Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The fallopian tube comes to the attention of the surgical pathologist under four general scenarios:
The tube has been interrupted for sterilization, and the pathologist must confirm this.
The tube has been removed as a component of the hysterectomy and is examined routinely.
The tube is removed for specific reasons, usually tubal pregnancy or, rarely, a tubal mass.
The tube becomes an important part of either ovarian cancer screening or staging a müllerian neoplasm.
Each of these scenarios requires the pathologist to have a working knowledge of the tubal anatomy and an appreciation of the subtle nature of early tubal cancer.
In revising this chapter, we have made several alterations, including the following:
Tubal intraepithelial carcinoma is discussed primarily in the context of its incidental discovery in otherwise normal specimens. Discussion of the role of the pathologist in serous cancer screening and detection in women with BRCA-1 or BRCA-2 mutations or others at high risk is addressed in greater detail in Chapter 24 . The latter is complete with detailed criteria for this diagnosis. The association between serous tubal intraepithelial carcinoma and pelvic serous cancer is further discussed in Chapter 25 in the context of determining the site of origin for pelvic serous carcinomas.
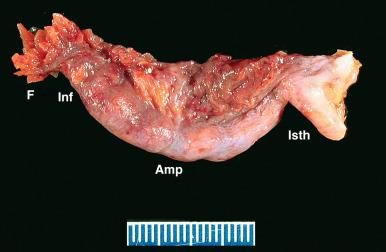
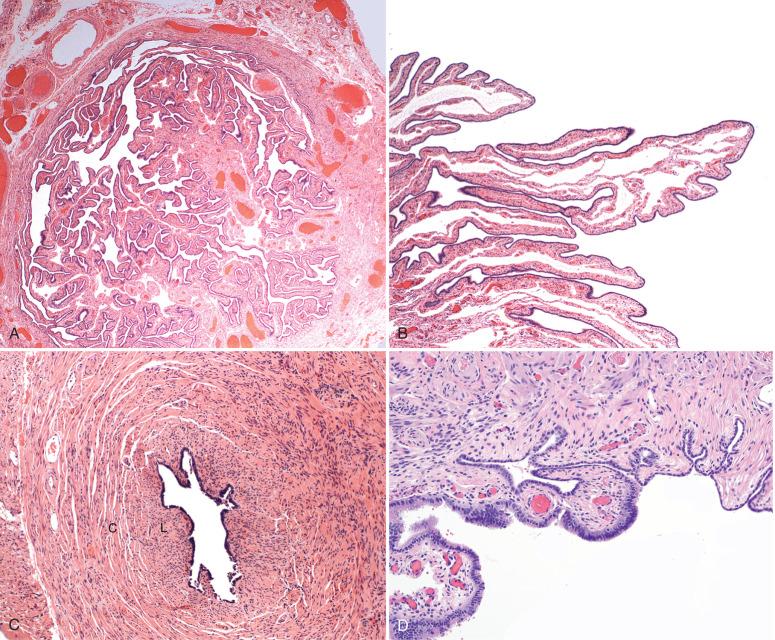
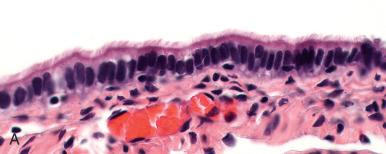
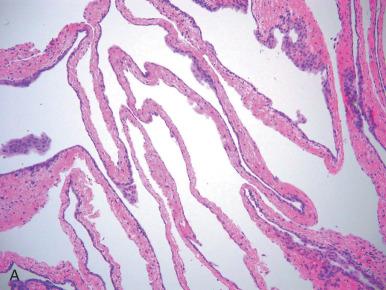
The fallopian tube is formed from the müllerian (paramesonephric ducts) and lies within the broad ligament located between the ovary and the uterus (see Chapter 1 ). It is roughly divided into three anatomic regions. The distal portion of the tube (infundibulum) is dilated, opens onto the peritoneal cavity, and is ringed by fimbrial projections ( Fig. 21.1 ). The infundibulum merges with the ampulla, gradually narrowing until it nears the isthmus, beyond which it terminates in the intramural region within the uterus. When viewed on cross section, the tube displays three distinct layers that are most prominent in the ampulla: the surface mucosa, the lamina propria, and the muscularis ( Fig. 21.2A-C ). The mucosa consists of a non-stratified epithelial lining composed of three cell types: ciliated, secretory, and intercalated cells. The most common are the ciliated cells, followed by secretory cells, which together constitute more than 90% of the cell population ( Fig. 21.3A ). The ciliated cells are critical to ovum transport. The secretory cells exhibit fusiform nuclei and apical secretory vacuoles, which often appear blue on hematoxylin and eosin staining. The intercalated cells are presumed to be a variant of the secretory cell and are seen as sporadic elongated nuclei between the ciliated cells. Similar cells can be visualized on immunofluorescence with antibodies to CD44, a marker linked to stem cells (see Fig. 21.3B ). The proportions of these individual cell types vary according to the location in the tube and account for wide variations in the appearance of the tubal epithelium (discussed later). Scattered lymphocytes are arranged at the epithelial–stromal interface and may signify a form of localized immune system.
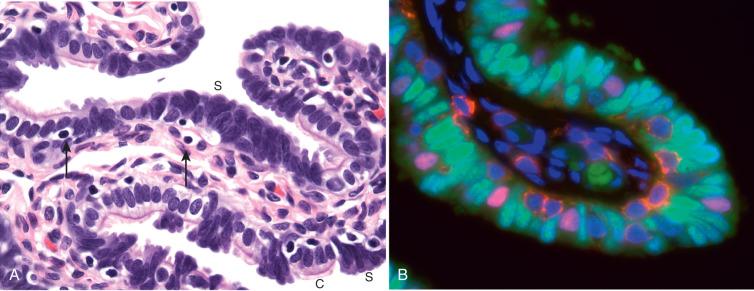
Beside predominantly CD8 + T cells, this first line of defense against precancerous lesions, also includes macrophages, dendritic cells and to a lesser extent natural killer (NK)-cells, CD4 + , and B cells.
Beneath this mucosa is the second layer, consisting of the lamina propria with loose connective tissue. In the normal tube, this portion is thrown into redundant slender folds known as plicae . The structural integrity of the plicae is relevant to ovum transport. The third layer consists of the muscularis, which is composed of circular smooth muscle fibers. The outer layer consists of the serosal surface and subserosal loose connective tissue (see Fig. 21.2C ). The isthmus portion of the tube also contains an additional inner longitudinal muscle layer (see Fig. 21.2C ). Finally, the müllerian epithelium joins the mesothelium on the fimbrial reflection (see Fig. 21.2D ).
The pathologist will encounter variations in cellular makeup of the tubal mucosa, depending on reproductive age, hormonal status, and location in the tube. With age, the plicae become progressively more blunt in appearance, owing to both contraction of the lining of the tube and a loss of epithelial complexity ( Fig. 21.4 ). This is analogous to the changes occurring in postmenopausal endometrium. A second variable is the epithelial thickness and composition. Ciliated cells are often more prominent in the fimbriated portion of the tube and may diminish in hypoestrogenic states. However, they are frequently encountered in all ages. The number and spacing of the secretory cells varies. In general, these cells alternate at regularly irregular intervals with ciliated cells. The explanation for this interplay of cell types is not altogether clear. However, it is best explained by the assumption that the secretory cells, or a subset thereof, are capable of undergoing terminal differentiation (ciliation). However, because both ciliated and secretory cells often appear anchored to the basement membrane, this process of differentiation seems to occur in the horizontal rather than vertical plane.
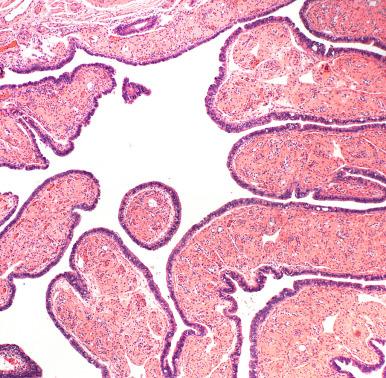
The fallopian tube can produce striking variations of epithelial growth, and these patterns can be impressive. In some areas, ciliated cells will predominate ( Fig. 21.5A ); others will exhibit a mixture of ciliated and secretory cells (see Fig. 21.5B ). In others, the ciliated cells may appear eosinophilic (see Fig. 21.5C ). Patterns that are regularly encountered include the following:
Physiologic epithelial changes: The most common and easily visualized is the admixture of ciliated and secretory cells to produce apparently stratified or tufting arrangements of both cell types suggesting focal hyperplasia. These may be prominent during pregnancy (see Fig. 21.5D ). Although these may be complex in appearance, their maintenance of both cell types is a reassuring reminder of benignity.
Benign epithelial proliferations include the following:
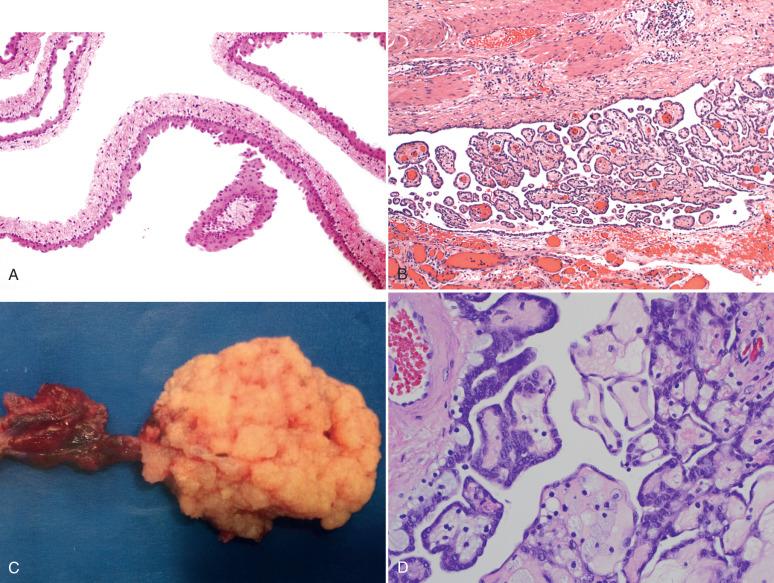
Benign serous tubal epithelial proliferations, also called p53 signatures: p53 signatures appear to result from inactivation and accumulation of p53 protein with corresponding reduction or loss of ability to mature into a ciliated population ( Fig. 21.6A ). They are most common in the fimbria. Currently the most popular explanation for this entity, which is found in 30% to 50% of normal fallopian tubes, is exposure to cytokines released during ovulation from the nearby ovarian cortex. p53 signatures are viewed as an early and, in most cases, self-limited phase of serous carcinogenesis in the fallopian tube. Like serous carcinomas, these early clonal proliferations contain p53 mutations, are in the distal tube, and manifest with evidence of DNA damage gamma-H2AX expression. However, they exhibit minimal atypias and at most a slightly elevated (10% to 15%) proliferative index. They are discussed again in Chapter 24 . Interestingly, women with germ line mutations in p53 (Li Fraumeni syndrome) harbor multiple p53 signatures in the fallopian tube (see Fig. 21.6B ).
![Fig. 21.6, A, Benign serous tubal epithelial lesion (so-called p53 signature). Note the focal intense staining for p53. B, Tubal section from a woman with Li Fraumeni syndrome displays multiple p53 signatures, a consequence of greater vulnerability to inactivation of the p53 gene in these women. C, Benign epithelial hyperplasia (type I secretory [stem] cell outgrowth). D, Note the presence of prominent ciliated differentiation. E and F, Benign epithelial hyperplasia (type II secretory cell outgrowth). These are distinctly endometrioid in appearance and contain altered expression of beta-catenin ( F, inset ). G, A type II secretory/stem cell outgrowth (SCOUT) with a squamous morule (atypical hyperplasias), indicating the presence of beta-catenin dysregulation. H, Type II SCOUTs with focal proliferative activity (left) are occasionally encountered. Coexisting endometrioid adenocarcinomas can occur with these but are rare. Fig. 21.6, A, Benign serous tubal epithelial lesion (so-called p53 signature). Note the focal intense staining for p53. B, Tubal section from a woman with Li Fraumeni syndrome displays multiple p53 signatures, a consequence of greater vulnerability to inactivation of the p53 gene in these women. C, Benign epithelial hyperplasia (type I secretory [stem] cell outgrowth). D, Note the presence of prominent ciliated differentiation. E and F, Benign epithelial hyperplasia (type II secretory cell outgrowth). These are distinctly endometrioid in appearance and contain altered expression of beta-catenin ( F, inset ). G, A type II secretory/stem cell outgrowth (SCOUT) with a squamous morule (atypical hyperplasias), indicating the presence of beta-catenin dysregulation. H, Type II SCOUTs with focal proliferative activity (left) are occasionally encountered. Coexisting endometrioid adenocarcinomas can occur with these but are rare.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/TheFallopianTubeandBroadLigament/4_3s20B9780323447324000212.jpg)
Benign secretory/stem cell outgrowths (SCOUTs): These overlap with secretory cell expansions (SCEs), are PAX2 negative, and fall into two categories. The first is type I SCOUTs, which are discrete expansions of stem cells undergoing ciliated differentiation (see Fig. 21.6B and C ). They are negative for ALDH1. The second is type II SCOUTs, exhibiting an endometrioid phenotype. Type II SCOUTs are ALDH1 positive and express both nuclear and cytoplasmic beta-catenin (see Fig. 21.6E and F ). Overall, SCOUTs are more commonly seen in aging fallopian tubes and are more commonly seen in benign tubes of women with high-grade serous carcinoma. However, whether this association is more a function of age association or an underlying stem cell vulnerability seen with age and serous cancer risk is unknown. The most important fact is that with rare exceptions, these proliferations do not function as direct precursors to fallopian tube malignancies. We occasionally encounter small proliferations associated with type II SCOUTs and have seen them in association with endometrioid adenocarcinomas of the tube, so they could rarely function as precursors to endometrioid neoplasms (see Fig. 21.40C and D , later). However, the overwhelming majority are benign—hence the term benign epithelial hyperplasia.
The purpose of tubal ligation is to eliminate the risk of subsequent pregnancy. In the United States, nearly one-quarter of reproductive-age women rely on sterilization of either gender to achieve contraception. The success of tubal sterilization is approximately 98%. Significantly, when failures do occur, the risk is greatest at 2 to 3 years following the procedure; 30% of these result in ectopic pregnancy. Disadvantages of tubal sterilization include pain, changes in menstruation, dyspareunia, changes in libido, and increased risk of gynecologic surgery. Benefits include a reduction in ovarian cancer risk and improved family life.
Many tubal sterilization procedures are carried out at the time of cesarean section. The tubes are transected and ligated; the clinician removes a segment of the tube under laparoscopic guidance and submits it to the pathologist to verify that (1) tube is present and (2) that a complete cross section is present. The pathologist verifies both and issues a report that a portion of oviduct is present and consists of a complete cross section. The pathologist must also report the presence of any tubal abnormalities, although most consist of evidence of old infection (in the form of chronic follicular salpingitis), tubal endometriosis, and peritubal adhesions. A common finding in tubal ligation specimens performed at the time of parturition is focal ectopic decidual change of the mucosa ( Fig. 21.7A ). These changes are occasionally encountered in specimens removed from women undergoing hormonal therapy. Another finding commonly seen in tubes that have been manipulated, either during tubal ligation or routine hysterectomy, is the presence of inflammatory cells that are incidental in nature. These include the margination of neutrophils from submucosal venules, associated with intraoperative manipulation (see Fig. 21.7B ), or the occasional presence of plasma cells in peripartum tubal ligation specimens. These are frequent and unassociated with other features of salpingitis.
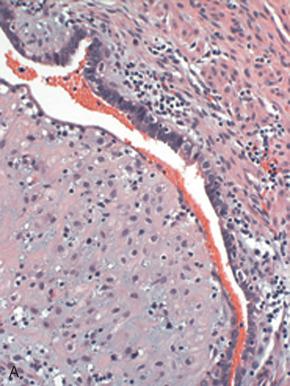
The pathologist faces four potential pitfalls when assessing tubal segments:
Diagnosis of tube in the absence of lumen . Because round ligament can mimic tube, the pathologist must require that a lumen be present ( Fig. 21.8A ).
Misclassification of a paratubal cyst as salpinx, by mistaking rudimentary plica as a segment of tube or a hydrosalpinx. Paratubal cysts typically contain a much more attenuated wall with minimal smooth muscle (see Fig. 21.8B ).
Misdiagnosing a segment of ureter as tube . This is unlikely to happen to either the surgeon or an experienced histologist, but it is included for completeness.
Mistaking thermal artifacts for neoplasia.
In some instances, the clinician will attempt a reanastomosis of the fallopian tube to restore fertility. This is successful in approximately 55% of cases. This procedure typically entails the removal of some segments, which are submitted to the pathologist. The most common findings in these segments are the absence of plica or small foci of hydrosalpinx associated with prior interruption. Other than confirming that no major abnormality (active infection or neoplasia) is present, the pathologist's role is to verify the origin of the tissues.
We have seen an increase in attention to pathologic evaluation of intra-tubal contraceptive devices, such as Essure. The pathologist's role is as follows: (1) carefully describe the findings, (2) obtain photographs as appropriate, and (3) separate the tissue from the device and submit for pathologic evaluation.
There is ample evidence that the distal fallopian tube is the principal site of early tubal malignancies, both in women at risk for pelvic cancer ( BRCA-1 or BRCA-2 mutations) and, to a lesser frequency, in the population. The Sectioning and Extensively Examining the FIMbraited End (SEE-FIM) protocol, which was developed in the Division of Women's and Perinatal Pathology at Brigham and Women's Hospital, calls for not only complete sectioning of the tubes, but also special attention to the fimbriated end. It is also discussed in Chapter 24 , and similar approaches have been recommended in other publications.
We use the SEE-FIM protocol or a variation in the following settings: (1) all risk-reducing salpingectomies, (2) tubes removed from all women with breast cancer, and (3) in the setting of any uterine or pelvic epithelial malignancy. In other specimens, we submit the entire distal 2 cm of the tube (fimbria and ampulla) and a single central section ( Fig. 21.9 ; Box 21.1 ). The reason for doing this is similar to that for submitting a section of the cervical transformation zone—that is, the fimbria is the region at greatest risk for tubal malignances and the site where one is most likely to discover an incidental early malignancy. We have already begun to receive cases or have been advised of cases in which an early carcinoma was discovered in the fimbria of an otherwise healthy individual for whom there were no known risk factors for pelvic cancer ( Fig. 21.10 ). Thus, the significance of examining the entire distal tube lies in the possibility that an occasional incidental discovery will not only permit appropriate early therapy if needed but will also uncover an individual at genetic risk (BRCA positive) and perhaps identify others in the family who could be tested and, if positive, benefit from early intervention. Some recent studies have validated this approach, although the precise number of cases of early cancer that will be unearthed remains to be determined. This concept is discussed in greater detail in Chapter 24 .
Tube is fixed for 1 to 2 hours.
Distal 2 cm are amputated (fimbria and ampulla).
Entire distal tube is sagittally sectioned at 1 to 2 mm and submitted in a single cassette.
Remainder of the tube is cross sectioned at 1- to 2-mm intervals and submitted in a separate cassette(s).
Tube is fixed for 1 to 2 hours.
Distal 2 cm are amputated (fimbria and ampulla).
Entire distal tube is sagittally sectioned finely at 1 to 2 mm and submitted with a single central tube cross section in the cassette.
SEE-FIM, Sectioning and Extensively Examining the FIMbraited End.
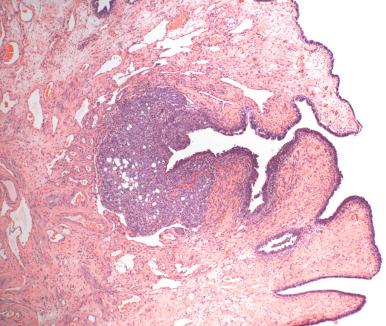
In the routine hysterectomy, fallopian tubes are examined with an eye toward excluding concurrent disease. Practically speaking, rests or benign proliferations ( Tables 21.1 and 21.2 ) and cysts may be seen.
| Entity | Figure | Notes |
|---|---|---|
| Arias-Stella effect (ASE) | 21.25 | Associated with pregnancy, seen in postpartum tubal sterilization specimens |
| Benign serous tubal intra-epithelia proliferation/lesion (p53 signature) | 21.6A and B | Benign self-limited clonal proliferations with p53 mutations |
| Benign epithelial hyperplasia (type I secretory/stem cell outgrowths [SCOUTs]) | 21.6C and D | Benign PAX2/ALDH1− clonal proliferations with both secretory and ciliated cell differentiation |
| Benign papillary epithelial hyperplasia | 21.23C; 21.34 | Type I SCOUT with papillary architecture. Occasionally associated with some low grade serous tumors, but causal role is unclear |
| Benign papilloma | 21.23A, B, and D | Discrete intraluminal papillary proliferation |
| Benign epithelial hyperplasia (type II SCOUTs) | 21.6E and F | Benign PAX2−/ALDH1+/beta-catenin+ proliferations with an endometrioid phenotype May be more common in tubes from women with serous or endometrioid neoplasia |
| Atypical (endometrioid) epithelial hyperplasia (type II SCOUTs with morules or glandular proliferation) | 21.6G and H | Uncommon; possible endometrioid precursor similar to counterpart in endometrium; rarely associated with endometrioid adenocarcinoma of the tube |
| Serous tubal epithelial proliferation/lesion of undetermined significance | 21.38A and B See Chapter 24 |
Clonal outgrowths of non-ciliated cells with p53 mutations, atypias, preserved polarity; undetermined relationship to malignancy risk |
| Serous tubal intraepithelial carcinoma | 21.38C, D, and E | Potentially malignant, 5% risk of later pelvic high grade serous carcinoma |
| Mucinous differentiation or metaplasia | 21.35 | Can occur as an isolated finding but can be related to mucinous tumors of the cervix, appendix, and elsewhere, including with Peutz-Jeghers syndrome |
| Entity | Figure | Comment |
|---|---|---|
| Benign Epithelial | ||
| Paratubal cyst | 21.12 | Common, rarely associated with malignancy |
| Polypoid endometriosis | Rare, presents as a nodular mass akin to an endometrial polyp; can recur and even more rarely associated with adenosarcoma | |
| Adenofibroma | 21.36 | Subtle microscopic foci quite common, larger lesions seen on a regular basis; rarely associated with malignancy |
| Benign papilloma | 21.23A, B, and D | Overlaps with papillary hyperplasia; usually seen on microscopic examination only |
| Serous cystadenoma/borderline serous tumor | 21.33 | Usually found in paratubal cysts |
| Tumor-Like Lesions | ||
| Salpingitis isthmica nodosa | 21.22 | Presents as a nodular lesion in the proximal tube |
| Tuberculous salpingitis | 21.29A and B | Rare |
| Amyloidosis | 21.31 | Rare |
| Ectopic granulosa cell rests or tumorlets | 21.11D and E | Peritubal |
| Ectopic adrenal tissue | 21.11C | Frequent, inconsequential |
| Hilar cell hyperplasia | Occasionally encountered in the mesosalpinx | |
| Ectopic pregnancy | See Chapter 29 | Approximately 1 : 80 pregnancies; frequency has increased, presumably due to assisted reproduction |
| Malakoplakia | See Chapters 11 and 15 | Extremely rare in the fallopian tube; involvement of lamina propria by macrophages; can simulate neoplasia |
| Torsion | 21.30 | |
| Mesothelial Lesions | ||
| Mesothelial hyperplasia | 21.15B | Reactive process, common |
| Solitary papillary mesothelioma | 21.15C and D | Isolated papillary mesothelial proliferation; if solitary and noninvasive, presumably benign |
| Adenomatoid tumor | 21.32 | Benign; may mimic signet ring cell carcinomas |
| Malignant Epithelial | ||
| High-grade serous carcinoma | 21.39 | Most common malignant epithelial tumor of the fallopian tube; arises from a serous tubal intraepithelial carcinoma or possibly from serous tubal epithelial proliferations/lesions |
| Endometrioid adenocarcinoma | 21.40 | Uncommon, may be encountered as small mucosal deposits originating from ovarian or endometrial tumors; rarely, primary of the tube |
| Clear cell, transitional cell, squamous, glassy cell, carcinosarcoma, undifferentiated carcinoma | 21.41, 21.44 | Extremely rare in the fallopian tube |
| Germ cell tumors (mature and immature teratomas), lymphomas | 21.45 | Rare |
| Female adnexal tumor of Wolffian origin (FATWO) | 21.46 | |
Walthard cell rests, consisting of transitional metaplasia of mesothelial cells or subcolumnar cells ( Fig. 21.11A ): These are most commonly seen adjacent to the fimbria but may be seen on or adjacent to the serosa in any segment of tube. They most likely occur via the development of reserve cells (see Fig. 21.11B ). This is supported by transitional metaplasia within mesothelial cysts within the mesosalpinx (discussed later). The lining cells characteristically contain small, uniform fusiform cell nuclei with nuclear grooves. A perhaps etiologically related but extremely uncommon condition is extensive squamous metaplasia of the peritoneal and ovarian surfaces associated with peritoneal dialysis.
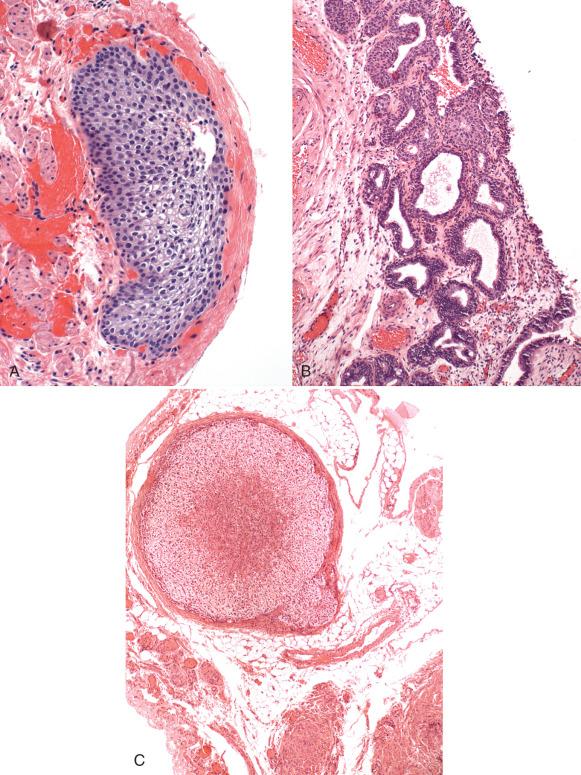
Adrenal rests consist of small discrete microscopic nodules of adrenal cortical cells, typically situated in the connective tissue of the mesosalpinx (see Fig. 21.11C ). Their presumed origin is from adrenal progenitor cells carried with the mesonephros during development. They are benign but may mimic steroid-producing cells, which occasionally are lipid rich and resemble adrenal cells. However, the latter are situated in the hilus, in contrast to the peritubal connective tissue.
Hilar type cells (Leydig): These can also be identified in the mesosalpinx.
Misplaced granulosa cells and rarely granulosa cell tumors (see Fig. 21.11D-F )
Focal mesothelial proliferations: These are usually inconsequential; but if florid, a localized mesothelioma must be excluded.
Paratubal cysts can be subdivided into the following categories.
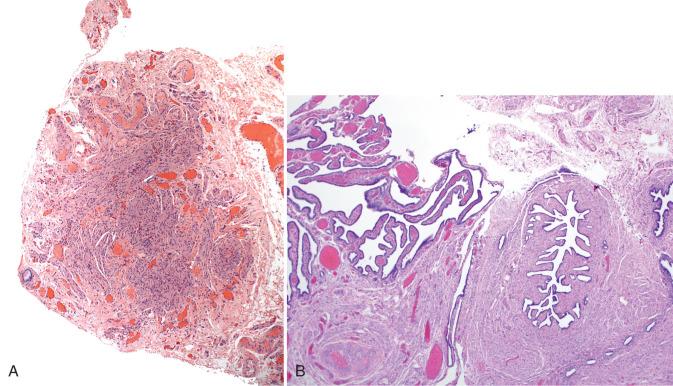
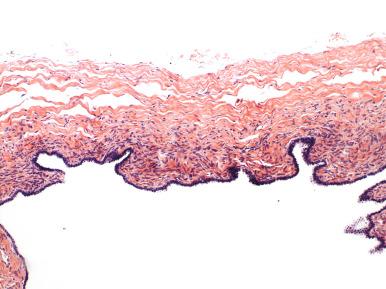
These cysts conceivably can arise via three different pathways. The first is müllerian transformation of the mesothelial surface. The second is detached and encysted epithelium from the fallopian tube that is ensconced in the mesosalpinx. The third is a vestigial remnant of the paramesonephric duct. The latter is most appealing, because these cysts are characteristically (if not invariably) closely associated with the fallopian tube, typically tethered by a thin band of connective tissue to the tube. Paratubal müllerian cysts characteristically contain an attenuated and translucent cyst wall ( Fig. 21.12A ) with delicate mesenchyme and variable smooth muscle (see Fig. 21.12B ). Plicae may be present but are sporadic, and the lining epithelium is ciliated (see Fig. 21.12C ). Paratubal cysts are distinguished from tube (hydrosalpinx) by the absence of a well-developed smooth muscle wall and from serous cystadenomas by the close similarity to tubal epithelium, occasional rudimentary plica, and, specifically, by the absence of a dense collagenized cyst wall that typifies cystomas. Cystadenomas are discussed later.
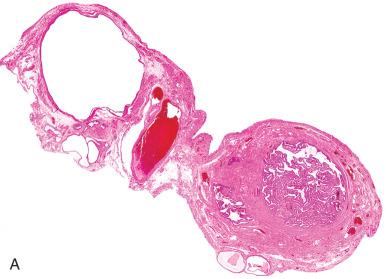
Endosalpingiosis are most likely implants of benign tubal epithelial cells that encyst in the mesosalpinx ( Fig. 21.13 ). Identical epithelial cysts can be seen in the ovarian cortex, mesentery, and lymph nodes, a distribution that precludes origin from paramesonephric (müllerian) duct remnants. These are distinguished from endometriosis by the well-developed ciliated lining and the absence of endometrial stroma or hemosiderin.
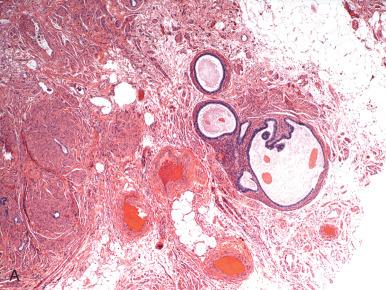
Cysts lined by mesothelium exhibit a range of histologic presentations. The first is cystic adhesions, which are simply complex adhesions with collections of clear fluid that may exhibit squamous metaplasia ( Fig. 21.14 ). A related lesion is the multilocular cyst with mesothelial proliferation, which is most likely a manifestation of inflammation ( Fig. 21.15A ). These cysts may also exhibit squamoid or transitional metaplasia similar to that seen in Walthard cell rests (see Figs. 21.14B and 21.15A ). Less commonly, papillary mesothelial hyperplasia is seen (see Fig. 21.15B ). These should be carefully scrutinized to exclude a mesothelioma, which can occur rarely in the fallopian tube (see Fig. 21.15C and D ). Another is the solitary cyst lined by cuboidal epithelium with a more collagenized fibrous wall, often termed simple cyst . Conceivably, many simple cysts are not mesothelial but epithelial, but in the absence of conspicuous cilia, the pathologist may elect to classify such cysts as simple cysts rather than unilocular cystadenomas ( Fig. 21.16 ).
Endometriosis is extremely common and is found in approximately 10% of tubal specimens. The possible origins of endometriosis are discussed in Chapter 23 and include transformation of coelomic epithelium and transtubal migration of uterine endometrium during menstruation. In the fallopian tubes, the importance of endometriosis lies in its relationship to infertility in younger women and an increased risk of malignancy with age.
In patients with endometriosis, the principal variable influencing fertility is the condition of the tube, with the rate of successful pregnancy diminishing from more than 50% with no abnormality to 0% with bilateral tubal occlusion. In contrast, ovarian and cul-de-sac lesions do not affect pregnancy rates. History and pelvic examination are of limited predictive value; many lesions are silent and detected by laparoscopy.
Tubal endometriosis has several morphologic presentations:
Classic endometriosis is characterized by endometrial glands and stroma in the muscularis or, more commonly, in the subserosa or mesosalpinx ( Fig. 21.17 ). Younger lesions closely resemble normal endometrium (see Fig. 21.17A ). Lesions composed of dilated glands surrounded by fibrosis and hemosiderin are more commonly encountered later in the natural history (see Fig. 21.17B ).
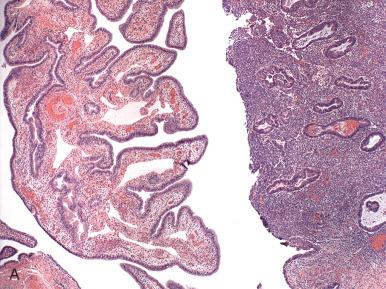
Polypoid endometriosis is another presentation, characterized by its tendency to mimic a neoplasm clinically, intraoperatively, or on gross examination. Microscopically, tubal epithelium hyperplasia and hyperchromasia may be mistaken for dysplasia. In this situation, identification of even a small focus of stroma is critical for such distinction.
In some cases, the process is more subtly present on the serosal surface of the fallopian tube and broad ligament, composed of scant epithelium and hemosiderin-laden macrophages (see Fig. 21.17C ).
A common, albeit nonspecific, feature of endometriosis is the presence of scattered plasma cells (see Fig. 21.17C inset ). However, this finding is not considered diagnostic. Similarly, in the absence of stroma or müllerian epithelium, hemosiderin is not sufficient for a diagnosis of endometriosis (see Fig. 21.17D ).
Of interest and possibly related to endometriosis are inflammatory changes in the fallopian tube lining, including follicular salpingitis. Some authors have proposed a relationship between salpingitis and endometriosis, suggesting that the former is a mechanism for tubal-associated infertility in these patients. This is consistent with the close relationship between tubal adhesions and pregnancy success rates.
An uncommon abnormality associated with endometriosis consists of expansion of the tubal plicae by pigmented histiocytes. This is alternatively termed pseudoxanthomatous salpingiosis or pseudoxanthomatous salpingitis . We prefer the term salpingiosis given the fact that inflammation is a minor component of this disorder. Pseudoxanthomatous salpingiosis is analogous to the xanthomatous pseudotumors seen elsewhere in the pelvic area in patients with endometriosis.
On gross examination, the tubal plicae are edematous and golden brown, a result of the numerous foamy macrophages ( Fig. 21.18A ).
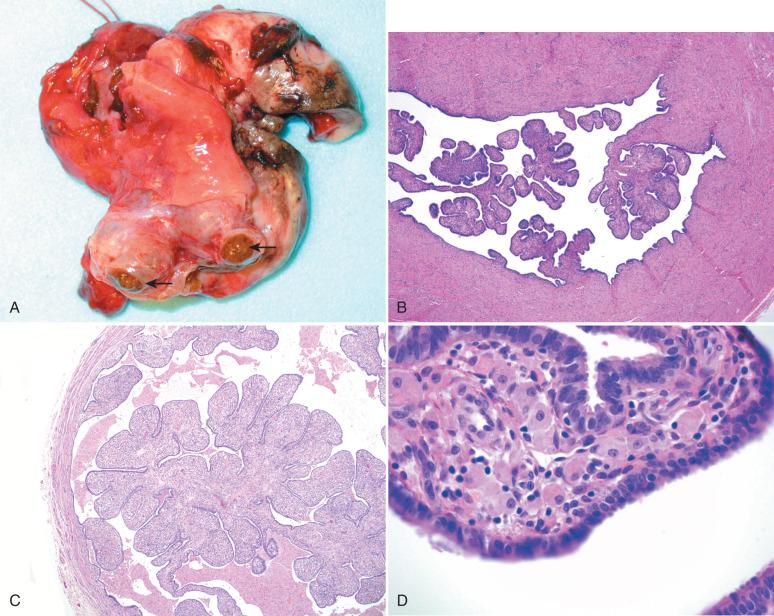
On microscopic examination, the foamy cells fill the interstitium of the plica (see Fig. 21.18B-D ). The origin of this process is not clear but may be due to hemorrhage directly into the fallopian tube lumen with a macrophage response.
Xanthogranulomatous salpingitis is distinguished from pseudoxanthomatous salpingiosis by its association with a frank inflammatory process of the tube and typically a long history of pelvic inflammatory disease (PID).
This disorder is characterized by foamy histiocytes and inflammatory cells in the wall of the tube, with the latter being the more prominent ( Fig. 21.19A-C ).
![Fig. 21.19, A and B, Xanthogranulomatous salpingitis. The lamina propria is consumed by inflammatory cells; foamy histiocytes are present on the luminal surface (left). C, At higher magnification, the dominant cell population is mononuclear, with scattered foamy histiocytes. D, Granulomatous salpingitis associated with contrast (ethiodized oil [Lipiodol]) material, present here as microscopic spaces, signifying lipid droplets that have dissolved during processing. Fig. 21.19, A and B, Xanthogranulomatous salpingitis. The lamina propria is consumed by inflammatory cells; foamy histiocytes are present on the luminal surface (left). C, At higher magnification, the dominant cell population is mononuclear, with scattered foamy histiocytes. D, Granulomatous salpingitis associated with contrast (ethiodized oil [Lipiodol]) material, present here as microscopic spaces, signifying lipid droplets that have dissolved during processing.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/TheFallopianTubeandBroadLigament/18_3s20B9780323447324000212.jpg)
Given the well-studied carcinogenicity of iron and iron-related compounds, one study showed a moderate increase in proliferation index of a subset of cases with pseudo-xanthomatous salpingitis (>10% of cases with mean index of 32%). The increase in Ki-67 proliferation index was disproportionate to the amount of inflammation, and it was suggested (but unproven) that pseudoxanthomatous salpingitis was a potential early event in fallopian tube serous carcinogenesis.
A variant of xanthogranulomatous salpingitis is associated with reaction to contrast agents (ethiodized oil [Lipiodol]) (see Fig. 21.19D ). In this condition, the lamina propria contains collections of discrete vacuoles corresponding to the foreign material, with a secondary granulomatous inflammation.
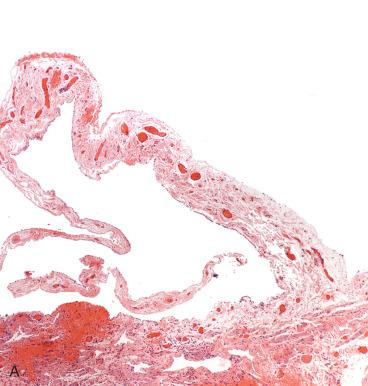
Adhesions are common and may follow prior salpingitis, surgery, or endometriosis. They may be confined to the tube (paratubal adhesions) ( Fig. 21.20A ), bridge the tube and ovary (tubo-ovarian adhesions) (see Fig. 21.20B ), or coalesce and form a cystic mass in which fluid collects (cystic adhesions) (see Fig. 21.14A ). As discussed earlier, adhesions are associated with infertility.
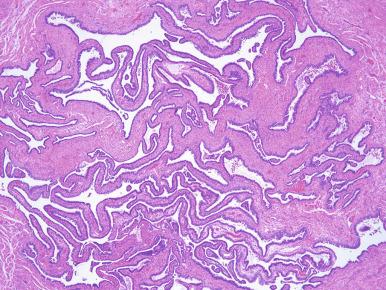
Chronic (follicular) salpingitis is not uncommon in hysterectomy specimens and reflects prior tubal infection (discussed later). The characteristic features are plical adhesions forming follicle-like architecture ( Fig. 21.21 ), often accompanied by hydrosalpinx (discussed later).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here