Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Moderate to severe congenital heart disease (CHD) occurs in approximately 6 per 1000 live births.
The incidence of CHD is much higher in the fetal population, estimated to be at least 15 per 1000.
The incidence of CHD is higher with positive family history, monochorionic twins, and in vitro fertilization.
Most fetuses with CHD have no known risk factors.
Fetal echocardiography is a safe and effective method of detecting CHD.
Early detection of CHD may have a substantial impact on care and prognosis, allowing intrauterine therapy, carefully planned delivery, and parental counseling.
Advancements in surgical techniques and perioperative management have significantly improved prognosis in many cases.
Sonographic evaluation of the fetal heart can identify cardiac abnormalities that affect obstetric care in a variety of ways, including mode of delivery, location of delivery, intrauterine therapy, parental reassurance, and opportunity for termination. Congenital heart disease (CHD) is a significant problem, with an incidence of moderate and severe CHD of approximately 6 per 1000 live births. If trivial lesions such as tiny muscular ventricular septal defects (VSDs) are included, the incidence is as high as 75 per 1000 live births. It is more difficult to determine the exact incidence of CHD in the fetal population. One large study suggested that CHD occurs in at least 15 per 1000 fetuses. More than 20% of perinatal deaths caused by congenital malformations are the result of a congenital heart defect. In 85% of CHD cases, both environmental and genetic factors are involved ( Table 37.1 ). The remaining 15% of cardiac anomalies are associated with a single gene or chromosomal abnormality. The risk of CHD increases to 2% to 3% with an affected sibling and to approximately 10% with two affected siblings or an affected mother—although the incidence is variable depending on the type of CHD in the affected relative. The risk to offspring of affected mothers is substantially higher than for those with affected fathers, suggesting that cytoplasmic inheritance may play a role in the genetics of CHD ( Tables 37.2 and 37.3 ). Only 50% of recurrent heart lesions are of the same type as the previously diagnosed defect.
| Factor | Frequency | Most Common Lesions |
|---|---|---|
| MATERNAL CONDITIONS | ||
| Diabetes | 3%-5% | TGA, VSD, coarctation |
| Lupus erythematosus | (1%-5%) | Heart block |
| Phenylketonuria | (12%-14%) | TOF, VSD, ASD, coarctation |
| Infection | Cardiomyopathy | |
| Rubella | (1%-2%) | TOF, PS, VSD, ASD, PDA, cardiomegaly |
| DRUGS | ||
| Accutane (retinoic acid) | 8%-20% | Truncus, TGA, TOF, DORV, VSD, AO arch interruption, or hypoplasia |
| Alcohol | 25%-30% | VSD, ASD, PDA, DORV, PA, TOF, dextrocardia |
| Amantadine | Single vent with PA | |
| Amphetamines | 5%-10% | VSD, TGA, PDA |
| Azathioprine | PS | |
| Carbamazepine | ASD, PDA | |
| Chlordiazepoxide | Unspecified CHD | |
| Codeine | Unspecified CHD | |
| Cortisone | VSD, coarctation | |
| Coumadin | Unspecified CHD | |
| Cyclophosphamide | TOF | |
| Cytarabine | TOF | |
| Daunorubicin | TOF | |
| Dextroamphetamine | ASD | |
| Diazepam | Unspecified CHD | |
| Dilantin (hydantoin) | AS, VSD, ASD, coarctation | |
| Lithium | <2% | Ebstein anomaly, TA, ASD, dextrocardia, MA |
| Methotrexate | Dextrocardia | |
| Oral contraceptives | Unspecified CHD | |
| Paramethadione | TOF | |
| Penicillamine | VSD | |
| Primidone | VSD | |
| Progesterone | TOF, truncus, VSD | |
| Quinine | Unspecified CHD | |
| Thalidomide | 5%-10% | TOF, VSD, ASD, truncus |
| Trifluoperazine | TGA | |
| Trimethadione | 15%-30% | ASD, VSD, TGA, TOF, HLHS, AS, PS |
| Valproic acid | TOF, coarctation, HLHS, AS, ASD, VSD, interrupted AO arch, PA without VSD | |
| Warfarin (Coumadin) | Unspecified CHD | |
| SYNDROMES | ||
| Apert | VSD, coarctation, TOF | |
| Arthrogryposis multiplex congenita | VSD, coarctation, AS, PDA | |
| Atrial myxoma, familial | Myxoma | |
| Beckwith-Wiedemann | Cardiomegaly | |
| C syndrome (Opitz trigonocephaly) | PDA | |
| Carpenter | VSD, PS, TGA, PDA | |
| Cat's eye (22 partial trisomy) | 40% | TAPVR, VSD, ASD |
| CHARGE | VSD, ASD | |
| CHILD | VSD, ASD | |
| Conradi-Hünermann (chondrodysplasia punctata) | VSD, PDA | |
| de Lange | 29% | VSD, TOF, DORV, PDA |
| DiGeorge | VSD, coarctation, truncus | |
| Ellis–van Creveld (chondroectodermal dysplasia) | 50% | ASD, single atrium |
| Fanconi pancytopenia | ASD, PDA | |
| Goldenhar | TOF, VSD, ASD | |
| Holt-Oram | ASD, VSD | |
| Kartagener | Dextrocardia | |
| Klippel-Feil | VSD, TGA, TAPVR | |
| Laurence-Moon (Bardet-Biedl) | VSD | |
| Leopard | PS | |
| Meckel-Gruber | VSD, ASD, coarctation, PS, PDA | |
| Noonan | PS, VSD, ASD, PDA | |
| Pallister-Hall | Unspecified CHD | |
| Pierre Robin | ASD | |
| Poland | TOF, ASD, PDA, VSD | |
| Refsum | A-V conduction defects | |
| Seckel | VSD, PDA | |
| Smith-Lemli-Opitz | VSD, PDA | |
| Treacher Collins | VSD, ASD, PDA | |
| Rubinstein-Taybi | ASD, VSD, PDA | |
| Silver | TOF, VSD | |
| Short rib polydactyly (non-Majewski type) | TGA, DOLV, DORV, HRH, AVSD | |
| Thrombocytopenia absent radius (TAR) | ASD, TOF, dextrocardia | |
| VACTERL | Unspecified CHD | |
| Waardenburg | VSD | |
| Weill-Marchesani | PS, VSD | |
| Williams | Supravalvular AS, PS, VSD, ASD | |
| Zellweger | VSD, ASD, PDA | |
| CHROMOSOMAL | ||
| Trisomy 13 (Patau) | 90% | VSD, ASD, dextroposition, PDA |
| Trisomy 18 (Edward) | 99% | Bicuspid AV, PS, VSD, ASD, PDA |
| Trisomy 21 (Down) | 50% | A-V canal, VSD, ASD, PDA |
| Triploidy | ASD, VSD | |
| 5p− (cri du chat) | 30% | Unspecified CHD |
| 9p− | VSD, PS, PDA | |
| Partial trisomy 10q | 50% | Unspecified CHD |
| 13q− | Unspecified CHD | |
| T 20p syndromes | VSD, TOF | |
| Turner (45,X) | 20% | Bicuspid AV, AS, coarctation, VSD, ASD, AVSD |
| 8 trisomy (mosaic) | 50% | VSD, ASD, PDA |
| 9 trisomy (mosaic) | 50% | VSD, coarctation, DORV |
| 13 q | 25% | VSD |
| +14q | 50% | ASD, TOF, PDA |
| 18q | 50% | VSD |
| XXXXY | 14% | ASD, ARCA, PDA |
| DISEASES AND CONDITIONS | ||
| Crouzon | Coarctation, PDA | |
| Neurofibromatosis | PS, coarctation | |
| Tuberous sclerosis | Rhabdomyoma, angioma | |
| Thalassemia major | Cardiomyopathy | |
| Defect | SUGGESTED RISK (%) | |
|---|---|---|
| If One Sibling | If Two Siblings | |
| Fibroelastosis | 4 | 12 |
| Ventricular septal defect | 3 | 10 |
| Patent ductus arteriosus | 3 | 10 |
| Atrioventricular septal defect | 3 | 10 |
| Atrial septal defect | 2.5 | 8 |
| Tetralogy of Fallot | 2.5 | 8 |
| Pulmonary stenosis | 2 | 6 |
| Coarctation of aorta | 2 | 6 |
| Aortic stenosis | 2 | 6 |
| Hypoplastic left heart | 2 | 6 |
| Transposition | 1.5 | 5 |
| Tricuspid atresia | 1 | 3 |
| Ebstein anomaly | 1 | 3 |
| Truncus | 1 | 3 |
| Pulmonary atresia | 1 | 3 |
a Combined data published during two decades from European and North American populations.
| Defect | AFFECTED PARENT | |
|---|---|---|
| Father | Mother | |
| Aortic stenosis | 3 | 13-18 |
| Atrial septal defect | 1.5 | 4-4.5 |
| Atrioventricular septal defect | 1 | 14 |
| Coarctation of aorta | 2 | 4 |
| Pulmonary stenosis | 2 | 4-6.5 |
| Tetralogy of Fallot | 1.5 | 2.5 |
| Ventricular septal defect | 2 | 6-10 |
Extracardiac malformations occur in 25%, and chromosomal anomalies occur in 13% of live-born neonates with CHD. About 50% of fetuses with nonimmune hydrops and cardiac anomalies have a chromosomal anomaly, and 10% will have extracardiac anomalies. Hydrops in the setting of CHD is predictive of a very poor prognosis.
Although the most common indications for formal fetal echocardiography are family history of CHD and fetal arrhythmia, the majority of these fetuses will have normal hearts. The highest incidence of CHD occurs in patients referred because of an abnormal four-chamber view, fetal hydrops, or significant polyhydramnios on a routine obstetric ultrasound. A routine ultrasound examination with a suspected fetal cardiac anomaly is associated with a 50% to 69% risk of CHD in live-born infants. Monochorionic twins are at a higher risk for CHD, with literature suggesting a ninefold increase in the incidence of CHD in monochorionic-diamniotic twin gestations. Lastly, there appears to be a slightly increased risk of CHD with in vitro fertilization (IVF), although at least some of this increased risk appears to be related to the increased incidence of twin pregnancies associated with IVF. Most fetuses with CHD have no known risk factors, which underscores the importance of a meticulous evaluation of the four-chamber heart views and outflow tracts on all routine obstetric ultrasound examinations. When severe structural cardiac anomalies are identified before viability, termination may be offered. Certainly, one of the most important aspects of fetal echocardiography is the psychological relief it affords parents whenever normal cardiac anatomy and function are documented in a fetus at risk.
The fetal heart is similar to that of the adult, with several anatomic and physiologic differences. The long axis of the fetal heart is perpendicular to the long axis of the body, such that a transverse section through the fetal thorax demonstrates the four cardiac chambers in a single view. The adult heart, in contrast, is obliquely oriented with its long axis along a line between the left hip and the right shoulder. The four-chamber view is important because 10% to 96% of structural anomalies are detectable on this view.
Abnormal heart on routine ultrasound
Hydrops
Polyhydramnios
Fetal arrhythmia
Fetal chromosomal anomalies
Fetal extracardiac anomalies
Family history (congenital heart disease [CHD], syndromes associated with CHD)
Maternal disease (diabetes, collagen vascular, phenylketonuria)
Maternal infection (rubella)
Teratogen exposure
Increased nuchal translucency on first-trimester screening
In vitro fertilization
Monochorionic twins
Monitoring response to intrauterine therapy
Monitoring fetus at risk for decompensation (persistent tachyarrhythmia, hydrops)
Cardiac axis and position are normally such that the apex of the heart points to the left and the bulk of the heart is in the left side of the chest ( Fig. 37.1A ). This is levocardia. In mesocardia the heart is central with the apex pointing anteriorly. In dextrocardia the apex is directed rightward, and the heart is primarily in the right side of the chest. This abnormality must be distinguished from dextroposition ( Fig. 37.1B ), in which the heart maintains a normal axis but is displaced to the right by an external process, such as a left chest mass or pleural effusion. Abnormal cardiac axis is associated with a 50% mortality and abnormal cardiac position with an 81% mortality.

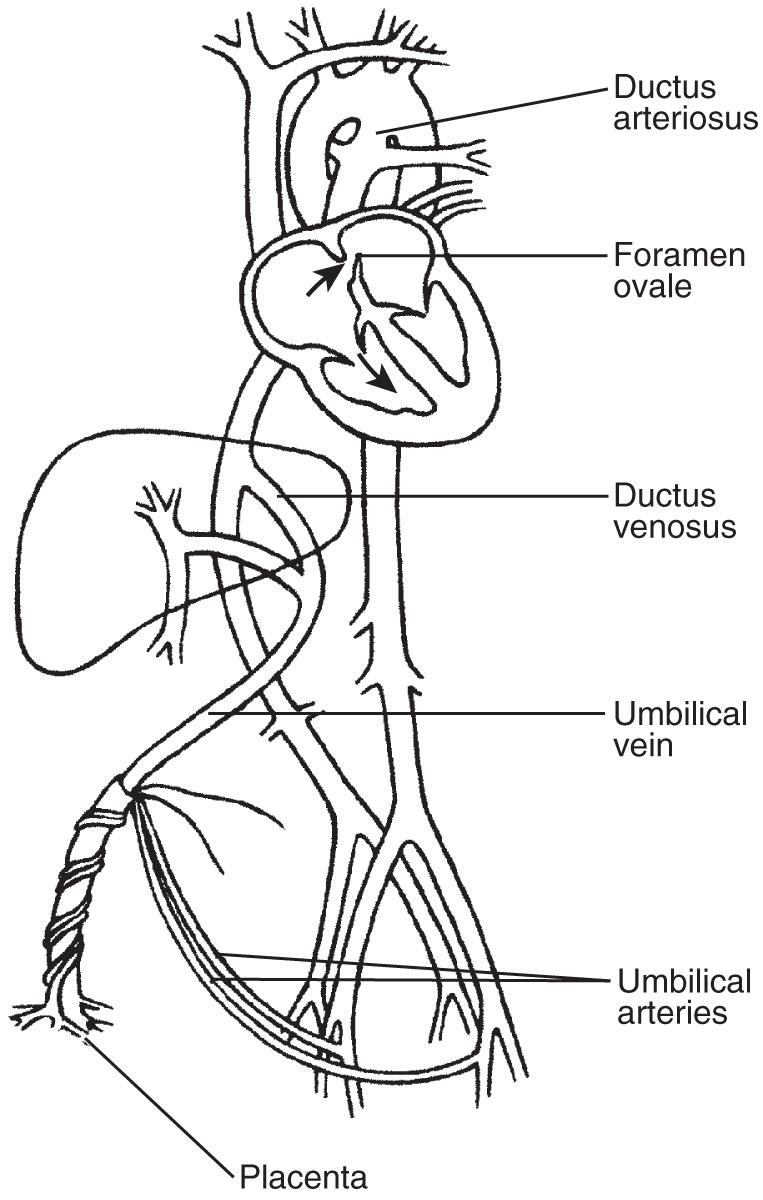
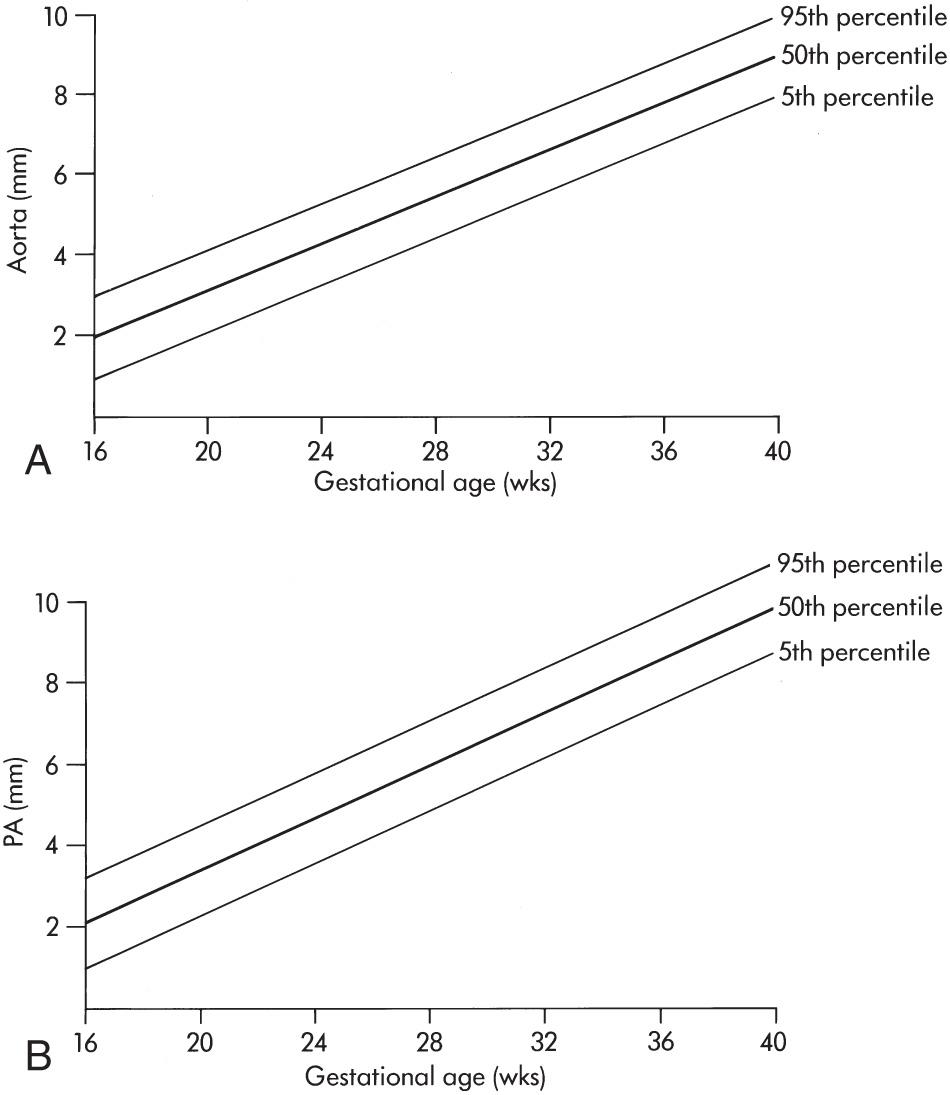
The fetal cardiovascular system contains several unique shunts: the ductus venosus, foramen ovale, and ductus arteriosus ( Fig. 37.2 ). Antenatally, the placenta rather than the lungs is the fetus's sole source of oxygen. Oxygenated blood leaves the placenta through the umbilical vein and travels through the hepatic vasculature and ductus venosus to the inferior vena cava (IVC) and then into the fetal right atrium. As a result of increased velocity, blood entering the IVC via the ductus venosus is shunted across the foramen ovale to the left atrium and then into the left ventricle, the aorta, and the fetal brain. Poorly oxygenated blood from the superior vena cava (SVC) also enters the right atrium, and mixes with the blood entering from the IVC, but continues to the right ventricle and pulmonary artery. Most of this blood is directed through the ductus arteriosus into the descending aorta. Thus these shunts function so that the majority of output from both ventricles enters the systemic circulation, rather than a substantial portion entering the pulmonary circulation, as in the adult. Blood that enters the pulmonary vasculature via the pulmonary artery is returned to the left atrium by the four pulmonary veins. From the left atrium it enters the left ventricle and ultimately the descending aorta, returning to the placenta via the iliac and umbilical arteries. Normal values for measurements of the fetal heart and great vessels are shown in Figs. 37.3 and 37.4 .
Fetal echocardiography is best accomplished at 18 to 22 weeks of gestation. Before 18 weeks, resolution is frequently limited by the small size of the fetal heart. After 22 weeks the examination may be compromised by progressive ossification of the fetal skull, spine, and long bones; the relatively smaller amniotic fluid volume; and unaccommodating fetal position. Importantly, some congenital cardiac abnormalities progress in utero and may be subtle or unrecognizable at or before 22 weeks but more obvious closer to term. Tachyarrhythmias may not become apparent until the third trimester. In some cases, first-trimester evaluation of the fetal heart may be accomplished with transvaginal ultrasound as early as 11 to 14 weeks. More recently, diagnostic results have been possible with a transabdominal approach at 11 to 13 weeks. However, first-trimester fetal echocardiography is limited and should be considered an adjunct to second-trimester evaluation, not a replacement.
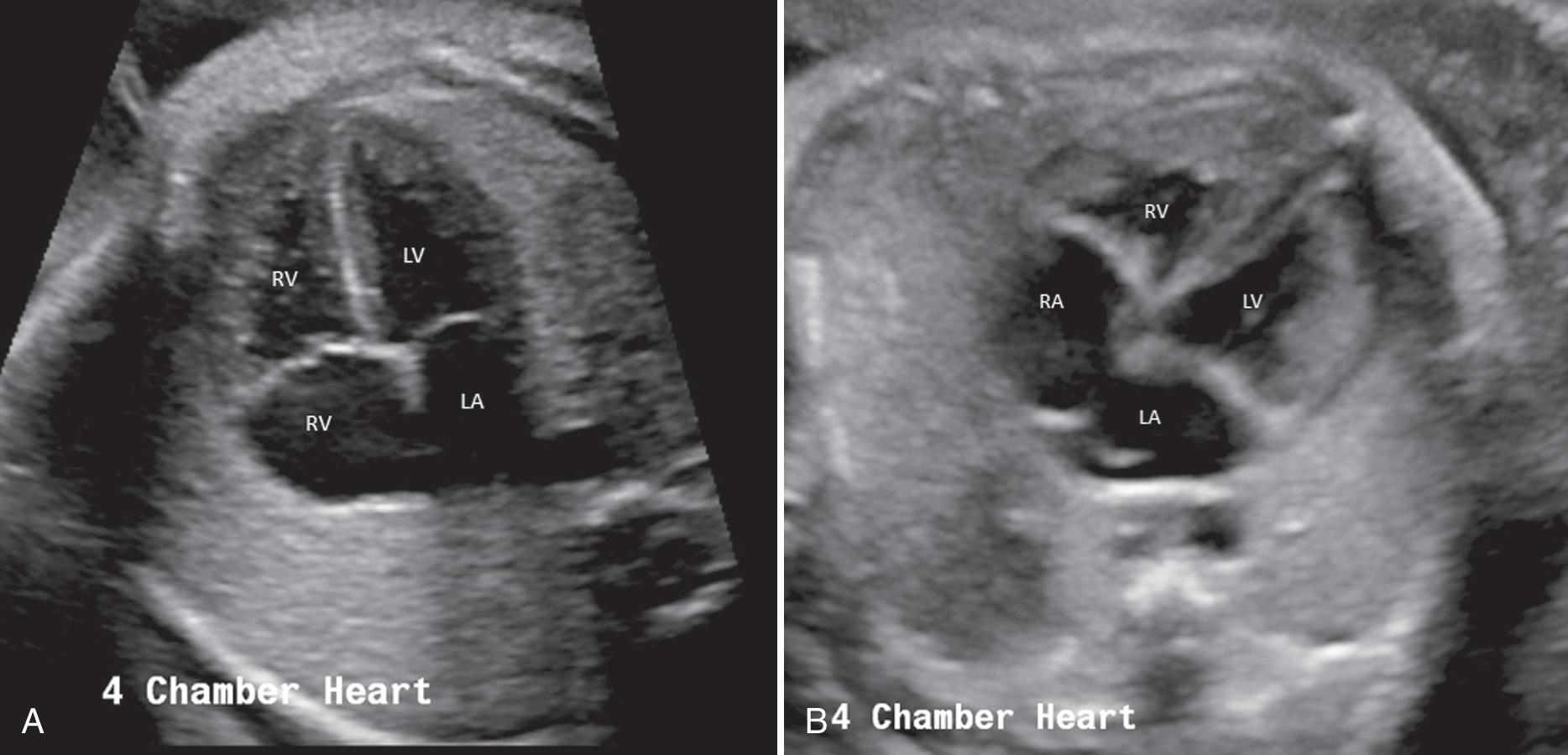
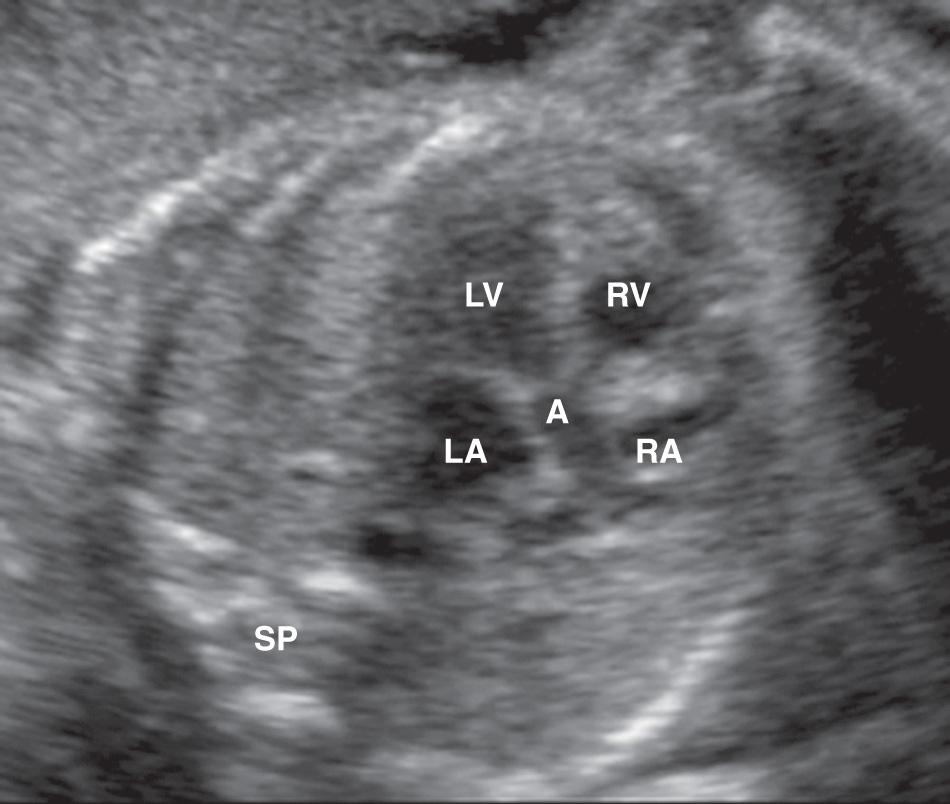
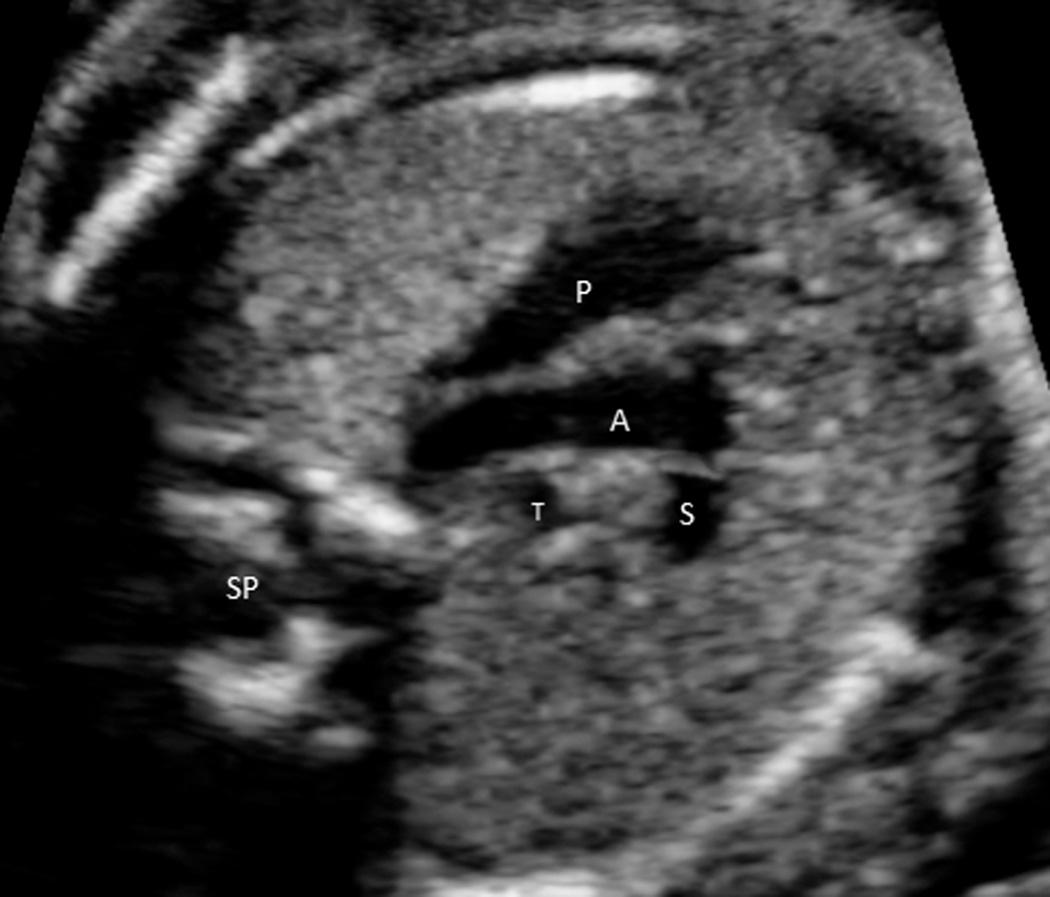
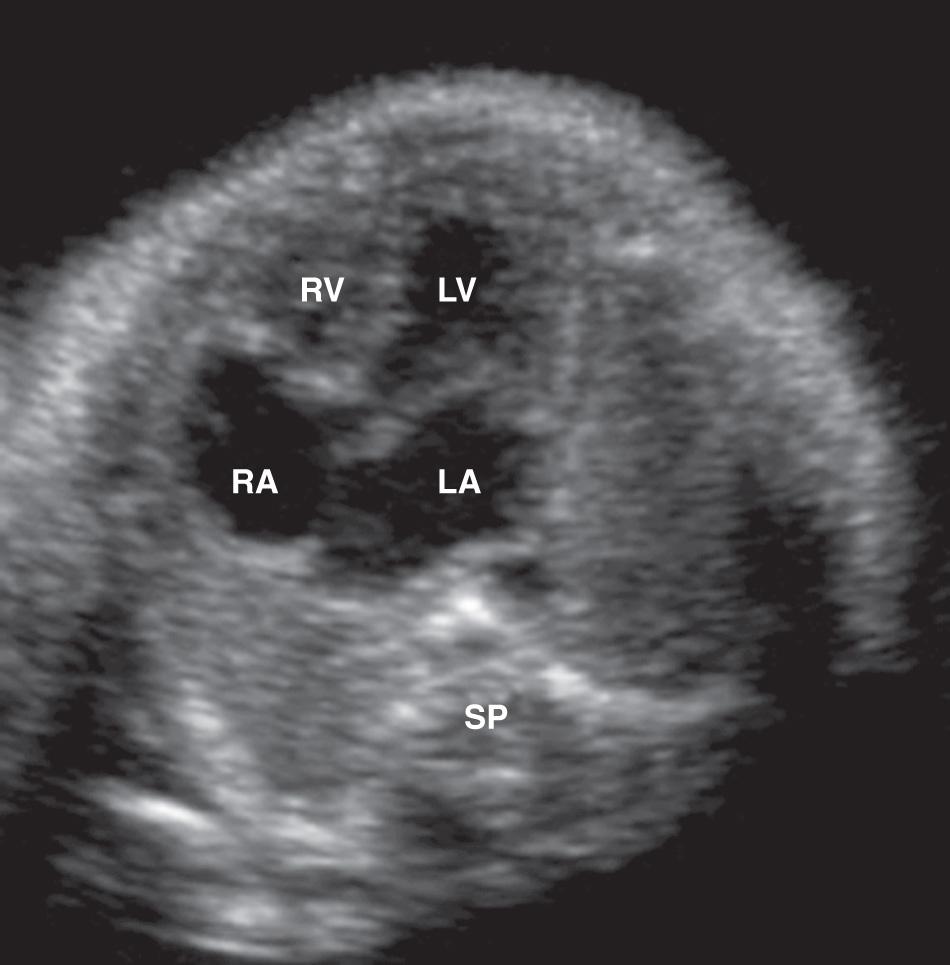
Scanning the fetal heart requires a systematic approach, beginning with determination of the position of the fetus within the uterus and the heart within the fetal chest. A transverse view through the fetal thorax above the level of the diaphragm demonstrates four cardiac chambers. Four-chamber views can be obtained with the angle of insonation parallel to the interventricular septum ( apical four-chamber view; Fig. 37.5A , ) or perpendicular to the septum ( subcostal four-chamber view; Fig. 37.5B , ). In a four-chamber view the echogenic foraminal flap of the foramen ovale can be observed moving into the left atrium. The two superior pulmonary veins may be seen entering the spherical left atrium. The atrioventricular valves are visible in the four-chamber view. The septal leaflet of the tricuspid valve inserts slightly more apically on the interventricular septum than the anterior leaflet of the mitral valve. The left ventricle has a relatively smooth inner wall, and a more elongated shape than the right ventricle. In the normal heart, the left ventricle is the apex forming ventricle. The internal surface of the right ventricle is coarse, particularly near the apex, where the moderator band of the trabecula septomarginalis is frequently recognized as a small, brightly echogenic focus. This helps identify the morphologic right ventricle.
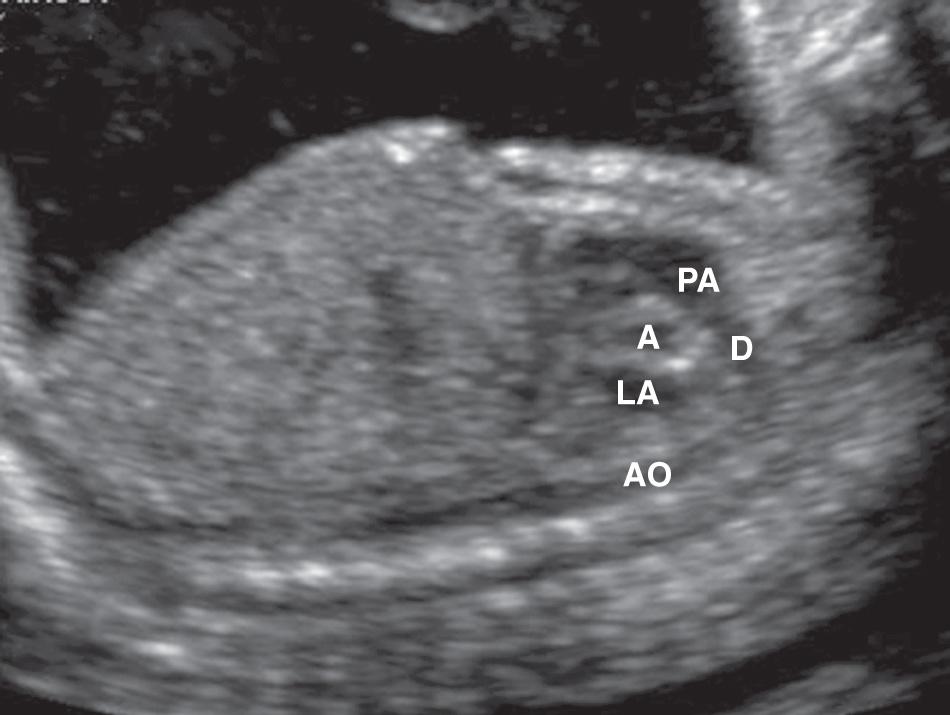
From the subcostal four-chamber view, angling the transducer toward the fetus's right shoulder permits evaluation of the continuity of the left ventricle with the ascending aorta ( Fig. 37.6 ). Further angulation in the same direction shows the right ventricle in continuity with the pulmonary artery ( Fig. 37.7 , and ). The diameter of the pulmonary artery is approximately 9% larger than that of the aorta between 14 and 42 weeks. The measured differences in these vessels and with M-mode versus two-dimensional (2-D) imaging are negligible (2%-5%) for both the pulmonary artery and the aorta. Further rightward rotation produces a sagittal view of the fetal thorax and a short-axis view of the ventricles ( Fig. 37.8 , ). Angulation toward the left fetal shoulder from this view shows the aorta as a central circle, with the pulmonary artery draping anteriorly and to the left ( Fig. 37.9 ).

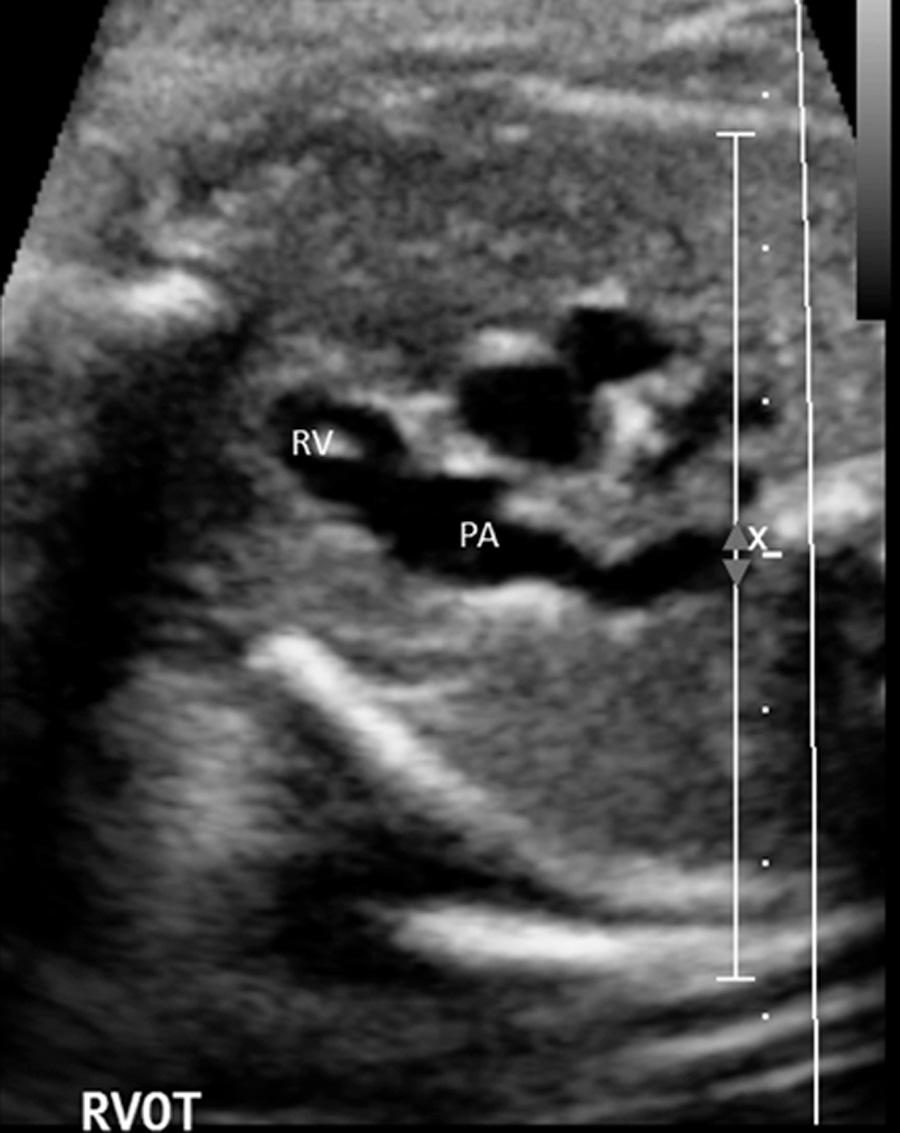
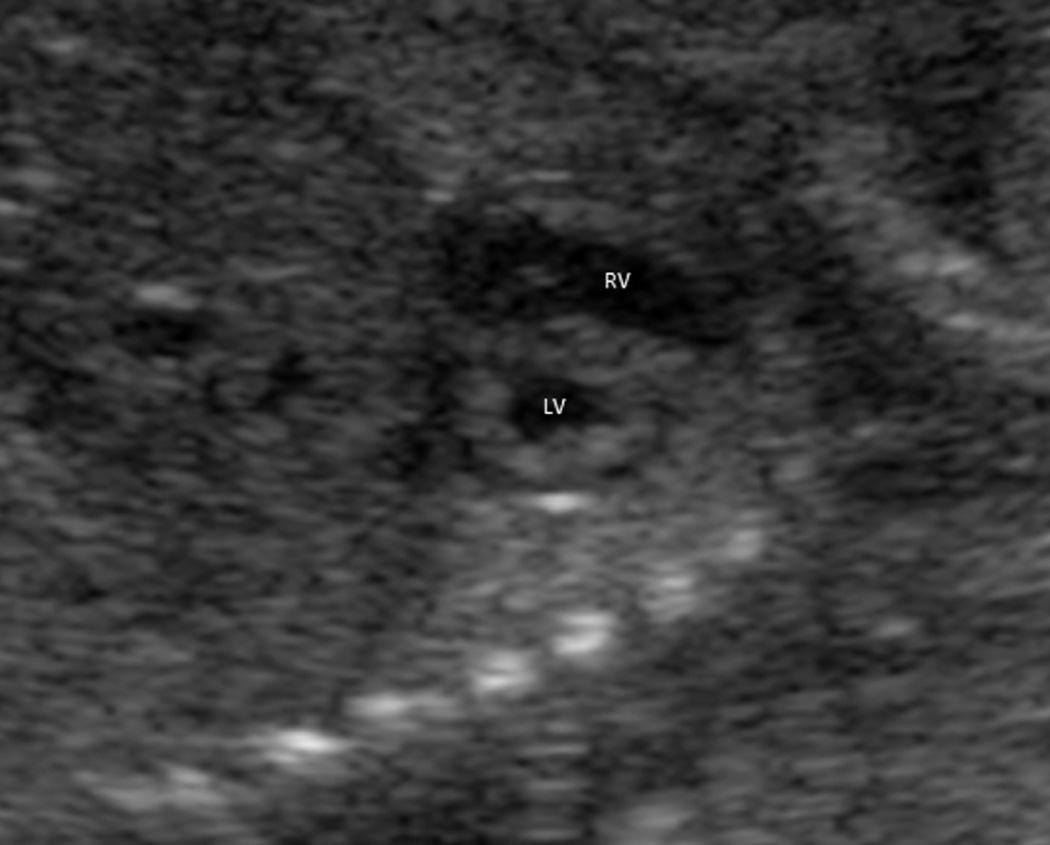
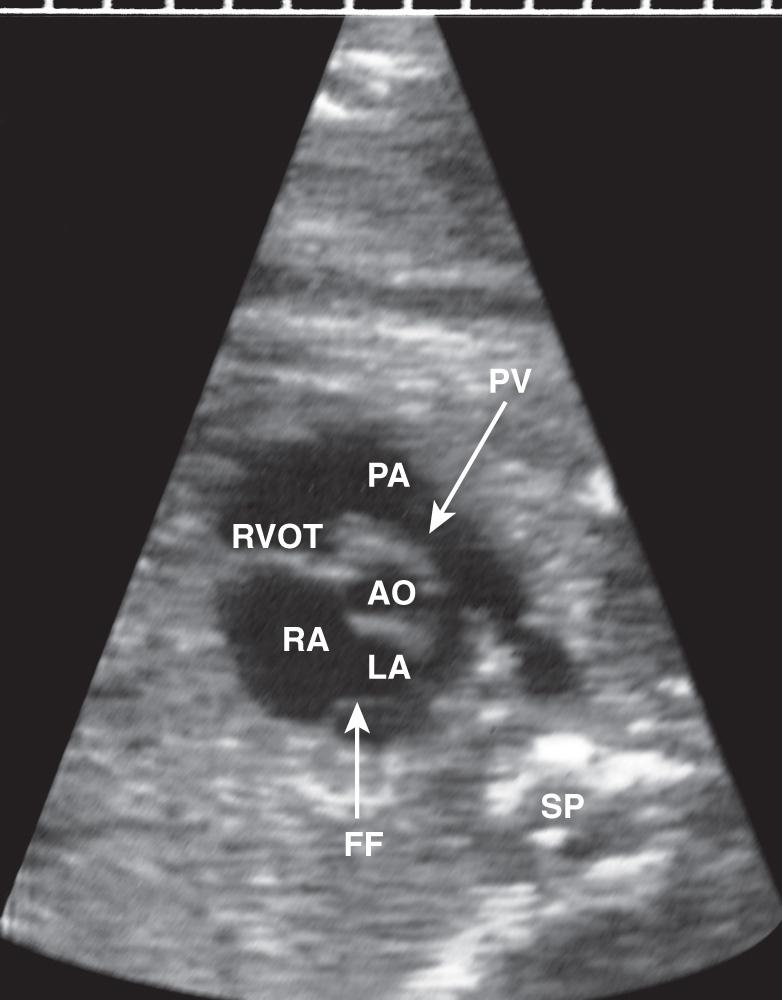
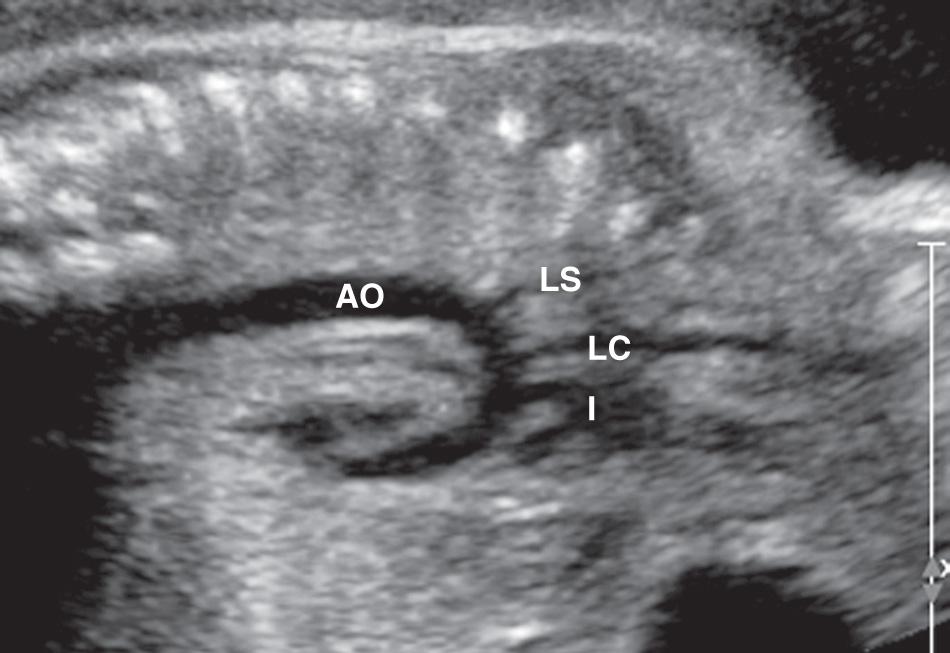
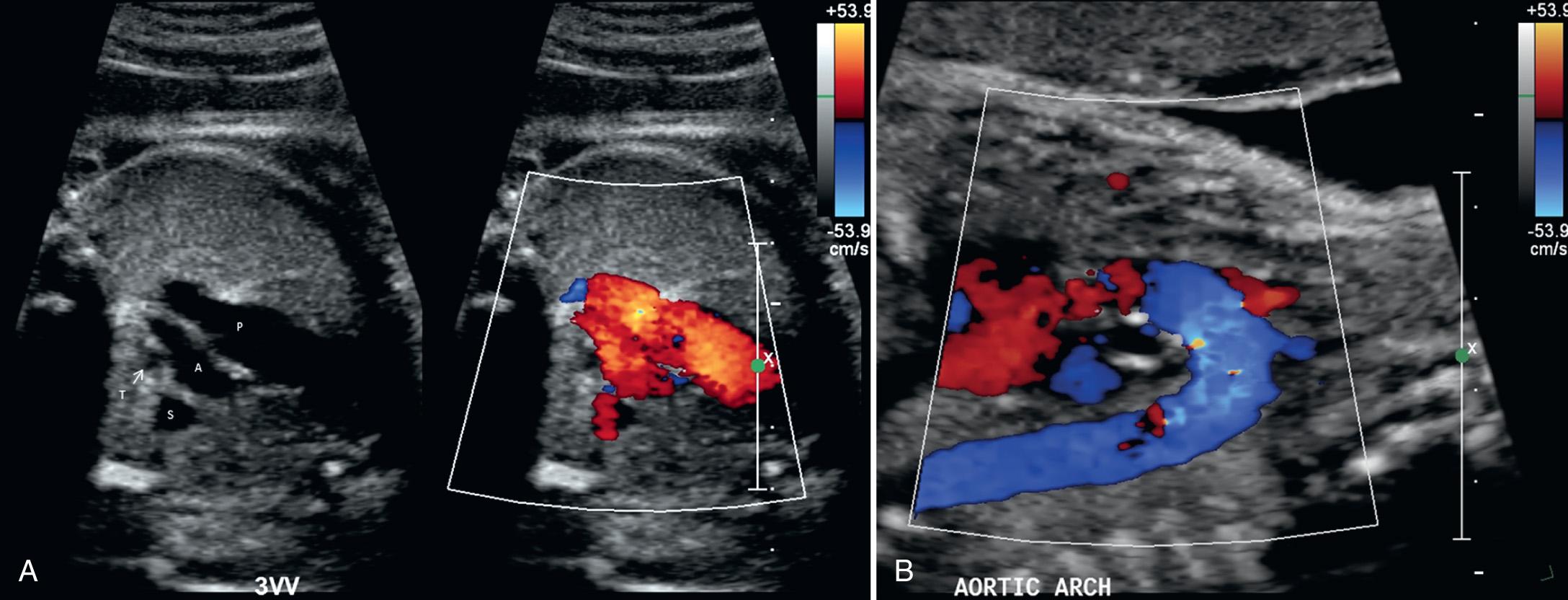
The apical four-chamber view may also be used as a starting point when evaluating normal cardiac anatomy. Yagel and colleagues described a series of planes arising from the apical four-chamber view, all accomplished by moving the transducer in a cephalad direction. A slight cephalad advancement will show an apical five-chamber view, which is useful in assessing continuity of the ascending aorta with the left ventricle ( Fig. 37.10 ). Continued cephalad movement should result in visualization of the bifurcating pulmonary artery and its relationship to the right ventricle. A three-vessel and trachea view should be visualized next ( Fig. 37.11 , ). This view allows evaluation of the main pulmonary artery–ductus arteriosus confluence, the transverse aortic arch, and the SVC. Comparison of vessel size, confirmation of vessel presence, and determination of blood flow direction with color Doppler can all be accomplished at this level. In addition, appropriate location of both great vessels to the left of the trachea can be confirmed. Returning to a sagittal plane of the fetus, directing the transducer from the fetal left shoulder to the right hemithorax demonstrates the distinctive candy-cane shape of the aortic arch ( Fig. 37.12 , and ). The three major vessels to the head and neck and the ductus arteriosus may be seen. The aortic arch should not be confused with the ductal arch ( Fig. 37.13 ), which is formed by the right ventricular outflow tract, pulmonary artery, and ductus arteriosus. The ductal arch is broader and flatter than the aortic arch. Lastly, sliding the transducer to the right while maintaining a sagittal plane on the fetus should allow visualization of the IVC and SVC entering the right atrium.
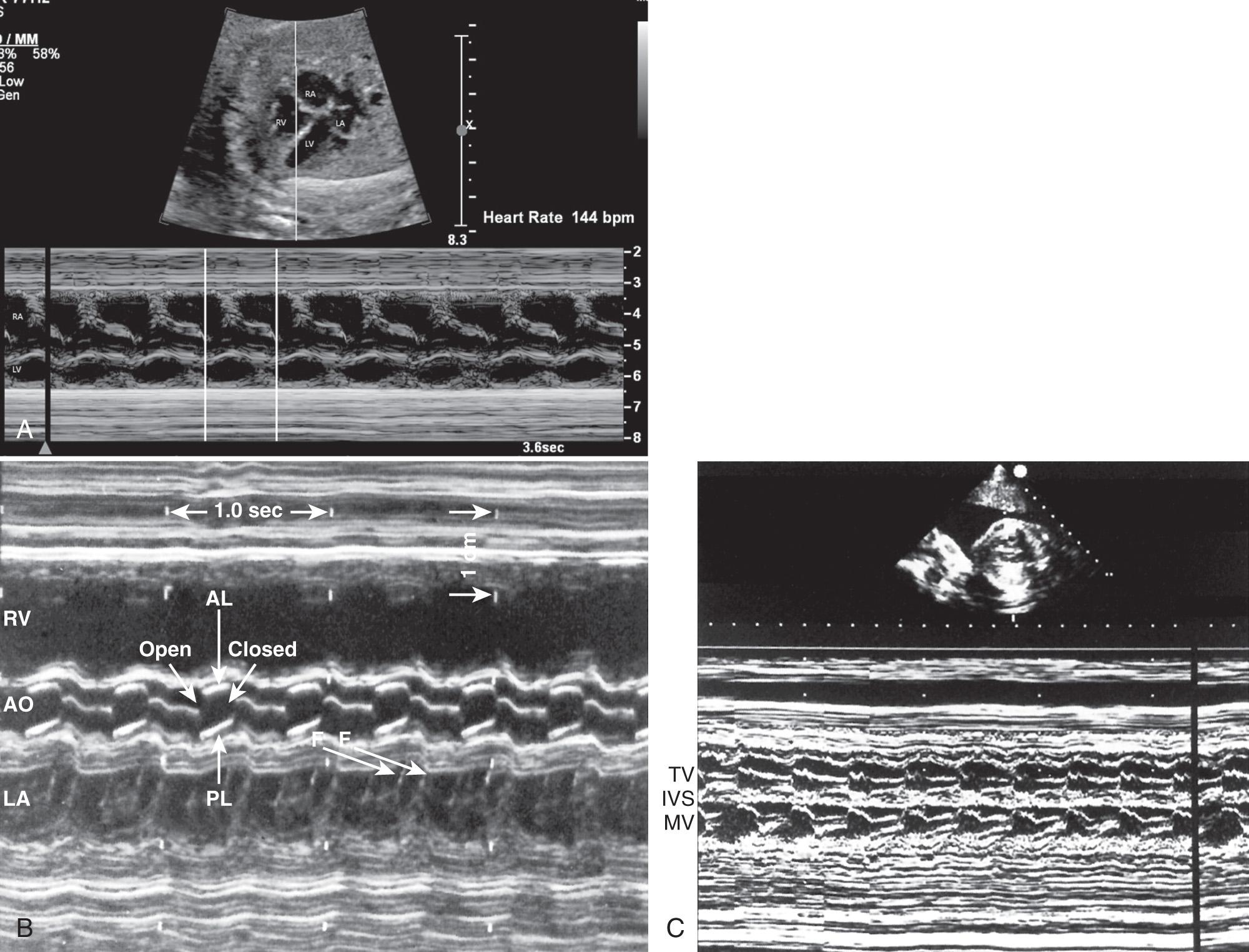
M-mode echocardiography provides a 2-D image of motion over time. It is useful in evaluating heart rate, chamber size, wall thickness, and wall motion ( Fig. 37.14 ). Simultaneous M-mode imaging through an atrium and ventricle is helpful in analyzing arrhythmias ( Fig. 37.15 ). Chamber size and function should be evaluated at the level of the atrioventricular (A-V) valves.
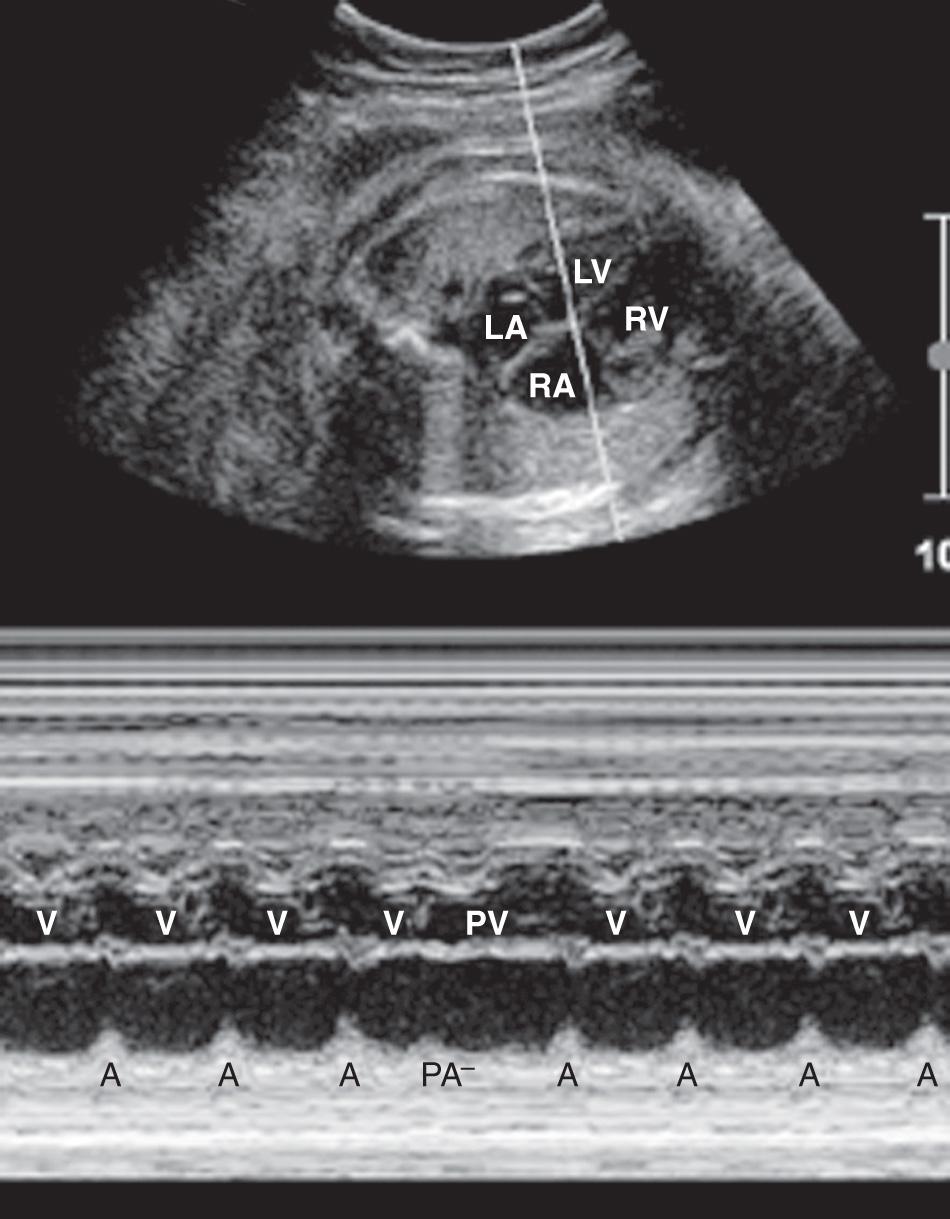
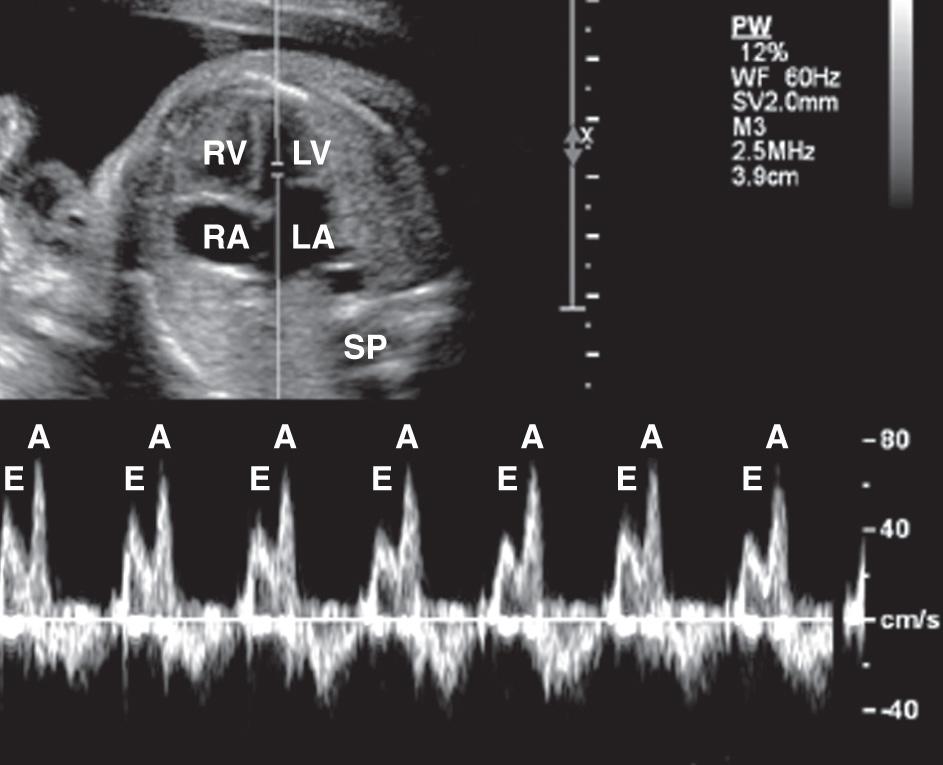
Spectral Doppler ultrasound evaluation of the fetal heart can be used to determine the velocity of flow through the vessels or valves ( Fig. 37.16 ) and to evaluate regurgitant flow through the valves of the heart ( Fig. 37.17 ). Variation in flow velocity may reflect structural or functional cardiac abnormalities. For example, a stenotic A-V valve will be associated with an abnormal flow pattern through the affected valve. Spectral Doppler ultrasound is useful in assessing the functional significance of structural abnormalities and arrhythmias.
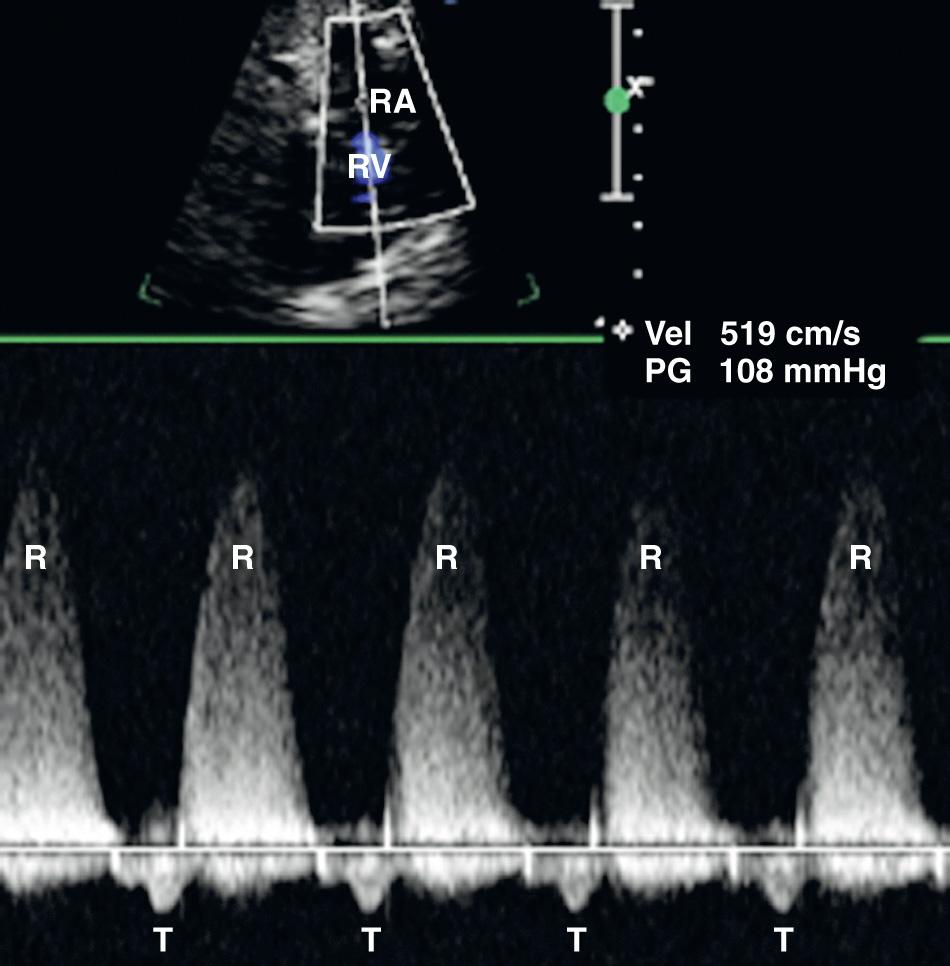
Color Doppler ultrasound permits a rapid interrogation of flow patterns within the heart and great vessels ( Fig. 37.18 ), allowing functional and structural abnormalities to be more rapidly characterized. For example, valvular stenosis is clearly demonstrated with color Doppler ultrasound, as is reversed flow through insufficient valves or in the great vessels. Color Doppler ultrasound often reduces the amount of time required for spectral Doppler ultrasound evaluation of the heart, particularly in the setting of complex cardiac anomalies. Subtle lesions such as small VSDs may be more reliably and easily identified with the use of color flow Doppler ultrasound.
The utility of several advanced technologies has been reported in the evaluation of the fetal heart, including three-dimensional (3-D) and four-dimensional (4-D) ultrasound, tissue Doppler imaging, strain and strain rate imaging, as well as fetal magnetocardiography and cardiovascular magnetic resonance imaging (MRI). However, many of these require specialized transducers or other equipment, sophisticated algorithms and specialized technical expertise. Additionally, limited resolution and cardiac motion are still considered major disadvantages in some settings.
An atrial septal defect (ASD) results from an error in the amount of tissue resorbed or deposited in the interatrial septum. It is the fifth most common form of CHD and is the most common form in adult patients. ASDs occur in 1 per 1500 live births and comprise 6.7% of CHD in live-born infants. ASDs occur twice as often in females as males. ASDs are associated with a variety of cardiac, extracardiac, and chromosomal abnormalities. ASDs can be classified by embryogenesis, size, or relationship to the fossa ovalis.
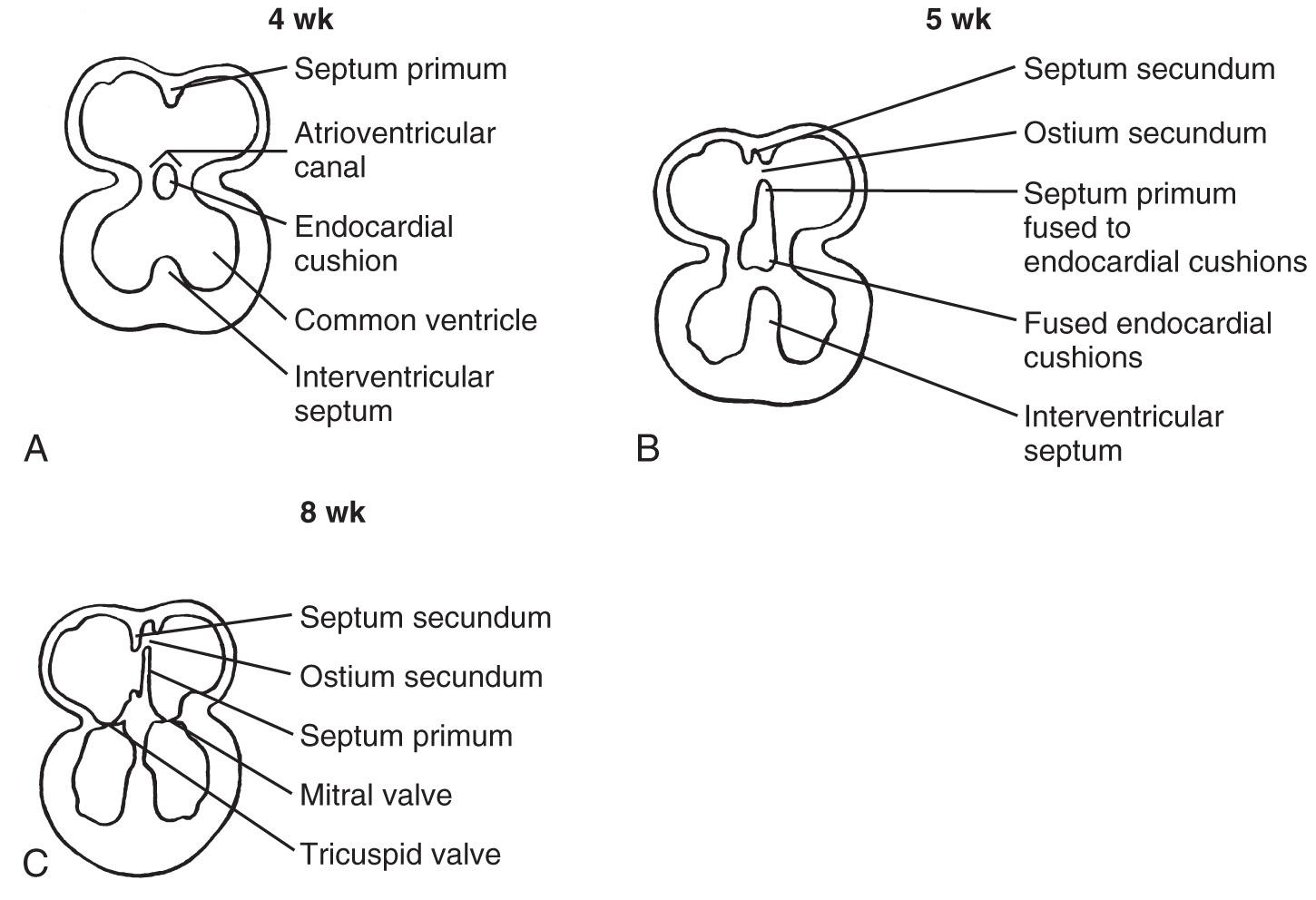
Embryologically, between the fourth and sixth weeks of gestation the primitive atrium is divided into right and left halves. The septum primum, a crescent-shaped membrane, develops along the cephalad portion of the atrium and grows caudally toward the endocardial cushions. The space between these two structures, termed the ostium primum, disappears when the septum primum fuses with the endocardial cushion. Before complete fusion, however, multiple small fenestrations develop in the septum primum, coalescing to form the ostium secundum.
A second crescent-shaped membrane subsequently develops just to the right of the septum primum. As this membrane grows toward the endocardial cushion, it partially covers the ostium secundum. Its crescent-shaped lower border never entirely fuses with the endocardial cushion, leaving an opening, the foramen ovale ( Fig. 37.19 ).
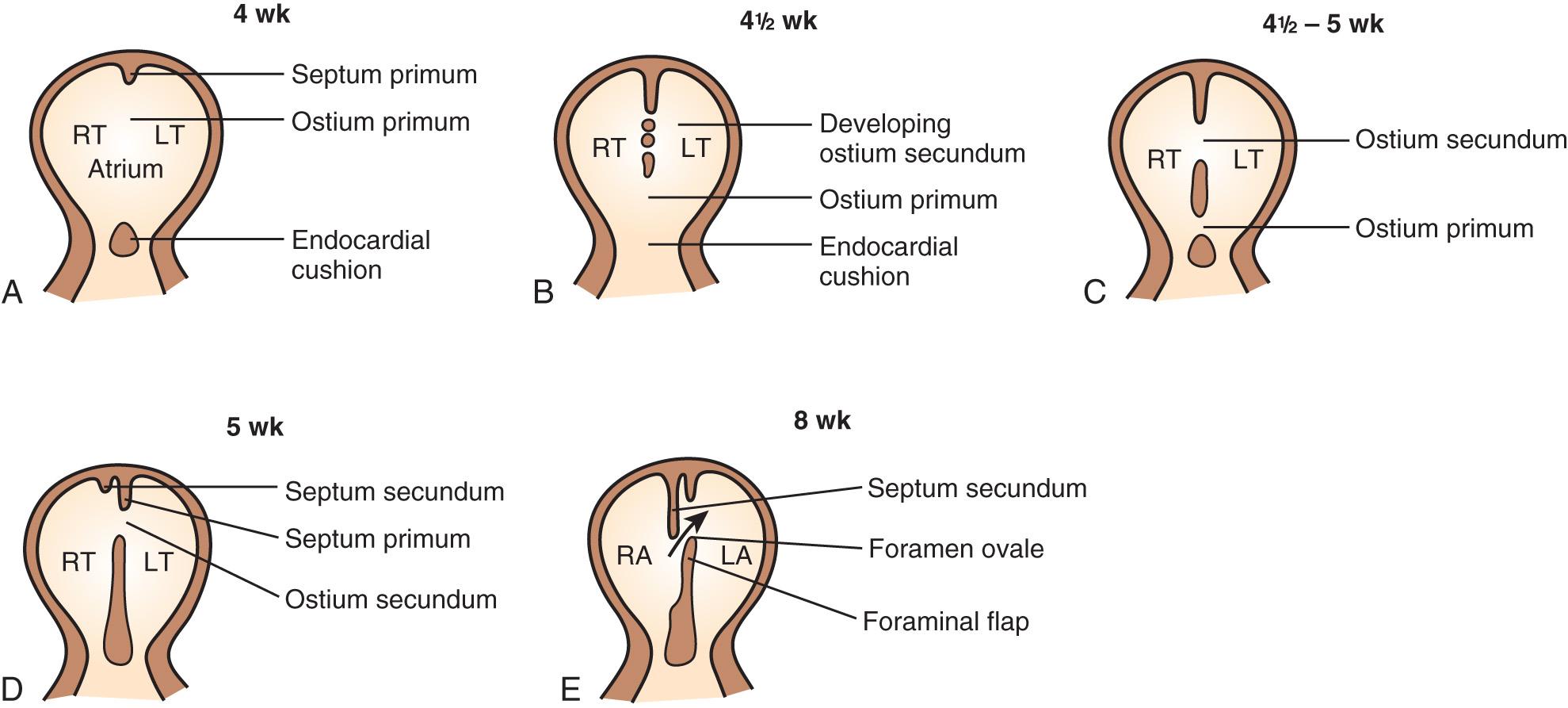
Ostium secundum ASDs make up more than 80% of all ASDs and generally occur in isolation. This ASD is caused by excessive resorption of the septum primum (foraminal flap) or by inadequate growth of the septum secundum ( Fig. 37.20A ). The ostium primum ASD is the second most common type and is located low in the atrial septum, near the atrioventricular (A-V) valves. Although the ostium primum ASD may occur alone, it is more frequently associated with a more complex congenital cardiac anomaly, the atrioventricular septal defect (AVSD) ( Fig. 37.20B ).
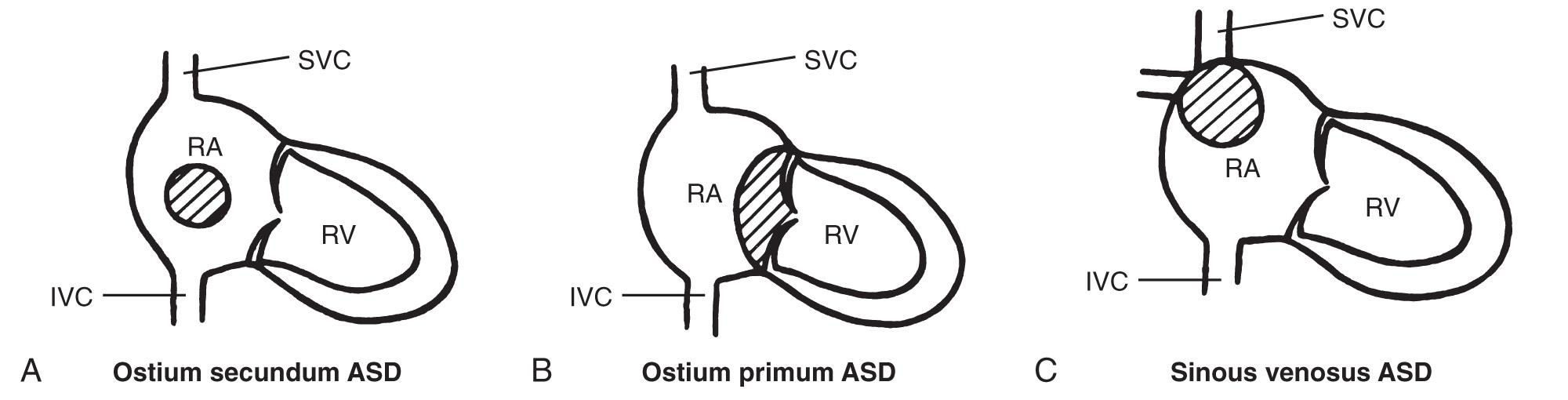
The sinus venosus ASD is a rare defect that can be divided into two types: (1) sinus venosus ASD of the SVC, with the defect adjacent to the SVC, and (2) sinus venosus ASD of the IVC, with the defect adjacent to the IVC. The first type is often associated with anomalous pulmonary venous return (APVR) ( Fig. 37.20C ). Coronary sinus ASDs, located at the ostium of the coronary sinus in the right atrium, may also occur but are exceedingly rare.
The prenatal sonographic diagnosis of ASD is difficult because the normal patent foramen ovale, which allows blood to flow from the right to the left atrium in utero, itself represents an ASD. It can be difficult to distinguish a small, pathologic ASD from the normal patent foramen ovale. The foraminal flap, or septum primum, is clearly visualized on the four-chamber view. It has a “loose pocket” configuration, appearing either circular or linear in shape as it opens into the left atrium ( Fig. 37.21 ).
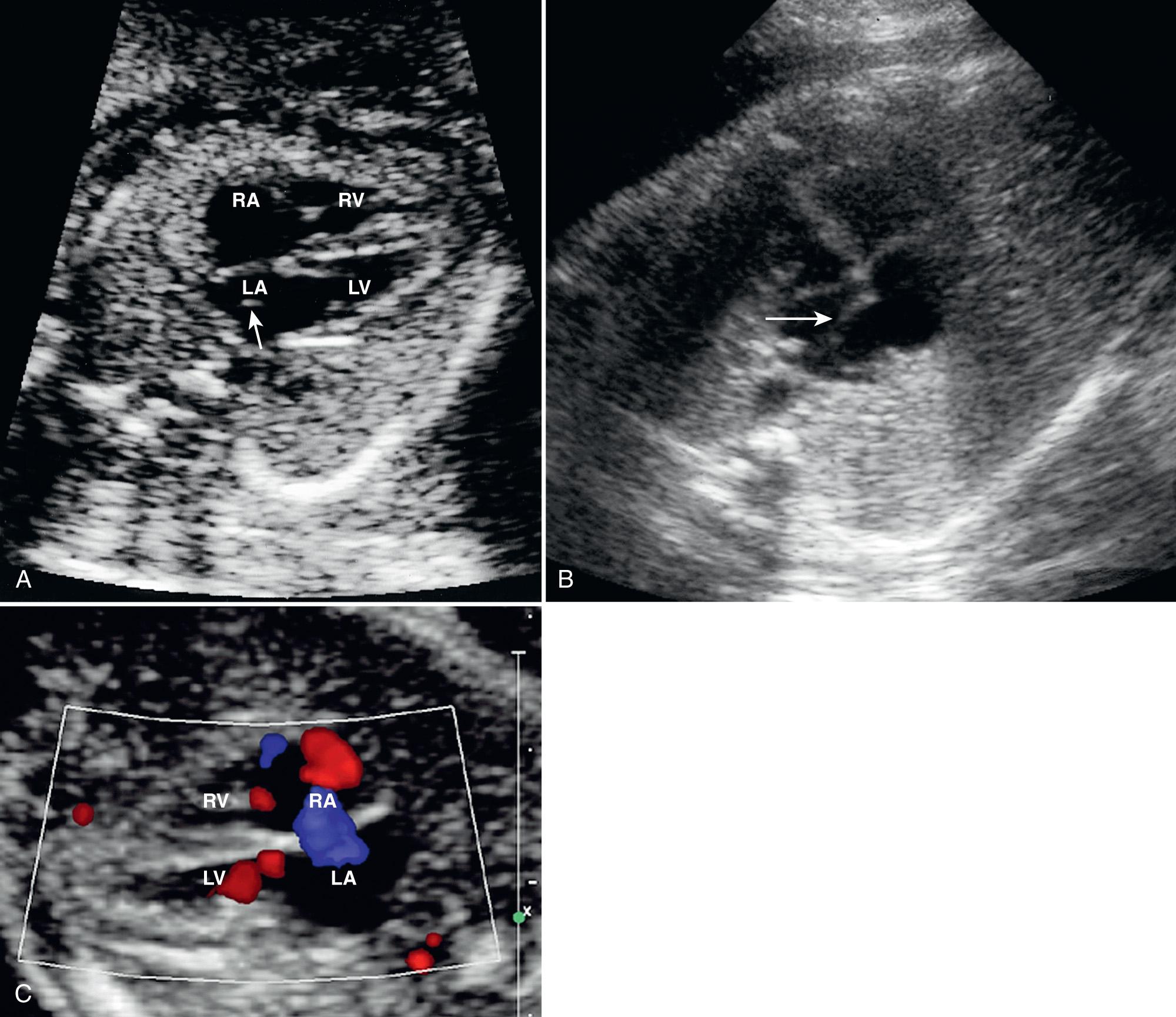
The septum secundum, which is thick and relatively stationary, makes up the majority of the atrial septum. The foramen ovale is an opening in the septum secundum. The septum secundum and foramen ovale are well visualized in the four-chamber views. The maximal size of the normal foramen ovale differs by 1 mm or less from the aortic root diameter at all gestational ages. An ostium secundum ASD appears as a larger than expected defect in the central portion of the atrial septum near the foramen ovale. Alternatively, it can appear as a deficient foraminal flap.
If the lowest portion of the atrial septum (just adjacent to the A-V valves) is deficient, an ostium primum defect should be suspected ( Fig. 37.22 ). Color Doppler ultrasound may be helpful in the diagnosis of larger ASDs. However, small ASDs are commonly obscured by the normal flow through the patent foramen ovale.
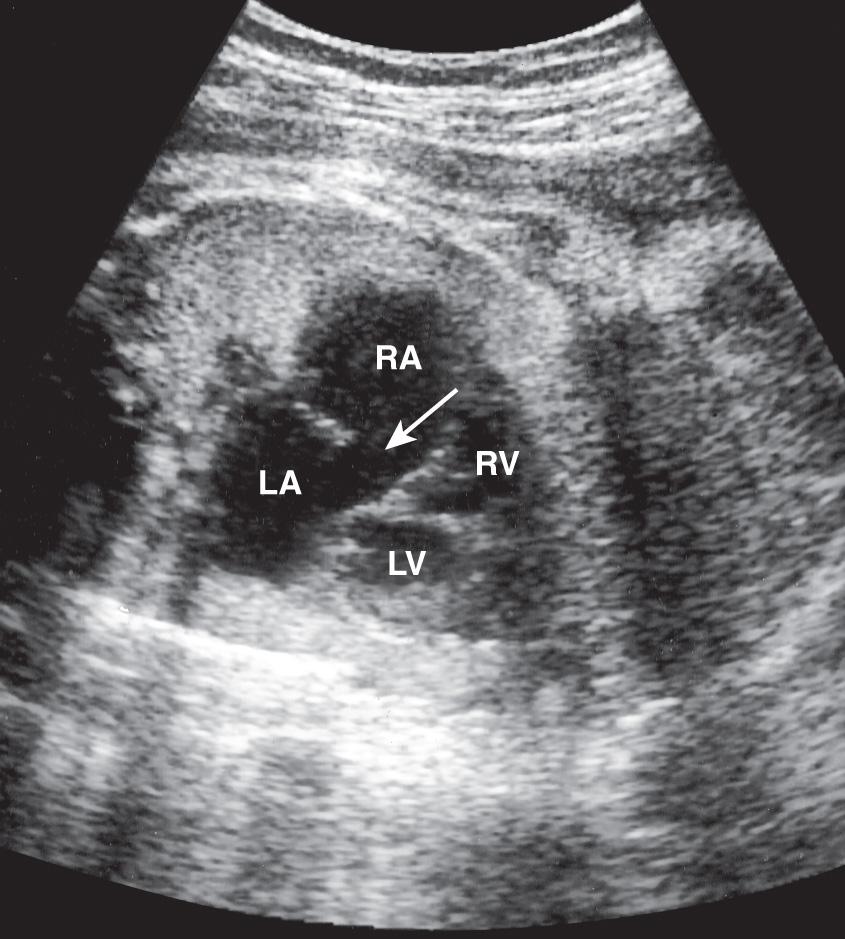
A large right-to-left shunt is physiologic in utero, and thus an ASD usually does not compromise the fetus hemodynamically. After birth, the shunt may cause right ventricular overload and pulmonary hypertension. Spontaneous closure of an ASD will occur in approximately two-thirds of patients. Patients with small ASDs may remain asymptomatic into their 50s.
Isolated VSD is the most common cardiac anomaly, accounting for 30% of heart defects diagnosed in live-born infants and 9.7% diagnosed in utero. VSDs are associated with other cardiac anomalies in 50% of cases. Of the structural cardiac defects, VSDs have the highest recurrence rate and the highest association with teratogen exposure. They are classified according to their position in the interventricular septum ( Fig. 37.23 ) as membranous or muscular VSD (inlet, trabecular, outlet).
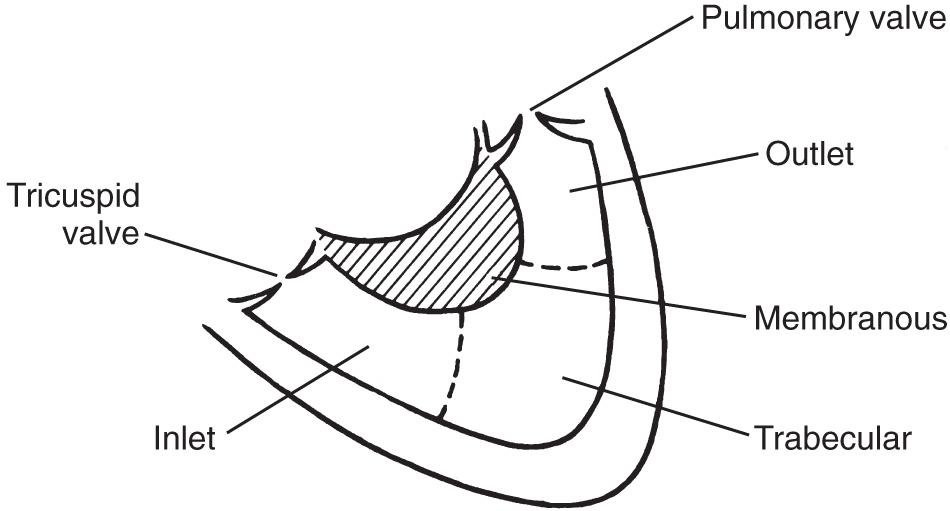
About 80% of VSDs occur in the membranous portion of the septum. However, because most membranous defects also involve a portion of the muscular septum, they are usually referred to as perimembranous defects. The subcostal four-chamber view provides optimal evaluation of the interventricular septum. At sonography, a VSD appears as an area of discontinuity in the interventricular septum. When the defect is small, this diagnosis is problematic, and at least one-third of VSDs are missed on the four-chamber view. Color Doppler imaging may improve the diagnostic accuracy for VSD. However, most are missed on fetal echocardiography. Small VSDs not detectable on gray-scale echocardiography may be documented with color Doppler ultrasound in some cases ( Fig. 37.24 ). In the setting of an isolated VSD, color Doppler ultrasound imaging typically shows bidirectional interventricular shunting, with a systolic right-to-left shunt and a late diastolic left-to-right shunt.
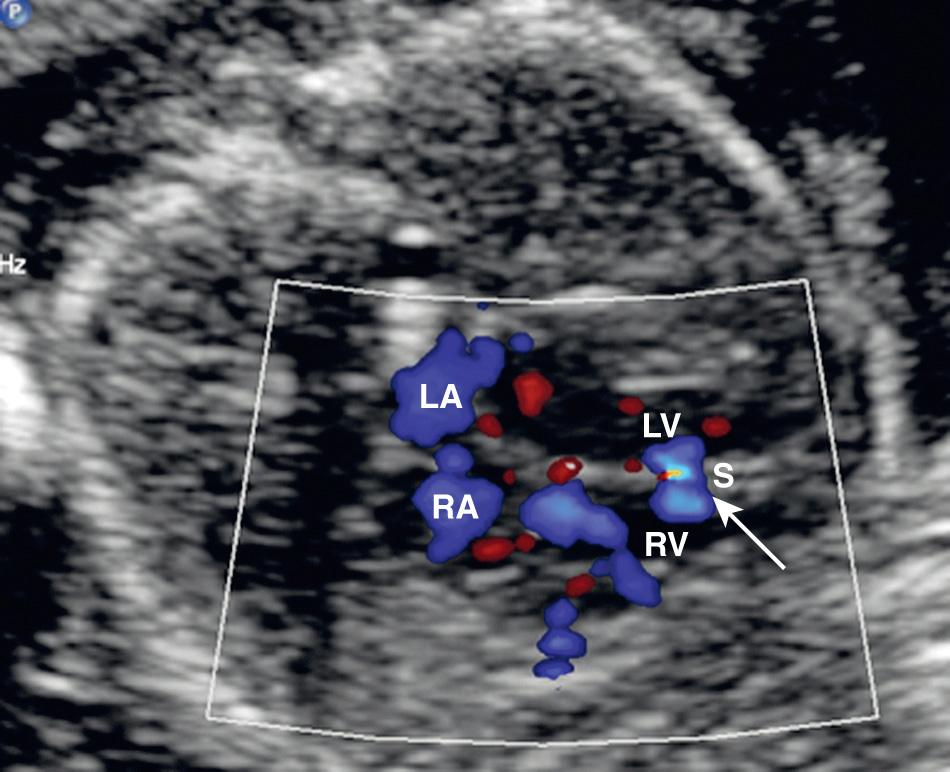
The prognosis for an infant with an isolated VSD is excellent, and many such defects go undetected. The rate of spontaneous closure of isolated muscular VSDs by 5 years of life is much higher (65%) than that for isolated perimembranous VSDs (20%). Overall about 40% of VSDs spontaneously close in the first year of life, and 60% resolve by 5 years of age. However, large defects detected in the fetus are associated with an 84% mortality. Concurrent cardiac, extracardiac, and chromosomal anomalies (trisomy 13, 18, 21, and 22) are associated with a worse prognosis.
VSDs may be extremely difficult to diagnose in utero, particularly when small in size. In addition, many small VSDs will close in utero or shortly after birth. A “pseudo” VSD in the membranous portion of the septum may be appreciated when evaluating the interventricular septum from an apical four-chamber view. This occurs when the angle of insonation is parallel to the septum, causing an artifactual dropout of the thin, membranous septum.
AVSD refers to a spectrum of cardiac abnormalities involving various degrees of deficiency of the interatrial and interventricular septa and of the mitral and tricuspid valves. These defects arise when the endocardial cushions fail to fuse properly and were previously called endocardial cushion defects or A-V canal defects. Almost two-thirds of fetuses with AVSD have additional cardiac anomalies. About one-third are associated with left atrial isomerism (both atria anatomically resemble the left), and of these the majority of affected fetuses have complete heart block. Chromosomal (especially trisomy 21 ) or extracardiac anomalies are associated in 78% of AVSDs.
Embryologically, in the primitive heart, the common atrium and ventricle communicate through the A-V canal. Development of the endocardial cushion results in division of the single, large A-V canal into two separate orifices, separating the atria from the ventricles ( Fig. 37.25 ). The interatrial and interventricular septa develop concurrently, eventually dividing the single atrium and ventricle into right and left portions. When the endocardial cushions fail to fuse properly, normal development of the mitral and tricuspid valves cannot occur, and an AVSD results ( Fig. 37.26 ).
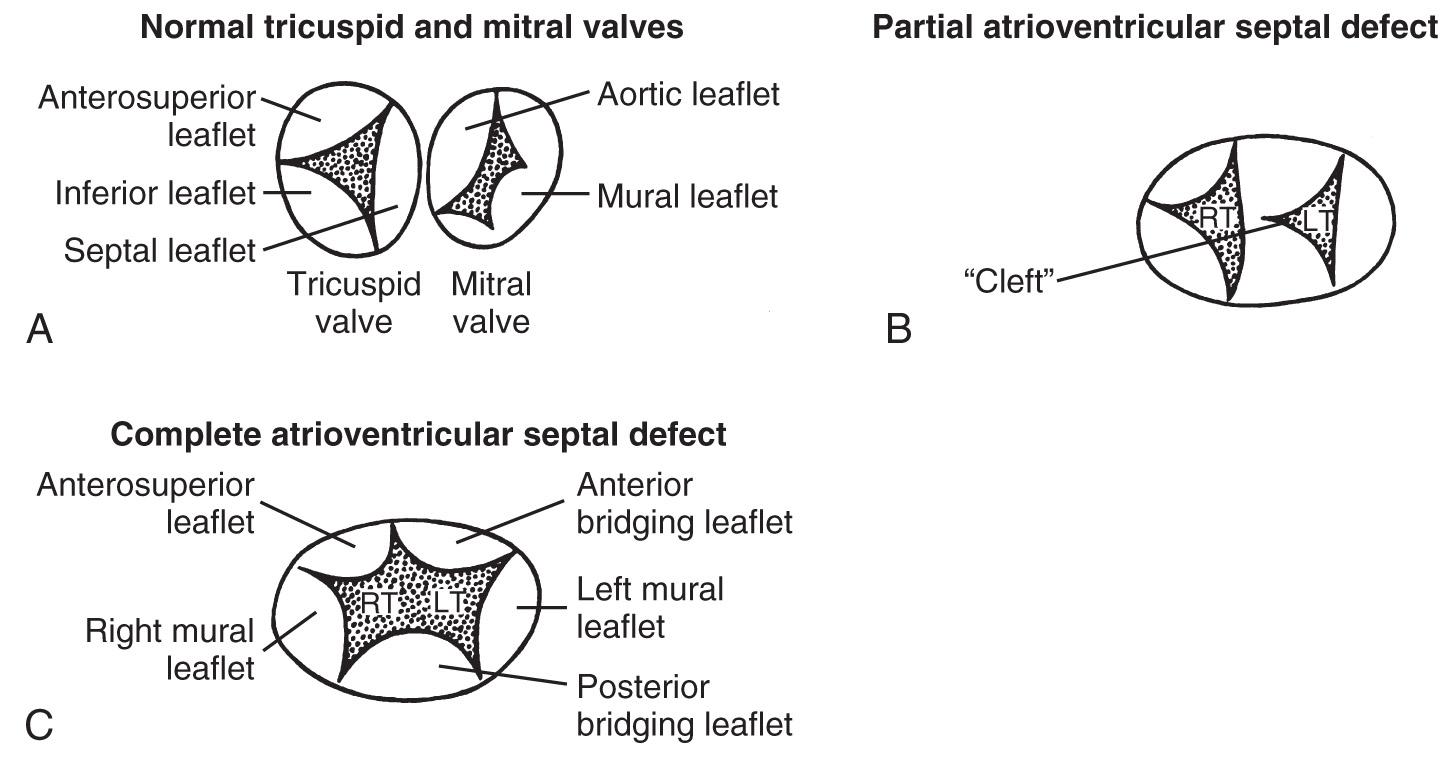
AVSDs are divided into complete and partial or incomplete forms. In both, the A-V valves are abnormal. In complete AVSD a single, multileaflet valve is present, whereas in incomplete AVSD two of the leaflets (bridging leaflets) are connected by a narrow strip of tissue, resulting in the appearance of two valve orifices. Complete AVSD has variable amounts of deficient tissue in the atrial and ventricular septa. The incomplete form is associated with an ostium primum ASD. At fetal echocardiography, 97% of AVSDs are complete, although after birth only 69% are complete. The fetal incidence of AVSD is four times greater than that in the live-born population, indicating a high incidence of in utero demise.
AVSDs are considered balanced when the A-V junction is connected to both the right and the left ventricle, such that blood flow is relatively evenly distributed. If this connection exists with primarily one ventricle, such as in the setting of a hypoplastic left ventricle, it is termed an unbalanced AVSD.
Sonographically, a defect in the atrial or ventricular septum with an associated single abnormal A-V valve is visible in a four-chamber view ( Fig. 37.27 , ). The abnormal valve should be suspected when the normal offset of the A-V valves is not visualized or when only a single A-V valve is appreciated in a short-axis view. Demonstration of two A-V valve orifices allows for differentiation between complete and incomplete forms of AVSD.
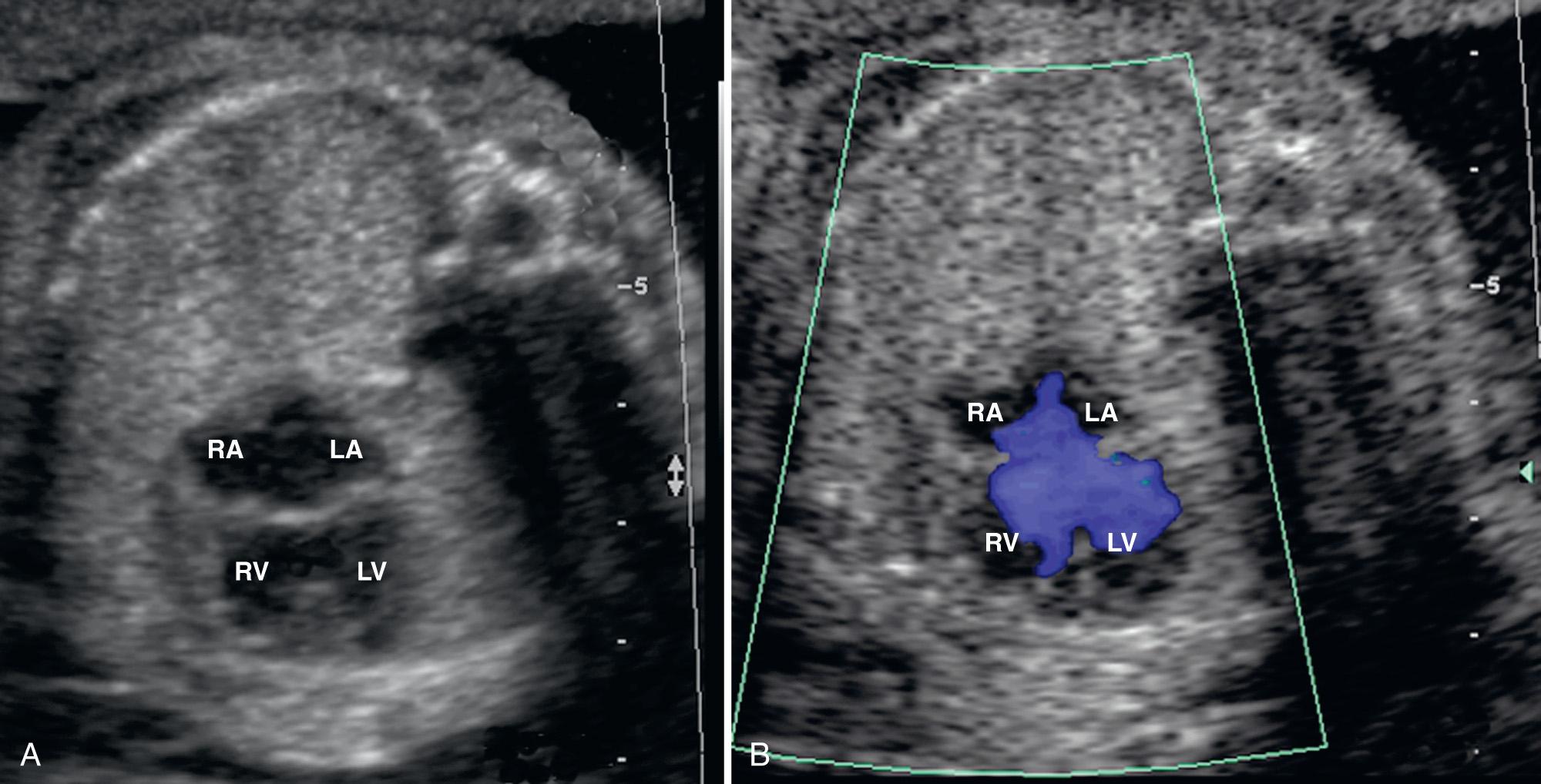
Color Doppler ultrasound demonstrates an open area of flow across the AVSD and the abnormal A-V valve. Color Doppler ultrasound imaging is particularly useful in the detection of valvular insufficiency. Holosystolic valvular insufficiency is closely associated with fetal hydrops and a worsening prognosis. Frequently, a left ventricular–to–right atrial jet can be identified across the ostium primum defect before the onset of holosystolic valvular insufficiency. Cardiac malformations associated with AVSD include septum secundum ASD, hypoplastic left heart syndrome (HLHS), valvular pulmonary stenosis, coarctation of the aorta, and tetralogy of Fallot (TOF). A meta-analysis of published cases of AVSD diagnosed prenatally confirmed that chromosomal anomalies are common, occurring in 25% to 58% of affected fetuses. Therefore karyotyping is indicated. Associated extracardiac anomalies are common, including omphalocele, duodenal atresia, tracheoesophageal atresia, facial clefts, cystic hygroma, neural tube defects, and multicystic kidneys.
The fetus with an AVSD and associated defects has a poor prognosis. When hydrops is present, few survive the neonatal period. Despite advances in pediatric cardiothoracic surgery, the overall outcome for antenatally diagnosed AVSD remains variable, with many studies reporting 5-year to 15-year survival rates below 50%. Others have reported excellent long-term results with newer surgical techniques such as the two-patch technique with early complete cleft closure for complete AVSD repair.
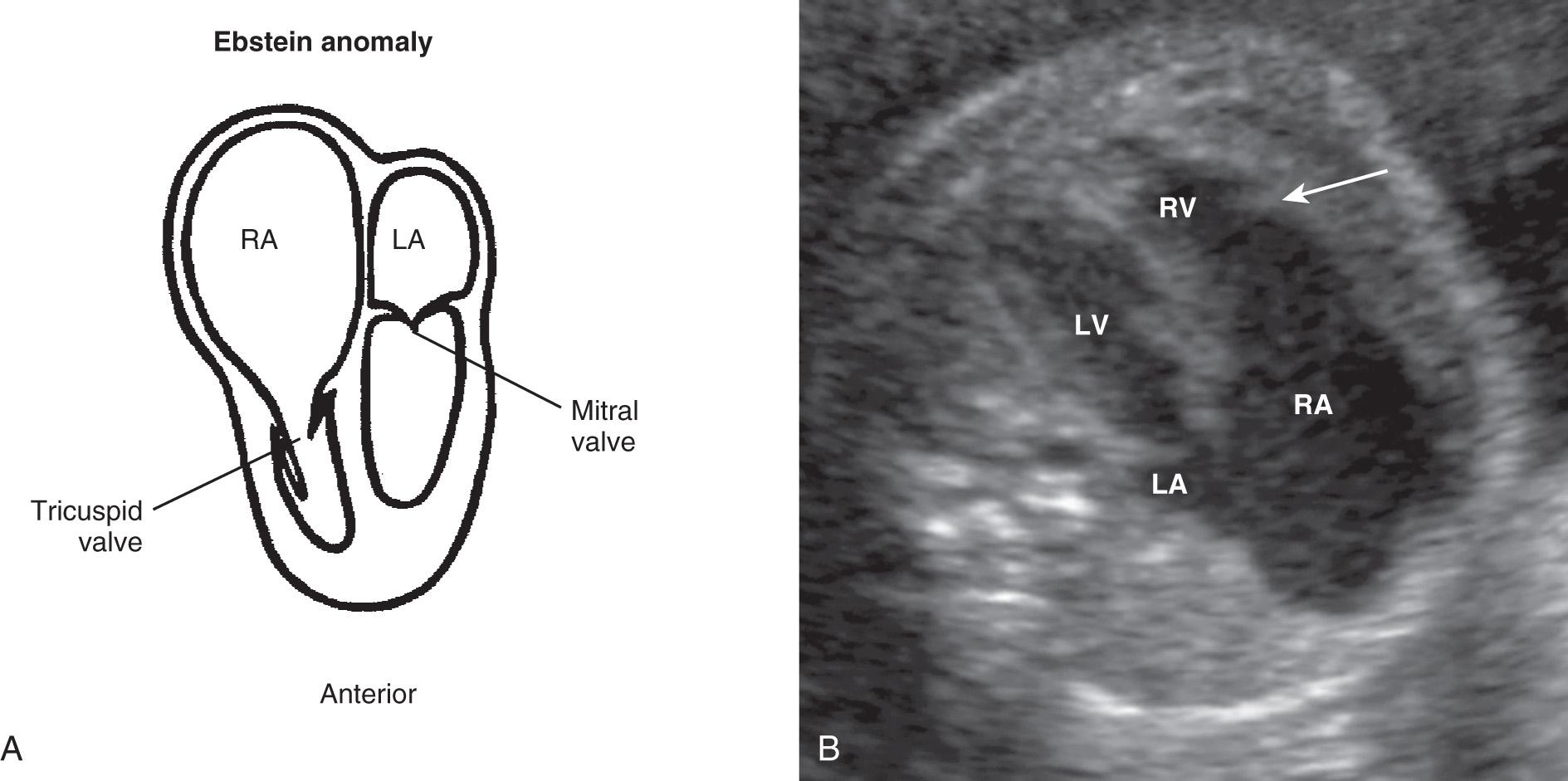
Ebstein anomaly is characterized by inferior displacement of the tricuspid valve, frequently with tethered attachments of the leaflets, tricuspid dysplasia, and right ventricular dysplasia ( Fig. 37.28 , ). Ebstein anomaly makes up approximately 7% of cardiac anomalies in the fetal population and has an incidence of 0.5% to 1% in high-risk populations. It occurs in approximately 1 per 20,000 live births.
Early data from biased retrospective studies suggested that lithium use during pregnancy was associated with an estimated 500-fold increase in the incidence of Ebstein anomaly in exposed fetuses. It is now clear that the increased risk is less than 2%. Ebstein anomaly may be associated with a variety of structural cardiovascular defects, particularly pulmonary atresia or stenosis, arrhythmias, and chromosomal anomalies.
Ebstein anomaly is readily detected in utero. The sonographic diagnosis rests on recognition of apical displacement of the tricuspid valve into the right ventricle, an enlarged right atrium containing a portion of the “atrialized” right ventricle, and a reduction in size of the functional right ventricle. Differential diagnosis includes tricuspid valvular dysplasia, Uhl anomaly, and idiopathic right atrial enlargement, but none of these has an inferiorly displaced tricuspid valve, the most reliable sign of Ebstein anomaly. Ebstein anomaly is one of the few structural defects that may cause substantial cardiac dysfunction in utero, frequently with cardiomegaly, hydrops, and tachyarrhythmias. Examination with spectral and color Doppler ultrasound is helpful in demonstrating tricuspid valve regurgitation, which causes further enlargement of the right atrium and ventricle. Tethered distal attachments of the tricuspid valve, marked right atrial enlargement, and left ventricular compression with narrowing of the pulmonary outflow tract are all associated with a poor prognosis. Fetuses diagnosed with Ebstein anomaly and tricuspid dysplasia have a poor prognosis, with an overall perinatal mortality (fetal demise or death before neonatal discharge) of 45%.
Arrhythmias, particularly supraventricular tachycardias (SVTs), are common with Ebstein anomaly and can further compromise the fetus. Overall, the 3-month mortality rate for patients diagnosed in utero is 80%. Surgical correction of Ebstein anomaly in young children is associated with a low mortality rate and an excellent quality of life. Because clinical presentation, treatment options, and prognosis are inconsistent, case-by-case management is variable.
In general, hypoplastic right ventricle occurs secondary to pulmonary atresia with intact interventricular septum. It has an incidence of 1.1% among stillbirths. Tricuspid atresia may be associated with a hypoplastic right ventricle, but this is not as common. Pathophysiologically, hypoplasia of the right ventricle develops because of a reduction in blood flow secondary to inflow impedance from tricuspid atresia or outflow impedance from pulmonary arterial atresia. Typical sonographic findings include a small, hypertrophic right ventricle and a small or absent pulmonary artery ( Fig. 37.29 , ). Spectral Doppler ultrasound may be helpful in demonstrating decreased flow through the tricuspid valve or pulmonary artery. Congestive heart failure and hydrops may develop from tricuspid regurgitation. After birth, closure of the ductus arteriosus frequently results in neonatal death. Prognosis improves with preoperative prostaglandin infusion to maintain the patency of the ductus.
In hypoplastic left heart syndrome, the left ventricular cavity is pathologically reduced in size. HLHS constitutes approximately 7% to 9% of all congenital cardiac lesions. It has a 2 : 1 male predominance and a recurrence risk of 0.5%. The small left ventricle results from decreased blood flow into or out of the left ventricle. The primary abnormalities include aortic atresia, aortic stenosis, and mitral valve atresia. It is associated with coarctation of the aorta in 80% of cases. The primary sonographic feature of HLHS is a small left ventricle ( Fig. 37.30 , ). The mitral valve is typically hypoplastic or atretic, as is the aorta. Color Doppler ultrasound is extremely helpful in the setting of HLHS, usually demonstrating the absence of flow through the mitral and aortic valves.

Three decades ago this syndrome had an extremely poor prognosis, with 25% mortality in the first week of life and most untreated infants dying within 6 weeks. Comfort care was provided, but little could be done to prolong survival. Currently it is expected that up to 70% of newborns with HLHS may reach adulthood owing to advancements in surgical techniques, perioperative management, and postoperative care. Prenatal diagnosis of HLHS is beneficial for preventing ductal shock and keeping affected infants stable in the preoperative stage. Monophasic blood flow across the mitral valve, restricted or absent flow through the foramen ovale, and retrograde flow through the aorta are all considered poor prognostic signs in utero. Despite significant advancements in medical and surgical management over the past 30 years, follow-up studies indicate that children with HLHS often experience major developmental delays and decreased exercise performance, even after heart transplantation. A recent meta-analysis found that although deficits remain, substantial improvement in neurodevelopment has occurred over the past 20 years in patients with surgically corrected HLHS.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here