Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Under the low power of the light microscope, normal liver is seen to have a regular structure based on portal tracts and efferent veins. The smallest portal tracts contain portal venules, hepatic arterioles and small interlobular bile ducts. Blood from both venules and arterioles passes through the sinusoidal system to reach efferent hepatic venules. From these, the blood drains into successively larger veins to reach the inferior vena cava. Bile flows from the smallest ducts into larger ducts, to reach the small intestine by way of the common bile duct.
The functional relationship between these various structures has been the subject of much debate. The most widely used models are the classic lobule and Rappaport’s acinus. The lobule has an efferent venule at its centre and portal tracts at its periphery ( Fig. 3.1 ). The acinus is based on a terminal portal tract, with blood passing from this, through successively less well-oxygenated parenchymal zones 1, 2 and 3, to efferent venules. It is worth emphasising that both lobules and acini are concepts rather than fixed anatomical structures. Several other models have been proposed, as well as modifications to the original lobular model. From a pathologist’s point of view, both lobular and acinar concepts have their merits in different situations. To give examples, the sinusoidal congestion of venous outflow obstruction is often more easily understood on the basis of the lobule, with maximum intensity at its centre. Bridging hepatic necrosis, however, is difficult to understand in terms of the lobule and has been explained as death of hepatocytes in acinar zones 3, the zones in which oxygen saturation is relatively low. In everyday practice it seems best to use words compatible with either model as far as possible. In this book we have therefore used the term ‘periportal’ to describe the part of the parenchyma lying nearest to a small portal tract, and ‘perivenular’ for the parenchyma near an efferent venule.

Portal tracts of different size may be seen in biopsies ( see Fig. 4.2 ). The smallest represent terminal tracts from which blood enters the parenchyma. Larger portal tracts contain vessels and ducts which convey blood and bile to and from the smaller tracts. Pathological processes do not necessarily affect large and small tracts to the same extent.
A typical small portal tract contains a bile duct, portal venule, hepatic arteriole and lymphatics, all embedded in connective tissue ( Fig. 3.2 ). A few lymphocytes and mast cells may be seen even in normal subjects and nerve fibres can be demonstrated by appropriate staining. The exact contents are variable, however, depending in part on the angle of sectioning. In a study of 16 needle biopsies from normal subjects, 38%, 9% and 7% of tracts did not contain a portal-vein branch, hepatic arteriole or bile duct, respectively. Most, but by no means all, hepatic artery branches are accompanied by bile ducts. These observations have obvious implications for the histological diagnosis of bile duct or blood vessel loss. A confident diagnosis requires examination of several portal tracts.

Near or at the margins of the small portal tracts, the bile canaliculi, formed as spaces between adjacent hepatocytes, communicate with the canals of Hering. These are lined partly by hepatocytes and partly by biliary epithelial cells. From the canals of Hering, bile drains into bile ductules lined entirely by biliary epithelium ( see Fig. 5.1 ). Neither canals of Hering nor ductules are easily seen in normal liver, but they may become apparent in disease ( Fig. 3.3 ). The exact location of the junction between the canals of Hering and bile ductules varies, the ductules sometimes having an intraparenchymal portion, seen in two-dimensional sections as apparently isolated ductules among hepatocytes. The canals of Hering and bile ductules have received much attention in recent years because they appear to be the site of a progenitor-cell compartment which becomes activated when a need for new hepatocytes and bile ducts cannot be adequately met otherwise. Progenitor cells can be immunostained for cytokeratins CK7 and CK19, EpCAM (epithelial cell adhesion molecule) and NCAM (neural cell adhesion molecule) and also stain with OV-6, an antibody used on frozen tissue to mark similar cells (oval cells) in rodents. The response to various types of liver injury may also involve participation of hepatoblasts derived from progenitor cells.
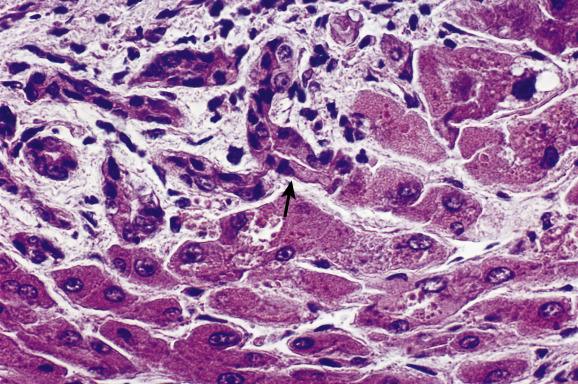
The interlobular ducts into which the ductules drain have an internal diameter of less than 100 μm and are more or less centrally located in the small portal tracts. They are lined by cuboidal or low columnar epithelium and have a basement membrane associated with diastase periodic acid–Schiff (DPAS)-positive material. Portal venules and hepatic arterioles usually lie close to these ducts but, as already noted, not all three structures are necessarily seen in a single plane of section. Positive identification of bile ducts in pathological states can be difficult, but is made easier by cytokeratin staining; ducts contain CK7 and CK19 in addition to CK8 and CK18; the latter two are also found in hepatocytes.
Bile drains from the interlobular ducts into septal bile ducts having an internal diameter of more than 100 μm. Septal ducts are lined by tall columnar epithelium, with basally located nuclei. These and larger ducts towards the hepatic hilum are sometimes associated with heterotopic exocrine pancreatic tissue. Around the largest intrahepatic ducts there are peribiliary glands.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here