Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Neural tube defects (NTDs) are a common congenital anomaly with clinically important associated morbidity and mortality rates.
Most open NTDs will have associated intracranial abnormalities, most commonly Chiari II malformation, detected by sonography.
Although much less common, closed NTDs are less likely to have associated intracranial abnormalities and require careful examination of the spine for detection.
Other spinal abnormalities such as scoliosis, sacral agenesis, caudal regression, sirenomelia, and sacrococcygeal teratoma have distinct sonographic appearances that aid in prenatal diagnosis.
Abnormalities of the spine are some of the most common congenital abnormalities. In the United States, overall incidence of neural tube defects (NTDs) was approximately 1 to 2 in 1000 births before 2000, but by 2007 it was less than 0.5 in 1000 pregnancies primarily as a result of the widespread use of folic acid before conception and the addition of folic acid to enriched grain products. Since the institution of folic acid supplementation, an estimated 1300 cases of NTDs each year have been prevented in the United States. At least 42 nations practice mandatory folic acid fortification to combat NTDs.
NTDs are associated with substantial morbidity and mortality rates. Many survivors have severe long-term morbidity that has a profound emotional, physical, and financial impact on their families. Fortunately, the birth incidence of spina bifida and anencephaly is decreasing in many areas of the world as a result of maternal screening programs (maternal serum tests and antenatal ultrasound) and, more recently, the administration of folic acid to women of child-bearing age.
In prenatal imaging, three-dimensional (3D) ultrasound and fetal magnetic resonance imaging (MRI) are making a positive impact, especially for precise localization of spina bifida and complete delineation of associated abnormalities. This precise information is useful for prognosis and possibly for prenatal surgery. Prenatal surgery for closure of myelomeningoceles is practiced in only a few centers at this time.
The precursors of the spinal cord and surrounding spinal column develop in the third and fourth weeks after conception (fifth and sixth menstrual weeks). During the third conceptual week, the bilaminar germ disc evolves into the trilaminar germ disc, which consists of the ectoderm layer (part of the amniotic cavity), the middle mesoderm layer, and the endoderm layer (part of the yolk sac cavity) ( Fig. 35.1A ). The mesoderm layer develops a midline central tube, the notochordal process, which runs along the long axis of the embryonic disc. The mesoderm lateral to the notochordal process has three components: paraxial mesoderm, intermediate mesoderm, and lateral plate mesoderm. By day 21 conceptual age, the hollow-tube notochordal process has evolved into a solid cord called the notochord, and the paraxial mesoderm has developed multiple discrete bumps called somites, of which there are 37 pairs when fully developed ( Fig. 35.1B ).
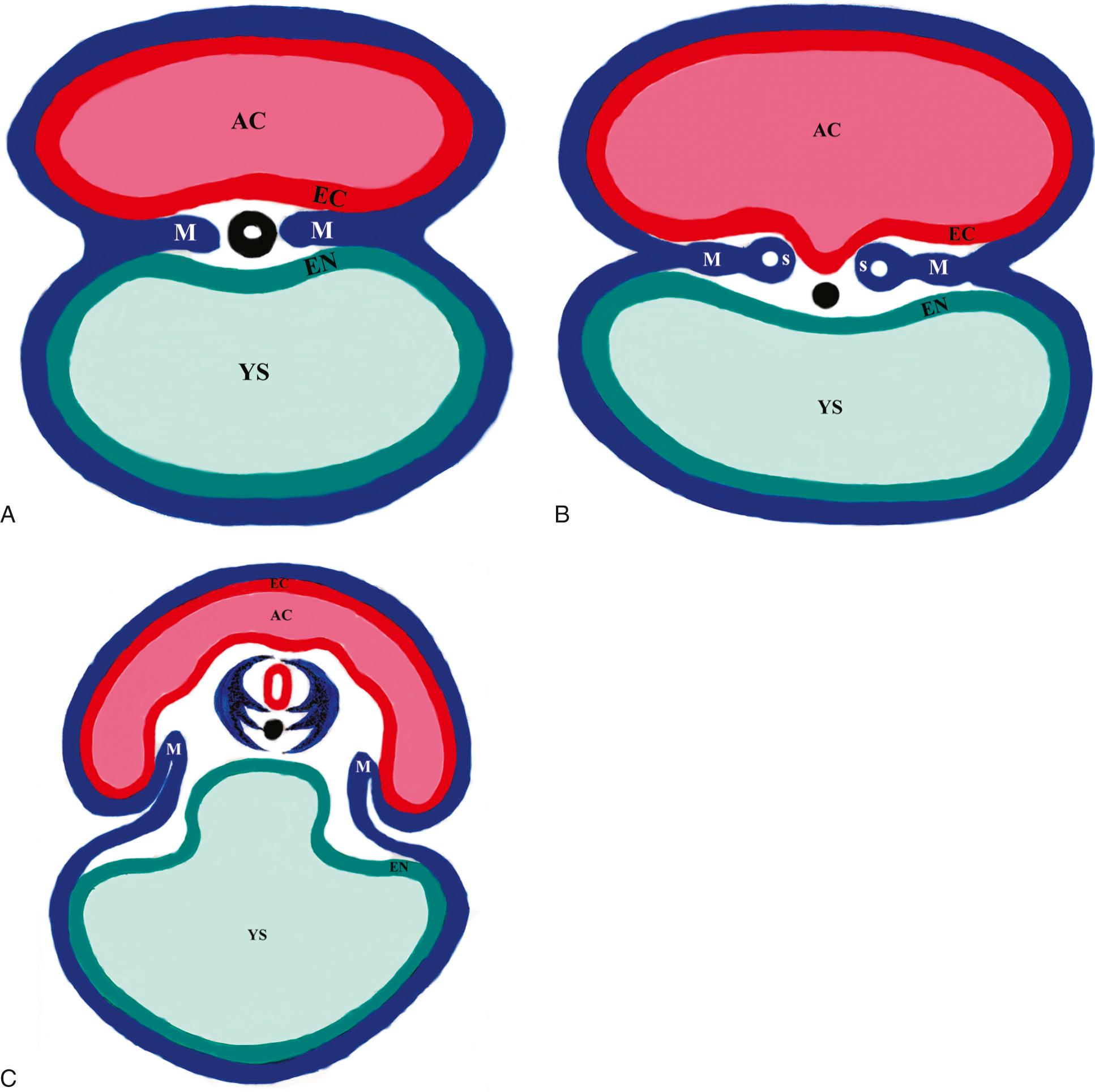
The notochord and the rest of the intraembryonic mesoderm induce the development of the neural plate in the ectoderm layer (amniotic cavity side of the germ disc), starting on conceptual day 18. The neural plate grows in length and breadth until conceptual day 21, when neurulation begins. Neurulation is the process of folding of the neural plate into the neural tube, probably induced by the adjacent notochord. The lateral edges of the neural folds begin to fuse dorsally into a closed neural tube in the occipitocervical region, leaving an opening at the cranial end (cranial neuropore) and the caudal end (caudal neuropore). The hollow center of the neural tube is called the neural canal, which will become the central canal of the spinal cord and ventricular system of the brain. By day 24 conceptual age, the cranial neuropore closes, and by day 25 the caudal neuropore closes ( Table 35.1 ).
| Menstrual Age (Days) | Conceptual Age (Days) | Embryo Length (mm) | Sac Diameter (mm) | Landmarks |
|---|---|---|---|---|
| 31 | 17 | 0.5 | 2 | Trilaminar disc Notochordal process Paraxial mesoderm ( Fig. 35.1A ) |
| 35 | 21 | 2 | 4 | Notochord Neural plate Somites ( Fig. 35.1B ) |
| 42 | 28 | 5 | 10 | Neural tube Notochord Sclerotome ( Fig. 35.1C ) |
The cranial end of the neural tube becomes the brain, and the caudal end becomes the spinal cord. In week 4 conceptual age, after the neural tube has formed, the adjacent 37 pairs of somites in the intraembryonic mesoderm give rise to the vertebral bodies and vertebral arches that will surround the spinal cord. A group of cells, the sclerotome, migrates from the somites and surrounds the adjacent neural tube and notochord. The ventral portion of the sclerotome surrounds the notochord and forms the rudiment of the vertebral body. The dorsal portion of the sclerotome surrounds the neural tube and forms the precursors to the vertebral arch ( Fig. 35.1C ). In the fetus, the notochordal remnant corresponds to the nucleus pulposus of the intervertebral discs.
Abnormalities of neural tube closure not only affect the spinal cord and brain but also interfere with normal development of surrounding vertebral arches, which are derived from adjacent mesodermal somites. Disturbances of neural tube closure underlie spina bifida and anencephaly defects.
Caudal regression defects may be related to defective development of the mesoderm layer in week 3 conceptual age, during transformation of the germ disc from two layers (bilaminar) to three layers (trilaminar). Various degrees of abnormal mesoderm development account for the wide spectrum of abnormalities found in caudal regression.
A failure of part of the neural tube to close, called spinal dysraphism, disrupts development of the nervous system and disrupts the induction of the overlying vertebral arches. The resulting open vertebral canal is called spina bifida. If dura and arachnoid protrude from the spinal canal, the result is a meningocele. If neural tissue and meninges protrude, the result is a myelomeningocele. In the most severe NTDs, the neural tube fails to form and fails to separate from the overlying ectoderm. In the spine, this condition is called rachischisis or myeloschisis; the open spinal cord is exposed along the dorsal surface of the fetus. If the defect involves the cranial neural tube, the brain is represented by an exposed dorsal mass of undifferentiated neural tissue, called exencephaly, anencephaly, or craniorachischisis. Differentiated brain and meninges may bulge from a nonossified gap in the skull (meningoencephalocele), but this is not related to failure of neural tube closure.
In animals, certain teratogens can induce NTDs: retinoic acid, insulin, and high plasma glucose levels. In humans, implicated factors include valproic acid (antiepileptic), maternal diabetes, and hyperthermia. Valproic acid may interfere with folate metabolism.
Prenatal sonography readily portrays the ossified portions of the fetal spine, whereas the nonossified cartilage is more difficult to delineate. It is therefore important for sonographers and sonologists to understand the temporal and spatial ossification patterns during fetal development in order to optimize spinal evaluation.
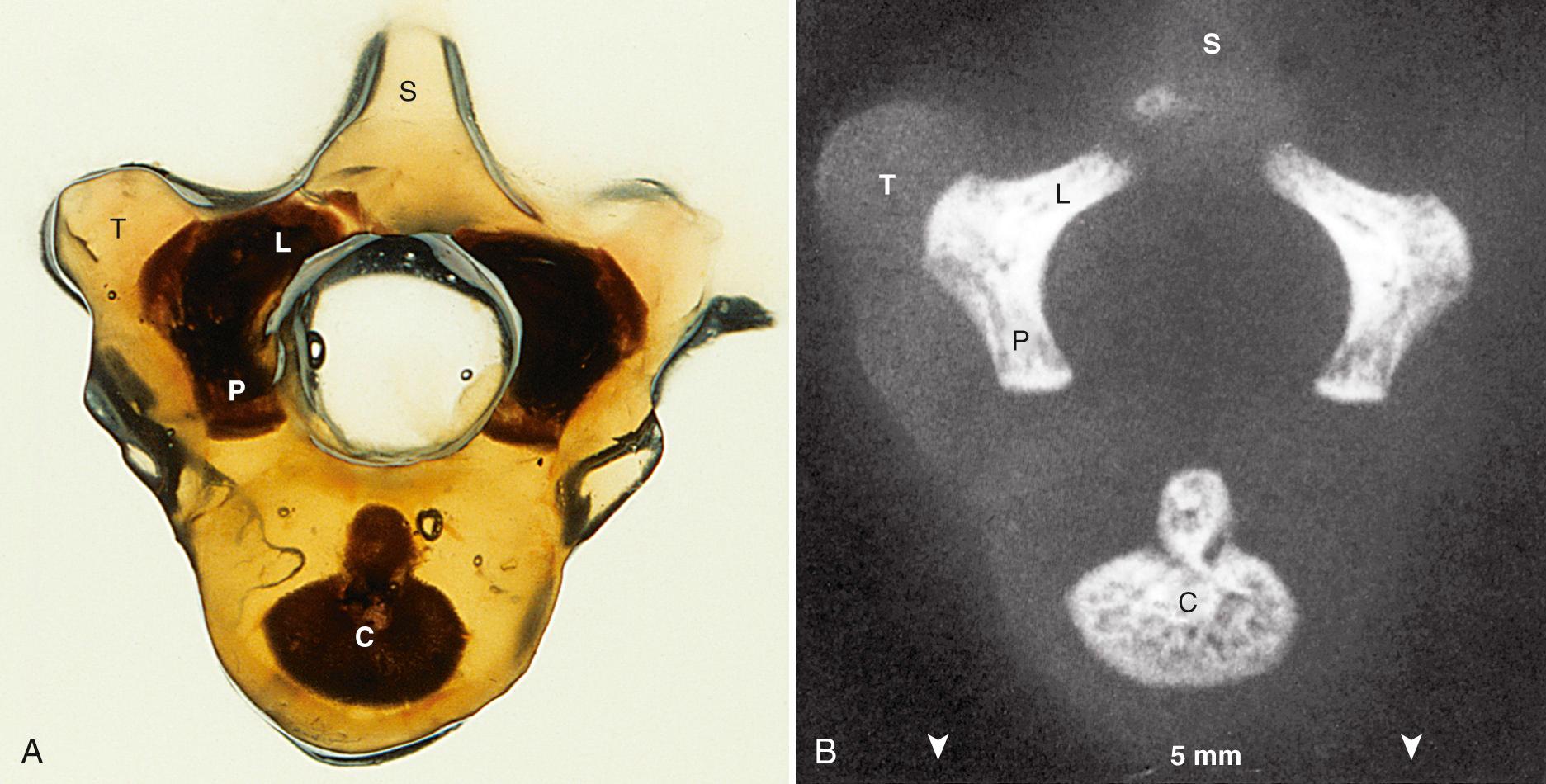
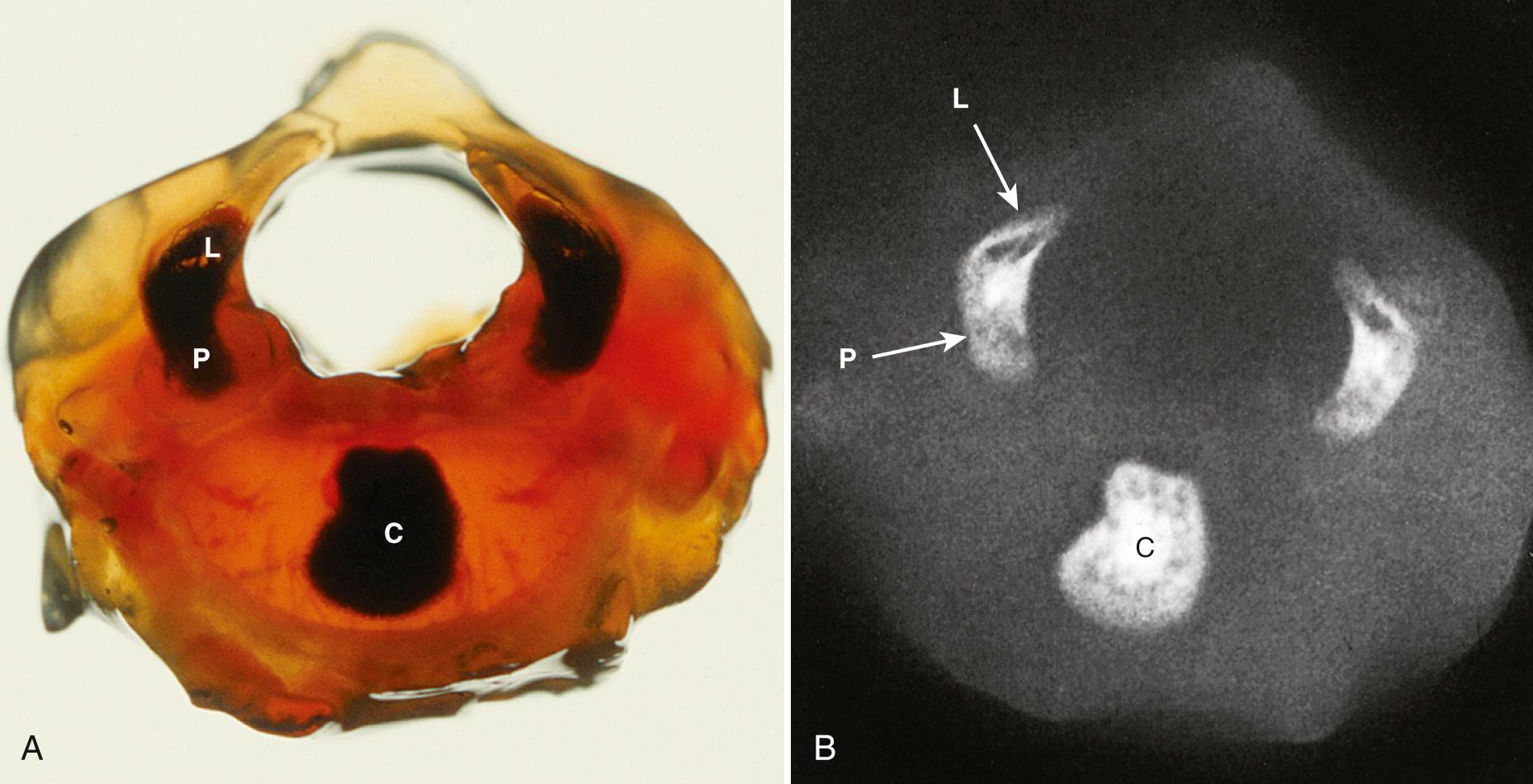
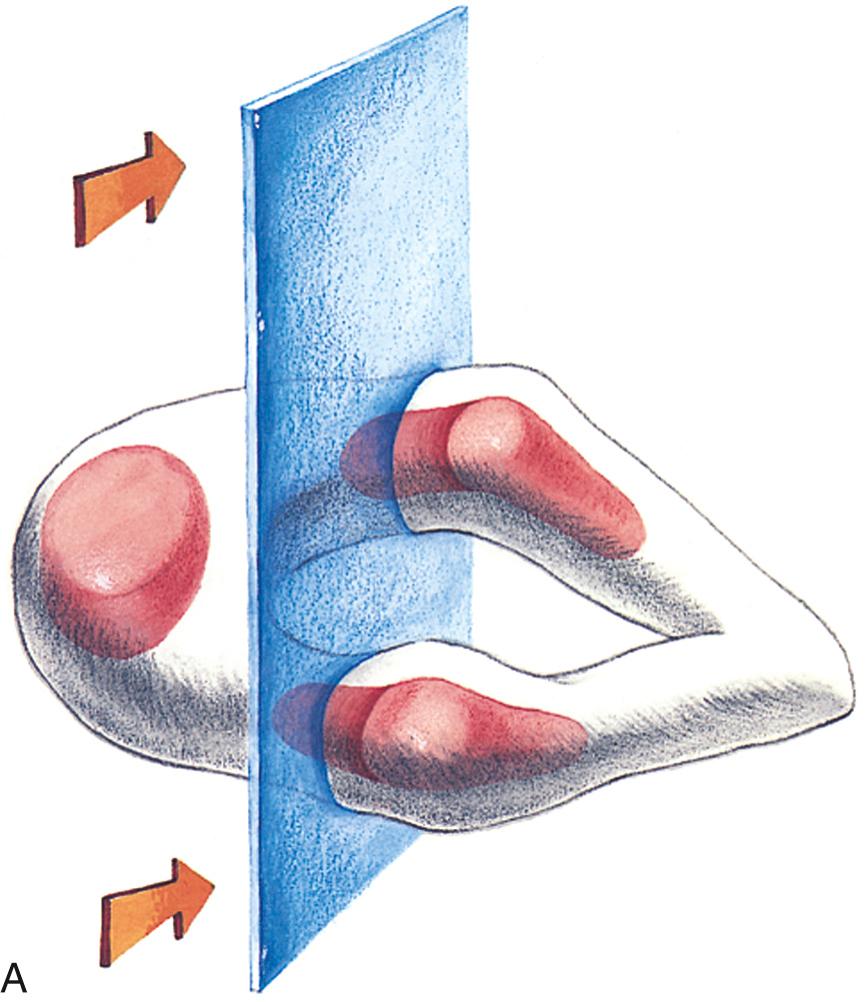
Each vertebra will develop three ossification centers: the centrum, right neural process, and left neural process. The centrum will form the central part of the vertebral body, and the neural process will form the posterolateral parts of the vertebral body and pedicles, the transverse processes, the laminae, and the articular processes.
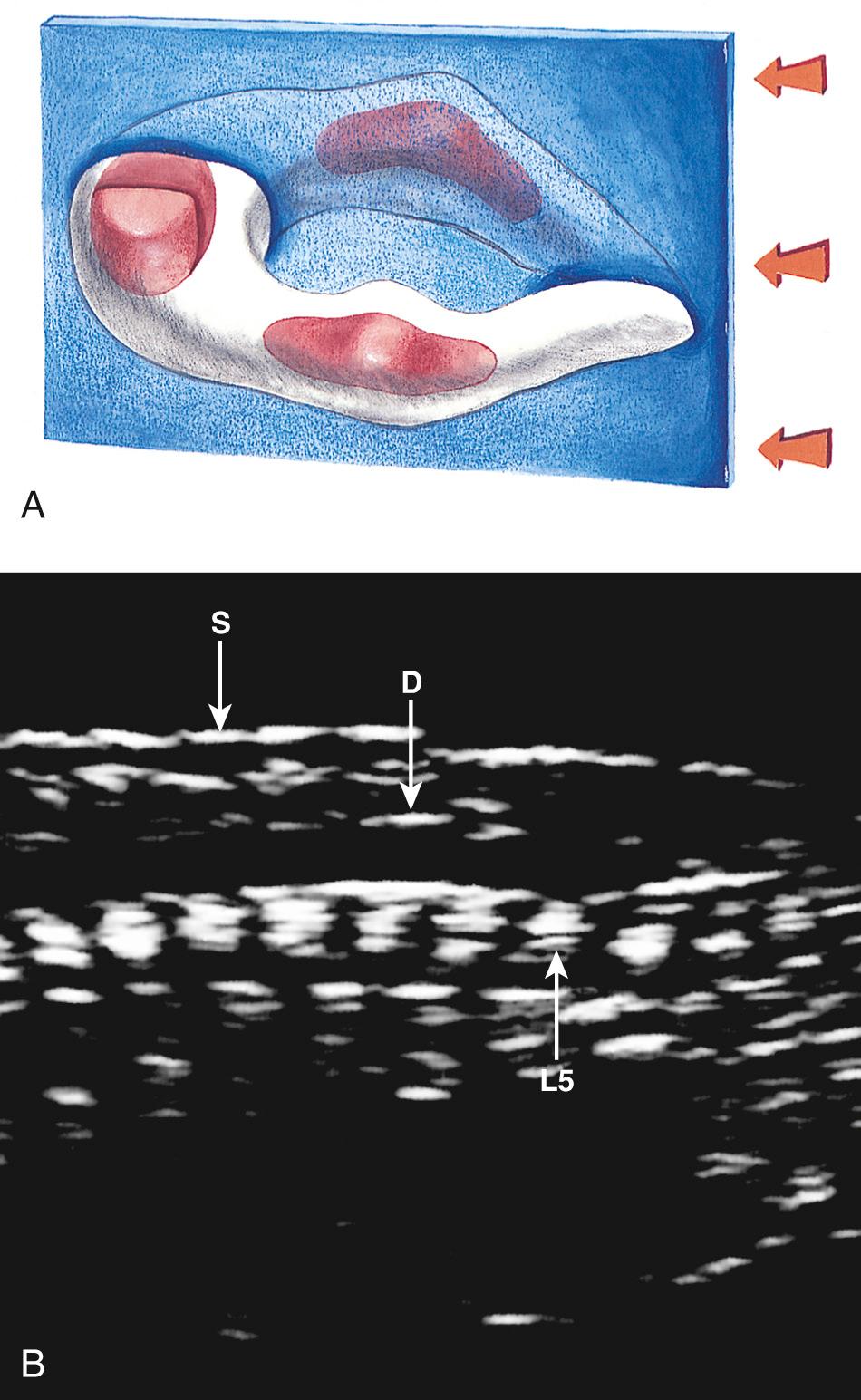
Ossification begins in the lower thoracic fetal spine at approximately 10 weeks' gestation (menstrual age). Ossification of the centra progresses in cranial and caudal directions simultaneously. Neural arch ossification proceeds caudally from the lower thoracic (T) spine to the lumbar (L) spine. It proceeds sequentially from L1 through L5 and then into the sacral (S) spine. By 13 weeks' menstrual age, there are three ossification centers in vertebrae C1 through L3 ( Fig. 35.2 ). Neural arch ossification begins as a small focus at the base of the transverse process and extends simultaneously into the pedicle anteriorly and into the lamina posteriorly ( Fig. 35.3 ).
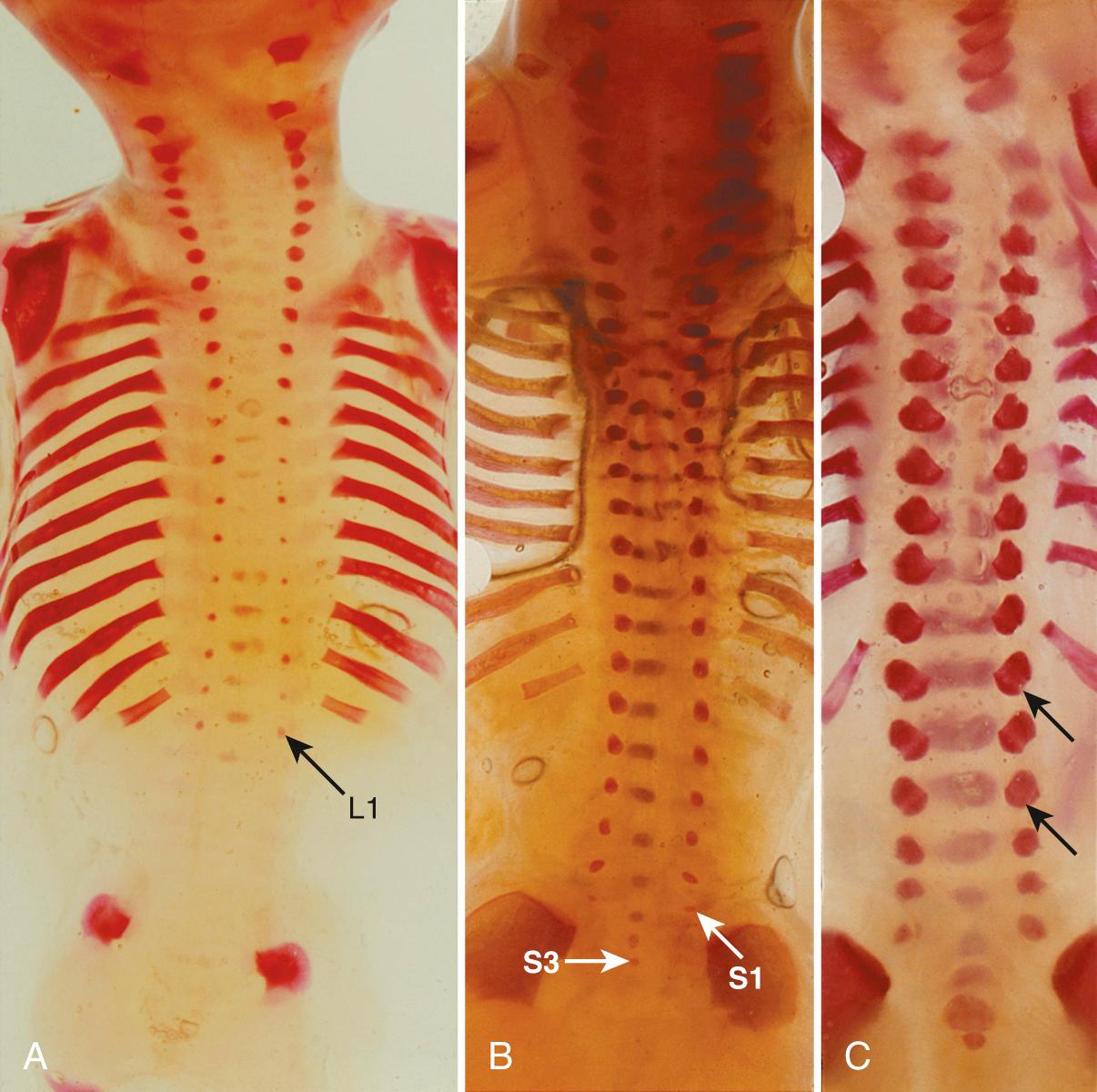
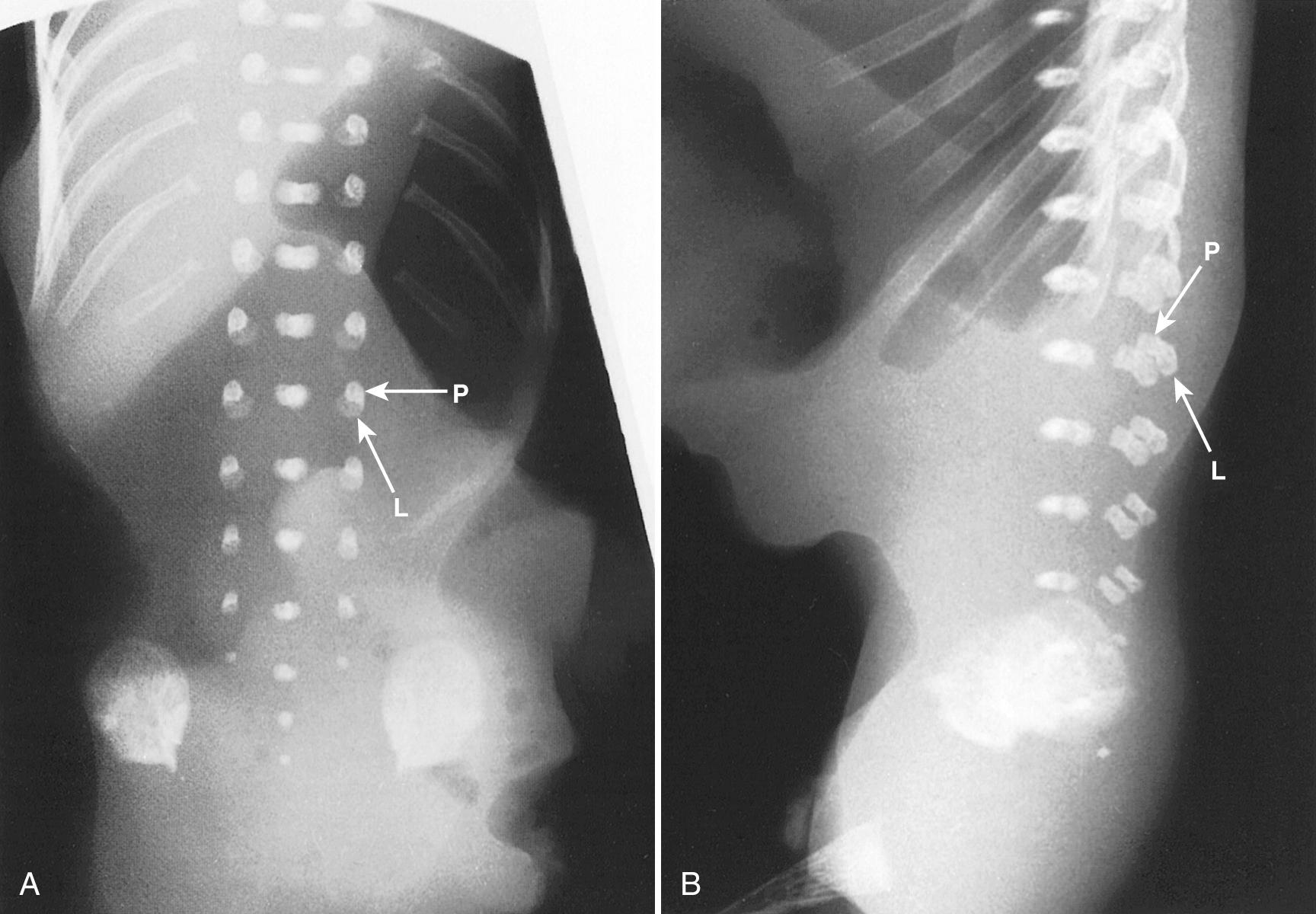
Ultrasound evaluation for spina bifida usually occurs between 16 and 22 weeks' gestation. By 16 weeks, there is enough ossification in the neural arches to assess for spina bifida to level L5, by 19 weeks to level S1, and by 22 weeks to level S2 ( Figs. 35.4 and 35.5 ). In some fetuses, there may be enough neural arch ossification to assess for spina bifida before these gestational ages. Braithwaite et al. assessed the fetal anatomy at 12 to 13 weeks' gestation by a combination of transabdominal and transvaginal sonography and reported successful examination of the vertebrae and overlying skin in both the transverse and the coronal plane in all cases. Others have reported successful prenatal diagnosis of spina bifida at 12 to 14 weeks' gestation on the basis of abnormal cranial findings. They caution that although the characteristic cranial findings may be present at 11 to 14 weeks, the prevalence of these findings in the first trimester remains to be determined ( Table 35.2 ). Furthermore, closed NTDs are less likely to be associated with abnormal cranial findings and therefore are more difficult to detect in the first trimester.
| Age (Wk) | Events |
|---|---|
| 10 | Ossification appears in lower thoracic spine vertebral bodies. |
| 13 | Some ossification is present from C1 to L5 vertebral bodies and arches. |
| 13-22 | Neural arch ossification simultaneously extends anteriorly into the pedicles and posteriorly into the laminae. |
| 16 | Enough neural arch ossification appears to assess for spina bifida to level L5. |
| 19 | Enough neural arch ossification appears to assess for spina bifida to level S1. |
| 22 | Enough neural arch ossification appears to assess for spina bifida to level S2. |
For fetuses at 19 to 33 weeks' gestation, the conus medullaris is normally situated at level L2-L3 or higher ( Fig. 35.6 ). Level L3 is taken to be indeterminate and L3-L4 or lower as abnormal. For those fetuses with tethered cord, the position of the conus medullaris is usually lower than normal. For earlier pregnancy (13-18 weeks' gestation), the conus medullaris may be normally as low as L4. At term, the conus is normally at or above L2.
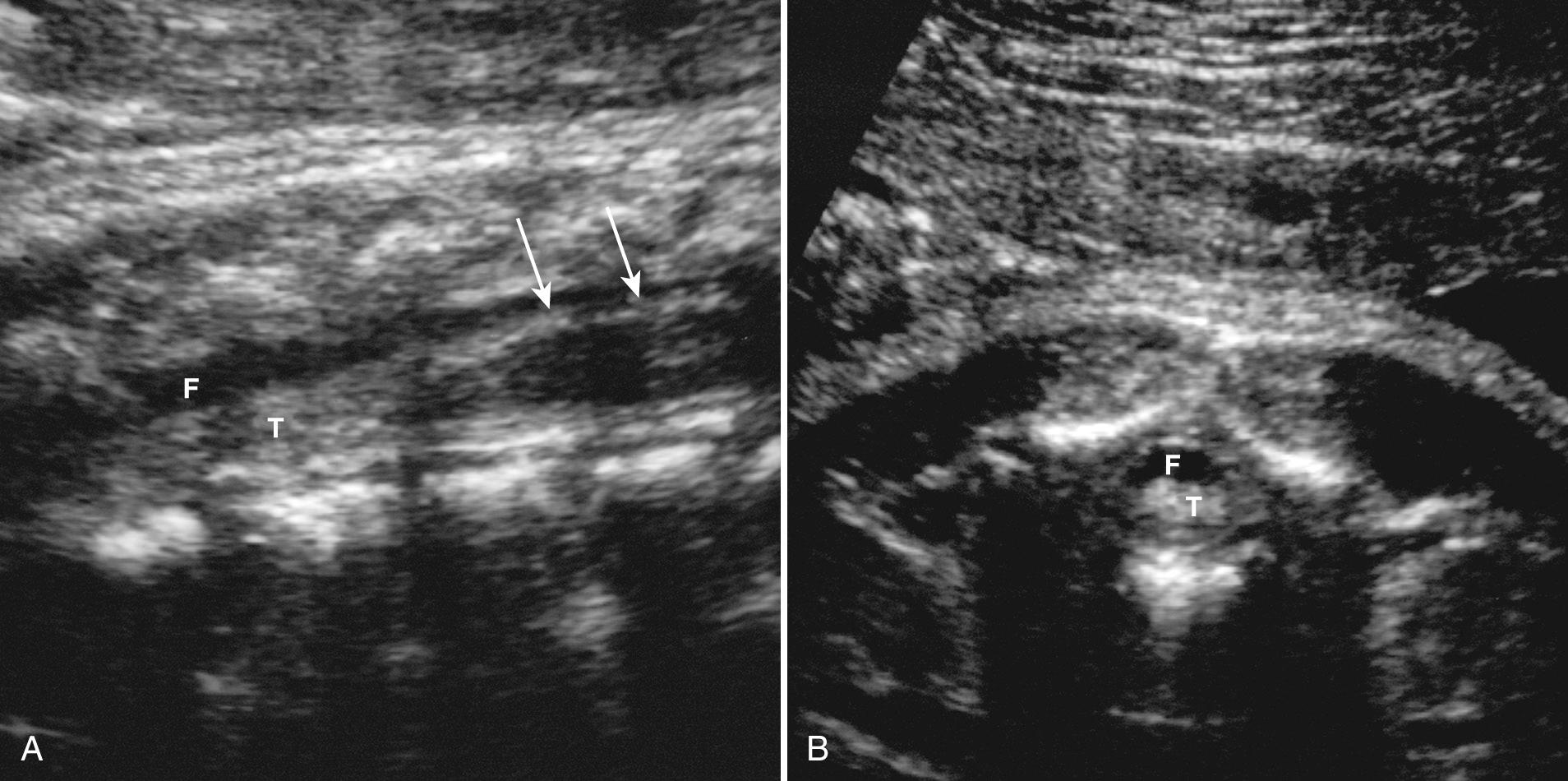
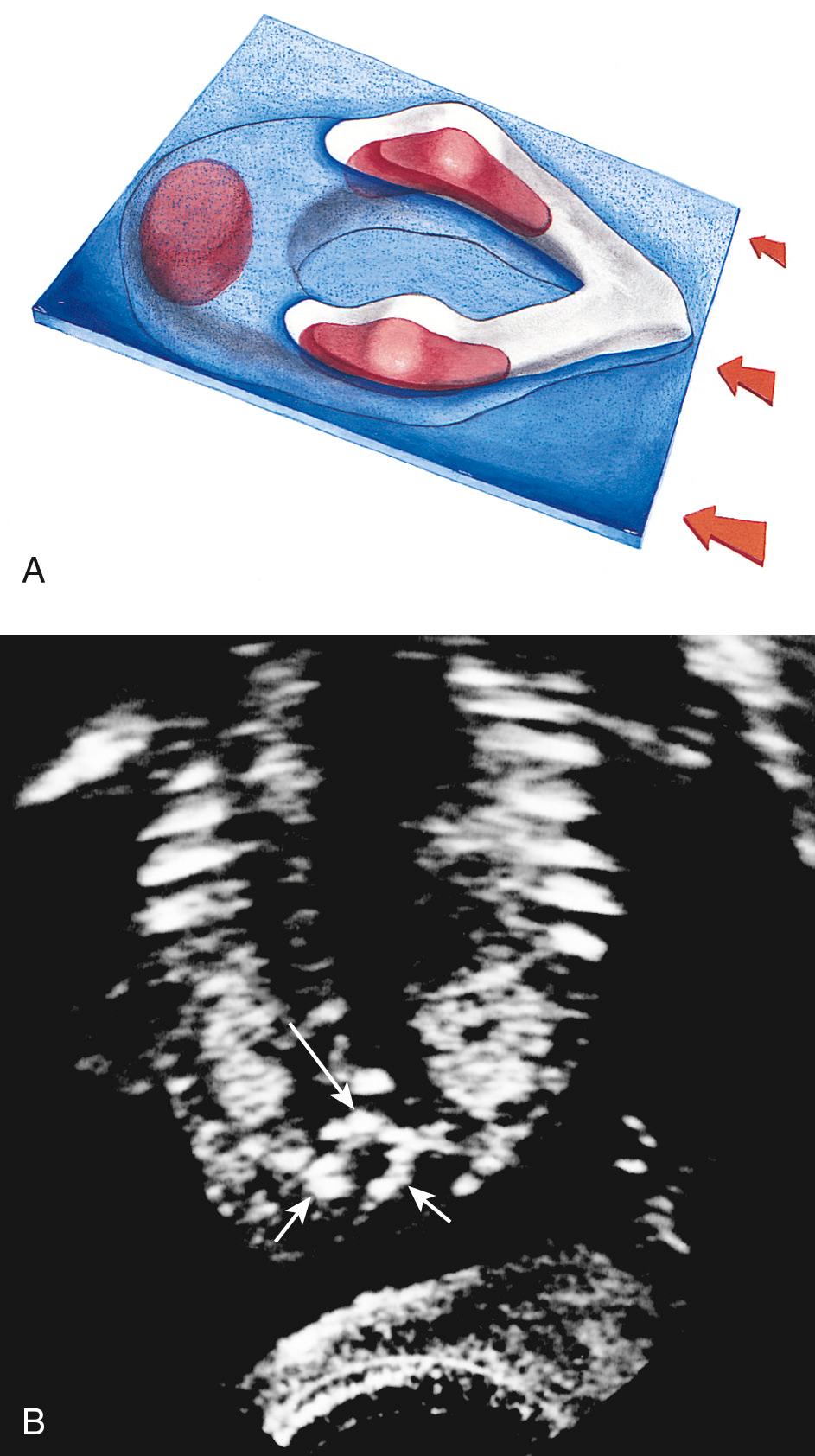
In clinical practice, the most useful scan planes to assess the posterior neural arches are posterior transaxial ( Fig. 35.7 ), lateral transaxial ( Fig. 35.8 ), lateral longitudinal (coronal) ( Fig. 35.9 ), posterior longitudinal (sagittal) ( Fig. 35.10 ), and posterior angled transaxial ( Fig. 35.11 ). The posterior angled transaxial scan plane is useful to visualize the pedicles and laminae simultaneously. Because the laminae course caudal to the transaxial plane, which contains the centrum and pedicles, only the angled scan plane can depict the pedicles and laminae simultaneously in their entirety.
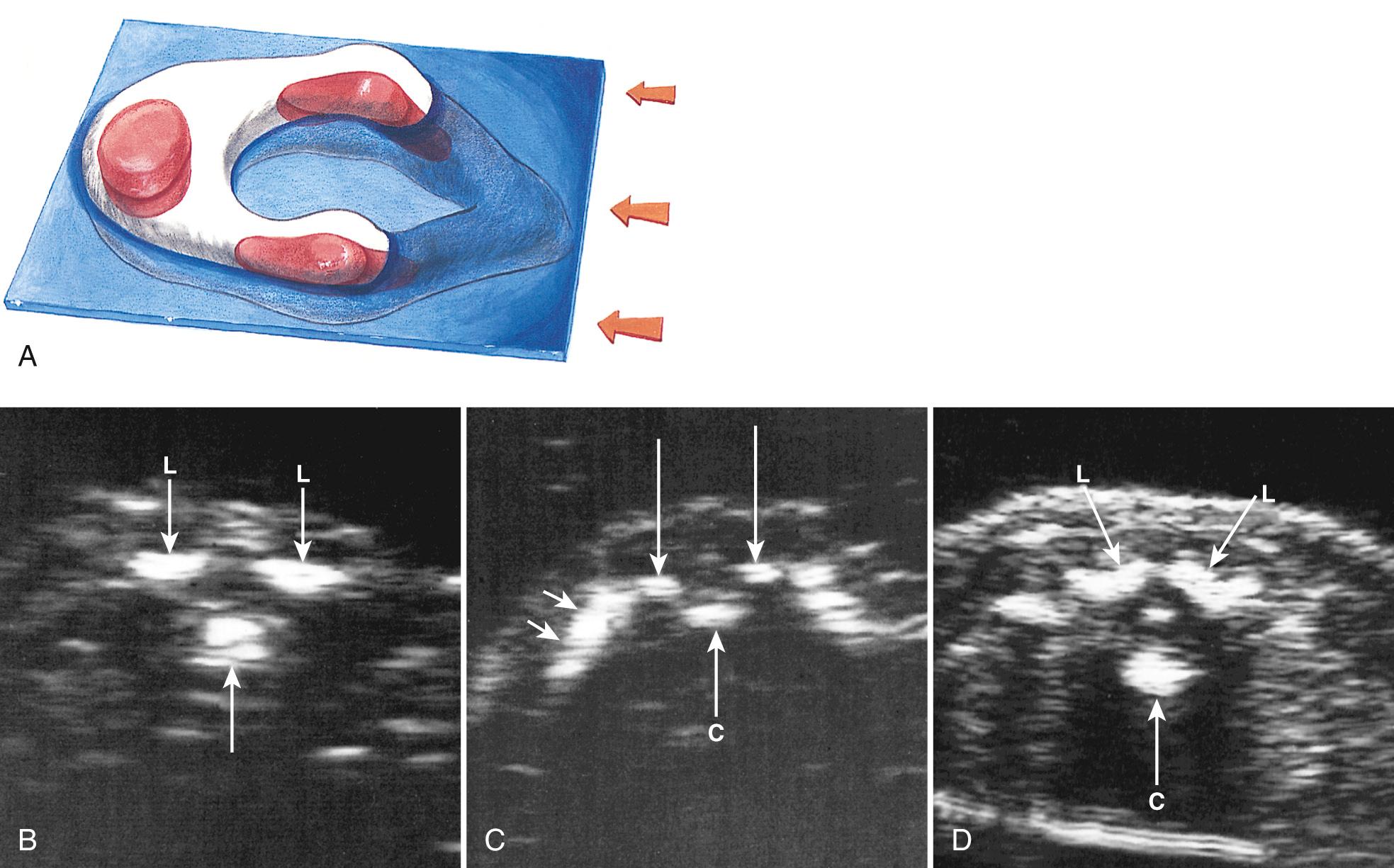
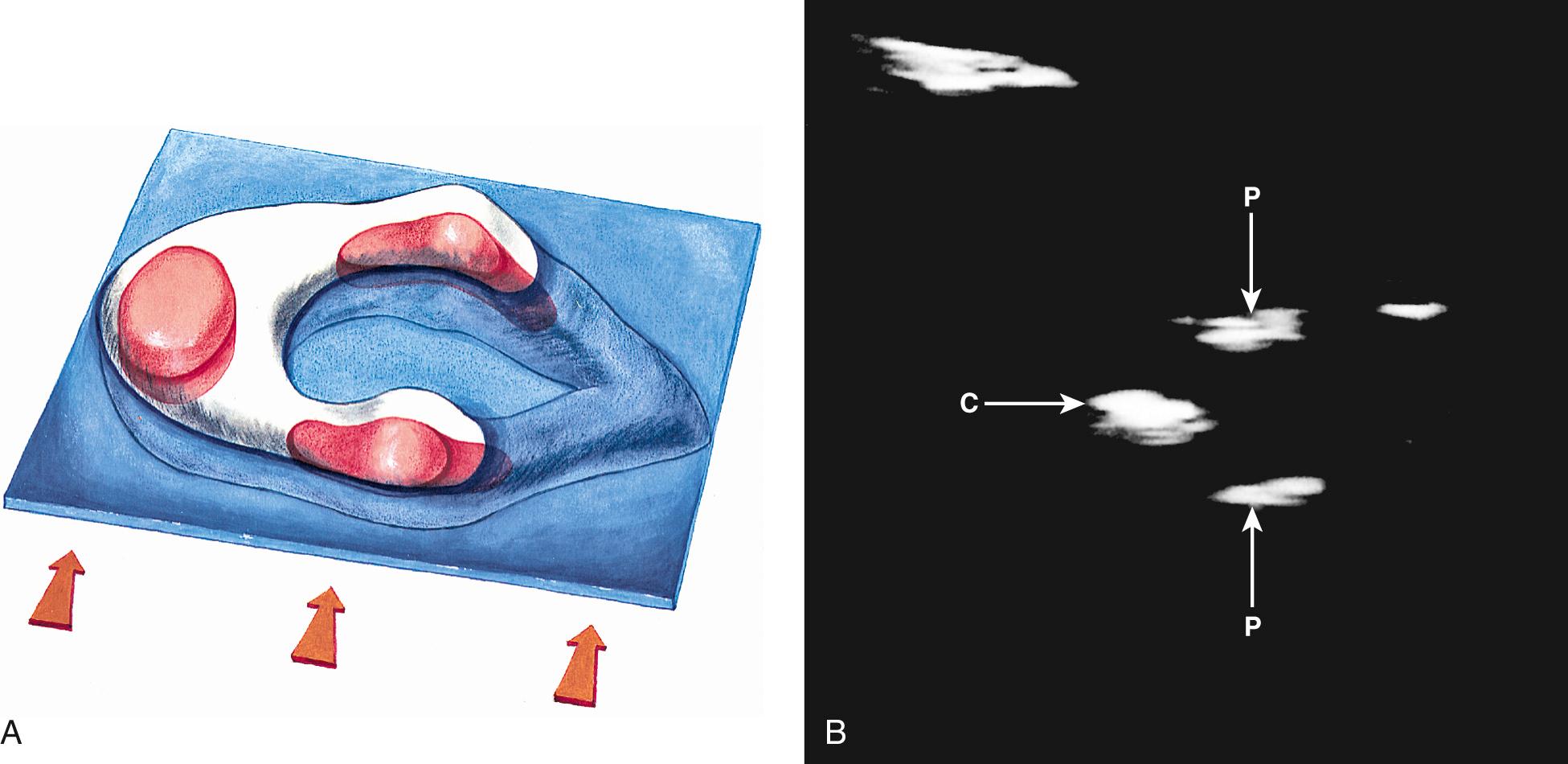
The detection rate of spina bifida at 18 to 20 weeks' gestation may be 80% or less during routine screening ultrasound, because the accuracy of ultrasound depends on the skill and experience of the operator. The accuracy of referral centers performing detailed targeted imaging because of a suspected NTD or high maternal serum alpha-fetoprotein (MS-AFP) level is close to 100%.
A detailed sonogram of the fetal spine may be requested for several reasons: previous suspicious ultrasound, family history of NTD, or raised serum or amniotic fluid AFP. To enhance detection of spina bifida, a detailed protocol should be consistently followed. The first step in assessing for spina bifida is scanning the head, because most fetuses with open spina bifida have signs of a Chiari II malformation in the brain at 16 to 22 weeks' gestation. These signs include obliterated cisterna magna (banana sign), concave frontal bones (lemon sign), and dilated lateral cerebral ventricles. The sensitivity of the banana sign for open spina bifida is close to 99%, and false-positive diagnoses are rare, although the lemon sign may occur in 1% to 2% of normal fetuses.
The next step is to determine the position of the fetal spine. The scan plane is placed perpendicular to the long axis of the fetal spine, either posterior transaxial or lateral transaxial (see Figs. 35.7 and 35.8 ). The sonographer should scan from one end of the spine to the other while maintaining the scan plane perpendicular to the spine ( Video 35.1). This is repeated several times. In the process, the sonographer builds up an impression of the 3D structures of the spine. The scan plane should then be repositioned parallel to the long axis of the fetal spine to obtain posterior longitudinal and lateral longitudinal views. The sonographer then examines all levels of the spine in posterior transaxial, lateral transaxial, lateral longitudinal, and posterior longitudinal scan planes ( Video 35.2). This may not be possible in a short time because of fetal position, but this usually changes enough in 30 to 45 minutes at 16 to 22 weeks to obtain all scan planes. If the spine cannot be visualized optimally, a repeat scan can be performed at a later gestational age. In both axial and longitudinal scan plans, it is important to visualize the skin covering the fetal back by ensuring that amniotic fluid is interposed between the fetus and the uterine wall.
Three-dimensional ultrasound imaging has shown promise in evaluating normal fetal structures and in providing additional information in abnormalities of many fetal structures, including the spine, hand, foot, and face. Bony structures can be visualized with maximum-intensity projection methods (see Fig. 35.9D ). In evaluation of spinal abnormalities, 3D ultrasound is most helpful in localizing spinal defects accurately by using simultaneous multiplanar imaging and referencing to the volume-rendered image. For determination of spinal level, T12 is taken to be the most caudal vertebra with a corresponding rib. Three-dimensional ultrasound may also show concomitant rib deformities in the setting of spinal abnormalities and is useful for counseling prospective parents by providing a more understandable visual demonstration of the skeletal abnormality.
Spina bifida implies a physical defect in the structure of the spinal canal that may result in a protrusion of its contents (meninges, cerebrospinal fluid, and neural tissue) ( Table 35.3 ). These defects usually occur along the dorsal midline (most often in the lumbosacral area) but in rare cases may occur anteriorly.
| Term | Definition | Comment |
|---|---|---|
| Spinal dysraphism (neural tube defect) | Failure of part of neural tube to close. | This disrupts both differentiation of central nervous system and induction of vertebral arches. |
| Spina bifida | Defect in posterior midline neural arch. | Arches fail to fuse along dorsal midline and fail to enclose vertebral canal. |
| Spina bifida occulta | Vertebral arches of a single vertebra fail to fuse. | Underlying neural tube differentiates normally; does not protrude from vertebral canal. |
| Meningocele | Dura and arachnoid protrude from vertebral canal through spina bifida defect in posterior midline neural arches. | |
| Myelomeningocele | Dura, arachnoid, and neural tissue protrude from vertebral canal through spina bifida defect in posterior midline neural arches. | |
| Rachischisis (e.g., myeloschisis) | Neural folds corresponding to future spinal cord fail to fuse and fail to differentiate (myeloschisis), invaginate, and separate from surface ectoderm. | The deformed underdeveloped spinal cord is exposed dorsally. This is the most severe form of spinal neural tube defect. |
| Cranioschisis (e.g., exencephaly, anencephaly) | Neural folds corresponding to future brain fail to fuse and fail to differentiate, invaginate (exencephaly, anencephaly), and separate from surface ectoderm. | The brain is represented by an exposed dorsal mass of undifferentiated neural tissue. |
| Inionschisis | Failure of neural tube to differentiate properly and close in occipital and upper spinal region. |
Open NTDs occur in less than 0.5 per 1000 births in North America and with higher frequencies in other geographic areas. Hispanic women in the United States have a higher incidence of births affected by NTDs, possibly because of a higher prevalence of a mutation in the methylenetetrahydrofolate reductase deficiency gene. In one area of China, the overall prevalence of NTDs in 2003 was 13.9 per 1000 live births. In recent years, however, there has been a decline in the incidence of NTDs. Some of this decline may be attributed to screening programs, which include measurement of MS-AFP and performance of second-trimester ultrasound.
Volume data are acquired from sagittal and transverse sweeps through the spine.
Volume data are reformatted to display standardized multiplanar views of the fetal spine.
Three-dimensional reconstruction of the fetal spine (with maximum-intensity projection filter) visualizes the ossified spinal elements.
To determine spinal level, the 12th thoracic (T12) is taken to be the most caudal vertebra with a corresponding rib.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here