Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Mammals face threats to life and limb on a daily basis. Survival hinges on multiple strategies, both learned and inborn. For example, zebras display stripes to help blend into the pack and avoid being isolated. Rodents forage for food under cover of darkness to minimize detection by predators. Parents teach their children to look both ways before crossing a busy street. All of these survival tactics developed to minimize the odds of bodily harm and maximize chances for finding food, reproducing, and raising young.
Despite these measures, mammals will inevitably sustain one or more injuries during their lifetime. Fortunately, evolution has also prepared for these occurrences through a multitude of organ systems and adaptations. The nervous system plays a prominent role in defending the host against environmental threats. The fight or flight response as described by Walter Cannon is a well-described example. Here the sympathetic arm of the autonomic nervous system (ANS) releases norepinephrine (NE) and epinephrine into the general circulation to prepare the host to fight or flee a perceived threat. Downstream effects of these stress hormones include inhibition of gastrointestinal function and increases in heart rate, blood pressure, respiratory rate, blood flow to muscles, and liberation of metabolic energy stores.
Our laboratory studies how the nervous and immune systems interact to protect against tissue injury and infection. We characterized the inflammatory reflex, a novel brain-to-immune pathway that monitors and regulates systemic inflammation through afferent and efferent vagus nerve signaling ( ; Fig. 130.1 ). Sensory information from the periphery is transmitted to the brain via afferent vagal fibers, which comprise 80% of total nerve fibers. As an example, injection of interleukin-1beta (IL-1β) into the peritoneal cavity of rodents elicits a fever response, but only in the presence of an intact vagus nerve ( ). Higher doses of cytokines can bypass this vagal pathway and elicit fever, which suggests that the vagus nerve is positioned as a sensitive and rapid detector of changes in systemic inflammation ( ).
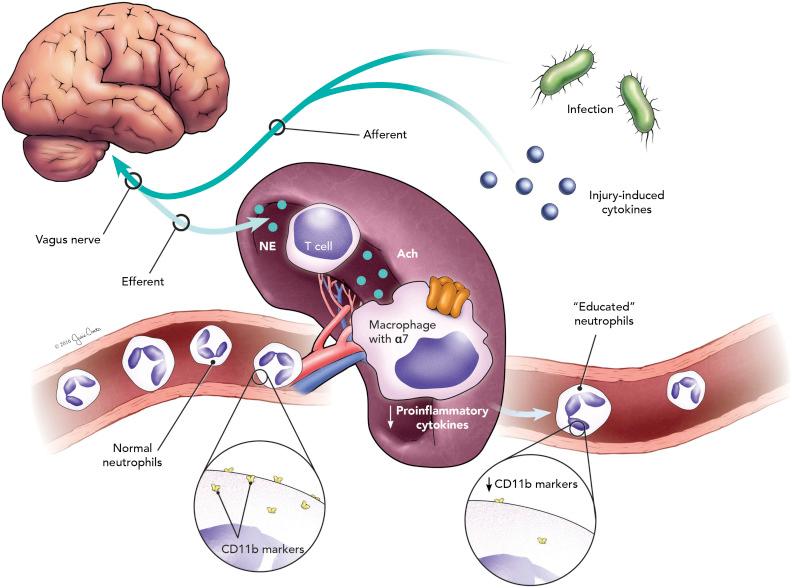
The brain can respond to changes in peripheral inflammation through release of counterregulatory stress hormones, including cortisol, into the circulation. Cortisol has numerous immunomodulatory effects, including suppression of cytokine production and inhibition of T cell proliferation. Identification of the inflammatory reflex highlighted that the brain also reacts to inflammation through efferent vagus nerve firing. This effector arm of the inflammatory reflex is termed the cholinergic antiinflammatory pathway (CAP) because acetylcholine (ACh) is the primary neurotransmitter of the vagus nerve. Initial studies in rodents demonstrated that electrical stimulation of the cervical vagus nerve significantly reduces systemic proinflammatory cytokine production following endotoxin challenge ( ). Follow-up studies showed that electrical vagus nerve stimulation limits organ specific cytokine production, with the greatest reduction coming in the spleen ( ). Moreover, splenectomy abolishes the systemic antiinflammatory effects of vagus nerve stimulation (VNS), reflecting the essential nature of this organ to the pathway ( ).
Elucidation of the vagal pathway to the spleen was initially challenging because autonomic input to the spleen is through the splenic nerve, which releases NE rather than ACh. Additional studies found that splenic neurectomy abolishes the effects of VNS, suggesting that the vagus nerve innervates the spleen by first synapsing with the splenic nerve in the celiac ganglion ( ). Cervical VNS causes release of NE in the spleen, which then modulates a specific subset of T lymphocytes containing choline acetyltransferase (ChAT) ( ). Absence of these cells through genetic knockout abolishes antiinflammatory vagus nerve signaling ( ). Finally, ACh from these ChAT-positive T cells downregulates cytokine-producing tissue macrophages in the spleen by binding to the α7 nicotinic acetylcholine receptor subunits on their cell surface ( ). As such, mice genetically deficient in α7 no longer respond to electrical VNS ( ).
While studying the inflammatory reflex, it became clear that an effective and proportional innate immune response is tantamount to survival. We reasoned, however, that uncontrolled inflammation is likely not the most immediate threat to life after injury. The most important initial priority after injury is achieving hemostasis. Uncontrolled bleeding from a major blood vessel can kill on the order of seconds to minutes. In comparison, the earliest and most robust proinflammatory cytokine responses, such as tumor necrosis factor (TNF) production, do not peak for an hour or longer following infection or injury. The body’s response to tissue injury reflects this priority, as the first cells to arrive at a wound are platelets, not neutrophils.
The substantial risks of traumatic hemorrhage are also reflected epidemiologically. Currently, the most common preventable cause of death following trauma is uncontrolled bleeding. This sobering statistic is amplified further by the notion that trauma is the third leading cause of death annually in the United States. In contrast to other leading causes of mortality that afflict older individuals, including heart disease, cancer and stroke, trauma is the most common cause of death of children and adults under the age of 45. Not surprisingly, the economic burden to society of losing individuals before or during their prime of age is astronomical.
Similar to these civilian statistics, trauma deaths in the military are also disproportionally caused by uncontrolled bleeding. Advances in fluid and blood product resuscitation are undoubtedly helping to improve overall outcomes from massive hemorrhage ( ). Nevertheless, noncompressible hemorrhage from penetrating torso injuries remains the most common preventable cause of death in this arena. Extremity hemorrhage also causes significant morbidity and mortality, although it is somewhat less problematic to treat in the field due to the use of tourniquets. This approach could still benefit from innovation, as tourniquet use remains relatively unchanged from its origins in the fourth century BC during the time of Alexander the Great.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here