Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Disclosures: The authors report no conflict of interest, financial or otherwise, concerning the material or methods used in this study or the findings specified in this paper.
Funding: There were no sources of financial or material support for this report.
Skeletal formation and growth occur as a process of sequential morphologic and biochemical events that take place during fetal development. During development, bone tissue is formed and grows through one of two processes. Bone can form directly from mesenchymal tissue, which is called intramembranous ossification. This occurs at the periosteal surfaces of all bones and in parts of the pelvis, scapula, clavicles, and skull. Alternatively, bone tissue can form by replacement of a cartilaginous model, which is referred to as endochondral ossification. Endochondral ossification occurs at the base of the skull, the vertebrae, and at the growth plates of the appendicular skeleton.
Proliferating cartilage constitutes the basis for much of the size increase of bones as organs, because bone tissue itself does not grow interstitially. In the long bones of the limbs the proliferating cartilage is located at the ends, in the form of what is known as a growth plate, physeal plate, or physis. The cartilage in the growth plate has a unique zonal structure, biochemistry, process of matrix mineralization, and blood supply that differs markedly from hyaline or articular cartilage. Other areas of the body that have proliferating cartilage of various configurations include the skull (sutures and the base), the spine (end plates and synchondroses), the pelvis (triradiate cartilages), and the carpus and tarsus.
Limb development has been studied in various organisms. Chick studies provided a wealth of information in the past because of the accessibility of the limb bud in ovo. However, molecular studies have focused largely on the mouse embryo. Cross-species transplants have indicated that many of the same signals control limb formation in both organisms. Additional information about limb patterning has been derived from the study of regenerating amphibian limbs.
The formation of the developing limb bud in vertebrates is initiated by the mesenchyme. Somites give rise to all limb muscle cells, whereas the lateral plate mesoderm gives rise to connective tissue and cartilage and thus determines the primary limb pattern. , In the absence of somites, the lateral plate mesoderm forms a limb with normal skeletal structure and tendons, which is devoid of any musculature.
The embryonic limb bud formation is initiated during the fourth week of gestation by the lateral plate mesoderm as a small projection on the ventrolateral body wall. This mesenchymal projection is covered by ectoderm, the tip of which thickens and becomes what is known as the apical ectodermal ridge (AER) ( Fig. 137.1 ), which drives limb outgrowth and proximodistal patterning. Underlying the AER are rapidly proliferating, undifferentiated mesenchymal cells that form what is known as the progress zone (PZ). Proliferation of these cells causes limb outgrowth. The events in upper and lower limb buds are similar in character and sequence. However, a slight craniocaudal time gradient exists, with the upper limb bud appearing and progressing 1 to 2 days before the lower limb bud. The molecular interaction between the AER and the underlying undifferentiated mesenchymal cells (PZ) drives limb development. Once the process of differentiation of all limb elements is complete, the AER disappears. Clinically, limb bud development is first identifiable by transvaginal ultrasound at 8 weeks.
![Fig. 137.1, A 5-week-old human embryo and enlargement of the upper and lower limb buds. The limb bud at this time is a jacket of ectoderm (apical ectodermal ridge [AER] ) covering undifferentiated mesenchymal cells (progress zone). The zone of polarizing activity, which is involved in anteroposterior patterning of the limb, is localized in a small area of mesoderm along the posterior border of the limb bud. Fig. 137.1, A 5-week-old human embryo and enlargement of the upper and lower limb buds. The limb bud at this time is a jacket of ectoderm (apical ectodermal ridge [AER] ) covering undifferentiated mesenchymal cells (progress zone). The zone of polarizing activity, which is involved in anteroposterior patterning of the limb, is localized in a small area of mesoderm along the posterior border of the limb bud.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/TheGrowthPlateEmbryologicOriginStructureandFunction/0_3s20B9780323712842001373.jpg)
As the limb bud grows, three major limb axes appear at different times and by different mechanisms ( Fig. 137.2 ). The limb bud develops asymmetries along the proximodistal (flank to digit tip), anteroposterior (thumb to small finger), and dorsoventral (back of hand to palm) axes. Some of the important signals for initiating and maintaining these axes have been described in literature and will be discussed later in this chapter. The genes that initiate and potentiate signals in different axes often interact and are regulated via feedback mechanisms, resulting in effects in multiple planes of growth.
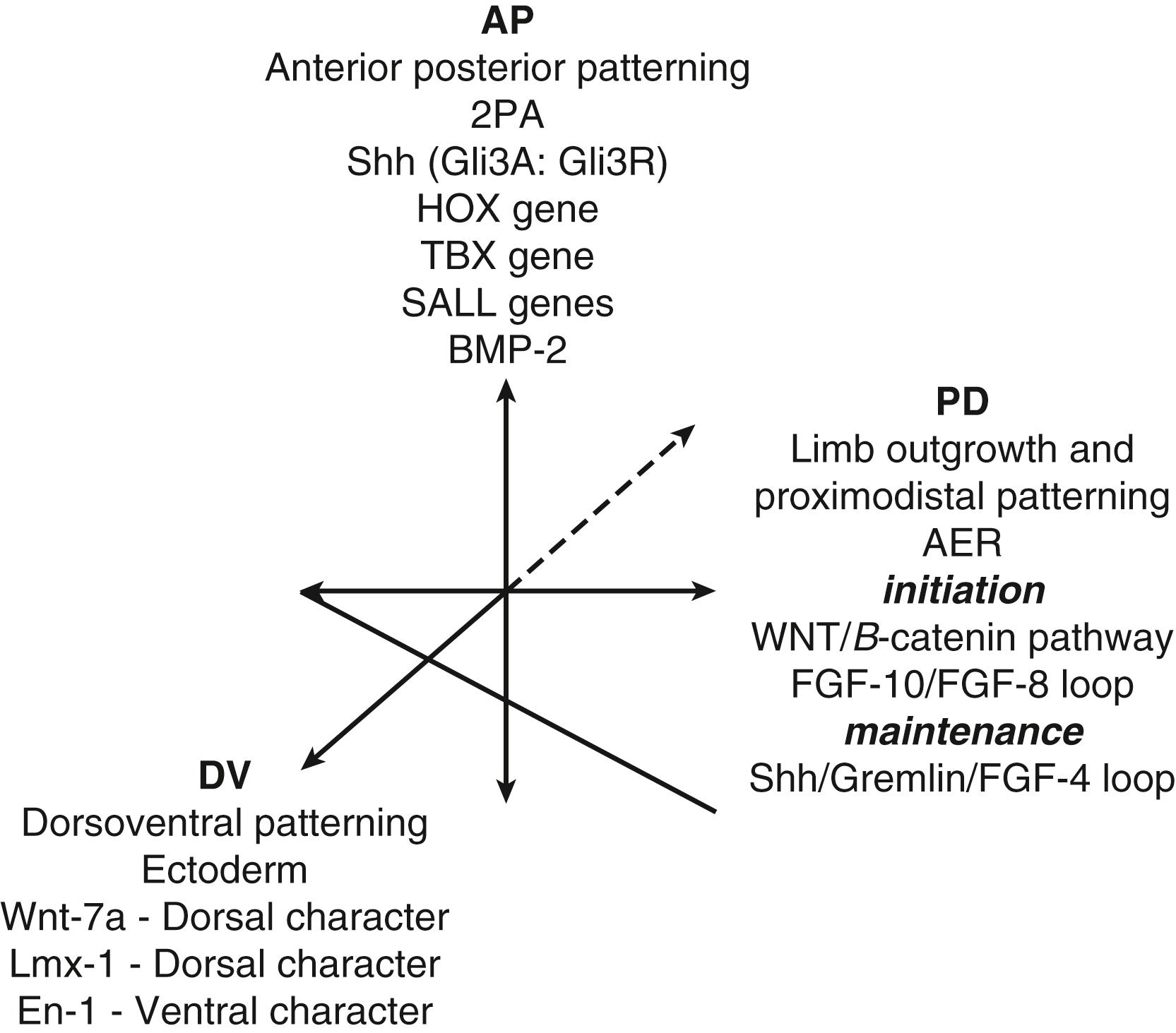
Limb bud outgrowth (proximodistal patterning) is dependent on signals emanating from the AER. The first signaling center to appear is AER and fibroblast growth factors (FGFs) produced in the AER stimulate cell proliferation in the underlying mesenchyme of the PZ. The expression of FGF-10 and FGF-8 is necessary to initiate limb bud outgrowth. The expression of each supports and promotes the expression of the other. This FGF-8/FGF-10 loop is mediated by Wnt-2b, a mammalian homologue of Drosophila segment polarity gene-wingless and Wnt-8c proteins, through a β-catenin (Wnt/β-catenin) pathway. FGF-2, FGF-4, FGF-8, FGF-9, and FGF-17 are expressed in the AER, and each is able to sustain limb bud outgrowth. A graft of an AER to an ectopic site on the bud leads to extra limb growth in the ectopic location. Removal of the AER or an abnormality in the AER stops limb bud growth and leads to proximal limb truncation. Similarly, disrupted FGF signaling leads to arrested limb development. Clinical examples of this are cleft hand and radial clubhand. However, limb bud outgrowth can be sustained after excision of the AER if FGF-impregnated beads are inserted in place of the AER. ,
Cell growth and proliferation of the mesenchyme are maintained through a regulatory loop, with FGFs and a protein called sonic hedgehog (Shh) , which maintains the integrity of the AER. The formin gene, which encodes several proteins that function as cytokines, is required to establish this Shh/FGF-4 feedback loop. Bone morphogenetic proteins (BMPs), members of the transforming growth factor-β (TGF-β) superfamily, play a complex role in the regulation of the AER (also in dorsoventral patterning). , BMPs are necessary to the induction of the AER. However, BMP signaling must be moderated by Gremlin (a BMP antagonist) to maintain the Shh/FGF-4 feedback loop necessary for limb outgrowth and patterning. The activation of Gremlin is dependent on the expression of the formin gene.
Anteroposterior axis determination is under the control of the zone of polarizing activity (ZPA), which is an area of tissue in the posterior aspect of the limb bud (see Fig. 137.1 ). Anteroposterior limb growth is also called radioulnar with ulnar signifying posterior and radius anterior location. Early chick studies showed that a mirror image digit duplication pattern developed when tissue from the posterior area was transplanted to the anterior portion of another wing bud. The number of these additional digits developed depended on the strength (number of cells) and duration of transplant (16 hours for 2 digits, 20 hours for 3 digits). This observation led to the development of the morphogen gradient model whereby anteroposterior patterning was determined by diffusion of a signal. The Shh gene is an important regulator of anteroposterior patterning. Normally there is a high concentration of Shh on the posterior (ulnar) side for small finger development and low concentration of Shh on anterior (radial) side for thumb development. Although early studies showed that exogenous application of retinoic acid to the anterior border of a normal limb could mimic results from ZPA transplant studies, this effect is now thought to occur via activation of the Shh gene. Shh represses Gli3R activity in the posterior limb bud, thus affecting the precise balance of Gli3A and Gli3R, which may be the basis for the graded response to Shh signaling.
Other genes important in anteroposterior patterning under investigation include the 5′Hoxd genes, Tbox, and Sall family genes (specifically Tbx2, Tbx3, Sall1, and Sall4), and defects in these genes cause digital alterations in humans. Clinically, abnormal upregulation of Shh in the ZPA results in polydactyly on the ulnar (posterior) side, while upregulation of Shh in the anterior aspect of the limb bud (where Shh concentration should be low) leads to an absent thumb.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here