Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Upon completion of this chapter, the student should be able to answer the following questions:
Describe the general anatomical components of the male and female reproductive system.
Map out the organization of the testis, with the Sertoli cells and developing sperm cells within the intralobular compartment, and the Leydig cells and capillary plexus within the interlobular/interstitial compartment.
Illustrate the processes of spermatogenesis and spermiogenesis.
List the functions of the Sertoli cell.
Diagram the process of testosterone synthesis within the Leydig cells, and the peripheral conversion of testosterone to estradiol or dihydrotestosterone.
Diagram the male hypothalamus/pituitary/testis axis, including all cell types and hormones involved.
Map out the organization of the ovary, and describe the various stages of follicular development, ovulation and corpus luteum formation.
List the stages and control of female germ cell progression from oogonia to egg.
Map out the steroidogenic pathways in the corresponding cell types that lead to androgen, estrogen, and progesterone synthesis.
Diagram the female hypothalamus/pituitary/ovarian axis during the menstrual cycle, including all cell types and hormones involved.
Explain changes in the female tract, with emphasis on the uterine endometrium, during the menstrual cycle.
List the events involved in fertilization.
Describe the development and function of the placenta.
Describe the development and function of the mammary glands.
The two most basic anatomical components of the reproductive system are the gonads and the reproductive tract. The gonads ( testes and ovaries ) perform an endocrine function that is regulated within a hypothalamic-pituitary-gonadal axis. The gonads are distinct from other endocrine glands in that they also perform gametogenesis. The reproductive tract is involved in several aspects of gamete development, function, and transport, and in women, allows fertilization, implantation, and gestation. Gametogenesis in the gonads, and development and physiology of the reproductive tract, are absolutely dependent on the endocrine function of the gonads. The clinical ramifications of this hormonal dependence include infertility in the face of low sex hormone production, ambiguous genitalia in dysregulated hormone or hormone receptor expression, and hormone-responsive cancers, especially uterine and breast cancer in women and prostate cancer in men.
The male reproductive system has evolved for continuous lifelong gametogenesis coupled with occasional internal insemination with a high density of sperm (>60 × 10 6 /mL in 3–5 mL of semen). In adult men the basic roles of gonadal hormones are (1) support of gametogenesis (spermatogenesis), (2) maintenance of the male reproductive tract and production of semen, and (3) maintenance of secondary sex characteristics and libido. There is no overall cyclicity of this activity in men.
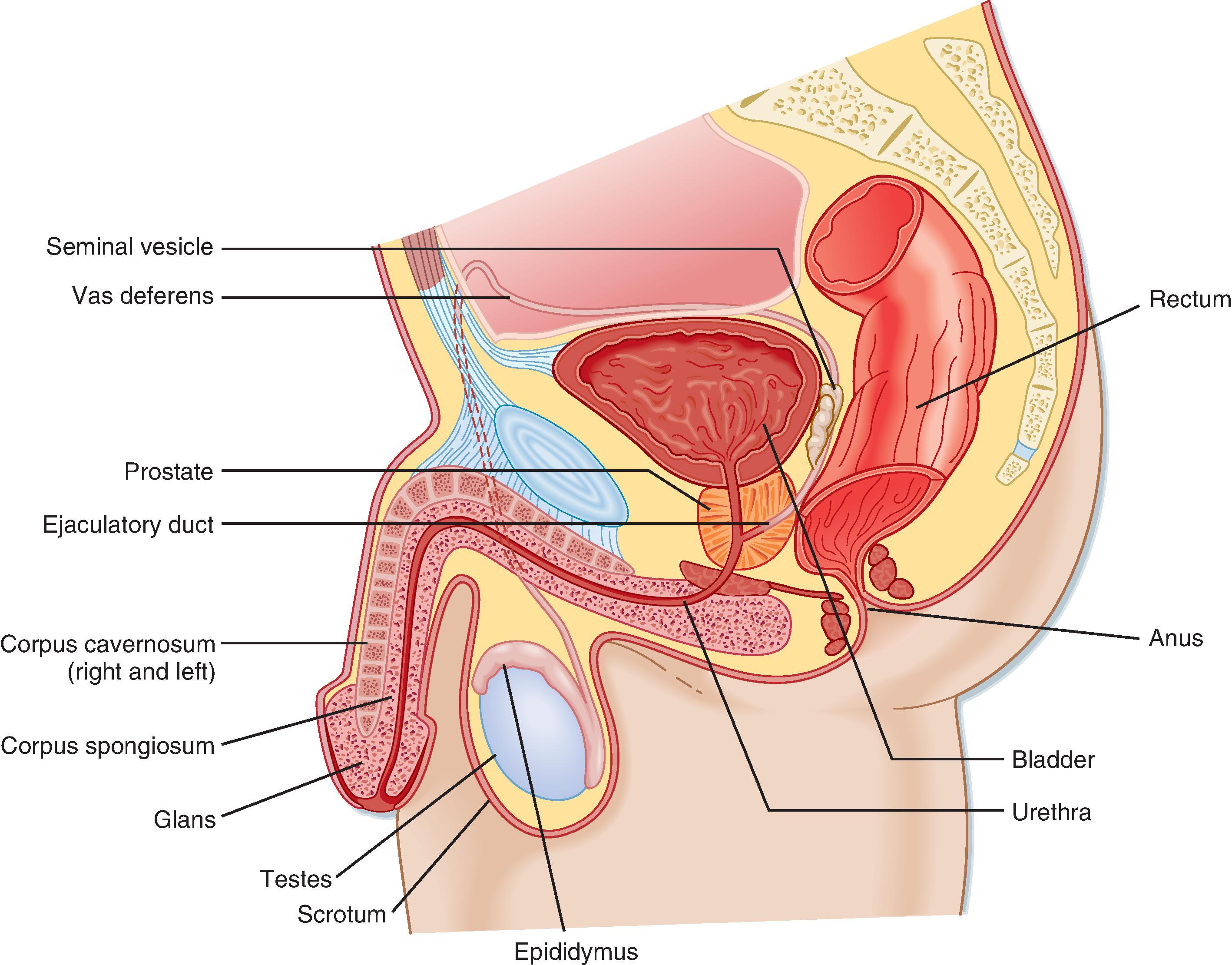
Unlike the ovaries, the testes reside outside the abdominal cavity in the scrotum ( Fig. 44.1 ). This location maintains testicular temperature at about 2°C lower than body temperature, which is crucial for optimal sperm development. The human testis is covered by a connective tissue capsule and divided into about 300 lobules by fibrous septa ( Fig. 44.2 ). Within each lobule are two to four loops of seminiferous tubules. Each loop empties into an anastomosing network of tubules called the rete testis. The rete testis is continuous with small ducts, the efferent ductules, that lead the sperm out of the testis into the head of the epididymis on the superior pole of the testis (see Fig. 44.2 ). Once in the epididymis, the sperm pass from the head, to the body, to the tail of the epididymis and then to the vas (ductus) deferens. Viable sperm can be stored in the tail of the epididymis and vas deferens for several months.
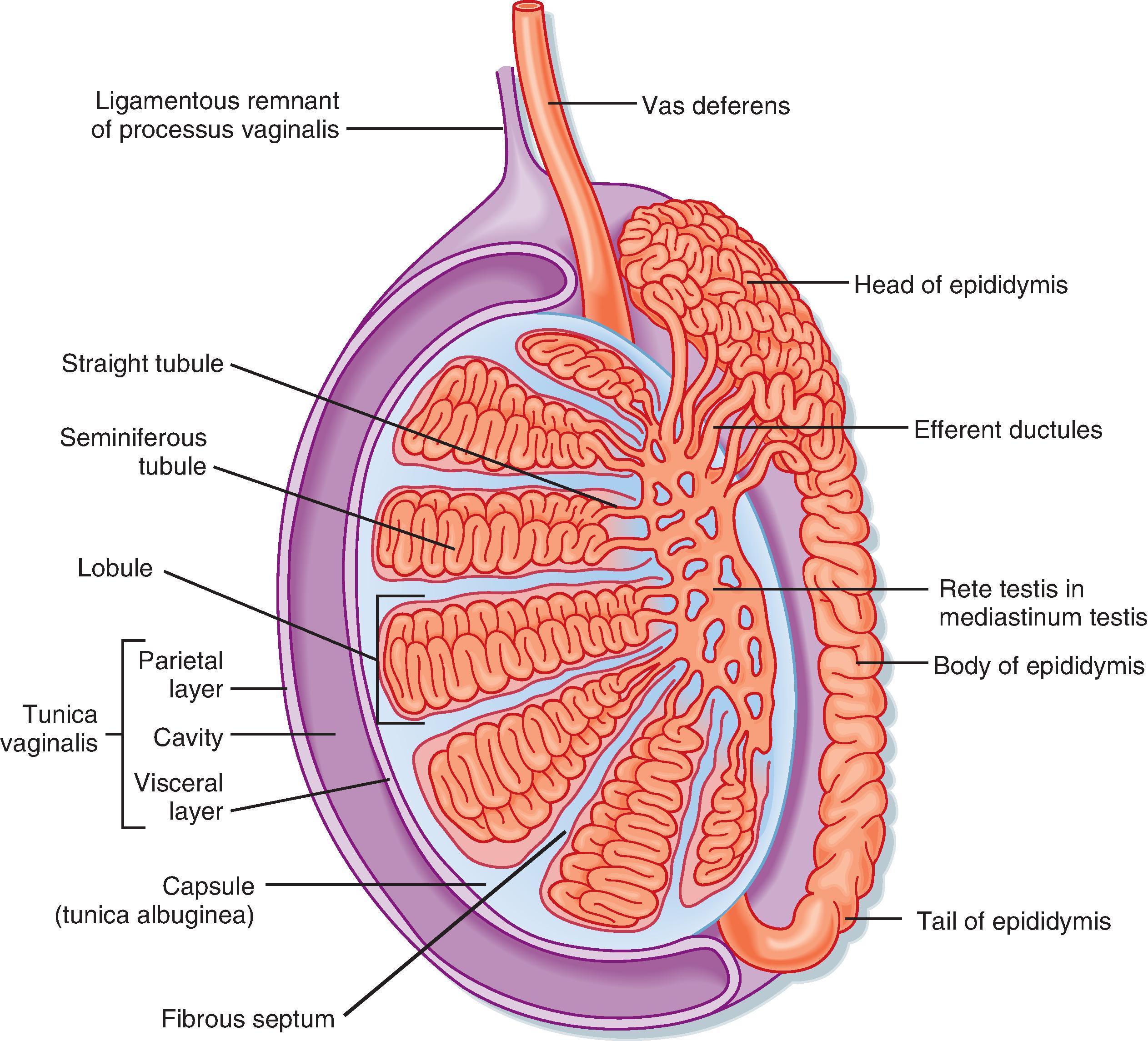
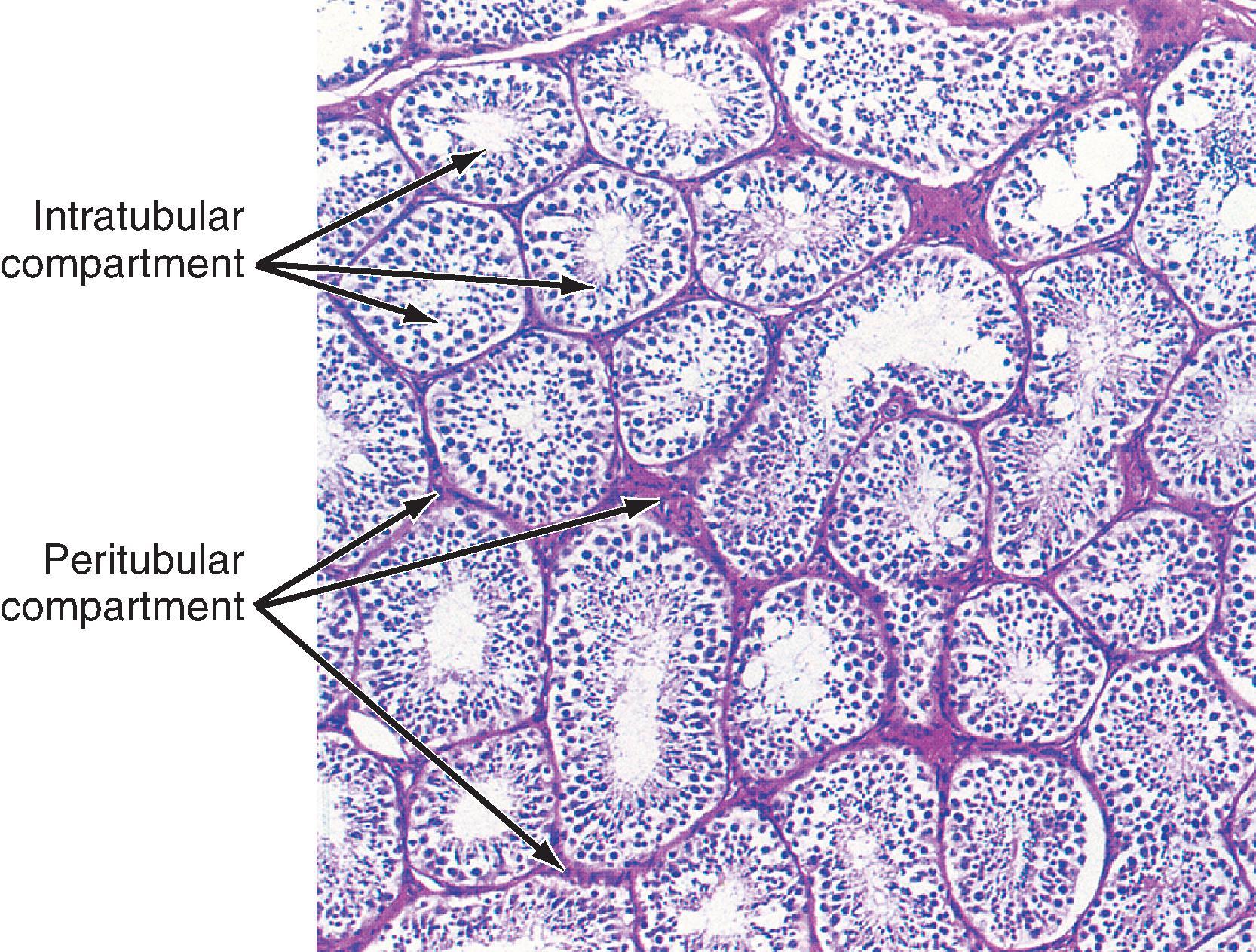
The presence of the seminiferous tubules creates two compartments within each lobule: an intratubular compartment, composed of the avascular seminiferous epithelium of the seminiferous tubule, and a peritubular compartment, composed of neurovascular elements, connective tissue cells, immune cells, and the interstitial cells of Leydig, whose main function is to produce testosterone ( Fig. 44.3 ).
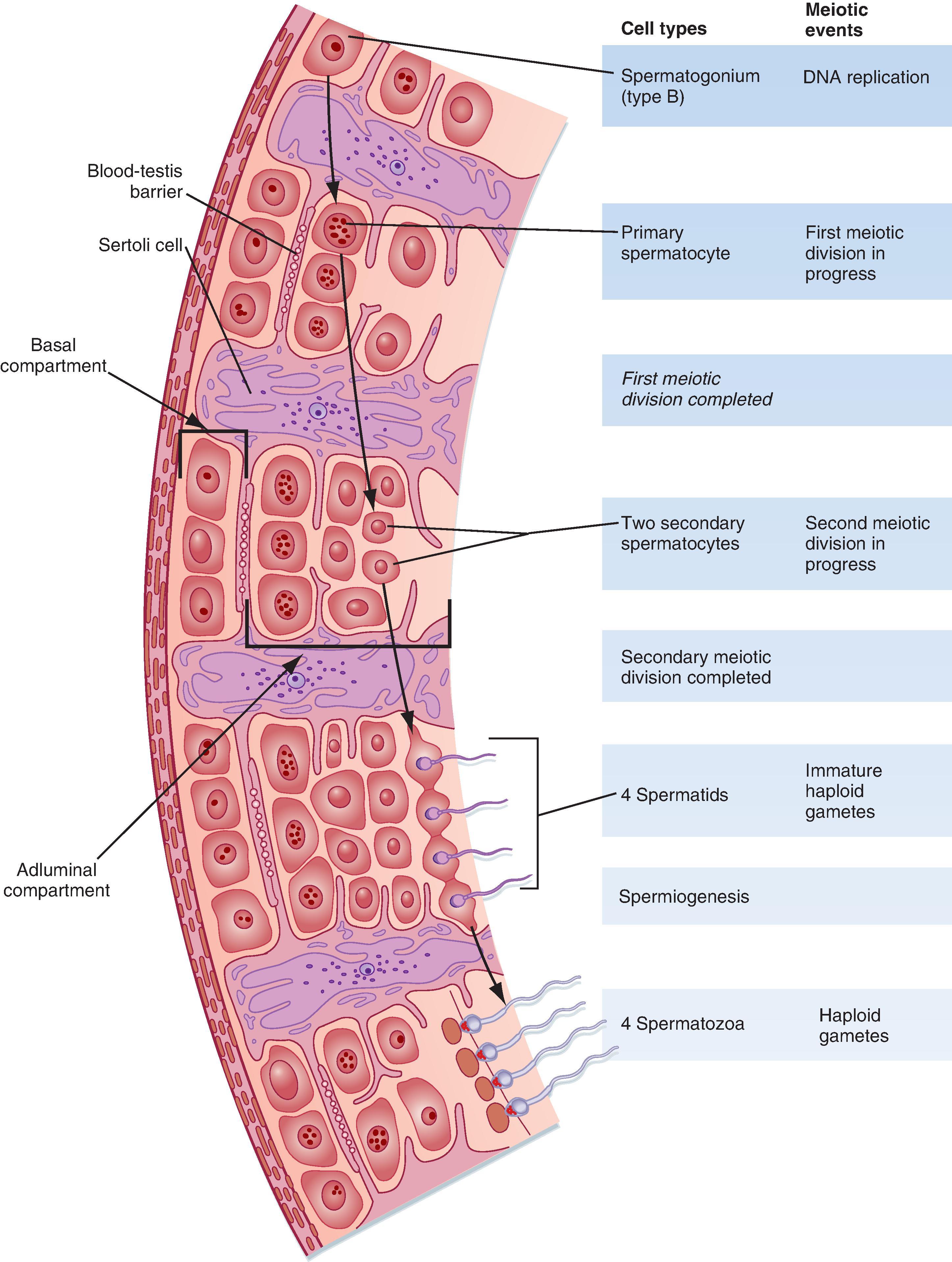
The seminiferous tubule is lined by a complex seminiferous epithelium composed of two cell types: sperm cells in various stages of spermatogenesis and Sertoli cells (“nurse cells”) that are in intimate contact with all sperm cells ( Fig. 44.4 ).
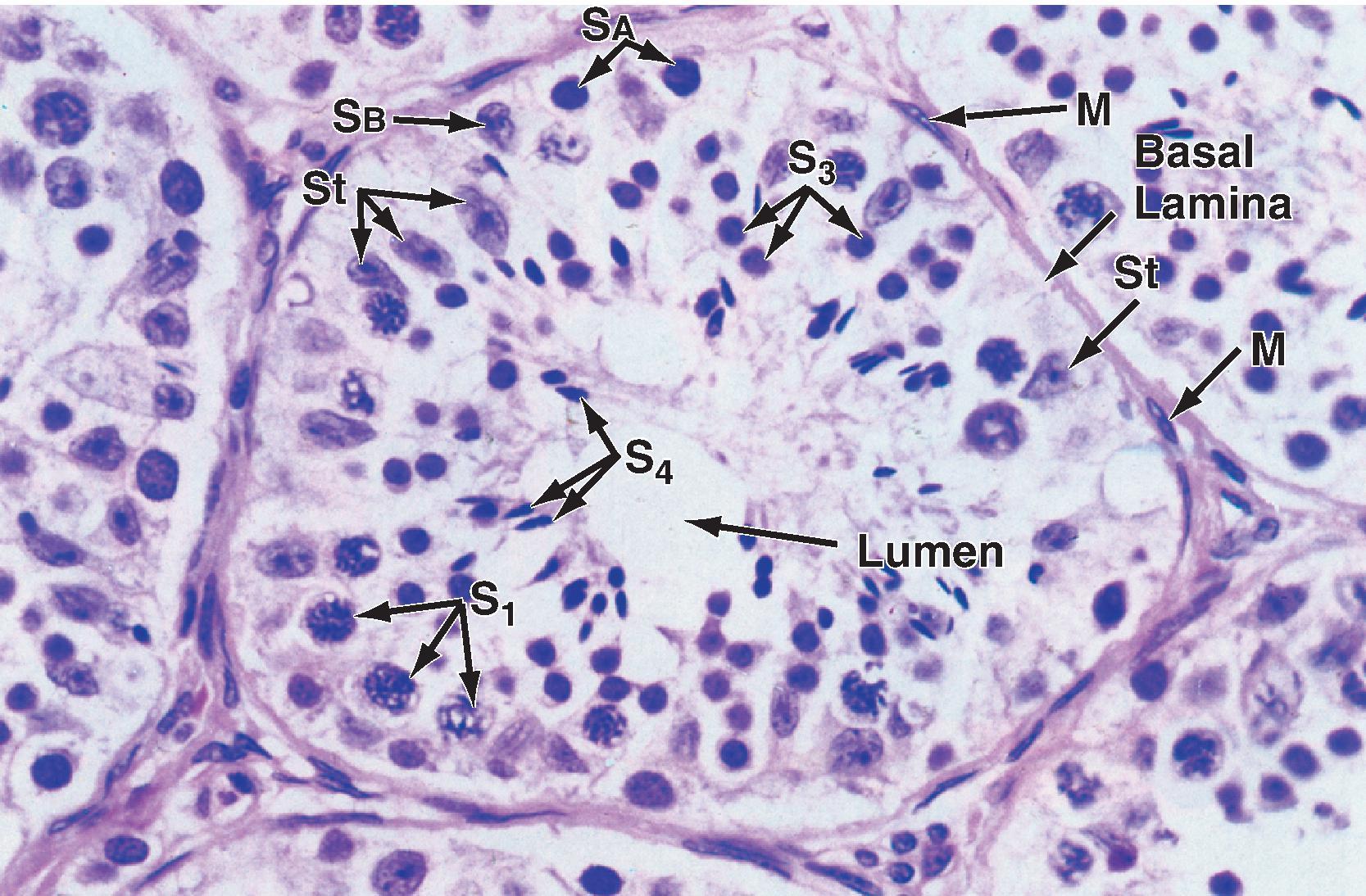
Spermatogenesis involves the processes of mitosis and meiosis. Stem cells called spermatogonia reside at the basal level of the seminiferous epithelium (see Fig. 44.4 , S A and S B ). Spermatogonia divide mitotically to generate daughter spermatogonia (spermatocytogenesis). One or more spermatogonia remain within the stem cell population, firmly adherent to the basal lamina. However, the majority of these daughter spermatogonia enter meiotic division, which results in haploid spermatozoa on completion of meiosis. These divisions are accompanied by incomplete cytokinesis such that all daughter cells remain interconnected by a cytoplasmic bridge. This configuration contributes to the synchrony of development of a clonal population of sperm cells. Spermatogonia migrate apically away from the basal lamina as they enter the first meiotic prophase. At this time they are called primary spermatocytes (see Fig. 44.4 , S 1 ). During the first meiotic prophase the hallmark processes of sexual reproduction involving chromosomal reduplication, synapsis, crossing over, and homologous recombination take place. Completion of the first meiotic division gives rise to secondary spermatocytes, which quickly (i.e., within 20 minutes) complete the second meiotic division. The initial products of meiosis are haploid spermatids (see Fig. 44.4 , S 3 ).
Spermatids are small round cells that undergo a remarkable metamorphosis called spermiogenesis ( Fig. 44.5 ). The products of spermiogenesis are the streamlined spermatozoa see Fig. 44.4 , S 4 ). As the spermatid matures into a spermatozoon, the size of the nucleus decreases and a prominent tail is formed. The tail contains microtubular structures that propel sperm, similar to a flagellum. The chromatin material in the sperm nucleus condenses, and most of the cytoplasm is lost. The acrosome is a membrane-enclosed structure on the head of the sperm that acts as a lysosome and contains hydrolytic enzymes that are important for fertilization. These enzymes remain inactive until the acrosomal reaction occurs (see Fertilization).
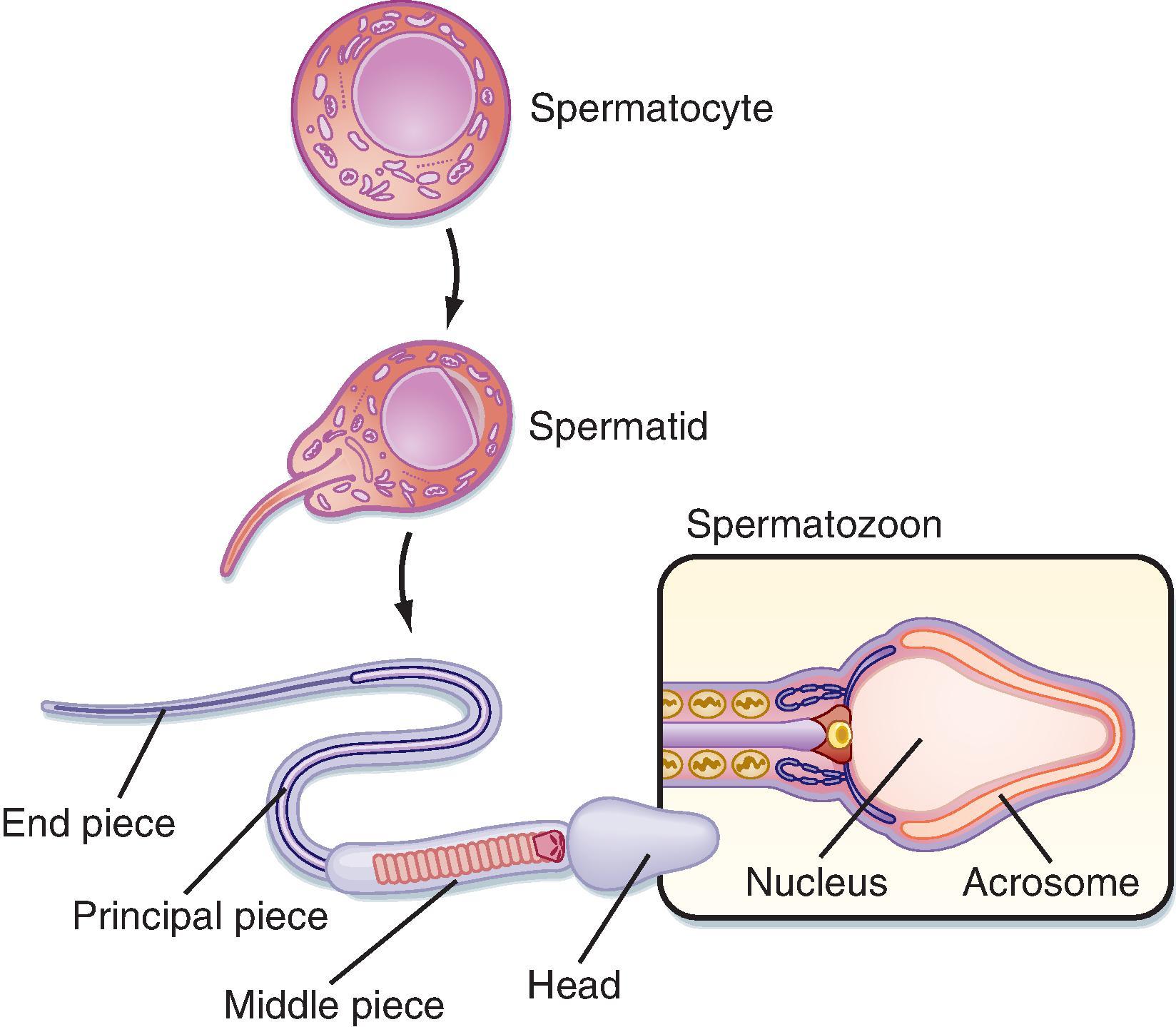
Spermatozoa (see Fig. 44.4 , S 4 ) are found at the luminal surface of the seminiferous tubule. Release of sperm, or spermiation, is controlled by Sertoli cells. The process of spermatogenesis takes about 72 days. A cohort of adjacent spermatogonia enter the process every 16 days so that the process is staggered at one point along a seminiferous tubule. In addition the process is staggered along the length of a seminiferous tubule (i.e., not all spermatogonia enter the process of spermatogenesis at the same time along the entire length of the tubule or in synchrony with every other tubule; there are about 500 seminiferous tubules per testis; see later). Because the seminiferous tubules within one testis are about 400 m in length, spermatozoa are continually being generated at many sites within the testis at any given time.
Sertoli cells are the true epithelial cells of the seminiferous epithelium and extend from the basal lamina to the lumen (see Fig. 44.4 , St). Sertoli cells surround sperm cells and provide structural support within the epithelium, and they form adhering and gap junctions with all stages of sperm cells. Through formation and breakdown of these junctions, Sertoli cells guide sperm cells toward the lumen as they advance to later stages of spermatogenesis. Spermiation requires the final breakdown of Sertoli–sperm cell junctions.
Another important structural feature of Sertoli cells is the formation of tight junctions between adjacent Sertoli cells ( Fig. 44.6 ). These Sertoli-Sertoli cell occluding junctions divide the seminiferous epithelium into a basal compartment containing the spermatogonia and early-stage primary spermatocytes and an adluminal compartment containing later-stage primary spermatocytes and all subsequent stages of sperm cells. As early primary spermatocytes move apically from the basal to the adluminal compartment, the tight junctions need to be disassembled and reassembled. These tight junctions form the physical basis for the blood-testis barrier (see Fig. 44.6 ), which creates a specialized immunologically safe microenvironment for developing sperm. By blocking paracellular diffusion the tight junctions restrict movement of substances between blood and the developing germ cells through a trans–Sertoli cell transport pathway. This organization enables Sertoli cells to control the availability of nutrients to germ cells.
Healthy Sertoli cell function is essential for sperm cell viability and development. In addition, spermatogenesis is absolutely dependent on testosterone produced by peritubular Leydig cells (see The Leydig Cell), yet it is the Sertoli cells that express the androgen receptor and respond to testosterone, not the developing sperm cells. Similarly, the pituitary hormone follicle-stimulating hormone (FSH) is also required for maximal sperm production, and again it is the Sertoli cell that expresses the FSH receptor, not the developing sperm. Thus testosterone and FSH support spermatogenesis indirectly through stimulation of Sertoli cell function.
Sertoli cells have multiple additional functions. They express the enzyme CYP19 (also called aromatase ), which converts Leydig cell–derived testosterone to the potent estrogen 17β-estradiol (see Intratesticular Androgen). This local production of estrogen may enhance spermatogenesis in humans. Sertoli cells also produce androgen-binding protein (ABP), which maintains a high androgen level within the adluminal compartment, the lumens of the seminiferous tubules, and the proximal part of the male reproductive tract. Sertoli cells also produce a large amount of fluid. This fluid provides an appropriate bathing medium for the sperm and assists in moving the immotile spermatozoa from the seminiferous tubule into the epididymis. Sertoli cells perform an important phagocytic function by engulfing residual bodies, which represent cytoplasm shed by spermatozoa during spermiogenesis.
Finally, the Sertoli cell has an important endocrine role. During development, Sertoli cells produce antimüllerian hormone (AMH; also called müllerian inhibitory substance ), which induces regression of the embryonic müllerian duct that is programmed to give rise to the female reproductive tract (discussed later). The Sertoli cells also produce the hormone inhibin. Inhibin is a heterodimer protein hormone related to the transforming growth factor-β family. FSH stimulates inhibin production, which then negatively feeds back on gonadotropes to inhibit FSH production. Thus, inhibin keeps FSH levels within a set point.
The peritubular compartment contains the primary endocrine cell of the testis, the Leydig cell ( Fig. 44.7 ). This compartment also contains the common cell types of loose connective tissue and an extremely rich peritubular capillary network that provides nutrients to the seminiferous tubules (by way of Sertoli cells) while transporting testosterone away from the testes to the peripheral circulation.
Leydig cells are steroidogenic stromal cells. These cells synthesize cholesterol de novo, as well as acquire it through low-density lipoprotein (LDL) receptors and high-density lipoprotein (HDL) receptors (also called scavenger receptor BI [SR-BI]), and store cholesterol as cholesterol esters as described for adrenocortical cells (see Chapter 43 ). Free cholesterol is generated by a cholesterol ester hydrolase and transferred to the outer mitochondrial membrane and then to the inner mitochondrial membrane in a steroidogenic acute regulatory (StAR) protein –dependent manner. As in all steroidogenic cells, cholesterol is converted to pregnenolone by CYP11A1. Pregnenolone is then processed to progesterone, 17-hydroxyprogesterone, and androstenedione by 3β-hydroxysteroid dehydrogenase (3β-HSD) and CYP17 ( Fig. 44.8 ). Recall from Chapter 43 that CYP17 is a bifunctional enzyme with 17-hydroxylase activity and 17,20-lyase activity. CYP17 displays a robust level of both activities in the Leydig cell. In this respect the Leydig cell is similar to the zona reticularis cell, except that it expresses a higher level of 3β-HSD, so the Δ4 pathway is ultimately favored. Another major difference is that the Leydig cell expresses a Leydig cell–specific isoform of 17β-hydroxysteroid dehydrogenase (17β-HSD type 3), which efficiently converts androstenedione to testosterone (see Fig. 44.8 ).
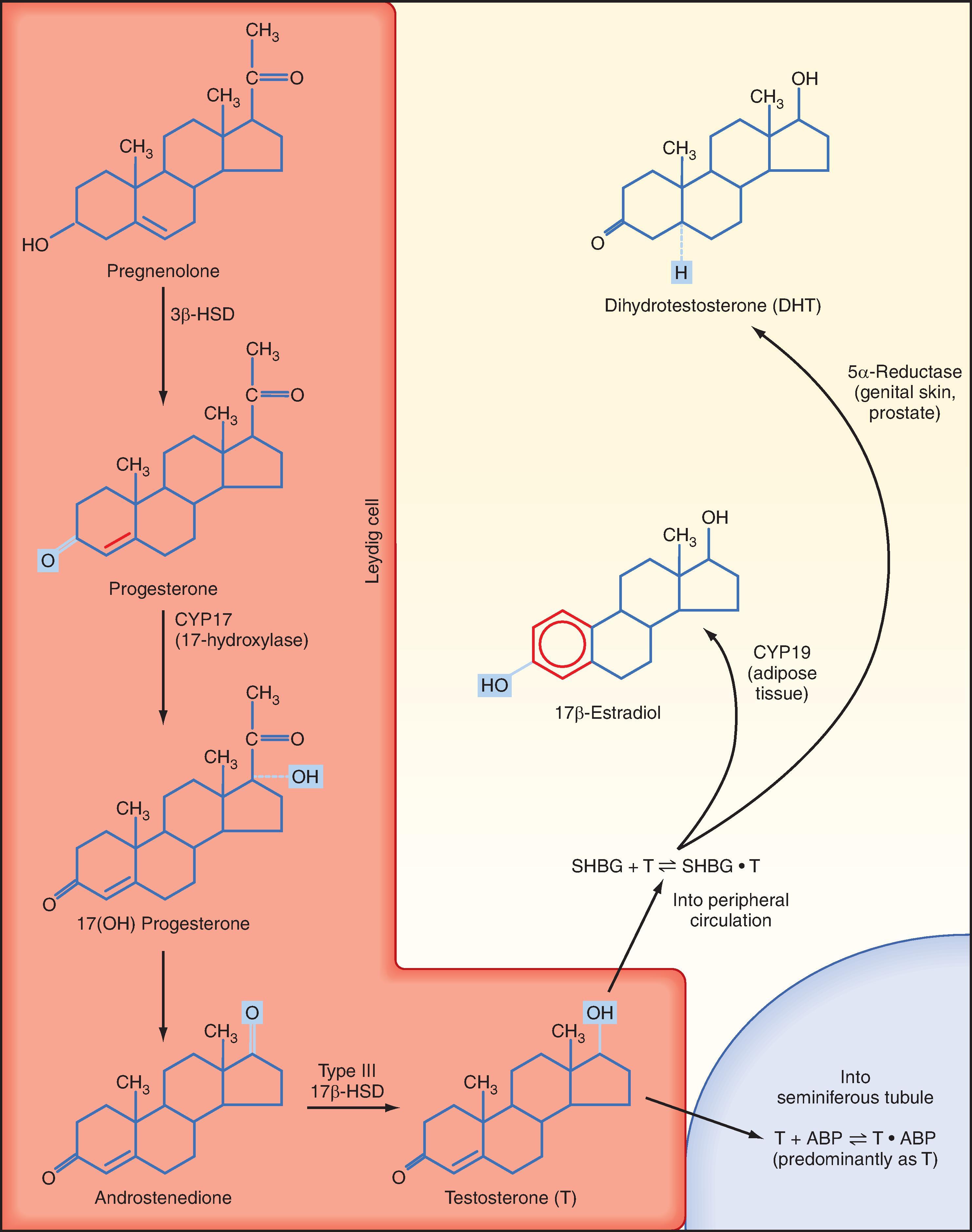
The testosterone produced by Leydig cells has several fates and multiple actions. Because of the proximity of Leydig cells to the seminiferous tubules, significant amounts of testosterone diffuse into the seminiferous tubules and become concentrated within the adluminal compartment by ABP (see Fig. 44.8 ). Testosterone levels within the seminiferous tubules that are greater than 100 times more concentrated than circulating testosterone levels are absolutely required for normal spermatogenesis. As mentioned earlier, Sertoli cells express the enzyme CYP19 (aromatase), which converts a small amount of testosterone into the highly potent estrogen 17β-estradiol. Human sperm cells express at least one subtype of the estrogen receptor, and there is some evidence from aromatase-deficient men that this locally produced estrogen optimizes spermatogenesis in humans.
In several tissues (especially adipose tissue), testosterone is converted to estrogen (see Fig. 44.8 ). Studies involving men with aromatase deficiency have shown that an inability to produce estrogen results in tall stature because of the lack of epiphyseal closure in long bones, as well as osteoporosis. Thus, peripheral estrogen plays an important role in both bone maturation and maintenance in men. These studies also implicated estrogen in promoting insulin sensitivity, improving lipoprotein profiles (i.e., increasing HDL, decreasing triglycerides and LDL), and exerting negative feedback on gonadotropins at the pituitary and hypothalamus.
Testosterone can also be converted into a potent nonaromatizable androgen, 5α-dihydrotestosterone (DHT), by the enzyme 5α-reductase (see Fig. 44.8 ). There are two isoforms of 5α-reductase, type 1 and type 2. Major sites of 5α-reductase 2 expression are the male urogenital tract, genital skin, hair follicles, and liver. 5α-Reductase 2 generates DHT, which is required for masculinization of the external genitalia in utero and for many of the changes associated with puberty, including growth and activity of the prostate gland (see Male Reproductive Tract), growth of the penis, darkening and folding of the scrotum, growth of pubic and axillary hair, growth of facial and body hair, and increased muscle mass ( Fig. 44.9 ). The onset of 5α-reductase 1 expression occurs at puberty. This isozyme is expressed primarily in the skin and contributes to sebaceous gland activity and the acne associated with puberty. Because DHT has strong growth-promoting (i.e., trophic) effects on its target organs, development of selective 5α-reductase 2 inhibitors has proven beneficial in the treatment of prostatic hypertrophy and prostatic cancer.
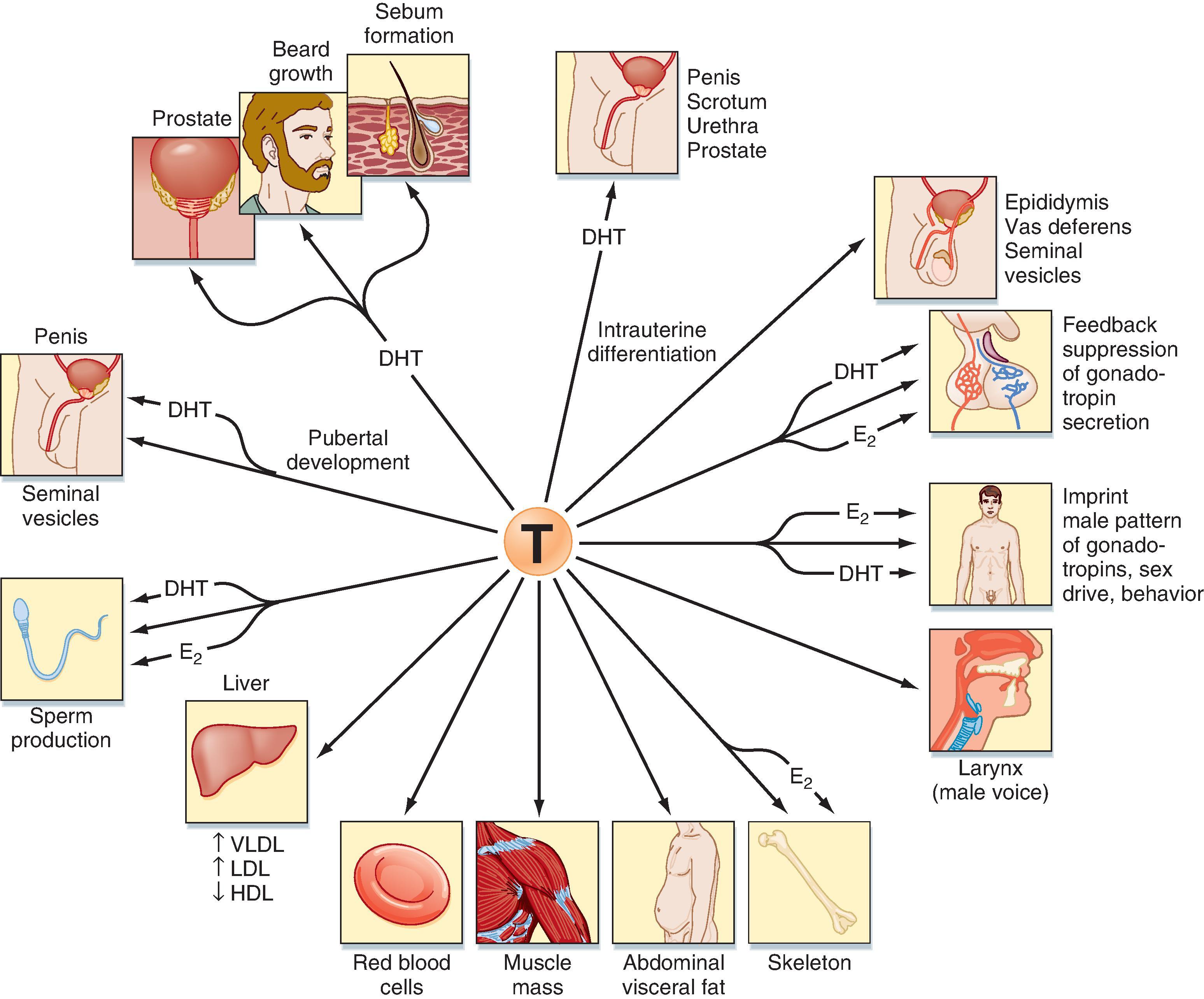
Testosterone has a direct action (i.e., without conversion to DHT) in several cell types (see Fig. 44.9 ). As mentioned earlier, testosterone regulates Sertoli cell function. It induces development of the male tract from the mesonephric duct in the absence of 5α-reductase. Testosterone has several metabolic effects, including increasing very low-density lipoprotein (VLDL) and LDL while decreasing HDL, promoting deposition of abdominal adipose tissue, increasing red blood cell production, promoting bone growth and health, and exerting a protein anabolic effect on muscle. Testosterone is sufficient to maintain erectile function and libido.
Testosterone and DHT act through the same androgen receptor (AR). The AR resides in the cytoplasm bound to chaperone proteins in the absence of ligand. Testosterone-AR binding or DHT-AR binding causes dissociation of the chaperone proteins, followed by nuclear translocation of the androgen-AR complex, dimerization, binding to an androgen response element, and recruitment of coactivator proteins and general transcription factors to the vicinity of a specific gene’s promoter. It remains unclear how testosterone and DHT differ in their ability to activate the AR in the context of different cell types. It has been proposed that the presence of different coactivator proteins in distinct cell types may exist, which might possess different affinities for the testosterone-activated conformation of the AR versus the DHT-activated AR.
As testosterone enters the peripheral circulation, it binds to and quickly reaches equilibrium with serum proteins. About 60% of circulating testosterone is bound to sex hormone–binding globulin (SHBG), 38% is bound to albumin, and about 2% remains as “free” hormone. Testosterone and its metabolites are primarily excreted in urine. Approximately 50% of excreted androgens are found as urinary 17-ketosteroids, with most of the remainder being conjugated androgens or diol or triol derivatives. Only about 30% of the 17-ketosteroids in urine are from the testis; the rest are produced from adrenal androgens. Androgens are conjugated with glucuronate or sulfate in the liver, and these conjugated steroids are excreted in urine.
The testis is regulated by an endocrine axis ( Fig. 44.10 ) involving parvicellular hypothalamic gonadotropin-releasing hormone (GnRH) neurons and pituitary gonadotropes that produce both luteinizing hormone (LH) and follicle-stimulating hormone (FSH).
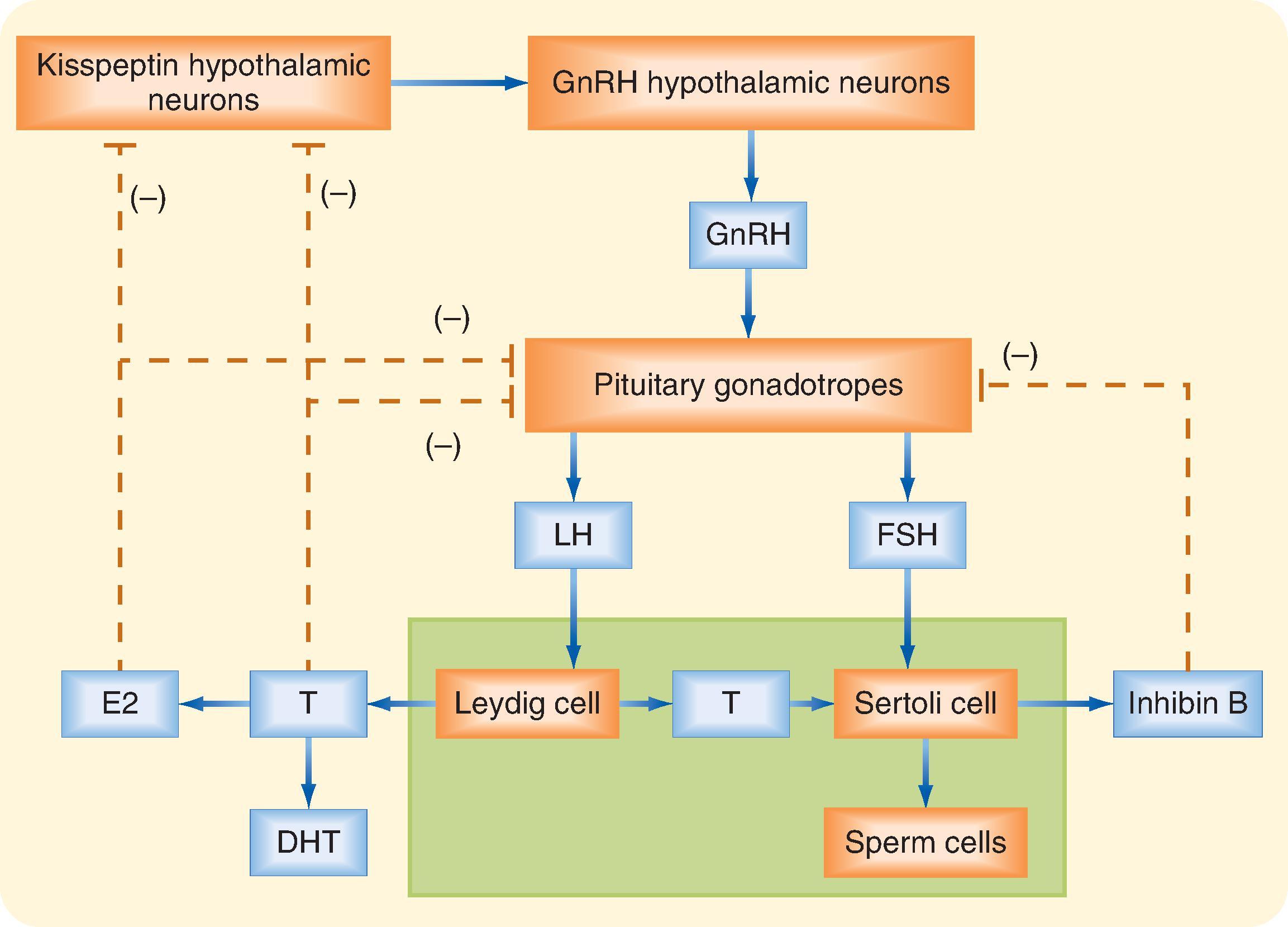
The Leydig cell expresses the LH receptor, and LH acts on Leydig cells much like adrenocorticotropic hormone (ACTH, or corticotropin) does on zona fasciculata cells in the adrenal cortex (see Chapter 43 ). Rapid effects include hydrolysis of cholesterol esters and new expression of StAR protein. Less acute effects include an increase in steroidogenic enzyme gene expression and expression of the low-density lipoprotein receptor (LDLR). Over the long term, LH promotes Leydig cell growth and proliferation.
Testosterone, and estradiol produced from peripheral conversion of testosterone, negatively feedback on GnRH hypothalamic neurons indirectly through inhibition of kisspeptin-producing neurons (see Fig. 44.10 ). Testosterone and estradiol also negatively feedback on pituitary gonadotropes. In contrast, DHT, the other major product of peripheral conversion of testosterone, has little effect on LH or FSH levels.
The Sertoli cell is stimulated by both testosterone and FSH. In addition to stimulating synthesis of proteins involved in the “nurse cell” aspect of Sertoli cell function (e.g., ABP), FSH stimulates synthesis of the dimeric protein inhibin. Inhibin is induced by FSH and negatively feeds back on the gonadotrope to selectively inhibit FSH production (see Fig. 44.10 ).
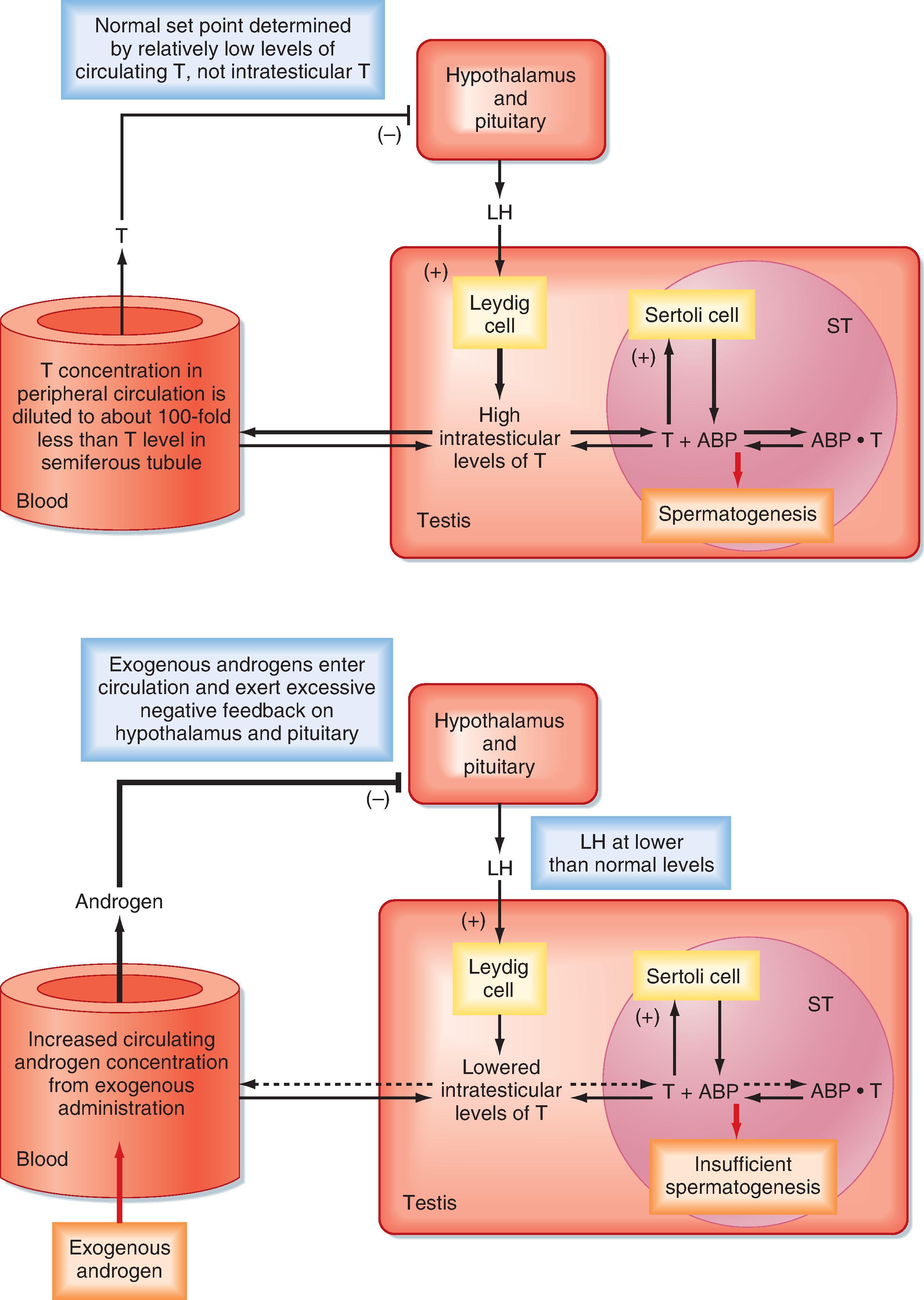
There is an important “loophole” in the male reproductive axis that is based on the fact that intratesticular levels of testosterone need to be greater than 100-fold higher than circulating levels of the hormone to maintain normal rates of spermatogenesis, yet it is the circulating levels of testosterone (and estradiol) that provide the negative feedback to the pituitary and hypothalamus. This means that exogenous administration of testosterone can raise circulating levels of testosterone and estradiol sufficient to inhibit LH but not sufficient to accumulate in the testis at the required concentration for normal spermatogenesis. However, the decreased LH levels will diminish intratesticular production of testosterone by Leydig cells, which results in reduced levels of spermatogenesis ( Fig. 44.11 ). This “loophole” is currently being investigated as a possible strategy for developing a male oral contraceptive. It is also the basis for sterility in some cases of steroid abuse in men.
Once spermatozoa emerge from the efferent ductules, they leave the gonad and enter the male reproductive tract (see Fig. 44.1 ). The segments of the tract are the: epididymis ( head, body, and tail ), vas deferens, ejaculatory duct, prostatic urethra , membranous urethra , and penile urethra. Unlike the female tract, there is a contiguous lumen from the seminiferous tubule to the end of the male tract (i.e., the tip of the penile urethra), and the male reproductive tract connects to the distal urinary tract (i.e., male urethra ). In addition to conveying sperm, the primary functions of the male reproductive tract are:
Sperm maturation. Sperm spend about a month in the epididymis , where they undergo further maturation. The epithelium of the epididymis is secretory and adds numerous components to the seminal fluid. Spermatozoa that enter the head of the epididymis are weakly motile but are strongly unidirectionally motile by the time they exit the tail. Spermatozoa also undergo the process of decapacitation, which involves changes in the cell membrane to prevent spermatozoa from undergoing the acrosome reaction before contact with an egg (see later). Sperm become capacitated by the female reproductive tract within the oviduct. The function of the epididymis is dependent on both luminal testosterone-ABP complexes that come from the seminiferous tubules and peripheral testosterone from blood.
Sperm storage and emission. Sperm are stored in the tail of the epididymis and vas deferens for several months without loss of viability. The primary function of the vas deferens, besides providing a storage site, is to propel sperm during sexual intercourse into the male urethra. The vas deferens has a very thick muscularis that is richly innervated by sympathetic nerves. Normally in response to repeated tactile stimulation of the penis during coitus, the muscularis of the vas deferens receives bursts of sympathetic stimulation that cause peristaltic contractions. Emptying of the contents of the vas deferens into the prostatic urethra is called emission. Emission immediately precedes ejaculation, which is the propulsion of semen out of the male urethra.
Production and mixing of sperm with seminal contents. During emission, contraction of the vas deferens coincides with contraction of the muscular coats of the two accessory sex glands, the seminal vesicles (right and left) and the prostate gland (which surrounds the prostatic urethra). At this point, sperm become mixed with all the components of semen. The seminal vesicles secrete approximately 60% of the volume. These glands are the primary source of fructose, a critical nutrient for sperm. The seminal vesicles also secrete semenogelins, which induce coagulation of semen immediately after ejaculation. The alkaline secretions of the prostate, which make up about 30% of the volume, are high in citrate, zinc, spermine, and acid phosphatase. Prostate-specific antigen (PSA) is a serine protease that liquefies coagulated semen after a few minutes. PSA can be detected in blood under conditions of prostatic infection, benign prostatic hypertrophy, and prostatic carcinoma and is currently used as one indicator of prostatic health. The predominant buffers in semen are phosphate and bicarbonate. A third accessory gland, the bulbourethral glands (also called Cowper’s glands ), empty into the penile urethra in response to sexual excitement before emission and ejaculation. This secretion is high in mucus, which lubricates, cleanses, and buffers the urethra. Average sperm counts are between 60 and 100 million/mL semen. Men with sperm counts below 20 million/mL, less than 50% motile sperm, or less than 60% normally conformed sperm are usually infertile.
Erection, penetration, and ejaculation. Emission and ejaculation occur during coitus in response to a reflex arc that involves sensory stimulation from the penis (via the pudendal nerve) followed by sympathetic motor stimulation to the smooth muscle of the male tract and somatic motor stimulation to the musculature associated with the base of the penis. However, for sexual intercourse to occur in the first place, the man has to achieve and maintain an erection of the penis. The penis has evolved as an intromittent organ designed to separate the walls of the vagina, pass through the potential space of the vaginal lumen, and deposit semen at the distal end of the vaginal lumen near the cervix. This process of internal insemination can be performed only if the penis is stiffened from the process of erection.
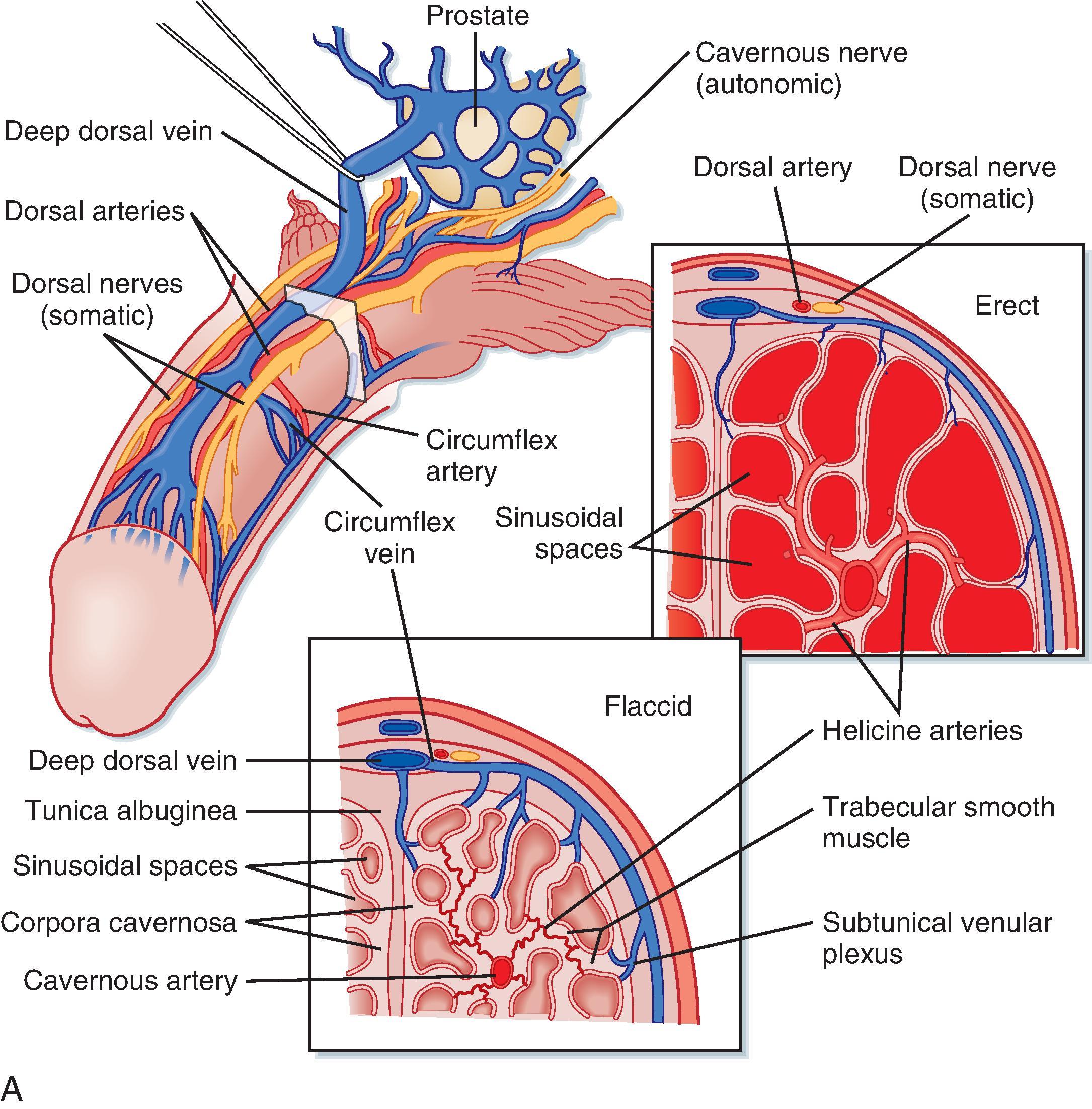
Erection is a neurovascular event. The penis is composed of three erectile bodies: two corpora cavernosa and one corpus spongiosum ( Fig. 44.12A ). The penile urethra runs through the corpus spongiosum. These three bodies are composed of erectile tissue —an anastomosing network of potential cavernous vascular spaces lined with continuous endothelia within a loose connective tissue support. During the flaccid state, blood flow to the cavernous spaces is minimal (see Fig. 44.12 A ). This is due to vasoconstriction of the vasculature (called the helicine arteries ) and shunting of blood flow away from the cavernous spaces. In response to sexual arousal the parasympathetic cavernous nerves innervating the vascular smooth muscle of the helicine arteries release nitric oxide (NO). NO activates guanylyl cyclase, thereby increasing cyclic guanosine monophosphate (cGMP), which decreases intracellular [Ca ++ ] and causes muscular relaxation (see Fig. 44.12 B ). Vasodilation allows blood to flow into the cavernous spaces to induce engorgement and erection. It also presses on veins in the penis and reduces venous drainage (see Fig. 44.12 B ).
Inability to achieve or maintain an erection is termed erectile dysfunction (ED) and is one cause of infertility in men. Multiple factors can lead to ED, including insufficient androgen production; neurovascular damage (e.g., from diabetes mellitus, spinal cord injury); structural damage to the penis, perineum, or pelvis; psychogenic factors (e.g., depression, performance anxiety); and prescribed medications and recreational drugs, including alcohol and tobacco. A major development in the treatment of some forms of erectile dysfunction is use of selective cGMP phosphodiesterase inhibitors that assist in maintaining an erection (see Fig. 44.12 B ).
There is no distinct andropause in men. However, as men age, gonadal sensitivity to LH decreases and androgen production drops. As this occurs, serum LH and FSH levels rise. Although sperm production typically begins to decline after age 50, many men can maintain reproductive function and spermatogenesis throughout life.
The female reproductive system is composed of the gonads, called ovaries, and the female reproductive tract, which includes the oviducts, uterus, cervix, vagina, and external genitalia.
The ovary is located within a fold of peritoneum called the broad ligament, usually close to the lateral wall of the pelvic cavity ( Fig. 44.13 ). Because the ovary extends into the peritoneal cavity, ovulated eggs briefly reside within the peritoneal cavity before they are captured by the oviducts.
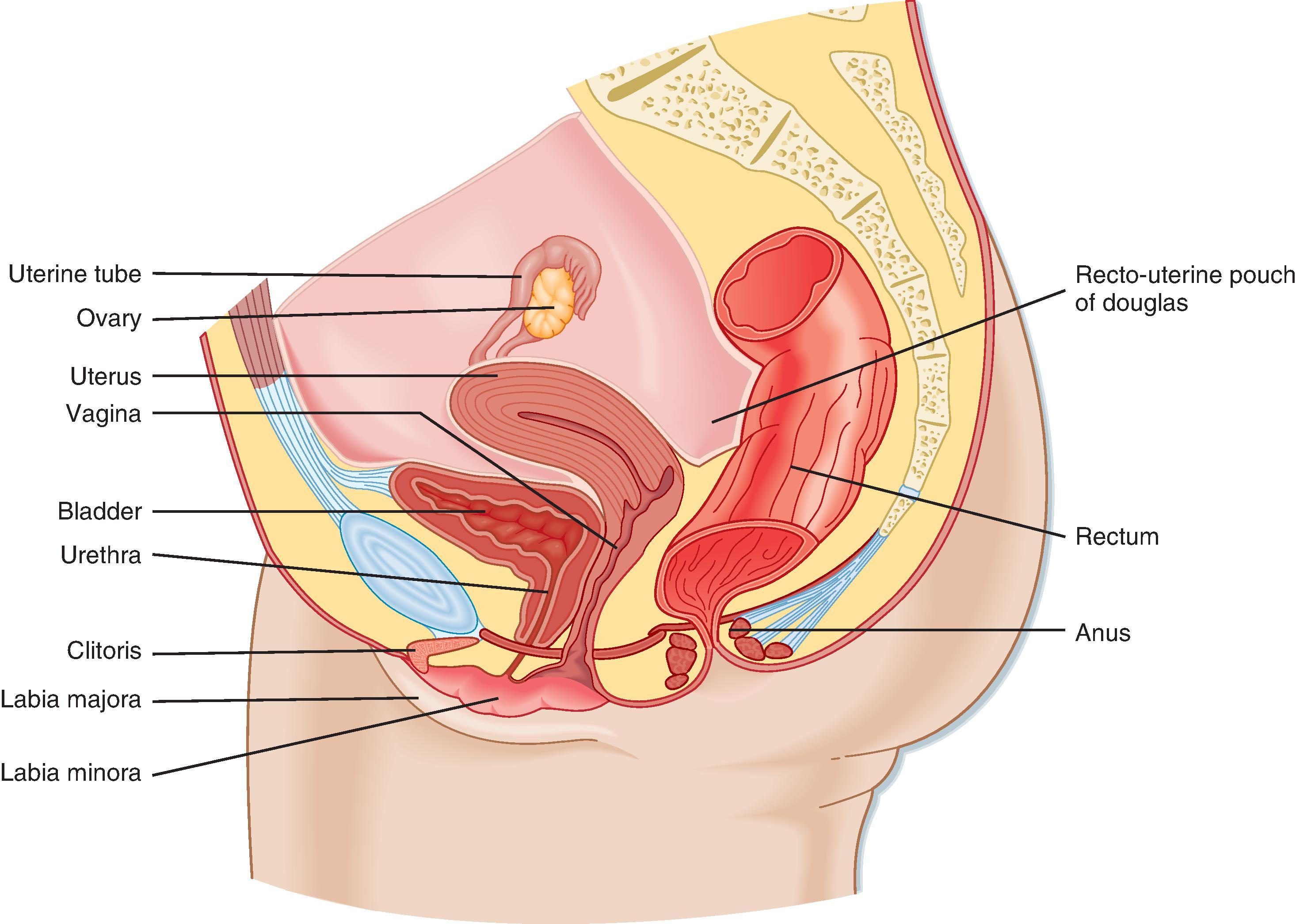
The ovary is divided into an outer cortex and inner medulla ( Fig. 44.14 ). Neurovascular elements innervate the medulla of the ovary. The cortex of the ovary is composed of a densely cellular stroma. Within this stroma reside the ovarian follicles (see Fig. 44.14 , F), which contain a primary oocyte surrounded by follicle cells. The cortex is covered by a connective tissue capsule, the tunica albuginea, and a layer of simple epithelium consisting of ovarian surface epithelial cells. There are no ducts emerging from the ovary to convey its gametes to the reproductive tract. Thus, the process of ovulation involves an inflammatory event that erodes the wall of the ovary. After ovulation the ovarian surface epithelial cells rapidly divide to repair the wall. The majority of ovarian cancer originates from this highly proliferative epithelium.
The ovarian follicle is the functional unit of the ovary, and it performs both gametogenic and endocrine functions. A histological section of the ovary from a premenopausal cycling woman contains follicular structures at many different stages of development. The life history of a follicle can be divided into the following stages:
Resting primordial follicle.
Growing preantral (primary and secondary) follicle.
Growing antral (tertiary) follicle.
Dominant (preovulatory, graafian) follicle.
Dominant follicle within the periovulatory period.
Corpus luteum (of menstruation or of pregnancy).
Atretic follicles.
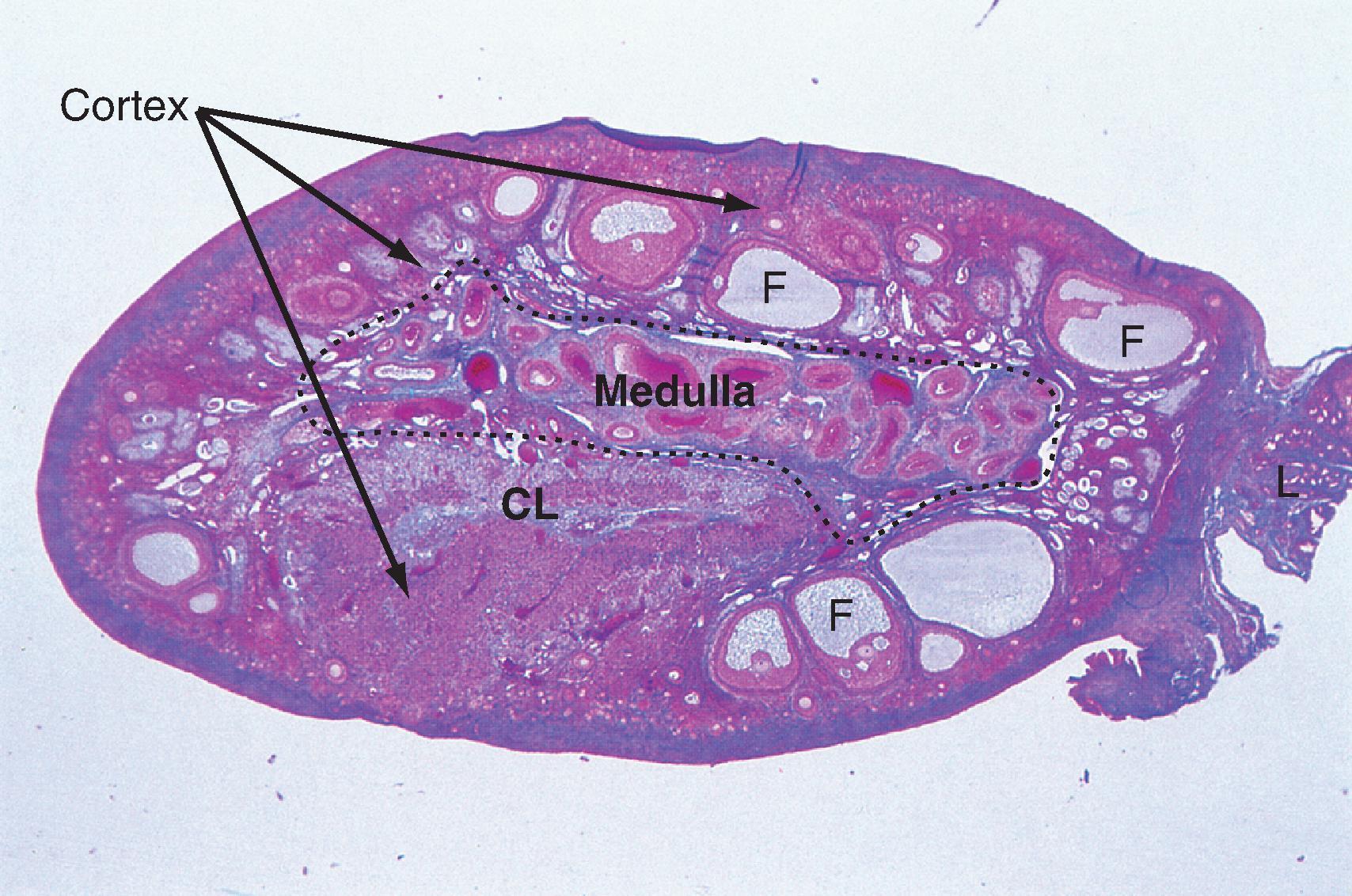
Resting primordial follicles ( Fig. 44.15 ) represent the earliest and simplest follicular structure in the ovary. Primordial follicles appear during midgestation through the interaction of gametes and somatic cells. Primordial germ cells that have migrated to the gonad continue to divide mitotically as oogonia until the fifth month of gestation in humans. At this point the approximately 7 million oogonia enter the process of meiosis and become primary oocytes. During this time the primary oocytes become surrounded by a simple epithelium of somatic follicle cells, thereby creating the primordial follicles (see Fig. 44.15 ). The follicle cells establish gap junctions with each other and the oocyte. The follicle cells themselves represent a true avascular epithelium surrounded by a basal lamina. Similar to Sertoli cell–sperm interactions, a subpopulation of granulosa cells remains intimately attached to the oocytes throughout their development. Granulosa cells provide nutrients such as amino acids, nucleic acids, and pyruvate to support oocyte maturation.
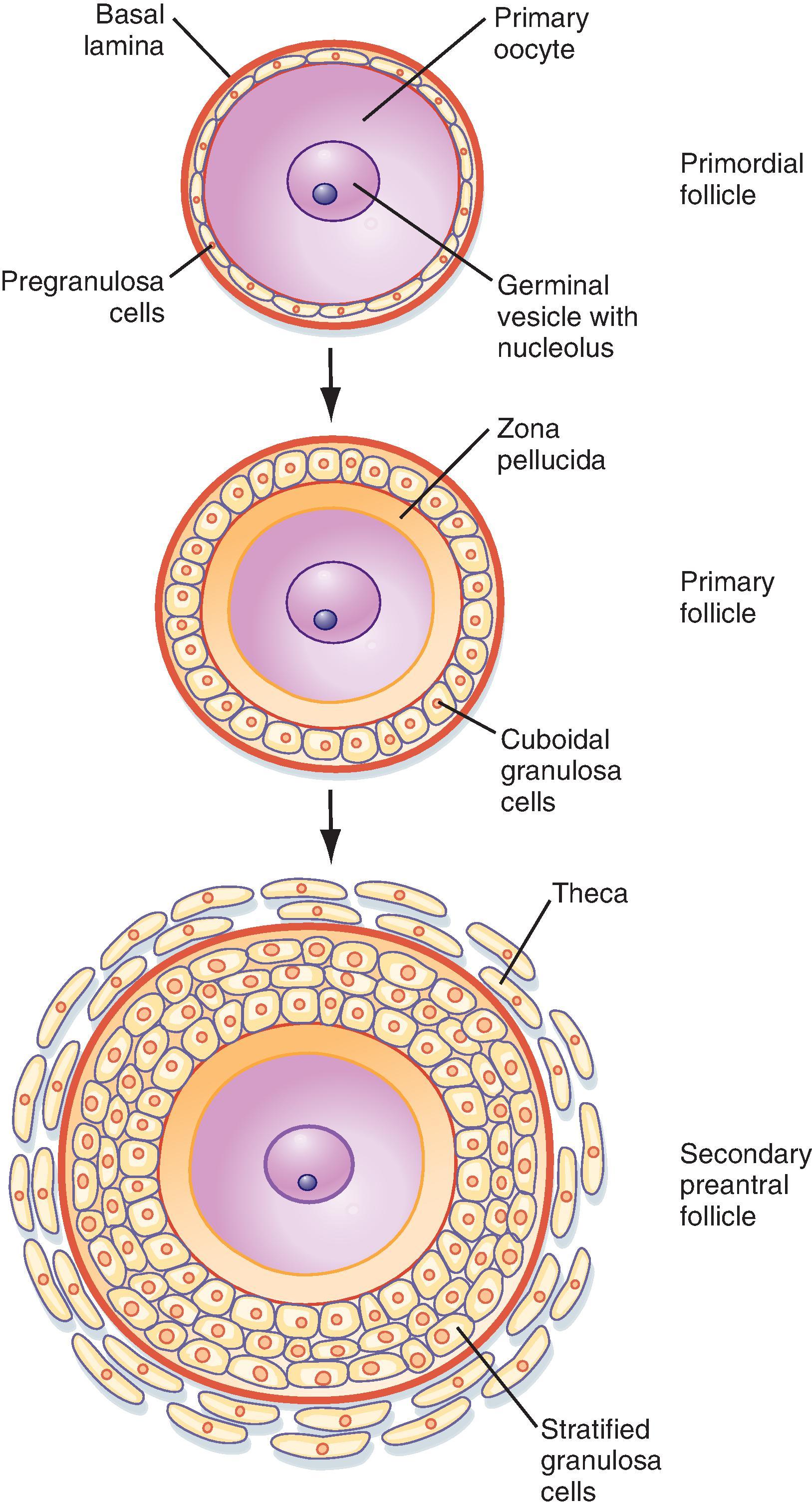
The primordial follicles represent the ovarian reserve of follicles ( Fig. 44.16 ). This reserve is reduced from a starting number of about 7 million to less than 300,000 follicles at reproductive maturity. Of these, a woman will ovulate about 450 between menarche (first menstrual cycle) and menopause (cessation of menstrual cycles). At menopause, less than 1000 primordial follicles are left in the ovary. Primordial follicles are lost primarily from death as a result of follicular atresia. However, a small subset of primordial follicles will enter follicular growth in waves. Because the ovarian follicular reserve represents a fixed finite number, the rate at which resting primordial follicles die or begin to develop (or both) will determine the reproductive life span of a woman. Age at the onset of menopause has a strong genetic component but is also influenced by environmental factors. For example, cigarette smoking significantly depletes the ovarian reserve. An overly rapid rate of atresia or development will deplete the reserve and give rise to premature ovarian insufficiency.
Pituitary gonadotropins maintain a normal ovarian reserve by promoting the general health of the ovary. However, the rate at which resting primordial follicles enter the growth process appears to be independent of pituitary gonadotropins. The decision of a resting follicle to enter the early growth phase is primarily dependent on intraovarian paracrine factors produced by both the follicle cells and oocytes.
In primordial follicles the gamete is derived from oogonia that have entered the first meiotic division; such oogonia are referred to as primary oocytes. Primary oocytes progress through most of prophase of the first meiotic division (termed prophase I ) over a 2-week period and then arrest in the diplotene stage. This stage is characterized by the decondensation of chromatin, which supports the transcription needed for oocyte maturation. Meiotic arrest at this stage, which may last for up to 50 years. This process appears to be due to “maturational incompetence,” or lack of the cell cycle proteins needed to support the completion of meiosis. The nucleus of the oocyte, called the germinal vesicle, remains intact at this stage.
The first stage of follicular growth is preantral, which refers to the development that occurs before the formation of a fluid-filled antral cavity. One of the first visible signs of follicle growth is the appearance of cuboidal granulosa cells. At this point the follicle is referred to as a primary follicle (see Fig. 44.15 ). As granulosa cells proliferate, they form a multilayered (i.e., stratified) epithelium around the oocyte. At this stage the follicle is referred to as a secondary follicle (see Fig. 44.15 ).
Once a secondary follicle acquires three to six layers of granulosa cells, it secretes paracrine factors that induce nearby stromal cells to differentiate into epithelioid thecal cells. Thecal cells form a flattened layer of cells around the follicle. Once a thecal layer forms, the follicle is referred to as a mature preantral follicle (see Fig. 44.15 ). In humans it takes several months for a primary follicle to reach the mature preantral stage.
Follicular development is associated with an inward movement of the follicle from the outer cortex to the inner cortex, closer to the vasculature of the ovarian medulla. Follicles release angiogenic factors that induce development of one to two arterioles that form a vascular wreath around the follicle.
During the preantral stage, the oocyte begins to grow and produce cellular and secreted proteins. The oocyte initiates secretion of extracellular matrix glycoproteins called ZP1, ZP2, and ZP3 that form the zona pellucida (see Fig. 44.15 ). The zona pellucida increases in thickness and provides a species-specific binding site for sperm during fertilization (see Pregnancy ). Importantly, granulosa cells and the oocyte maintain gap junctional contact via cellular projections through the zona pellucida. The oocyte also continues to secrete paracrine factors that regulate follicle cell growth and differentiation.
Granulosa cells express the FSH receptor during this period, but they are primarily dependent on factors from the oocyte to grow. They do not produce ovarian hormones at this early stage of follicular development. The newly acquired thecal cells are analogous to testicular Leydig cells in that they reside outside the epithelial “nurse” cells, express the LH receptor, and produce androgens. The main difference between Leydig cells and thecal cells is that thecal cells do not express high levels of 17β-HSD. Thus the major product of theca cells is androstenedione as opposed to testosterone. Androstenedione production at this stage is minimal.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here