Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Infants with neonatal jaundice should be imaged before 2 months of age to optimize surgical treatment of biliary atresia, if present.
Steatosis and cirrhosis both attenuate the ultrasound beam, making imaging challenging, but it is important for determining the underlying cause of disease.
Approximately 40% of liver tumors in children are benign, and hemangiomas are the most common of these.
Among malignant liver tumors in children, hepatoblastoma is most common.
Doppler assessment of the liver provides critical information used to differentiate among numerous causes of hepatic disease.
Knowledge of common spontaneous portosystemic collateral pathways facilitates the sonographic examination and a search for root causes of cirrhosis.
Effective examination of pediatric segmental liver transplants requires familiarity with their substantially altered anatomy.
The anatomy of the liver can be explored in many planes with sonography. The usual course of intrahepatic vessels and their normal variants can be traced. There is a simple sonographic approach to the segmental anatomy of the liver based on the nomenclature of the French surgeon Couinaud, who described the segments according to the distribution of the portal and hepatic veins. Each segment has a branch (or a group of branches) of the portal vein at its center and a hepatic vein at its periphery. Each lobe of the liver contains four segments. The segments are numbered counterclockwise: 1 through 4 make up the left lobe, and 5 through 8, the right lobe. Segment 1 is the caudate lobe or Spiegel lobe. The right and left lobes are separated by the main hepatic fissure, a line connecting the neck of the gallbladder and the left side of the inferior vena cava (IVC) ( Fig. 51.1 ).
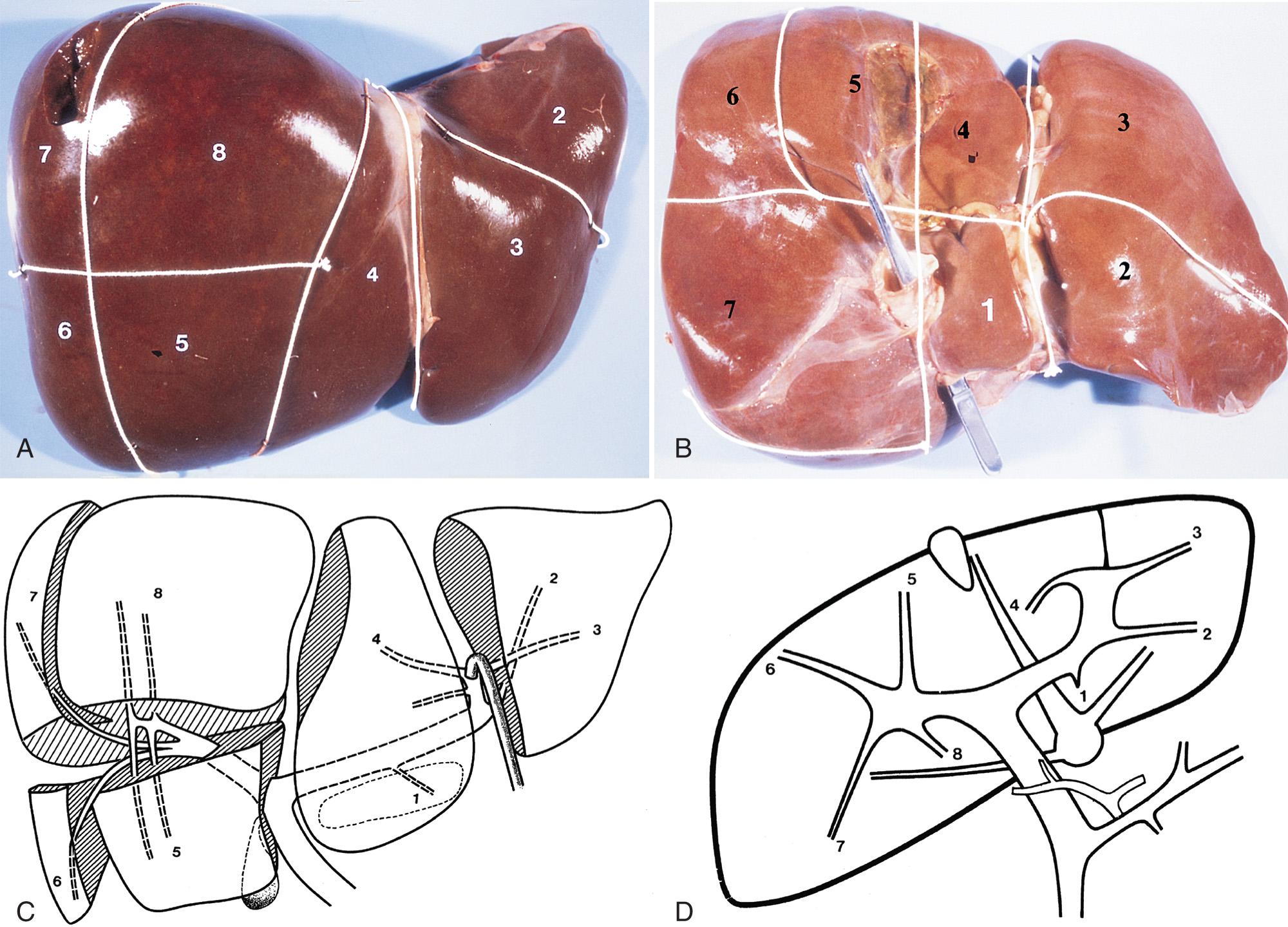
The segmental branches of the portal vein (each one of which leads into a segment) can be outlined in the form of two H s turned sideways, one for the left lobe (segments 1-4) and one for the right lobe (segments 5-8) ( Fig. 51.2 ).
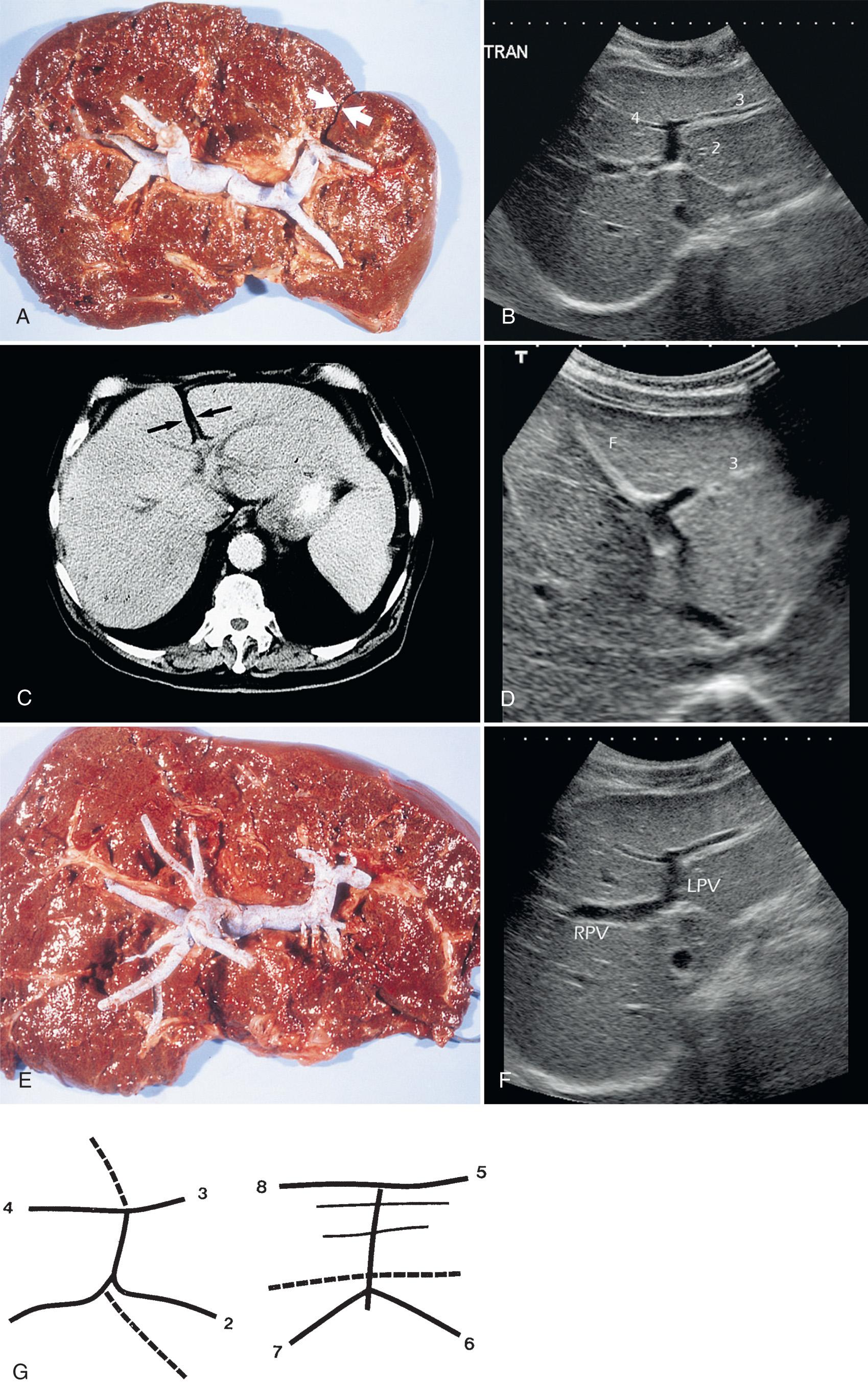
The H of the left lobe is visualized with an oblique, upwardly tilted subxiphoid view. The H is formed by the left portal vein, the branch entering segment 2, the umbilical portion of the left portal vein, and the branches to segments 3 and 4. To this recumbent H are attached two ligaments, the ligamentum venosum, also called the “lesser omentum” or the “hepatogastric ligament,” and the falciform ligament. The ligamentum venosum separates segment 1 from segment 2. The falciform ligament is visible between the umbilical portion of the left portal vein and the outer surface of the liver. It separates segment 3 from segment 4.
Segment 1, the caudate lobe, is bordered posteriorly by the IVC, laterally by the ligamentum venosum, and anteriorly by the left portal vein. Unlike the other segments of the liver, segment 1 may receive branches of the left and right portal veins. The portal veins to segment 1 are usually small and are rarely seen sonographically. The caudate lobe has one or more hepatic veins that drain directly into the IVC, separately from the three main hepatic veins. This special vascularization is a distinctive characteristic of segment 1.
The portal vein leading to segment 2 is a linear continuation of the left portal vein, completing the lower horizontal limb of the H . Segmental branches to segments 3 and 4 form the other horizontal limb. Segments 2 and 3 are thus located to the left of the umbilical portion of the left portal vein, the ligamentum venosum, and the falciform ligament. Segment 4, the quadrate lobe, is situated around the right anterior limb of the portal venous H , to the right of the umbilical portion of the left portal vein and the falciform ligament. Segment 4 is separated from segment 5 by the main hepatic fissure and from segments 5 and 8 by the middle hepatic vein. The quadrate lobe is separated from segment 1 by the left portal vein.
The right portal vein and its branches are best seen with a sagittal or oblique midaxillary intercostal approach. In some patients a subcostal approach is also useful. The right portal vein follows an oblique or vertical course, directed anteriorly.
The branches leading to the segments of the right lobe of the liver are also distributed in the shape of a sideways H . The right portal vein forms the crossbar of the H . The branches to segments 5 and 8 form the upper limb of the H , and the branches to segments 6 and 7 form its lower portion. The branches of segments 6 and 7 are more obliquely oriented, and the transducer should be rotated slightly upward for segment 7 and downward in the direction of the right kidney for segment 6.
The middle hepatic vein separates segments 5 and 8 from segment 4. The right hepatic vein separates segments 5 and 8 from segments 6 and 7 ( Fig. 51.3 ). Segment 5 is bordered medially by the gallbladder and the middle hepatic vein and laterally by the right hepatic vein. The right portal vein serves as a landmark for the separation of segment 5 from segment 8. Segment 8 is separated from segment 7 by the right hepatic vein and from segment 4 by the middle hepatic vein.
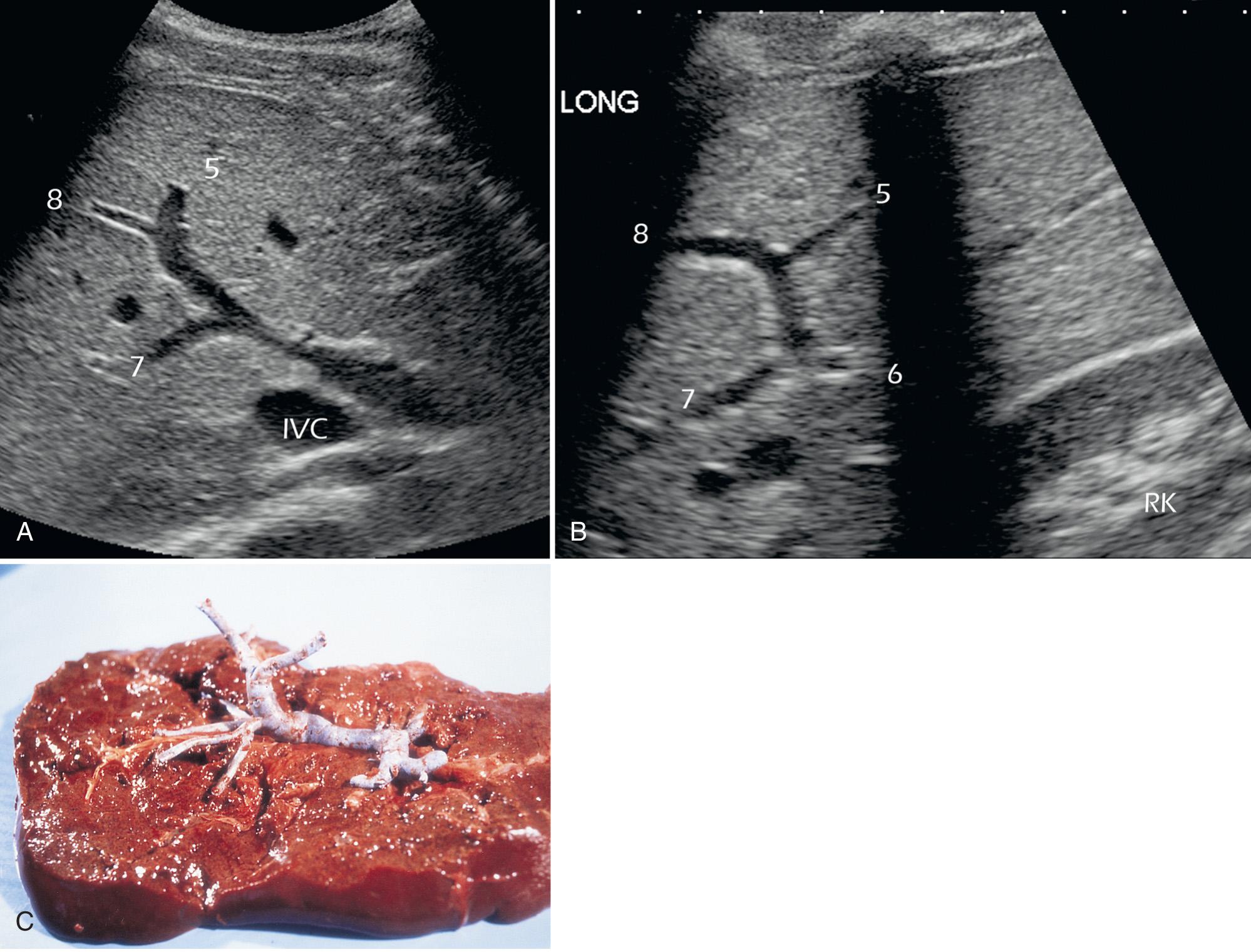
Segments 6 and 7 are separated from segments 5 and 8 by the right hepatic vein. Segment 6 is the part of the liver closest to the kidney; its lateral border is the rib cage. Segment 7 is separated from segment 8 by the right hepatic vein and is bordered laterally by the rib cage and cephalically by the dome of the diaphragm.
When seen with an oblique coronal subxiphoid view, the three hepatic veins form a W , with its base on the IVC. The left and middle hepatic veins join the left anterior part of the IVC ( Fig. 51.4 ). The hepatic veins separate the following segments: the left hepatic vein separates segment 2 from segment 3; the middle hepatic vein separates segment 4 from segments 5 and 8; and the right hepatic vein separates segments 5 and 8 from segments 6 and 7. With the oblique subxiphoid view, the right portal vein is seen en face, which helps separate the superficial segment 5 from the more deeply situated segment 8.
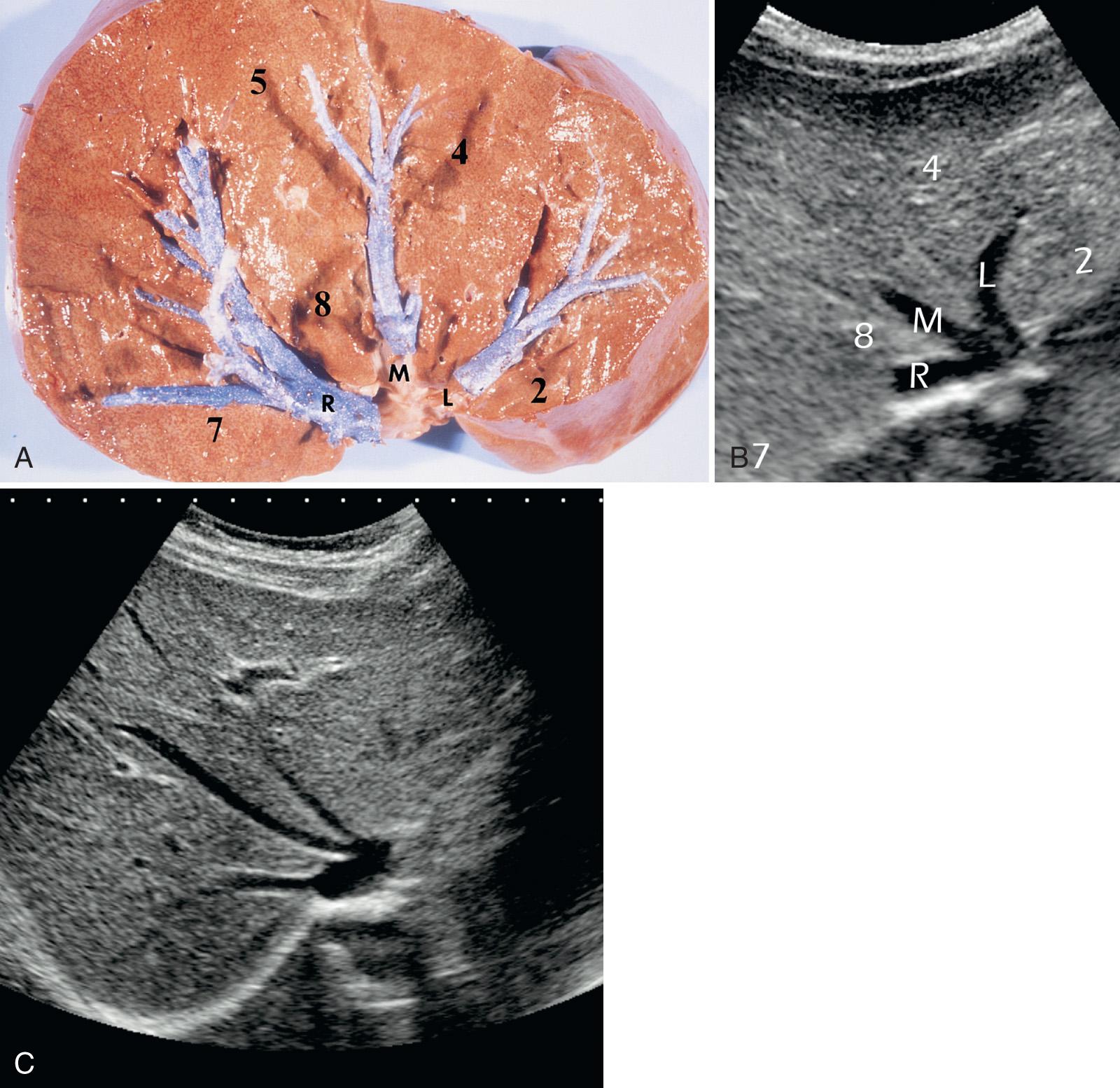
The sonographic examination of the child's liver should include visualization of the right and left portal veins and their segmental branches, as well as the hepatic veins. Not only can focal lesions be identified and accurately localized, but thrombosis, compression, or tumor invasion of vessels can be outlined. Doppler sonography is added when the presence and direction of blood flow within these veins need to be assessed. Exploring the liver through its vessels is an excellent way to ensure that the sonographic examination is complete and not just an arbitrary glance at this otherwise homogeneous organ with variable contours and few landmarks except for its veins. Because the branches of the hepatic artery and the bile ducts are neighbors of the portal veins, the examination of the lobar and segmental portal veins ensures a complete assessment of these structures as well.
The cause of persistent jaundice in the newborn is often difficult to define because clinical and laboratory features may be similar in hepatocellular and obstructive jaundice. If bile obstruction, biliary atresia, or metabolic diseases such as galactosemia and tyrosinemia are to be treated effectively with surgery or specific diet and medication, the diagnosis must be made early, in the first 2 to 3 months, before irreversible cirrhosis has occurred.
Sonography plays an important role in defining causes of extrahepatic obstruction to bile flow that may be effectively treated with early surgery, including choledochal cyst, biliary atresia, and spontaneous perforation of the bile ducts. (Other causes of bile duct obstruction, such as cholelithiasis, tumors of the bile ducts or pancreas, and congenital stenosis of the common bile duct (CBD), usually appear later in childhood.) Intrahepatic causes of neonatal jaundice include hepatitis (bacterial, viral, or parasitic) and metabolic diseases (e.g., galactosemia, tyrosinemia, fructose intolerance, α 1 -antitrypsin deficiency, cystic fibrosis, paucity of interlobular bile ducts, North American Indian cirrhosis). Systemic diseases that cause cholestasis include heart failure, shock, sepsis, neonatal lupus, histiocytosis, and severe hemolytic disease.
Choledochal cyst
Biliary atresia
Spontaneous perforation of the bile ducts
Paucity of interlobular bile ducts (Alagille syndrome)
Syphilis
Listeria
Staphylococcus
Hepatitis B
Hepatitis C
Cytomegalovirus
Human immunodeficiency virus
Rubella
Herpesvirus
Epstein-Barr virus
Toxoplasma
Shock
Sepsis
Heart failure
Neonatal lupus
Histiocytosis
Hemolytic diseases
Galactosemia
Tyrosinemia
Fructose intolerance
α 1 -Antitrypsin deficiency
Cystic fibrosis
The infant with jaundice is usually screened with sonography. When dilation of the bile ducts is found, percutaneous cholangiography or cholecystography may be performed if the cause and anatomy of the obstruction are unclear. If sonography fails to outline an anatomic abnormality, hepatobiliary scintigraphy may define patency of the CBD, unless hepatocyte damage is extensive. If no radionuclide reaches the gut, liver biopsy is typically performed. Both scintigraphy and the sonographic search for the gallbladder are enhanced by the bile-stimulating effect of phenobarbital administered for 3 to 5 days before the test. The triangular cord sign, an echogenic cone-shaped density just cranial to the portal vein bifurcation on longitudinal or transverse scans, is highly predictive of biliary atresia. An absent or small gallbladder (<1.5 cm in length) coupled with the triangular cord sign is even more specific for the diagnosis.
Dilation of varying lengths and severity of the CBD, termed “choledochal cyst,” usually manifests as jaundice in infancy, clinically mimicking neonatal hepatitis and biliary atresia ( Fig. 51.5 ). Todani's classification describes five types. Type I, cylindrical or saccular dilation of the common bile duct, is most common (80%-90%) and is thought to be caused by an abnormal insertion of the CBD into the pancreatic duct, forming a common channel and facilitating reflux of enzymes into the CBD, with consequent inflammation. Because choledochal cysts have been detected in 15-week gestational age fetuses, when amylase is not yet present, and because surgically treated cysts in the newborn period show minimal inflammation, there must be causative factors (as yet unknown) other than the common-channel theory. Two rare but well-documented causes of bile duct dilation (choledochal cyst) in the newborn are localized atresia of the CBD and multiple intestinal atresias in which the CBD empties into a blind pouch of bowel. A choledochal cyst manifesting later in childhood may have a different pathogenesis. It is usually complicated by cholangitis and classically causes abdominal pain, obstructive jaundice, and fever. In some cases the cyst is palpable as a mass.
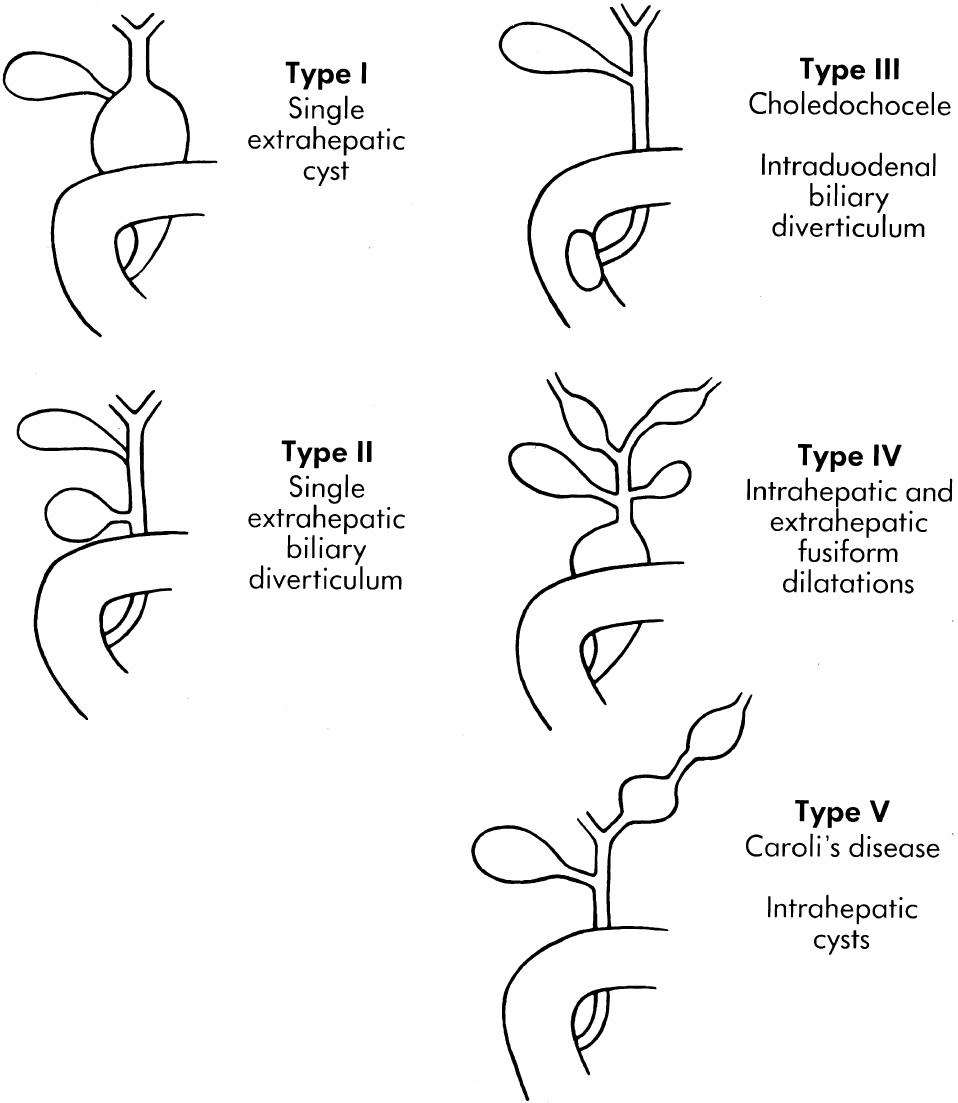
Choledochal cyst type II consists of one or more diverticula of the CBD (2% of cysts). Choledochocele (type III) is a dilation of the intraduodenal part of the CBD (1%-5%). Multiple intrahepatic and extrahepatic cysts make up type IV (10%), sometimes subcategorized into types IVa and IVb, which has only extrahepatic cysts. Caroli disease (type V) affects intrahepatic bile ducts.
Sonographic screening of the jaundiced infant shows one or several thin-walled cysts at the liver hilum or within the liver (choledochal cyst type I; Fig. 51.6 ). The gallbladder is identified separately. Dilation of intrahepatic ducts, as well as stones, may occur later as a result of bile stasis and cholangitis. Scintigraphy is used to document bile flow into the cyst, and percutaneous cholecystography/cholangiography or endoscopic retrograde cholangiopancreatography is performed if detailed mapping of the bile system is deemed necessary before surgery.
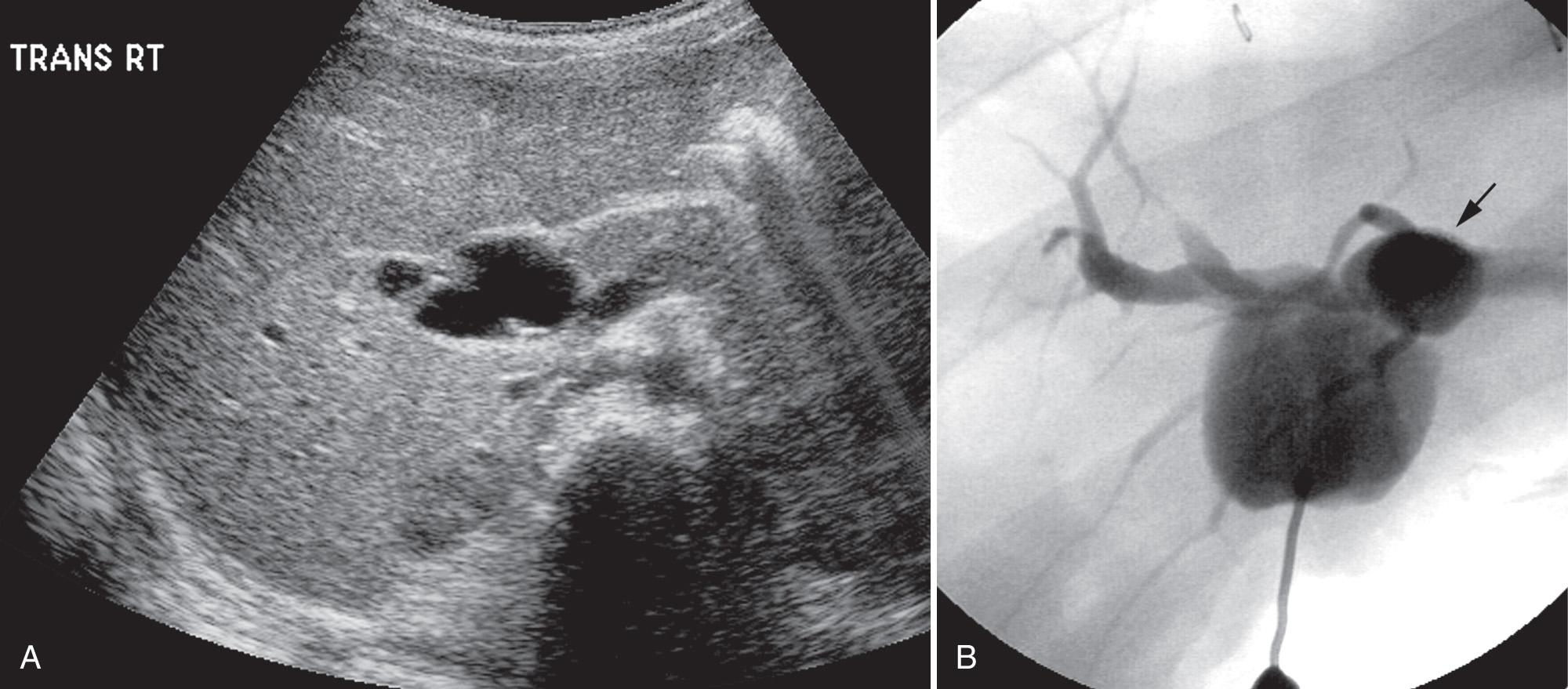
Caroli disease (type V choledochal cyst) consists of nonobstructive dilation of intrahepatic bile ducts and is often associated with congenital hepatic fibrosis and autosomal recessive polycystic kidney disease. The disease is caused by the arrest of or derangement in embryologic remodeling of ducts, resulting in segmental dilation. Patients tend to seek medical attention later than with other types of choledochal cysts, usually after cholangitis and stones have formed in childhood ( Fig. 51.7 ). At sonography, Caroli disease has dilated ducts surrounding branches of the portal vein. Sludge and stones are often visible within the dilated ducts. Abscesses complicating cholangitis are seen as cavities with walls thicker than those of the ducts and filled with heterogeneous material. Polycystic kidneys, when present, are another clue to the diagnosis of Caroli disease; Caroli disease and autosomal recessive polycystic kidney disease may occur together because both are associated with mutation in the PKHD1 gene.
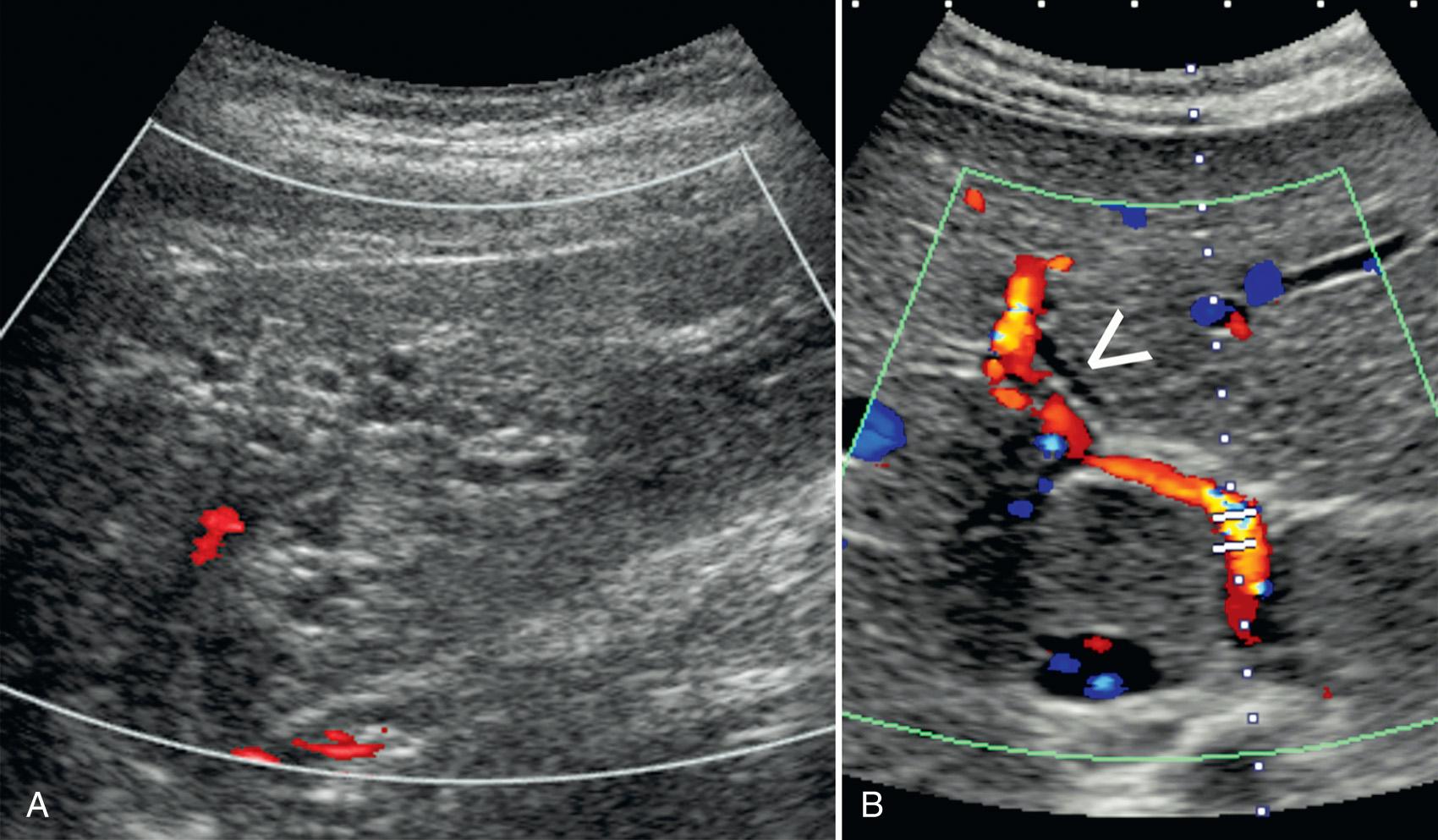
Rupture of the bile duct is rare. Rupture in newborns leads to jaundice, abdominal distention, and substantial morbidity. Because the site of rupture is usually the junction of the cystic duct and CBD, it is believed that a developmental weakness at this site leads to the rupture. The biliary system is undilated, but there are ascites and loculated fluid collections around the gallbladder. Although the condition was previously treated surgically, the recent trend is toward percutaneous drainage and conservative management.
Manifesting with chronic cholestasis, usually within the first 3 months of life, ductal paucity and arteriohepatic dysplasia (Alagille syndrome [ALGS]) are diagnosed histologically by noting a reduced number of interlobular bile ducts compared with the total number of portal areas. Because of cholestasis, the gallbladder may be very small (disuse). The CBD is normal ( ). The liver is usually enlarged, especially the left lobe. Portal hypertension ensues, with splenomegaly and esophageal varices. In children with ALGS, paucity of bile ducts is associated with a characteristic facies, pulmonary artery stenosis, butterfly vertebrae, segmentation anomalies, and, infrequently, renal abnormalities (renal tubular acidosis). ALGS is inherited as an autosomal dominant disease with variable penetrance, most commonly due to mutation in JAG1 (ALGS type 1), but in a small proportion of cases mutation in NOTCH2 (ALGS type 2).
The incidence of biliary atresia varies from 1 in 8000 to 1 in 10,000 births. Boys and girls are equally affected. Biliary cirrhosis occurs early, often in the first weeks after birth. Biliary atresia is characterized by absence or obliteration of the lumen of the extrahepatic and intrahepatic bile ducts ( Fig. 51.8 ). It is associated with the polysplenia syndrome (biliary atresia, situs inversus, polysplenia, symmetrical liver, interrupted IVC, preduodenal portal vein) in about 20% of patients, as well as with trisomy 17 or 18. Because the disease is extremely rare, and because the pancreatic duct, which develops with the bile ducts, is normal in affected children, biliary atresia likely develops after the bile ducts have formed. The etiology of biliary atresia is still unclear, although there is evidence pointing to viral, toxic, and multiple genetic factors. An in utero insult to the hepatobiliary system may result in a progressive sclerosis of the extrahepatic and intrahepatic bile ducts. In addition, certain drugs (e.g., carbamazepine) have been associated with biliary atresia.
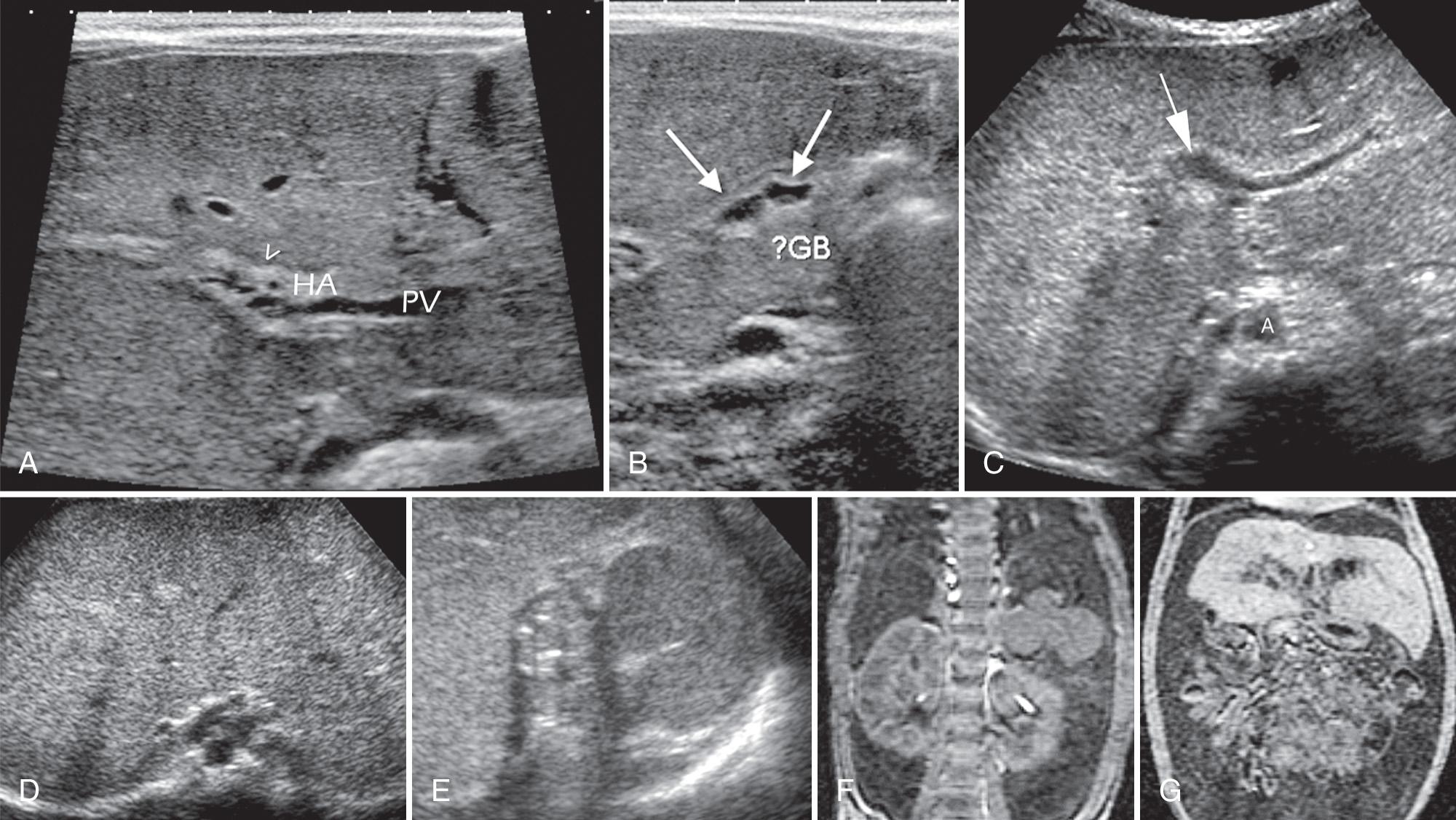
Jaundice classically develops gradually, 2 to 3 weeks after birth. The diagnosis is readily made if there are radiologic or sonographic signs of the polysplenia syndrome (see Fig. 51.8C-G ). Because the flow of bile is interrupted, the gallbladder is typically very small (20%) or absent (80%). After having the infant fast for 4 to 6 hours, a specific search with a high-frequency transducer will demonstrate a very small gallbladder (microgallbladder) in about 20% of patients. When the gallbladder is visible, the “ghost triad” appearance has been reported to help in making the correct diagnosis of biliary atresia. The ghost triad consists of gallbladder length less than 1.9 cm, irregular or incomplete mucosal lining, and irregular or lobular contour. The echogenic fibrotic remnant of the CBD seen adjacent to the portal vein has been called the triangular cord sign. The combination of a small gallbladder, less than 1.5 cm in length, and the triangular cord sign are very specific for the diagnosis of biliary atresia. Any intrahepatic bile duct remnants may dilate and be visible at sonography as bile duct dilation or small cysts. In addition, the cholangitis that complicates biliary atresia may result in cystic areas within the liver.
Surgical treatment creates communication between a loop of jejunum (transposed to the liver after a Roux-en-Y anastomosis) and any patent bile “ductules” at the liver hilum. This is the classic hepatoportoenterostomy described by Kasai in 1959. Even if there is no mucosal contact between intestine and the bile ducts, the procedure permits bile drainage, with complete clinical remission in 30% and partial drainage in 30% of affected children. The prognosis becomes much more guarded when the Kasai procedure is delayed to more than 60 days after birth. Despite successful Kasai procedures, 75% of patients require liver transplantation before age 20 years. Biliary atresia remains the most common reason for liver transplant in pediatric patients.
Defined as an infection of the liver occurring before the age of 3 months, neonatal hepatitis is now considered an entity distinct from toxic or metabolic diseases affecting the neonate. The causative agent (bacterium, virus, or parasite) reaches the liver through the placenta, via the vagina from infected maternal secretions, or through catheters or blood transfusions. Transplacental infection occurs most readily during the third trimester, and syphilis, Toxoplasma, rubella, and cytomegalovirus (CMV) are the most common agents ( Fig. 51.9 , ).
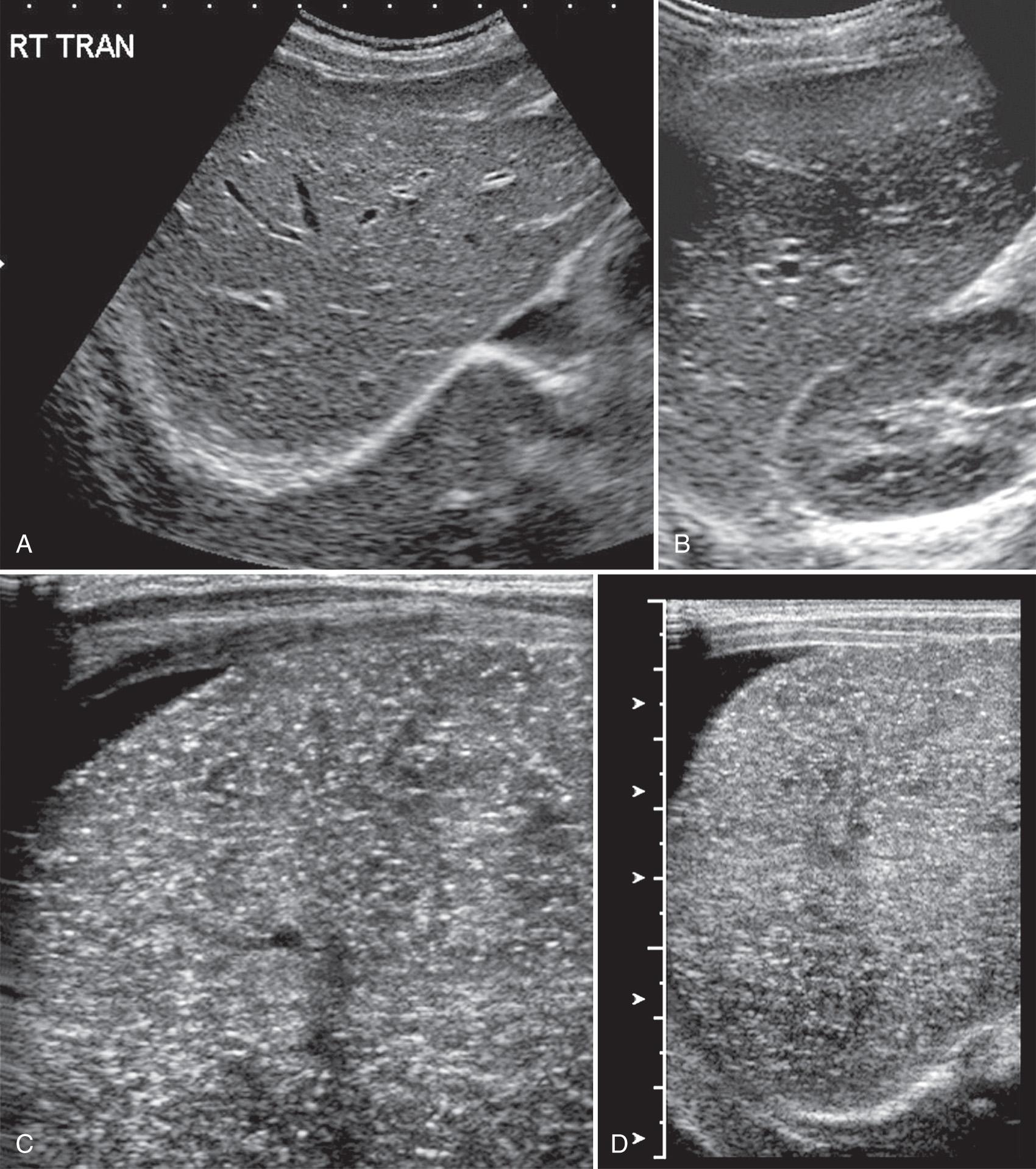
Neonatal bacterial hepatitis is usually secondary to upward spread of organisms from the vagina, infecting endometrium, placenta, and amniotic fluid. In twin pregnancies, the fetus nearest the cervix is more frequently affected. Listeria and Escherichia coli are the usual organisms. During vaginal delivery, direct contact with herpesvirus, CMV, human immunodeficiency virus (HIV), and Listeria organisms may lead to hepatitis. Blood transfusions may contain hepatitis virus B or C, CMV, Epstein-Barr virus, and HIV. Infected umbilical vein catheterization usually results in bacterial hepatitis or abscesses.
With the exception of diffuse hepatomegaly, there are no specific sonographic signs of hepatitis, unless abscesses (usually bacterial in origin) occur. The gallbladder wall may be thickened, probably from hypoalbuminemia. Dystrophic calcifications in the hepatic parenchyma may be seen.
The association of jaundice with urinary tract infection or sepsis occurs more often in male than female newborns. Jaundice, hepatomegaly, and vomiting are common clinical signs. Urinary tract symptoms are uncommon, as are shock and fever. A thorough examination of the kidneys, ureters, and bladder should therefore accompany sonography of the liver in the infant with jaundice. Similarly, the diaphragm and lung bases should be examined to look for pleural effusions and pneumonia, which may be accompanied by sepsis and jaundice in the newborn.
Because these disorders cause liver damage in the newborn, some rapidly destroying the liver if untreated, and because several can be treated effectively with diet or drugs once diagnosed, pediatricians and radiologists should be well acquainted with inborn errors of metabolism. Liver damage is caused by storage of a hepatotoxic metabolite or by absence of an essential enzyme that impairs the detoxification process of the liver.
Steatosis is especially prominent in the glycogen storage diseases, galactosemia, tyrosinemia, and cystic fibrosis. Cirrhosis eventually develops in all the diseases that cause liver damage, and portal hypertension then follows. The risk of hepatocellular carcinoma is significantly increased in α 1 -antitrypsin deficiency, in tyrosinemia, and in glycogen storage disease type I. Liver adenomas also develop in the last two entities, as does renal tubular disease, which is usually characterized by acidosis and nephrocalcinosis.
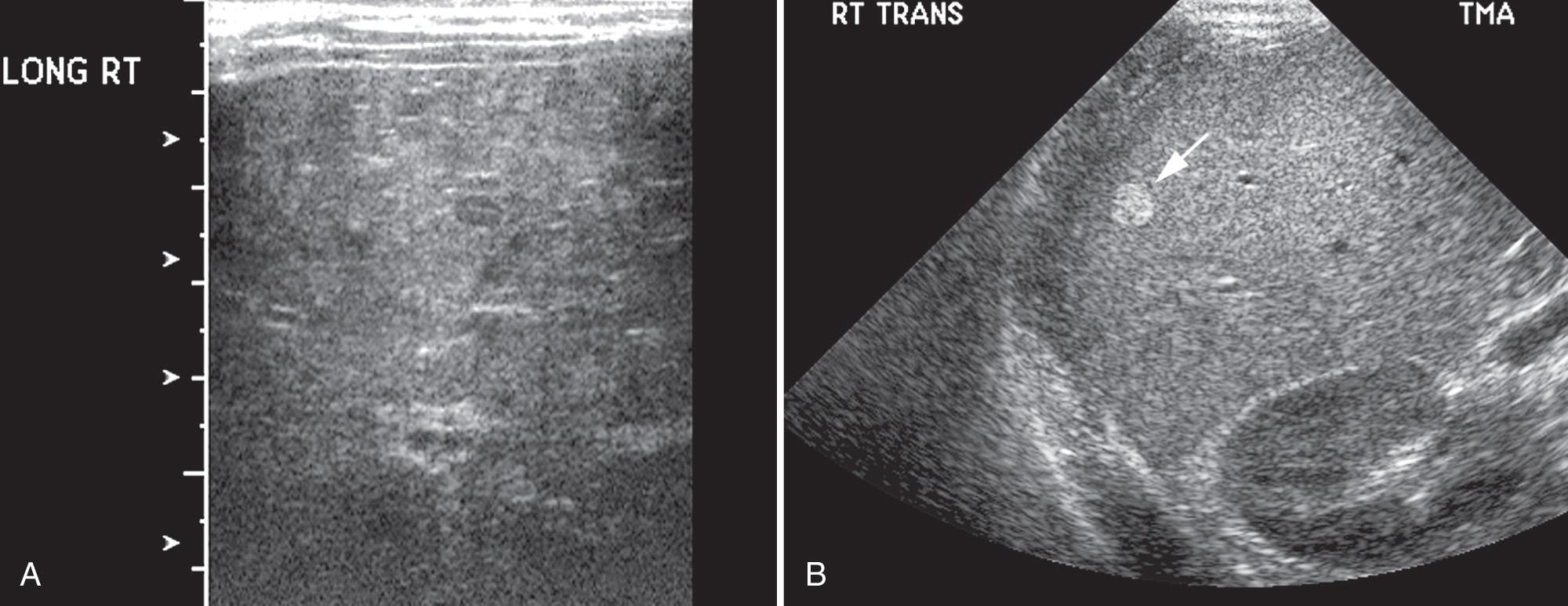
Tyrosinemia is best treated by transplantation. Until drug therapy became available for the treatment of the acute neurologic crises in infants with acute tyrosinemia, transplantation was performed as a lifesaving procedure as soon as surgically feasible. Currently, transplantation is done once liver nodules appear because hepatocellular carcinoma develops in about 30% of children with tyrosinemia who survive the neonatal period. A review of livers dissected at liver transplantation found that preoperative sonograms and computed tomography (CT) scans were not accurate in distinguishing regeneration nodules, adenomas, and carcinomas, nor was alpha-fetoprotein (AFP) analysis ( Fig. 51.10 ).
Glycogen storage disease type IV
Galactosemia
Fructose intolerance
Tyrosinemia
Wolman disease
Zellweger syndrome
Neonatal iron storage disease
Wilson disease
α 1 -Antitrypsin deficiency
Cystic fibrosis
Glycogen storage disease (I and III)
Mucopolysaccharidoses
Gaucher disease
The sonographic examination of children with possible metabolic disease includes a careful analysis of liver size and architecture, searching for steatosis, cirrhosis, and nodules; an analysis of renal architecture, searching for increased size and nephrocalcinosis; and a Doppler examination of the abdomen searching for signs of portal hypertension.
Fat accumulates in hepatocytes after cellular damage (fatty degeneration), through the overloading of previously healthy cells with excess fat (fatty infiltration), or in certain enzyme deficiency syndromes, through the inability of fat to be mobilized out of the liver. Drugs (acetylsalicylic acid, tetracycline, valproate, warfarin [Coumadin]) and toxins (aflatoxin, hypoglycine) as well as alcohol abuse lead to fatty degeneration of liver cells. Steatosis is also seen in metabolic liver disorders such as galactosemia, fructose intolerance, and Reye syndrome. Obesity ( ), corticosteroid therapy, hyperlipidemia, and diabetes are examples of increased fat mobilization and its entry into the liver. In malnutrition, nephrotic syndrome, and cystic fibrosis, not only does excess fat enter the liver, but mobilization of fat out of the hepatocyte is deficient as well. When parenteral nutrition does not include lipids, steatosis results from a deficiency of essential fatty acids. Most inherited disorders of the liver mentioned previously involve an enzyme deficiency and result in steatosis.
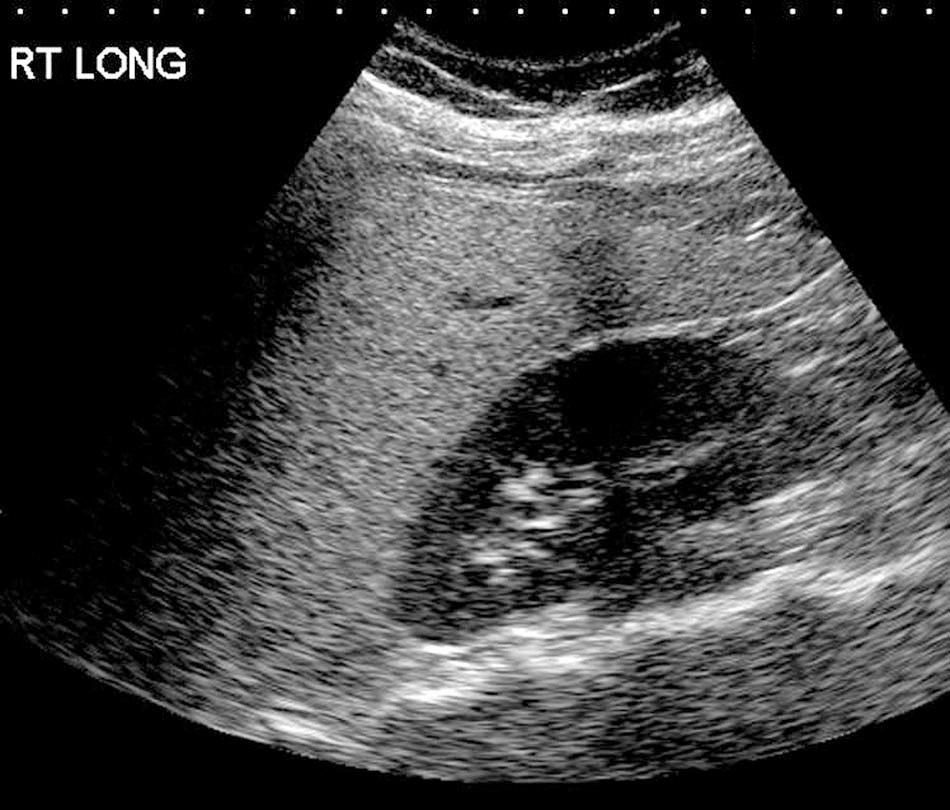
Fatty changes are reversible, may be diffuse or focal, and are often detected on ultrasonography before clinically suspected. On sonography, areas of steatosis are highly echogenic, blurring vessel walls. The nearby kidney cortex appears much less echogenic. When focal, steatosis usually has smooth, geometric, or fingerlike borders ( Fig. 51.11 ). Intervening normal liver may appear hypoechogenic and masquerade as mass lesions (metastases or abscesses), especially if the ultrasound gain is adjusted by using the fatty areas as a normal reference. Segments 4 and 5 are often spared in steatosis, perhaps because of favorable blood supply by the gallbladder and its vessels. Nodules of steatosis may mimic metastases on CT. Areas of abnormal echotexture on sonographic studies can be further evaluated with CT; the two modalities are complementary in this situation. Ultrasound grading of steatosis is subjective; magnetic resonance spectroscopy can reliably quantify fat content. Magnetic resonance imaging (MRI) sequences and various MRI contrast agents can characterize focal lesions in the liver. Despite sophisticated imaging, occasionally biopsy may be necessary.
Drugs
Acetylsalicylic acid
Tetracycline
Valproate (and other antiepileptic medications)
Warfarin (Coumadin)
Toxins
Aflatoxin
Hypoglycine
Alcohol abuse
Metabolic liver disease
Galactosemia
Fructose intolerance
Reye syndrome
Obesity
Corticosteroid therapy
Hyperlipidemia
Diabetes
Malnutrition
Nephrotic syndrome
Cystic fibrosis
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here