Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The parathyroid glands were the last endocrine glands to be discovered. The earliest reference to the parathyroid glands is Sir Richard Owen’s description of a small compact yellow glandular body that was attached to the thyroid gland in the Indian rhinoceros. Ivar Victor Sandstrom, a 25-year-old Swedish medical student at the University of Uppsala, has been credited with first identifying the parathyroid glands in human cadavers in 1880, naming them “glandulae parathyreoideae” and providing a detailed gross anatomic and microscopic description.
Anton Wolfer first described postoperative tetany in 1879 in Theodore Billroth’s first survivor of total thyroidectomy. Eugene Gley, a professor of physiology at the College de France in Paris, was the first to recognize the association of tetany with the parathyroid glands. In 1891, he reported tetany and death in rabbits and rats following thyroidectomy and removal of the glands described by Sandstrom. ,
Also in 1891, Friedrich von Recklinghausen, Professor of Pathology in Strasbourg, reported a patient with multiple fractures, bending of the long bones, fibrosis, cystic changes, and brown tumors. This constellation of findings was termed osteitis fibrosa cystica of von Recklinghausen. , In 1904, the association of osteitis fibrosa cystica and parathyroid disease was identified by Max Askanazy, a German-Swiss pathologist who reported two patients with severe bone changes and a parathyroid tumor. At that time it was hypothesized that the changes in the parathyroid glands were the result of primary bone disease, and patients with severe bone disease were treated with parathyroid extract.
It was not until 1915 that the severe bone disease was considered to be the result of changes in the parathyroid glands. Dr. Friedrich Schlagenhaufer, a professor of pathology in Vienna, reported autopsy results in two patients with osteomalacia who were found to have a solitary parathyroid tumor. It was then that enlargement of the parathyroid gland was considered to be the primary event in the development of bone disease and Schlagenhaufer suggested that some patients may benefit from parathyroid tumor excision.
In 1905, William MacCallum, a pathologist at Johns Hopkins Hospital, demonstrated that administration of calcium or parathyroid extract could reverse or prevent postoperative tetany. Likely influenced by the work of MacCallum, William Halstead used intravenous calcium gluconate to treat tetany in patients following thyroidectomy and emphasized the importance of preventing injury to the parathyroid glands at the time of thyroidectomy to prevent tetany. However, the prevailing view at the time was that the primary function of the parathyroid glands was to detoxify the blood, much like the liver.
It was not until 1925 when James B. Collip, a Canadian biochemist at the University of Alberta, used hot acid extraction to produce parathyroid hormone (PTH) extracts that were used to successfully reverse tetany in parathyroidectomized animals. Collip and colleagues also reported that repeated injections of parathyroid extract in dogs produced hypercalcemia, vomiting, dehydration, atony, and eventually death. Collip suggested that the function of the parathyroid glands was to regulate calcium metabolism.
One of the most important events in the history of the parathyroid glands occurred in Vienna in 1925, when Felix Mandl, a Viennese surgeon, treated Albert Jahne, a 38-year-old tramcar conductor who had severe bone disease from primary hyperparathyroidism (HPT). Mandl, influenced by Friedrich Schlagenhaufer’s finding of a single parathyroid tumor in two patients with osteitis fibrosa cystica, performed a neck exploration on Albert Jahne under local anesthesia on July 30, 1925, and excised a 2.5-cm parathyroid gland that resulted in dramatic improvement of his bone disease. This was an epic event because it established the relationship of osteitis fibrosa cystica to enlargement of a parathyroid gland and helped dispel the belief that the severe bone disease was the result of parathyroid deficiency.
In the United States, Captain Charles Martell was the first and most well-recognized case of primary HPT. He was a merchant marine sea captain who presented with severe progressive bone disease manifested by loss of height, multiple fractures, generalized demineralization, and cysts. He also had kidney stones, lethargy, and fatigue and was found to have elevated serum calcium, low serum phosphorus, and hypercalciuria. Martell underwent six unsuccessful cervical explorations before Edward Churchill, with the assistance of Oliver Cope, performed a mediastinal exploration at the Massachusetts General Hospital in 1932 and removed a 3-cm parathyroid tumor just medial to the superior vena cava.
In 1929, Barr, Bulger, and Dixon from Barnes Hospital in St. Louis introduced the term hyperparathyroidism to describe a syndrome characterized by rarefaction of bone and cystic bone tumors, kidney stones, muscle weakness, hypotonia, and high levels of calcium in the serum and urine. This was the direct result of their care of Elva Dawkins, who was diagnosed with HPT and underwent the first successful parathyroidectomy in the United States by Isaac Y. Olch at Barnes Hospital in St. Louis on August 1, 1928.
In the 1930s, Fuller Albright, a Harvard physician, noted that 80% of patients with osteitis fibrosa cystica had nephrocalcinosis and nephrolithiasis, which were the hallmarks for primary HPT. He further defined primary HPT as “a condition in which PTH is higher than needed” and identified single adenoma, multiple adenomas, diffuse hyperplasia, and cancer as causes for primary HPT. He also described secondary and tertiary HPT and distinguished them from primary HPT.
The modern era of parathyroid history began with the invention of the autoanalyzer in 1951 by Leonard Skeggs, an American biochemist. Multichannel autoanalyzers were introduced into clinical use in the 1970s, and this eventually led to rapid serum analysis and diagnosis of hypercalcemia. Over time, more and more patients with HPT were incidentally diagnosed with hypercalcemia on routine blood work and were less likely to have the severe bone disease.
A second major development was the successful purification and isolation of PTH by Auerbach and later by Rasmussen and Craig. More effective measurement of serum PTH followed in the second half of the twentieth century and resulted in an improvement in the diagnosis of HPT. In 1963, Berson and colleagues reported the use of an immunoassay to measure human PTH. This was followed by the successful sequencing and synthesis of PTH in the early 1970s by Potts and colleagues at Harvard University. Further refinement in the diagnosis of HPT occurred as a result of the development of radioimmunoassays by Rosalyn Yalow for measurement of peptide hormones for which she won the Nobel Prize in 1977. , , In 1987, Nussbaum and colleagues developed a two-site immunoradiometric assay that measured the active intact PTH.
Initially, a minimum of 24 hours was required for measurement of intact PTH using the two-site immunoradiometric assay. Recognizing that the half-life of PTH was on average 3 to 5 minutes, it was not long until a “quick” intraoperative PTH (IOPTH) assay was developed by modifying reagents and reducing incubation time that could produce an intact PTH result in 10 minutes. This led to the development and use of IOPTH monitoring for determining curative parathyroidectomy, which was championed by Irvin and colleagues in the early 1990s. This, in combination with advances in parathyroid localization paved the way for minimally invasive or focused parathyroidectomy.
The pharyngeal pouches in the human embryo give rise to the face, neck, and surrounding structures ( Fig. 38.1 ). The two pairs of parathyroid glands develop from the endoderm of the third and fourth branchial pouches and neural crest mesenchyme. The paired inferior parathyroid glands and the thymus develop from the third branchial pouch. The paired superior parathyroid glands develop from the fourth branchial pouch along with the lateral lobes of the thyroid gland and the ultimobranchial bodies ( Fig. 38.2 ).
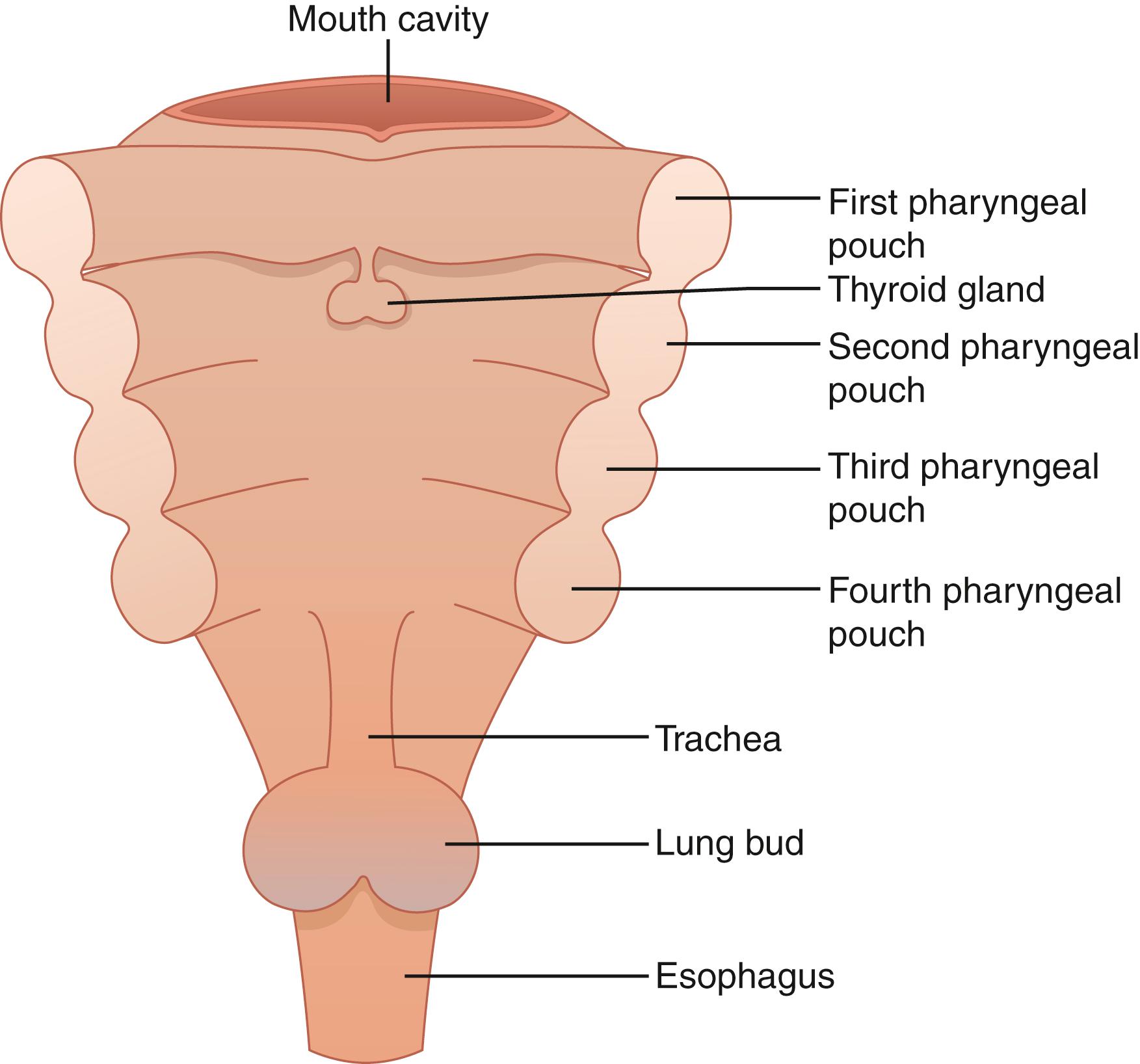
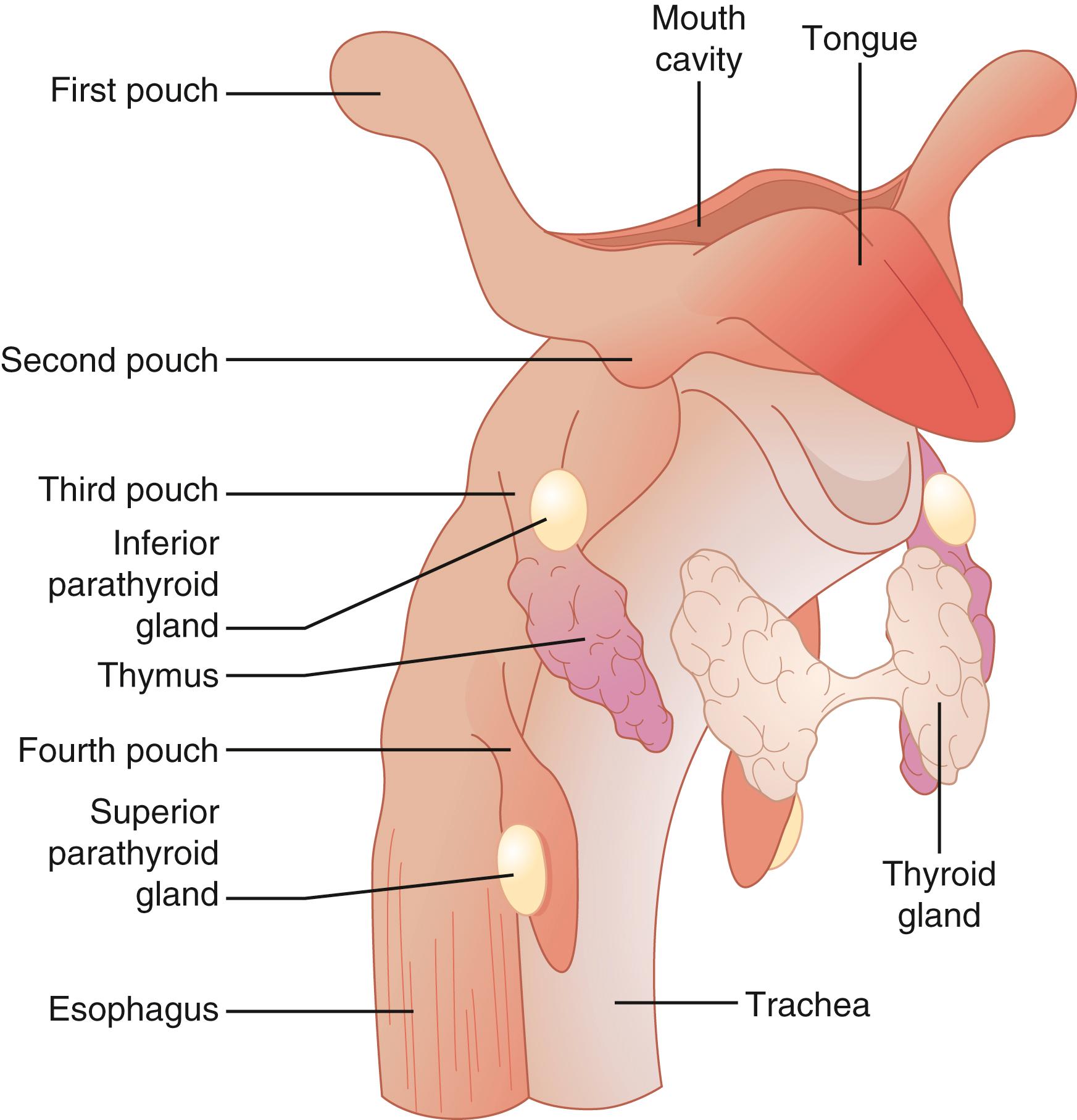
In the fifth week of embryologic development, the dorsal and ventral aspects of the third pharyngeal pouch differentiate into the inferior parathyroid glands and the thymus, respectively. At the sixth week of embryologic development, the inferior parathyroid glands and the primordia of the thymus lose their attachment with the pharynx and migrate caudally. The inferior parathyroid glands eventually locate on the dorsal surface of the inferior poles of the thyroid gland.
The dorsal aspect of the fourth pharyngeal pouch forms the superior parathyroid glands. The fourth pharyngeal pouch is also thought to give rise to the lateral lobes of the thyroid gland. When the superior parathyroid glands lose their attachment with the pharynx, they are thought to attach themselves to the thyroid, which descends caudally.
The more extensive embryologic migration of the inferior parathyroid glands is the explanation for their more frequent ectopic location. The embryologic migration of the superior parathyroid glands is more limited and, as a result, they are more likely to be found in the predictable anatomic location.
The mammalian homolog of drosophila glial cell missing gene ( GCM2 ) has been identified as a key regulatory gene in parathyroid differentiation and development. GCM2 has been shown to regulate the expression of key functional genes including PTH and may also be involved with the regulation of PTH gene expression. The factors involved in the upregulation of GCM2 , as well as the transcription factors and signaling pathways that are involved in the embryologic development of the parathyroid glands, have yet to be elucidated. Mutations in the GCM2 gene have also been associated with familial isolated HPT and a higher risk of parathyroid cancer.
Most individuals have paired superior and inferior parathyroid glands, although supernumerary and less than four glands have been reported in autopsy studies. Approximately 84% of individuals have four glands, 13% have more than four glands, and 3% have three glands. Normal parathyroid glands are oval, spherical, or bean shaped; they vary in color from yellow-tan to reddish brown, and on average are 5 × 3 × 2 mm in size and weigh 35 to 40 mg ( Fig. 38.3 ). , Parathyroid glands are typically surrounded by adipose tissue anteriorly, laterally, and posteriorly and the vascular pedicle is medial in location.
The inferior thyroid artery is the predominant vascular supply for both the superior and inferior parathyroid glands in 80% of cases. The inferior thyroid artery is a branch of the thyrocervical trunk that originates from the subclavian artery. Alternatively, the parathyroid glands may receive their blood supply from the superior thyroid artery or from small arterial branches directly from the thyroid gland. Each parathyroid gland has one or more fine end arterial branches that are sensitive to devascularization during parathyroid and thyroid surgery. In 70% to 80% of cases, the ipsilateral and contralateral parathyroid glands are located in symmetrical positions in the neck. Approximately 16% of patients with primary HPT will have parathyroid glands in an ectopic location.
The parathyroid glands are usually found within 5 mm of the superior and inferior margins of the tubercle of Zuckerkandl along the posterior capsule of the lateral lobes of the thyroid gland. The superior parathyroid glands are most commonly located on the posterior surface of the upper pole of the thyroid gland, posterior and superior to the recurrent laryngeal nerve ( Fig. 38.4 ). Because of their shorter embryologic migration compared to the inferior parathyroid glands, they have a more predictable anatomic location and are less likely to be ectopic. Eighty percent of the superior glands are found in their expected anatomic location, approximately 1 cm superior to the junction of the inferior thyroid artery and the recurrent laryngeal nerve at the level of the cricoid cartilage where the recurrent laryngeal nerve enters the larynx. The superior parathyroid glands are often found within the capsule of the thyroid gland.
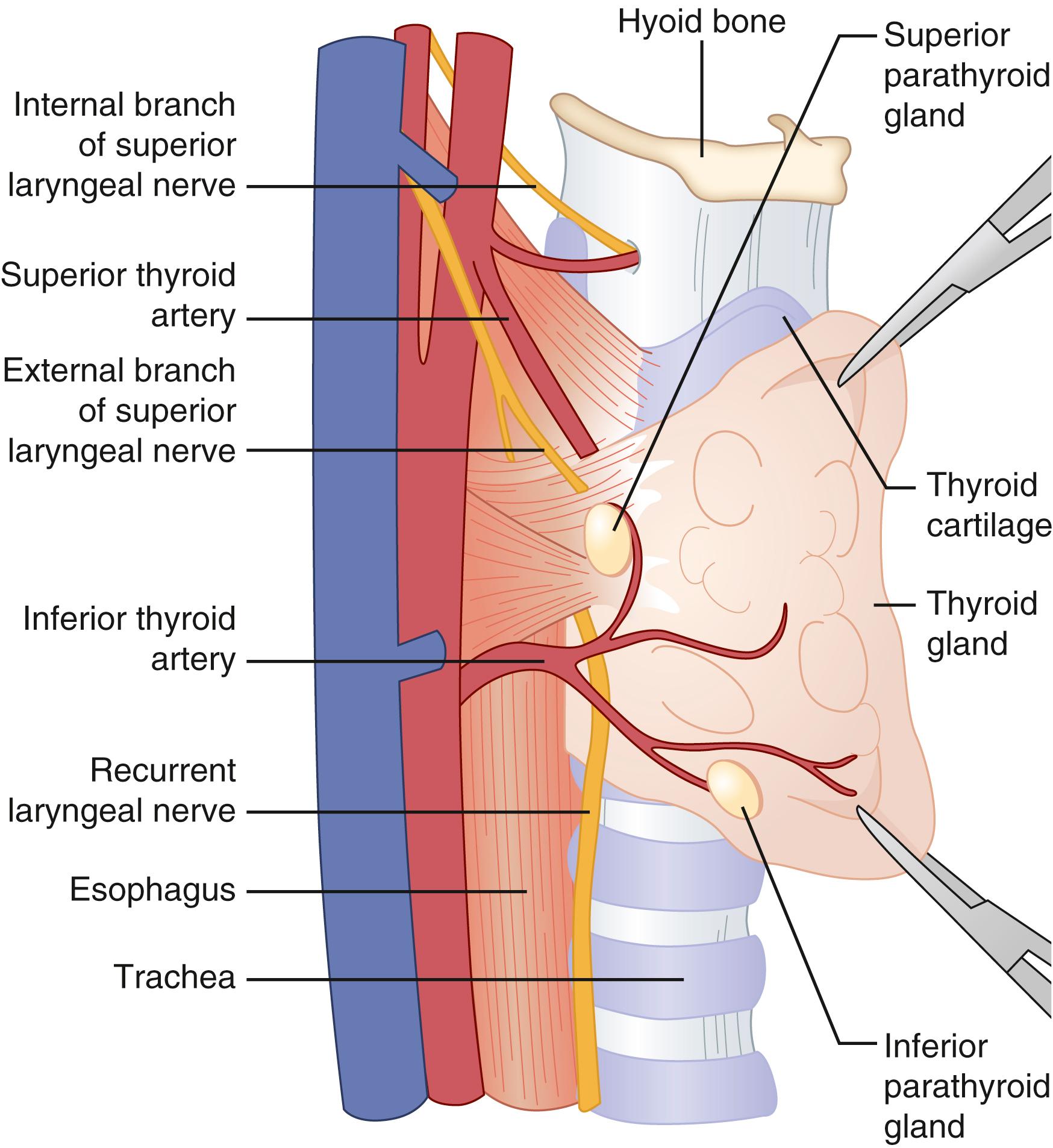
The challenge in identifying the superior parathyroid glands at the time of surgery is usually due to a lack of adequate mobilization and anteromedial rotation of the thyroid lobe to access their posterior location. In such a situation, ligation and division of the superior pole vessels may be helpful in optimizing exposure of an abnormal superior parathyroid gland. Ectopic locations of the superior parathyroid glands include the tracheoesophageal groove, behind the esophagus or the pharynx, in the posterior mediastinum and intrathyroidal.
The inferior parathyroid glands are most commonly found 1 cm inferior to the junction of the inferior thyroid artery and the recurrent laryngeal nerve, anterior and medial to the recurrent laryngeal nerve on the posterolateral aspect of the inferior pole of the thyroid lobe ( Fig. 38.4 ). The inferior parathyroid glands are less often found in a subcapsular location. Due to their more extensive embryologic migration, the inferior parathyroid glands are more commonly ectopic and can be found anywhere from the angle of the mandible to the pericardium. The most common ectopic location for an inferior gland is within the thymus ( Fig. 38.5 ). Other sites for an ectopic inferior parathyroid gland include the anterior superior mediastinum, the aortopulmonary window, the carotid sheath, intrathyroidal, and undescended in a submandibular location. Ectopic intrathyroidal parathyroid glands occur in 0.7% to 3.6% of patients with primary HPT.
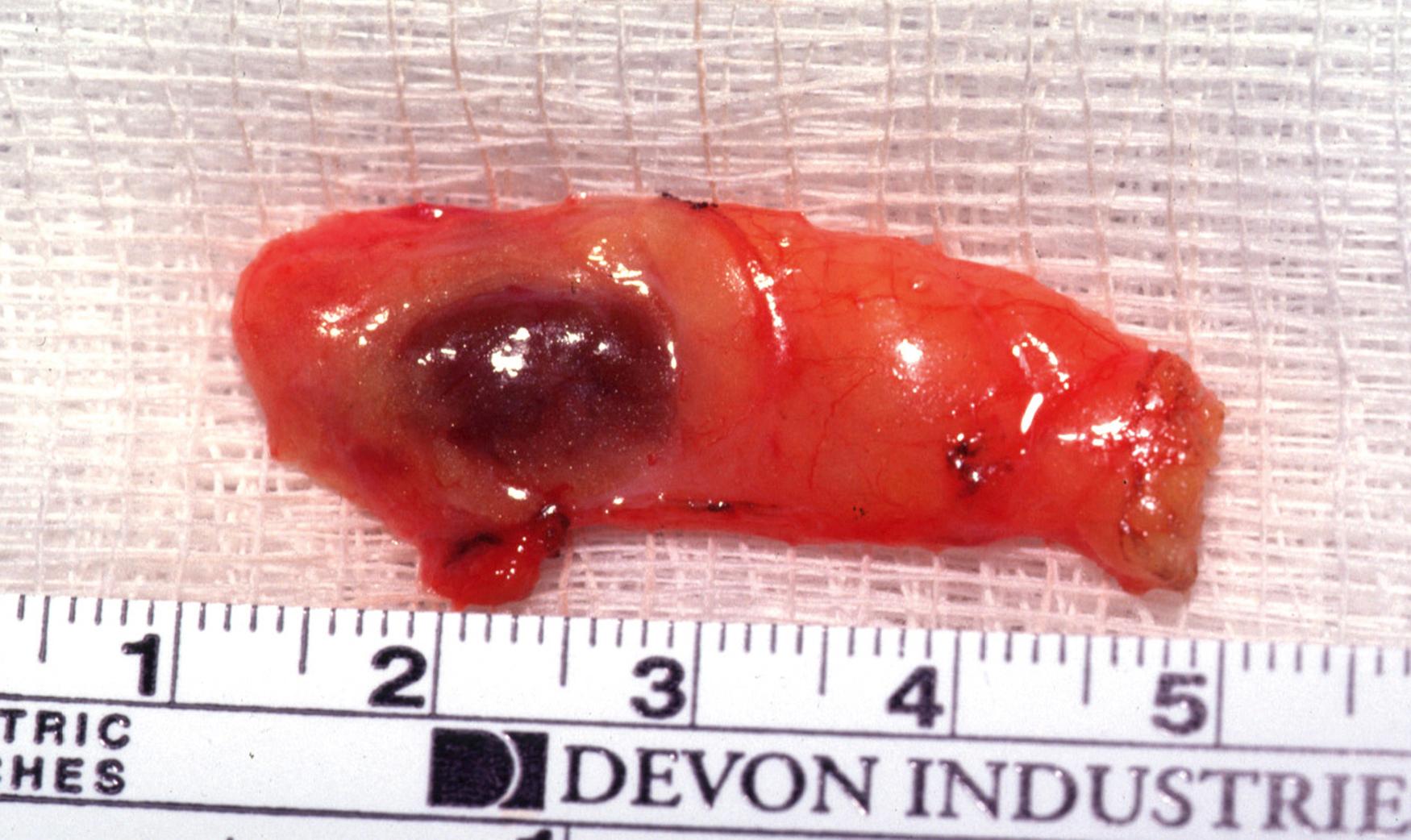
Each parathyroid gland has a connective tissue capsule from which fibrous septa develop that support and separate clusters of parenchymal cells. A variable number of adipose cells are interspersed between the clusters of parenchymal cells. A normal parathyroid gland consists of approximately 25% to 40% adipose cells. With age, the percent of adipose cells within the parathyroid gland increases. The color of the parathyroid glands is affected by the amount of fat. In older patients, the parathyroid glands are more often light yellow or tan in color because of a greater percentage of fat, whereas in young patients, the parathyroid glands are darker with a reddish-brown color because of a lesser percentage of fat.
The parenchymal cells of the parathyroid gland consist of two principal cell types: the chief cells and the oxyphil cells. Clear cells are a third cell type, which may occasionally be seen and are more often present with parathyroid hyperplasia. Chief cells are present in the greatest number in the parathyroid gland and are responsible for synthesis and secretion of PTH. The cytoplasm of the chief cells has secretory granules seen on electron microscopy that contain PTH.
The oxyphil cells are larger than chief cells and can be distinguished by their large, acidophilic granules in their cytoplasm. On electron microscopy, the acidophilic granules correspond to a large concentration of mitochondria. The number of oxyphil cells in the parathyroid gland increases with age. The function of the oxyphil cells is unknown. It has been previously postulated that the success or failure of technetium-99m-sestamebi imaging in identification of a parathyroid adenoma may, in part, be related to the oxyphil cell content of the abnormal parathyroid tissue.
It is important to recognize that histologic evaluation is unable to definitively differentiate a parathyroid adenoma from parathyroid hyperplasia and even normal from abnormal parathyroid tissue. The only expectation that a surgeon should have from intraoperative pathologic evaluation is to determine whether or not the tissue submitted is parathyroid tissue. It is sometimes important to ensure that excised tissue, presumed to be parathyroid, is not thyroid, nodal, or thymic tissue. Intraoperative pathologic examination of an excised parathyroid gland is usually unnecessary if IOPTH monitoring is used.
Extracellular calcium concentration is the predominant regulator of PTH synthesis and secretion by the chief cells of the parathyroid glands. Calcium binds to calcium-sensing receptors (CaSRs) on the surface of the chief cells and inhibits PTH secretion and PTH gene expression. High calcium also enhances PTH degradation into fragments. Conversely, when calcium is reduced, PTH gene expression and secretion are increased. 1,25-Dihydroxyvitamin D (1,25[OH] 2 D), the active form of vitamin D, binds to vitamin D receptors on chief cells and inhibits PTH gene expression and parathyroid cell proliferation. Other regulators of PTH secretion include lithium, transforming growth factor-alpha, prostaglandins, inorganic phosphate, and fibroblast growth factor 23.
PTH synthesis begins with the production of a 115-amion acid pre-pro-PTH polypeptide, which is cleaved in the chief cells to the active 84–amino acid intact-PTH peptide. The NH2 terminus, a 34-amino acid residue and the biologically active part of the intact-PTH peptide, which binds to the G-protein-coupled PTH/PTHrP receptor type 1 (PTHR1). PTHR1 is highly expressed in bone and kidney.
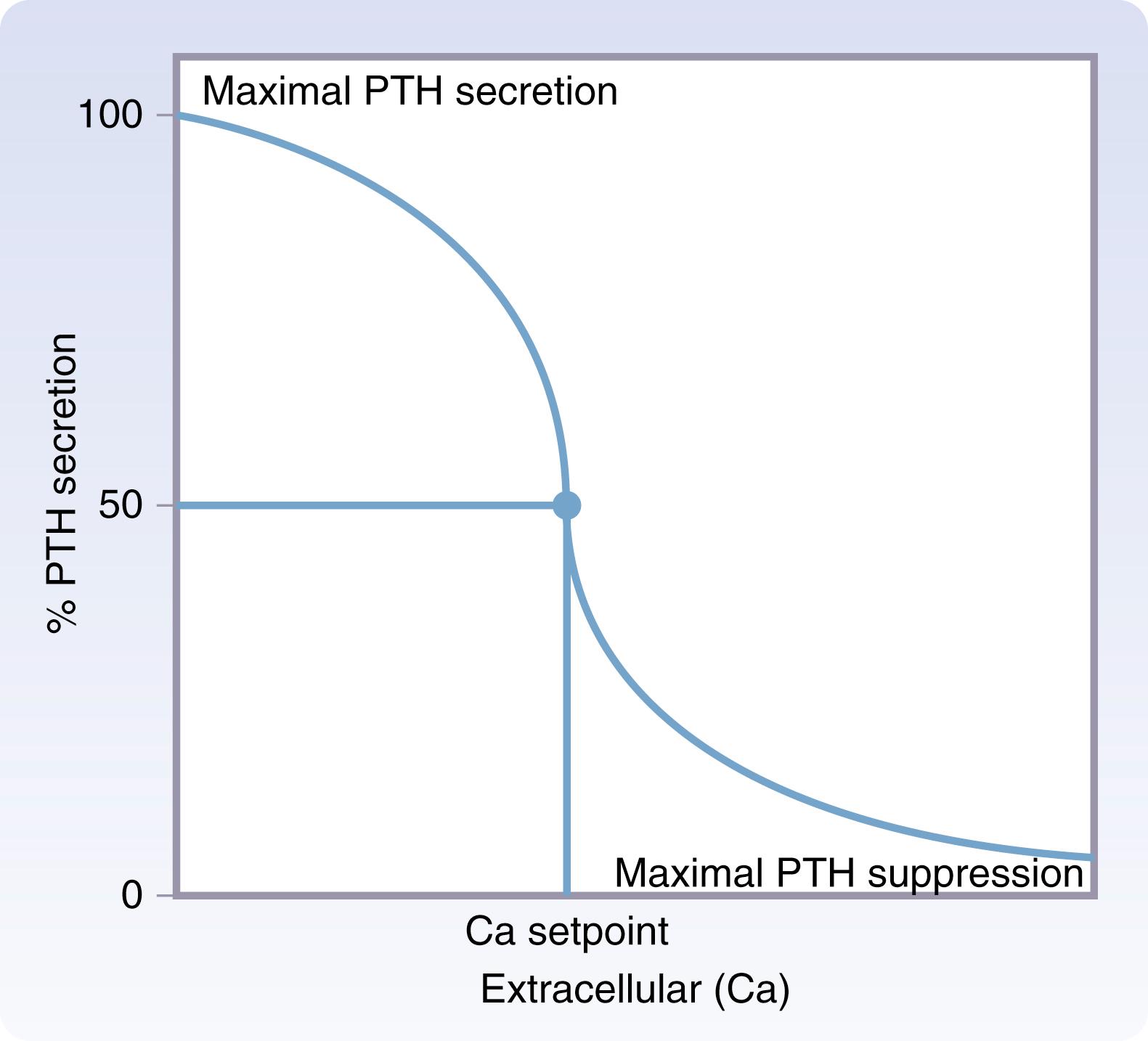
PTH is essential in the regulation of calcium and phosphate metabolism. Extracellular calcium concentration is the principal stimulus regulating PTH secretion. A reverse sigmoidal relationship exists between extracellular calcium concentration and PTH secretion ( Fig. 38.6 ). Maximum PTH secretion occurs at low extracellular calcium concentrations and maximal suppression occurs at high extracellular calcium concentrations. Extracellular calcium levels are tightly maintained at a calcium set point, which is defined as the extracellular calcium concentration where PTH secretion is half-maximally suppressed. The calcium set point occurs on the steep portion of the PTH curve such that large increases in PTH secretion occur in response to small reductions in extracellular calcium concentration and large reductions in PTH secretion occur with small increases in extracellular calcium concentration detected by the CaSR on parathyroid chief cells.
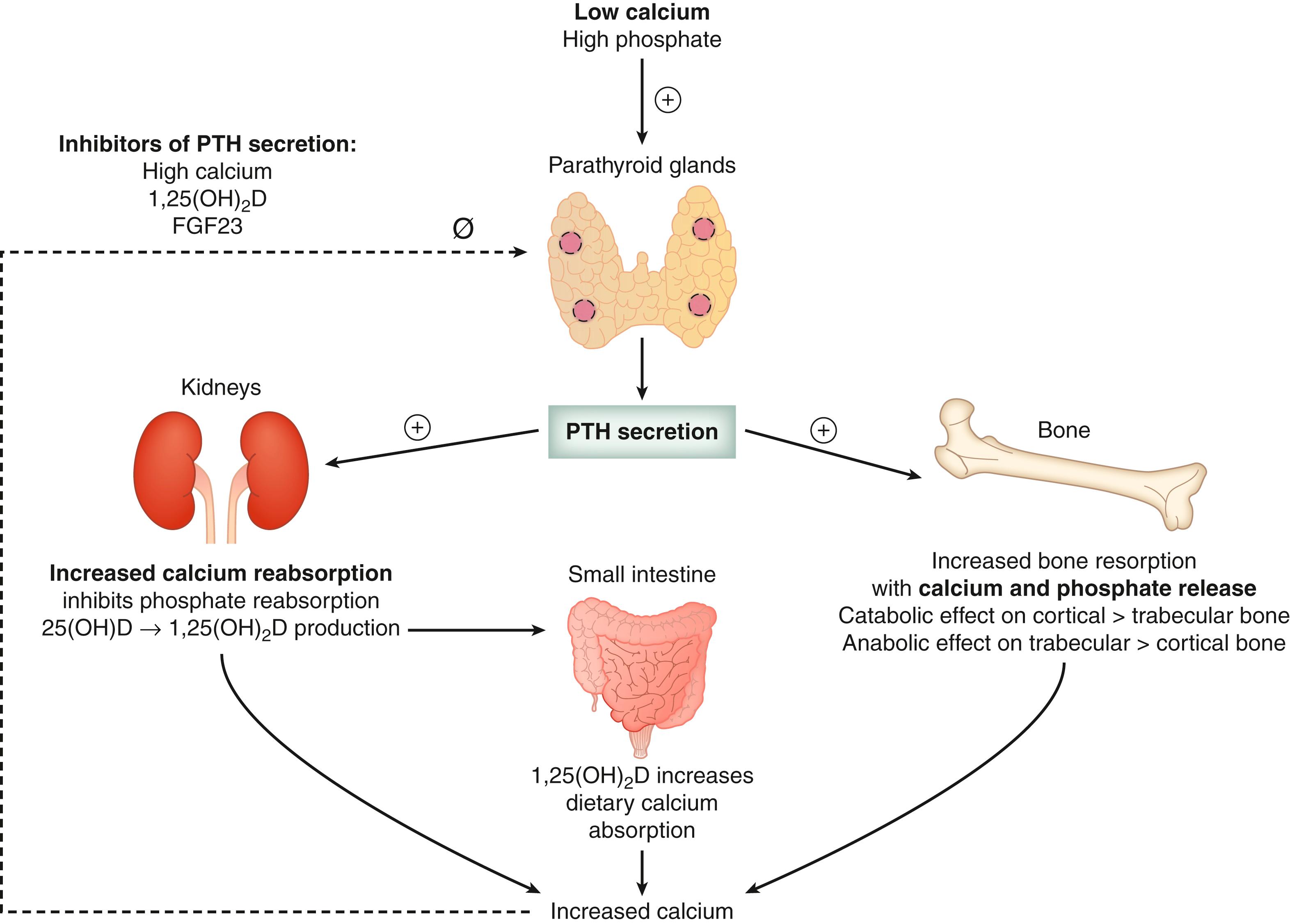
PTH acts on the ascending limb of the loop of Henle and the distal convoluted tubule of the kidneys to enhance calcium reabsorption, inhibit phosphate reabsorption, and increase phosphate excretion causing phosphaturia. Also in the kidneys, PTH stimulates the conversion of 25-hydroxyvitamin D (25[OH]D) to the active metabolite 1,25[OH] 2 D by activating the gene that encodes for 25-hydroxyvitamin D-1α hydroxylase enzyme ( Fig. 38.7 ). 1,25[OH] 2 D increases intestinal absorption of dietary calcium in the small intestine ( Fig. 38.7 ). PTH increases bone remodeling and release of calcium and phosphate from the skeleton. The PTH-mediated renal effects to maintain calcium homeostasis are rapid, taking minutes to hours, compared to the skeletal effects, which take hours to days.
PTH has a complex effect on bone remodeling, with both anabolic and catabolic effects. PTH binds to the PTHR1 receptor that is found on cells of the osteoblastic lineage, which ultimately leads to the formation of new bone matrix and mineralization. However, osteoblast-lineage cells also produce regulators of osteoclast formation, such as the receptor activator of nuclear factor-κB, receptor activator of nuclear factor-κB ligand, and osteoprotegerin. PTH increases osteoclast activity indirectly through the effects of receptor activator of nuclear factor-κB ligand and osteoprotegerin, leading to increased bone resorption with calcium and phosphate ion release from hydroxyapatite, the major mineral component of bone. The net effect of PTH on bone varies based on the severity and chronicity of PTH excess. In patients with chronically elevated PTH levels, such as primary and secondary HPT, the net effect is bone resorption leading to osteopenia and osteoporosis. However, intermittent administration of recombinant human PTH has been shown to stimulate bone formation more than resorption and is currently in clinical use for treatment of osteoporosis.
The half-life of PTH is 3 to 5 minutes, although it can vary from 1 to 21 minutes. PTH fragments are produced in the liver and in the parathyroid cells and are excreted by the kidney. The renal clearance of PTH fragments is slower than the PTH breakdown into fragments. Thus, 80% of circulating PTH consists of fragments that are inactive, while only 20% is the intact biologically active PTH peptide.
HPT refers to excess synthesis and secretion of PTH by abnormal parathyroid glands. Depending on the underlying cause of excess PTH, HPT is classified as primary, secondary, or tertiary. Primary HPT is caused by autonomous overproduction of PTH by one or more abnormal parathyroid glands. Most patients with primary HPT present with hypercalcemia and an elevated or inappropriately high-normal (nonsuppressed) PTH level. It affects 1 out of every 500 women and 1 out of every 2000 men. It occurs most commonly in postmenopausal women between 50 and 60 years of age. The incidence of primary HPT increases with age and the prevalence is about 1% in postmenopausal women and 0.86% in the general population.
Primary HPT and malignancy are the most common causes of hypercalcemia in adults, accounting for approximately 80% of all cases of hypercalcemia. Less common causes account for the other 20% ( Table 38.1 ). Unlike primary and tertiary HPT, most other causes of hypercalcemia are associated with a low serum PTH level, with the exception of prolonged lithium therapy and familial hypocalciuric hypercalcemia (FHH). Patients on prolonged lithium therapy may develop lithium-induced HPT.
| Endocrine | Primary hyperparathyroidism |
| Tertiary hyperparathyroidism | |
| Familial hypocalciuric hypercalcemia | |
| Hyperthyroidism | |
| Malignancy | Tumors producing PTHrP (SCC of lung, bladder cancer, renal cell cancer) |
| Osteolytic bone metastasis | |
| Hematologic malignancies (lymphoma, leukemia, multiple myeloma) | |
| Granulomatous Disease | Sarcoidosis |
| Tuberculosis | |
| Fungal infection | |
| Medications | Calcium |
| Thiazide diuretics | |
| Lithium | |
| Vitamin A and D intoxication | |
| Milk alkali syndrome | |
| Miscellaneous | Paget and other bone diseases with prolonged immobilization |
FHH is a rare autosomal dominant disorder affecting the renal CaSR that results in a higher set point for renal calcium excretion, hypocalciuria, asymptomatic hypercalcemia, and normal or mildly elevated PTH levels. Patients with FHH have none of the clinical manifestations of primary HPT. Because FHH has a high penetrance, patients typically develop chronically elevated serum calcium levels before the age of 30, and thus, it is uncommon for a postmenopausal woman to be newly diagnosed with FHH without a history of chronic mild hypercalcemia. , No treatment is required for FHH.
Primary HPT occurs when one or more abnormal parathyroid glands enlarge and secrete PTH inappropriately relative to serum calcium levels. The three pathologic conditions that result in primary HPT are parathyroid adenoma, hyperplasia, and carcinoma. Increased serum calcium levels occur as a result of PTH-induced increases in bone resorption, renal calcium reabsorption, and indirectly by increased dietary calcium absorption from the gastrointestinal tract. Under normal conditions, a high serum calcium level should inhibit excess PTH production ( Figs. 38.6 and 38.7 ). However, the parathyroid cells in a parathyroid adenoma have a lower-than-normal sensitivity to the inhibitory action of calcium, with a higher calcium set point, such that a higher circulating level of calcium is maintained. In parathyroid hyperplasia, there is an overall increase in the number of parathyroid cells, which produce excess PTH but maintain their normal sensitivity to calcium. Both conditions give rise to primary HPT and cause hypercalcemia.
The frequency of multigland disease (double adenoma or four gland hyperplasia) is variable and is dependent on how it is defined. Historically, multigland disease has been defined based on gland size, morphology, and histology. More recently, multigland disease has been defined as persistent elevation of IOPTH levels despite the removal of a single hypersecreting parathyroid gland or persistent postoperative HPT after removal of a single parathyroid gland. Multiglandular disease occurs in approximately 10% to 15% of all patients with primary HPT, ranging from 4% to 33% in the literature, whereas a single parathyroid adenoma occurs in approximately 85% to 90% of patients with primary HPT. Parathyroid cancer is found in less than 1% of cases. ,
Exposure to ionizing radiation in the cervical region is a risk factor for developing primary HPT. This includes external beam radiation for benign and malignant conditions, exposure during nuclear accidents and radioactive iodine ablation for thyroid disease. The latency period is approximately 25 to 40 years. Lithium therapy is a well-known cause of hypercalcemia and HPT. Up to 15% of patients who are on chronic lithium for more than 10 years develop HPT. Lithium decreases the parathyroid cells’ sensitivity to calcium and alters the calcium set point for PTH secretion. Lithium-associated HPT is also associated with a comparatively higher incidence of parathyroid hyperplasia. Thiazide diuretics, although not a risk factor for primary HPT, may unmask preexisting HPT by causing increased renal calcium reabsorption and increased serum calcium levels. Patients with primary HPT who are taken off thiazides remain hypercalcemic.
Primary HPT is most commonly a sporadic disease. In approximately 3% to 5% of patients, primary HPT occurs as part of a familial syndrome including multiple endocrine neoplasia (MEN) 1, MEN2A, MEN4, HPT-jaw tumor syndrome (HPT-JT), and familial isolated HPT ( Table 38.2 ). Some of the mutations that cause sporadic primary HPT overlap with mutations found in familial HPT. Patients with inherited HPT are typically younger, may have a family history of endocrinopathies, and may exhibit other associated findings of a particular syndrome (see “Familial Primary HPT” section).
| Disorder | Genes | Inheritance | Phenotype | Age ∗ | Surgical Approach † |
|---|---|---|---|---|---|
| Sporadic | |||||
| Parathyroid adenoma and hyperplasia | PRAD1, CCND1 (20%–40%), MEN1 (12%–35%), CDC73 , CDKN1B , AIP | Sporadic | Isolated pHPT with single adenoma, double adenoma or four-gland hyperplasia | >45 | Minimally invasive parathyroidectomy with IOPTH or bilateral exploration |
| Parathyroid carcinoma | RB1, CDC73, MEN1 , microRNA-296 | Sporadic | pHPT secondary to parathyroid carcinoma | >45 | En bloc resection |
| Inherited | |||||
| MEN1 | MEN1 | AD | pHPT (95%) in third decade, pNETs (40%), pituitary adenomas (30%), adrenocortical and thyroid tumors, meningioma, facial angiofibromas, lipomas | 25–45 | Subtotal parathyroidectomy and transcervical thymectomy |
| MEN2A | RET | AD | pHPT (15%–35%), MTC (99%), pheochromocytoma (50%), lichen planus, amyloidosis, Hirschsprung disease | 38 | Resection of only visibly enlarged parathyroid glands. Address or rule out pheochromocytoma first |
| MEN4 | CDKN1B | AD | pHPT (80%), pituitary adenoma (40%), pNETs, adrenal, thyroid, gonadal and renal tumors | 50 | Same as MEN1 |
| Familial isolated HPT | MEN1, CDC73, CASR, GCM2, CDKN1B | AD | Isolated pHPT, parathyroid carcinoma ( GCM2 mutation) | 39 | Bilateral neck exploration with resection of enlarged glands only versus subtotal parathyroidectomy En bloc resection for parathyroid cancer |
| HPT-JT | CDC73 | AD | pHPT in second or third decade, parathyroid carcinoma (35%), ossifying fibromas of mandible and maxilla, renal cysts, hamartomas and Wilms tumor, and uterine tumors | 32 | Minimally invasive parathyroidectomy with IOPTH or bilateral exploration En bloc resection for parathyroid cancer |
Up to 80% of patients with primary HPT are diagnosed as a result of incidental hypercalcemia found on routine bloodwork. However, patients with primary HPT can have diverse symptoms, which are the result of calcium effects on multiple organ systems, including the renal, skeletal, gastrointestinal, cardiac, and neuromuscular systems ( Table 38.3 ). In a recent cohort study of over 9000 patients, 35% of patients had objective findings of end-organ effects at the time of diagnosis, including osteoporosis, nephrolithiasis, or hypercalciuria. In addition, 62% of patients developed end-organ effects within 5 years of diagnosis. Up to 20% of patients with primary HPT have symptomatic nephrolithiasis, 10% of patients have recurrent kidney stones, and up to 12% of patients have “silent nephrolithiasis” when they are screened with abdominal imaging. ,
| Target Organ or System | Symptoms | Comments |
|---|---|---|
| Renal | Nephrolithiasis, nephrocalcinosis, polyuria, polydipsia, renal insufficiency | 15%–20% of patients have kidney stones |
| Skeletal | Fragility fractures | Unrelated to significant trauma |
| Osteopenia/osteoporosis | Cortical bone > trabecular bone (distal third of radius most affected) | |
| Bone pain | Common | |
| Osteitis fibrosa cystica | Rare, but may occur with advanced disease, characterized by bone pain and multiple skeletal deformities including salt and pepper appearance of the skull, bone cysts, and brown tumors of bone | |
| Neuromuscular | Proximal muscle weakness, muscular atrophy, gait disturbance | Rare |
| Easy fatigability, generalized weakness | Common | |
| Gastrointestinal | Gastroesophageal reflux, constipation, abdominal pain, peptic ulcer disease | Common Rare |
| Nausea, vomiting, acute pancreatitis | Rare, can be seen in cases of severe hypercalcemia | |
| Neuropsychiatric | Fatigue, depression, anxiety, emotional lability, sleep disturbances, lethargy, memory loss, inability to concentrate, mental status change, psychosis, obtundation, and coma | Often reported |
| Rare, can be seen with severe hypercalcemia | ||
| Cardiovascular | Exacerbation of hypertension, valvular disease, myocardial calcifications, premature atherosclerosis, left ventricular hypertrophy, shortened QT interval, conduction abnormalities, and heart block | Conflicting data on improvement of cardiac parameters after parathyroidectomy |
Primary HPT results in loss of bone mass, which is most pronounced at sites of cortical bone in the distal third of the radius and the femoral neck. However, recent studies using high-resolution peripheral quantitative CT (HRpQCT) have demonstrated that both cortical and trabecular bone are affected by primary HPT, with increased fractures seen in the vertebral bodies in patients with primary HPT compared to a control population. , Up to 15% of patients with HPT have osteopenia in the lumbar spine. In a longitudinal observational study, patients with untreated primary HPT followed for 15 years had a decline in bone mineral density of 35% at the distal radius and 10% at the femoral neck. All patients with primary HPT should be screened for osteopenia and osteoporosis with measurement of bone mineral density of the distal third of the radius, the lumbar spine, the femoral neck, and the hip.
Neuromuscular and neuropsychiatric symptoms are often reported by patients with primary HPT. Many of these symptoms may improve with surgical intervention, and include easy fatigability, generalized weakness, depression, anxiety, memory loss, and inability to concentrate. Three randomized controlled trials show neurocognitive benefits of surgery when compared with observation in patients with primary HPT.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here