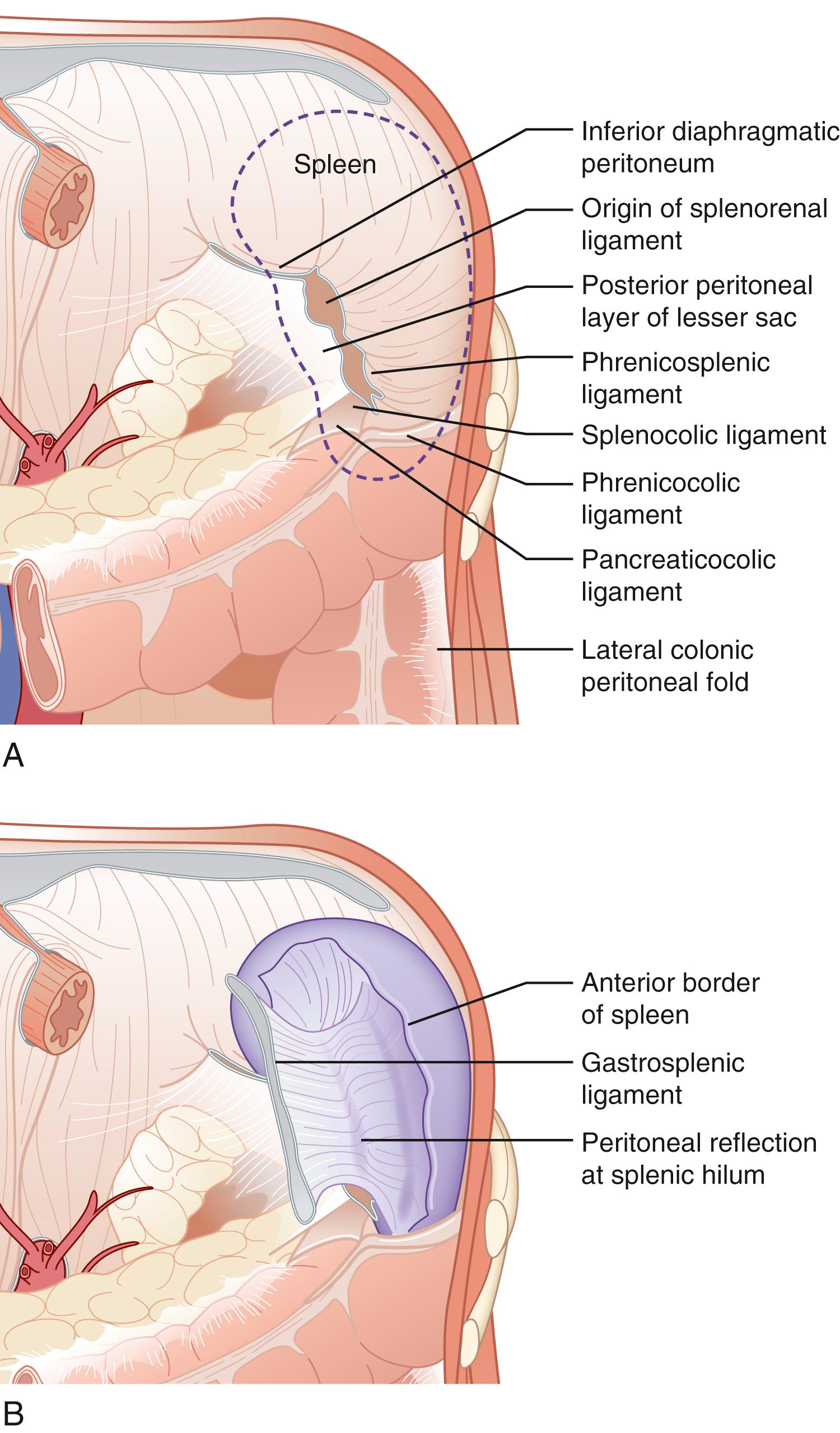
The spleen is the largest lymphoid tissue mass in the body; it measures 7 to 13 cm in length and weighs up to 250 g. The spleen develops from mesenchymal cells in the dorsal mesogastrium during week 5 of embryogenesis. It is initially adherent to dorsal pancreatic bud and ultimately separates from the pancreatic bud and settles into the left uppermost aspect of the abdomen in the intraperitoneal cavity. Anatomically, it comprises of two surfaces: the diaphragmatic surface and visceral surface. The diaphragmatic surface is roofed by the diaphragm, separating it from the pleura. However, the costodiaphragmatic recess extends to the inferiormost aspect of a normal-sized spleen. The spleen’s visceral surface is in close proximity to the greater curvature of the stomach, splenic flexure of the colon, apex of the left kidney, and tail of the pancreas ( Fig. 57.1 ). On topographic anatomy, it lies in the left lower thorax and it is normally protected by ribs 9, 10, and 11. In healthy adult individuals, it is not palpated below the costal margin. However, in infants it is palpated below the costal margin at the midaxillary line. This relationship is crucially important in trauma patients who present with fractured left lower ribs as risk of splenic injury is high. Spleen is an intraperitoneal organ and is suspended in the peritoneal cavity by multiple peritoneal reflections misreferred to as “ligaments” ( Fig. 57.2 ); at the diaphragmatic surface is the splenophrenic ligament, the visceral surface, and the gastrosplenic, splenorenal, and splenocolic ligaments. In patients without portal hypertension, the splenophrenic and splenocolic ligaments are relatively avascular. The gastrosplenic ligament carries the short gastric vessels in its superior aspect and the left gastroepiploic in its inferior aspect. The splenorenal ligament houses the splenic artery and vein as well as the tail of the pancreas. The tail of the pancreas abuts the splenic hilum in 30% of individuals and is within 1 cm of the hilum in 70% of cases, thus it is important to ligate the splenic vessels within 1 cm from the splenic hilum to avoid injury to the tail of the pancreas.
The splenic artery, a branch of the celiac trunk together with its branches of the short gastric arteries provide the arterial blood supply of the spleen. The splenic artery is a tortuous vessel that gives off multiple branches (16–18 branches) to the pancreas as it travels along its posterior aspect ( Fig. 57.3 ). Understanding the variant anatomy of the splenic artery helps surgical planning to avoid potential intraoperative bleeding. There are two common variations of the splenic artery with regards to the relation between the splenic artery branches and the hilum. The magistral type, which branches into terminal and polar arteries near the hilum of the spleen; and the distributed type, which, as the name implies, gives off its branches early and distant from the hilum. The magistral type of splenic arterial anatomy occurs in 30% of individuals compared with the distributed type (70%). The splenic artery branches to approximately five to six polar arteries and six short gastric arteries. The superior polar artery which sometimes communicates with the short gastric arteries, the superior, middle, and inferior terminal arteries, and an inferior polar artery. Knowing these variable distributions are necessary in performing resections, especially a spleen-preserving distal pancreatectomy, in an effort to preserve splenic function.
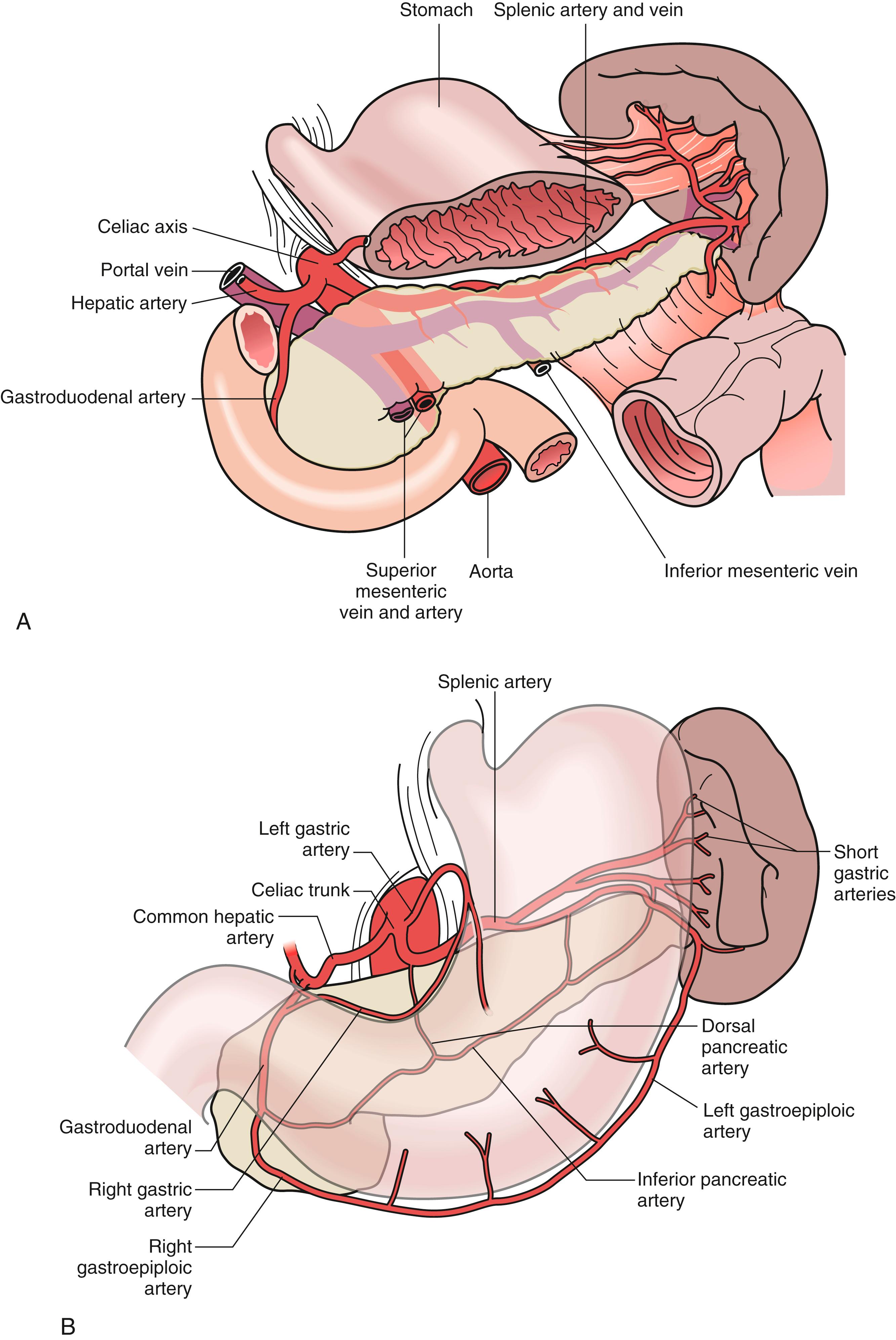
The splenic vein created by the union of several splenic veins and the left gastroepiploic vein then travels posteriorly to the pancreas, joining with pancreatic branches and often the inferior mesenteric vein to join the superior mesenteric vein to form the portal vein.
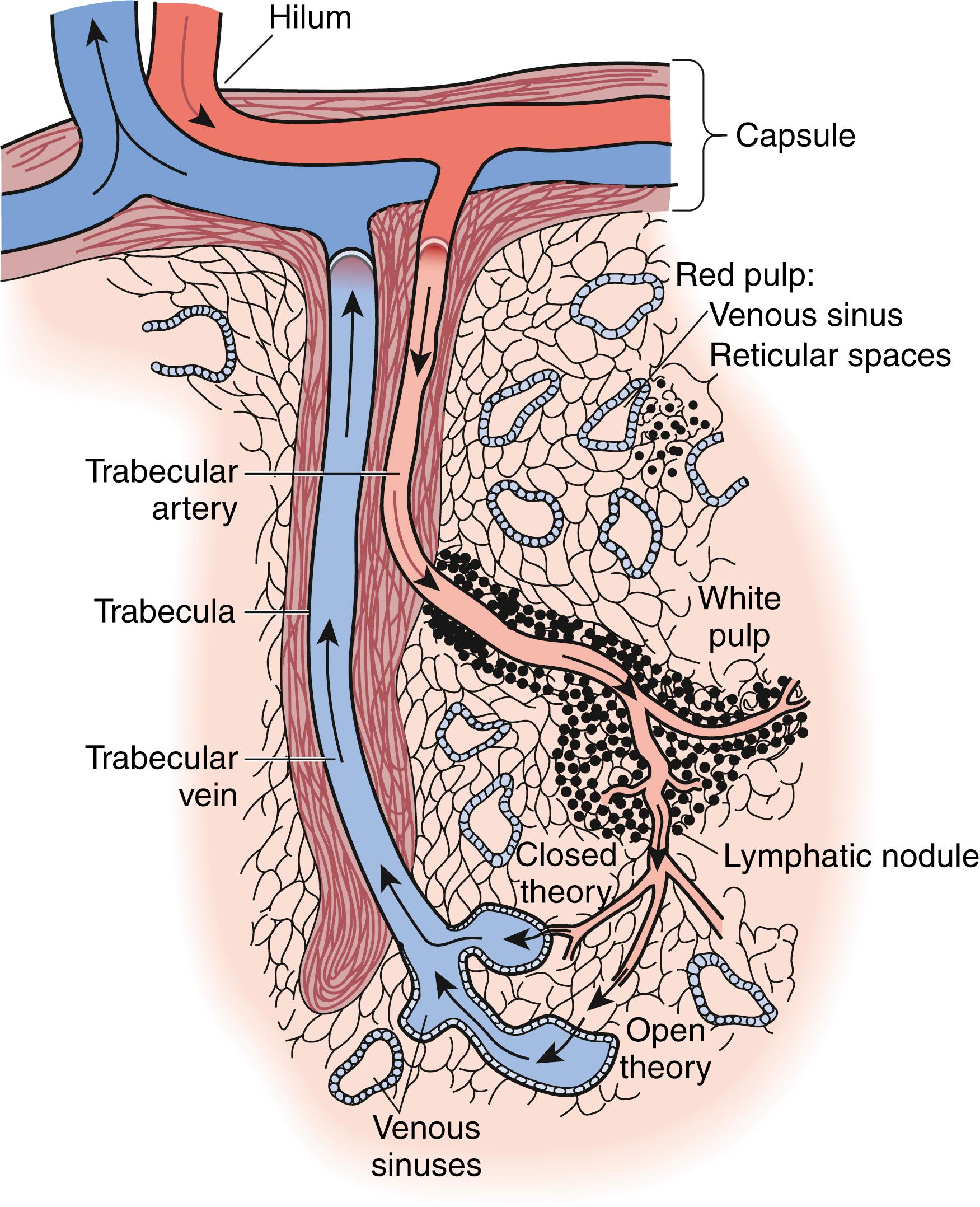
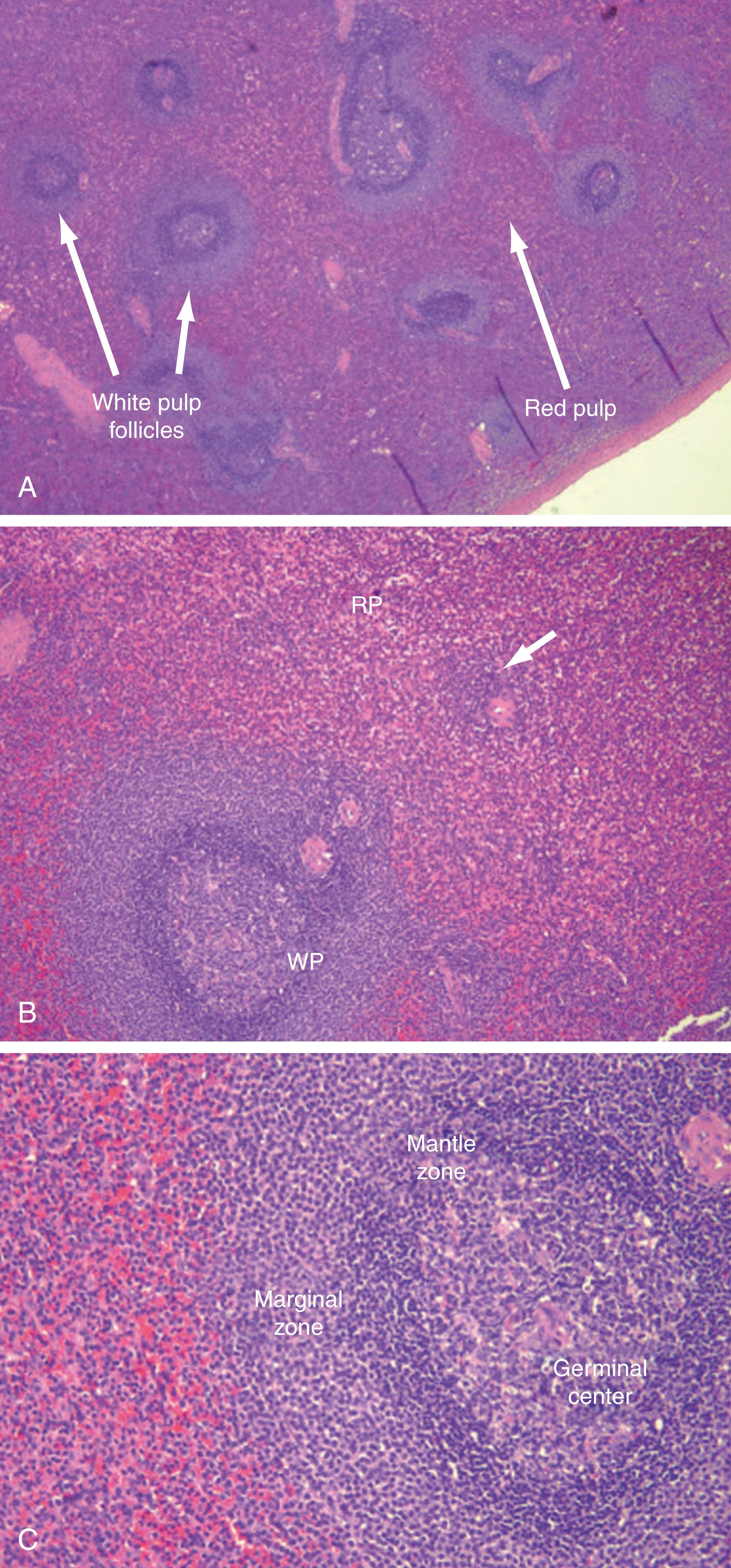
The spleen is encased within a fibroelastic capsule. From the capsule trabeculae extend and compartmentalize the spleen. The spleen is also segmented by the divisions of the splenic vessels as they branch within the organ and merge with these trabeculae ( Figs. 57.4 and 57.5 ). The arterioles branch into even smaller vessels and leave these trabeculae to merge with the splenic pulp, where their adventitia is replaced by a covering of lymphatic tissue that continues until the vessels thin to capillaries. These lymphatic sheaths make up the white pulp of the spleen and are interspersed among the arteriolar branches as lymphatic follicles. The white pulp then interfaces with the red pulp at the marginal zone. It is in this marginal zone that the arterioles lose their lymphatic tissue and the vessels evolve into thin-walled splenic sinuses and sinusoids. The sinusoids then merge into venules, draining into veins that travel along the trabeculae to form splenic veins that mirror their arterial counterparts.
Splenic function can be summarized into hematopoietic, reservoir, filtration, and immunity.
During fetal development between 3 to 5 weeks of fetal life, the spleen has important hematopoietic functions, which include white and red blood cell (RBC) production. This production is assumed by the bone marrow during the fifth month of gestation, and under normal conditions, the spleen has no significant hematopoietic function beyond this point. However, in certain pathologic conditions such as myelodysplastic syndrome, the spleen is one of the main organs involved in extramedullary erythropoiesis.
The spleen functions as a reservoir, where it pools in platelets. Normally one third of the platelets are pooled within the spleen. Thus, patients with splenomegaly are able to sequestrate large volume of platelets (up to 80%) with resultant thrombocytopenia. Due to that, after splenectomy, patients usually present with thrombocytosis which could be one of the factors that play a role in increasing thrombotic complications after splenectomy.
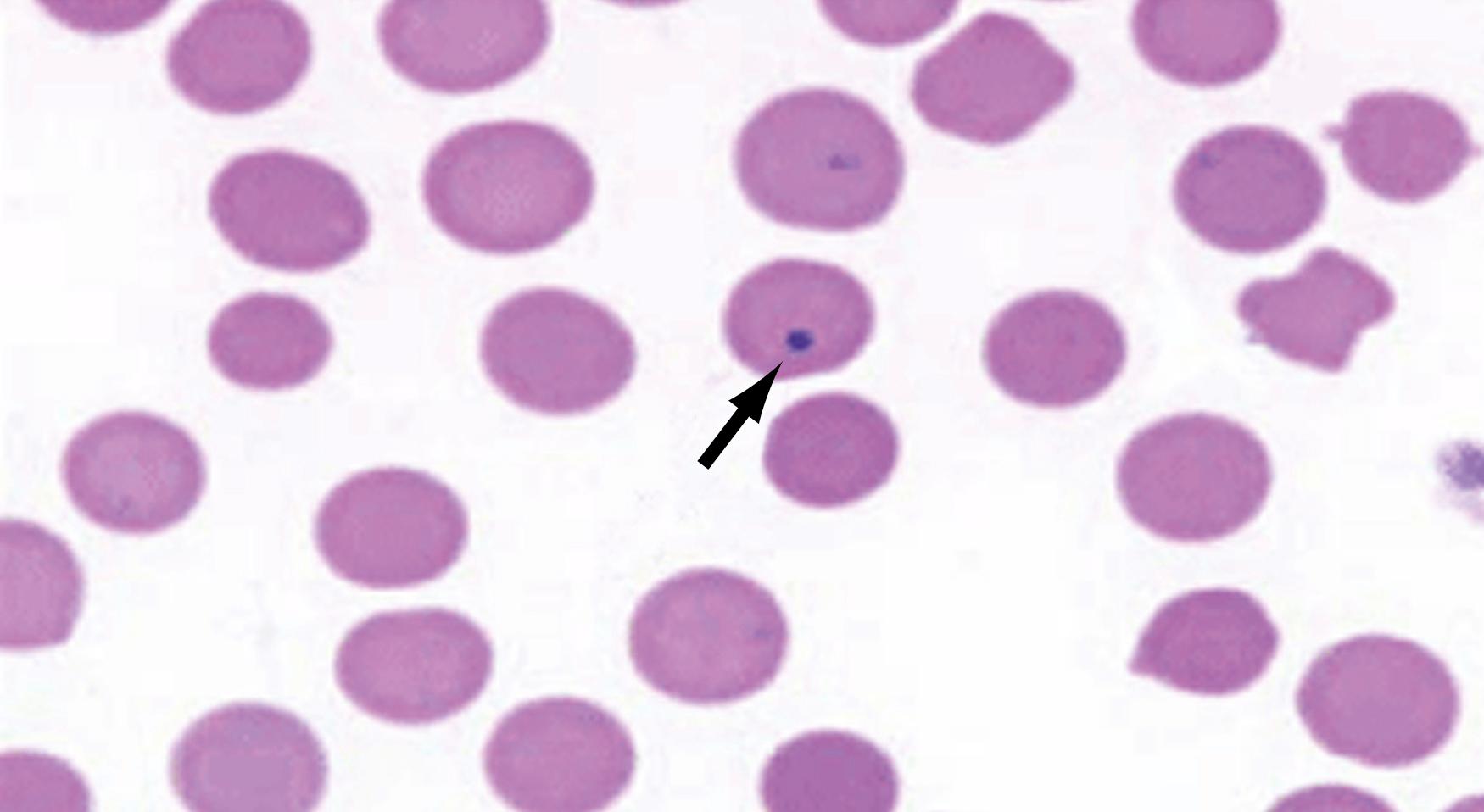
The splenic filtration process consists of two methods of blood flow, the closed and open systems. In the closed system, blood flows directly from arteries to veins. In the open system, the blood flows through the arterioles and then trickles through a sieve-like parenchyma made up of reticuloendothelial cells into the splenic sinuses before draining into the venous system ( Figs. 57.4 and 57.5 ). The cellular elements are directed toward these reticuloendothelial cells, in which cellular cleansing processes take place. These include removal of senescent cells, cellular inclusions (e.g., RBC nucleoli), and parasites and the sequestration of RBCs (for maturation) and platelets (reservoir). The plasma is directed to the lymphoid tissue, where soluble antigens stimulate the production of antibodies. RBC morphology, and thus RBC function, is maintained by splenic filtration. Normal RBCs are biconcave and deform easily. This plasticity allows passage through the microvasculature and optimizes the exchange of oxygen and carbon dioxide. Imperfect RBCs with inclusions such as nucleoli, Howell-Jolly bodies (nuclear remnant), Heinz bodies (denatured hemoglobin), Pappenheimer bodies (iron granules), acanthocytes (spur cells), codocytes (target cells), and stippling cause these RBCs to undergo cleansing in the spleen. The presence of Howell-Jolly bodies on a peripheral blood smear is one of the most characteristic finding for asplenia; surgical or medical (hemoglobinopathies) ( Fig. 57.6 ). Howell-Jolly bodies are strongly basophilic inclusion bodies found in the cytoplasm of RBCs and represent nuclei remnants that were unable to be cleared by a functioning spleen. Aged RBCs with decreased plasticity (>120 days) become trapped and destroyed in the spleen. Abnormal erythrocytes that result from hemoglobinopathies such as sickle cell anemia, hereditary spherocytosis, thalassemia, or pyruvate kinase deficiency (PKD) are also trapped and destroyed by the spleen. The overall effect is worsening anemia, splenomegaly, and sometimes autoinfarction of the spleen. Similarly, the spleen is involved in platelet destruction in immune thrombocytopenia (ITP), formerly known as idiopathic thrombocytopenia purpura.
It functions by antibody synthesis and phagocytosis. Asplenic patients have been found to express subnormal immunoglobulin M levels, and their peripheral blood mononuclear cells exhibit a suppressed immunoglobulin response. Other factors involved in the immune response are opsonins, such as properdin and tuftsin. Opsonins, produced in the spleen, exhibit reduced serum levels after splenectomy. Properdin, a globulin protein also known as factor P, initiates the alternate pathway of complement activation; this increases the destruction of bacteria and abnormal cells. Tuftsin, a tetrapeptide, enhances the phagocytic activity of mononuclear phagocytes and polymorphonuclear leukocytes. Absence of a circulating mediator appears to result in suppressed neutrophil function. The spleen also plays a key role in cleaving tuftsin from the heavy chain of immunoglobulin G; thus, circulating levels of tuftsin are subnormal in asplenic patients. Additionally, splenic filtration may be particularly important for removal of microorganisms for which the host does not have a specific antibody ( Box 57.1 ).
Red blood cell membrane
Red blood cell surface pits and craters
Howell-Jolly bodies
Heinz bodies
Pappenheimer bodies
Acanthocytes
Senescent red blood cells
Particulate antigen
Spherocytes (hereditary spherocytosis)
Sickle cells, hemoglobin C cells
Antibody-coated red blood cells
Antibody-coated platelets
Antibody-coated white blood cells
The immune functions of the spleen become evident after splenectomy, when patients are noted to be at risk for specific infections related to encapsulated bacteria Streptococcus pneumoniae , Haemophilus influenza, and Neisseria meningitidis . Asplenia and hyposplenism could be a result of surgical absence of spleen or lack of functioning of an anatomically present spleen. The most serious sequela is overwhelming postsplenectomy infection (OPSI). OPSI is discussed in detail at the end of this chapter.
Splenectomy may be indicated for conditions other than trauma. These indications encompass mainly hematologic disorders in addition to other mass lesions and splenic vascular lesions that are discussed elsewhere in this textbook.
ITP is the most common hematologic indication for splenectomy and is discussed here in detail. ITP was previously known as idiopathic thrombocytopenic purpura. In 2009, the ITP workgroup (IWG) published guidelines and defined the abbreviation to be ITP and dropped idiopathic and purpura as pathophysiology is better understood and the majority do not present with purpura. ITP is characterized by a low platelet count below 100 × 10 9 /L despite normal bone marrow and the absence of other causes of thrombocytopenia that could be responsible for the finding. The pathogenesis is not fully understood. However, immunoglobulin G autoantibodies directed towards the platelet membranes are believed to be responsible for platelet destruction within the reticuloendothelial system by macrophages and cytotoxic T cells. In addition to the destruction, there is dysfunction of megakaryocytes with low level of thrombopoietin. It is classified as primary ITP when there is no clear etiology. Primary ITP is further classified into three subtypes based on disease chronicity: newly diagnosed (within 3 months), persistent ( 3–12 months), and chronic (greater than 12 months). Secondary ITP is due to a known cause such as medication-induced, infectious, or rheumatologic conditions (i.e., systemic lupus erythromatosis). The typical presentation of ITP is characterized by purpura, epistaxis, and gingival bleeding. Less commonly, gastrointestinal bleeding and hematuria are noted. Intracerebral hemorrhage is a rare but sometimes fatal presentation.
The diagnosis of primary ITP involves the exclusion of other relatively common causes of thrombocytopenia—pregnancy, drug-induced thrombocytopenia (e.g., heparin, quinidine, quinine, sulfonamides), viral infections, and hypersplenism ( Box 57.2 ). Mild thrombocytopenia may be seen in approximately 6% to 8% of otherwise normal pregnancies and in up to 25% of women with preeclampsia. Drug-induced thrombocytopenia is thought to occur rarely, in approximately 20 to 40 cases/million users of common medications, such as trimethoprim-sulfonamide and quinine. Other medications, such as gold salts, have a higher incidence, almost 1% of users. Viral infection (e.g., hepatitis C, human immunodeficiency virus (HIV) infection, rarely Epstein-Barr virus infection) can be responsible for thrombocytopenia independent of splenic sequestration. Once again, other processes must be ruled out, but healthcare providers can be confident of these causative factors if platelet counts improve with successful treatment of the responsible infection. Bacterial infection, specifically Helicobacter pylori, has also been linked to infection-related thrombocytopenia that improves with eradication. Other causes are listed in Box 57.2 ; spurious laboratory values caused by platelet clumping or the presence of giant platelets should not be ignored.
In vitro platelet clumping caused by ethylenediaminetetraacetic acid (EDTA)–dependent or cold-dependent agglutinins, insufficiently anticoagulated specimen, glycoprotein IIb/IIIa inhibitors (e.g., abciximab)
Giant platelets that are miscounted as WBC by automated counters rather than platelets
Pregnancy (gestational thrombocytopenia, preeclampsia, HELLP syndrome)
Drug-induced thrombocytopenia (common drugs include heparin, quinidine, quinine, sulfonamides, acetaminophen, cimetidine, ibuprofen, naproxen, ampicillin, piperacillin, vancomycin, linezolid, glycoprotein IIb/IIIa inhibitors
Viral infections, such as HIV, HCV, EBV (infectious mononucleosis), rubella
Malaria
Hypersplenism caused by chronic liver disease
Alcohol
Nutrient deficiencies (e.g., vitamin B12, folate, copper)
Rheumatologic/autoimmune disorders (e.g., systemic lupus erythematosus, rheumatoid arthritis)
Myelodysplasia
Congenital thrombocytopenias
Thrombotic thrombocytopenic purpura and hemolytic-uremic syndrome
Chronic disseminated intravascular coagulation
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here