Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
During embryonic patterning, individual cells divide, migrate, differentiate, and respond to environmental cues. The extracellular matrix (ECM) is effectively involved in all these dynamic processes during development, maintenance, and disease. Cells are continuously connected with the ECM, a latticework of glycoproteins that provides structural support and spatial arrangement and directs tissue morphogenesis. The ECM provides a specialized microenvironment that regulates cell behavior by interacting with cell surface receptors known as integrins. This allows regulation of extracellular and intracellular signaling emanating from extrinsic factors, including growth factors (GFs), hormones, and biomechanical forces. Proteolysis of the ECM also generates neoepitopes that confer functions on cells and tissues distinct from those specified by their nonproteolyzed counterparts. In this chapter, we provide a review of the complex structure of the ECM and its essential role in normal development and pathophysiology, and elaborate on ECM-integrin signaling with tissue-specific examples during embryonic development and adult tissue maintenance.
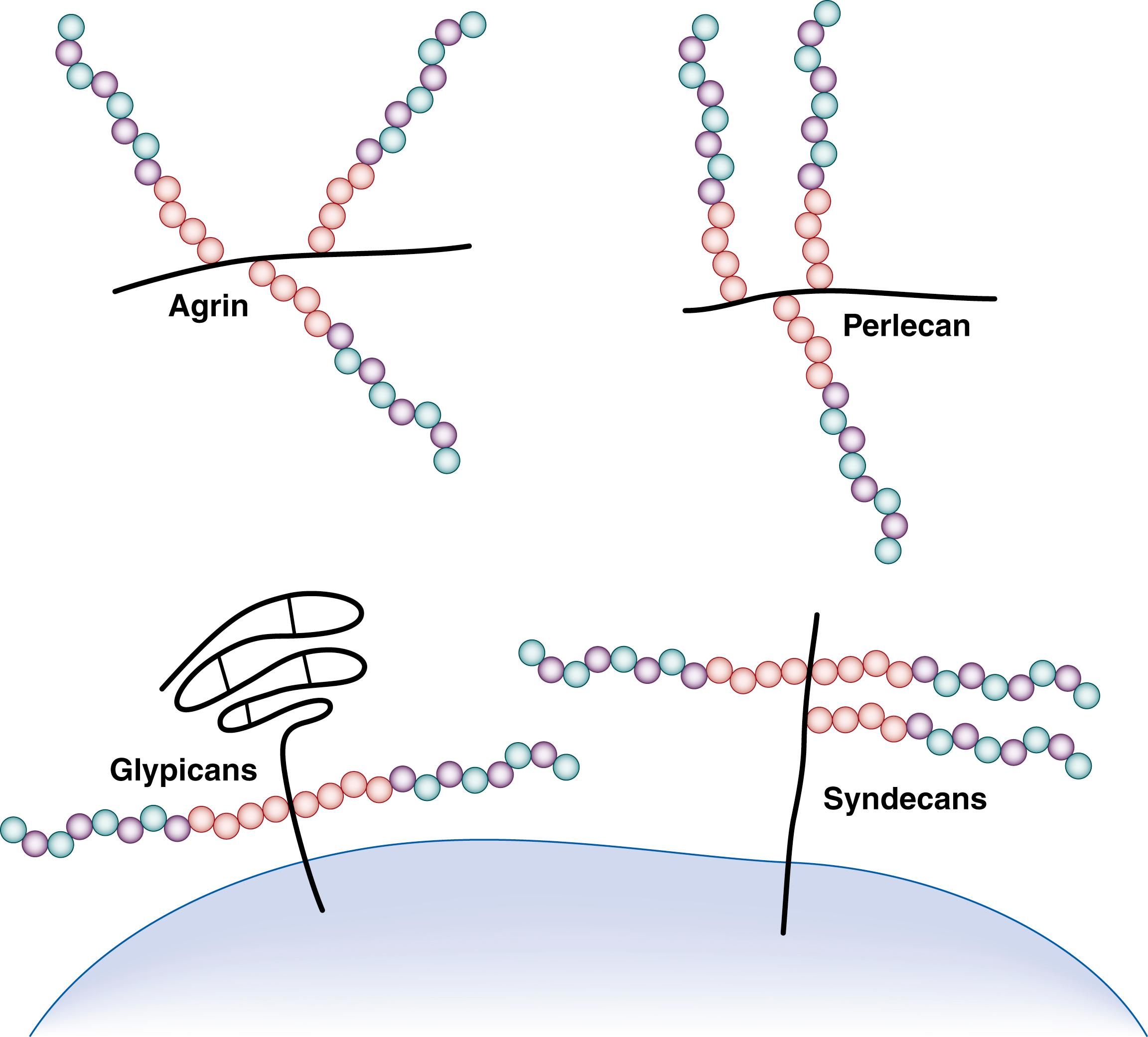
To decipher how the ECM confers numerous different functions, an appreciation of its complex structure is essential. The ECM is an oligomeric, three-dimensional network composed of four major protein components: collagens, structural glycoproteins (e.g., fibronectin, laminin, tenascin-C), proteoglycans (e.g., heparan sulfate [HS], chondroitin sulfate, syndecans), and elastic fibers (e.g., elastin, microfibrillar proteins). Matrix proteins are secreted by the epithelial and stromal cells and bind each other as well as cell adhesion molecules. Developmental processes, including the response of unpatterned tissue to morphogen gradients, are regulated by glycosaminoglycans, whereas the surface of most cells and the ECM are decorated by HS proteoglycans (HSPGs). HSPGs are composed of a core protein to which long linear glycosaminoglycan HS chains are covalently linked ( Fig. 5.1 ).They engage in numerous cell-matrix interactions and function by binding and regulating local concentrations of GFs and morphogens, and mediate a wide range of functions in early vertebrate development, for example, left-right patterning and cardiovascular and neural development. , The distribution and organization of the ECM is both dynamic and tissue-specific. For example, mesenchymal cells are surrounded by an interstitial stromal ECM, which includes type I and type II collagen, fibronectin, and proteoglycans. The basement membrane of endoderm-derived organs such as the lung represents another specialized ECM that is composed predominantly of laminin, type IV collagen, and HSPGs. Alternatively, basement membrane material may separate distinct cell layers, as is the case in the kidney glomerulus, where the basement membrane separating epithelial and endothelial cells also functions as a filter. In the case of elastic tissues such as skin and arteries, the ECM is reinforced with elastin fibers to provide additional structural stability for resilience to mechanical forces. Within these different extracellular matrices, additional structural and functional diversity is generated using alternative gene promoters and RNA splicing, and by posttranslational modifications, including glycosylation and sulfation of newly synthesized matrix proteins. Once secreted into the extracellular space, ECM proteins require integration into a functional network. Identifying binding partners for a specific ECM protein is therefore a prerequisite to ascertaining its biochemical and cell-signaling properties. For example, the ECM glycoprotein tenascin-C (TNC) controls cell adhesion, migration, differentiation, and synthesis of ECM molecules via interacting in a tissue-specific manner with fibronectin, perlecan, neurocan, heparin, phosphacan, syndecan, glypican, and periostin. , Accordingly, understanding the biology of a single ECM component requires an appreciation of the structure and functions of numerous other affiliated proteins. Because of the number of steps involved in coordinating ECM expression, secretion, and assembly, deciphering how individual ECM proteins contribute to structural morphogenesis during developmental processes has been a challenging task.
Normal development requires precise temporal and spatial coordination of cellular proliferation, migration, differentiation, and apoptosis. Deciding which of these programs a cell will ultimately elect is determined, to a large extent, by the ECM. Promotion or suppression of cellular proliferation by the ECM results in either activation or silencing of genes involved in the regulation of the cell cycle. To counteract uncontrolled cellular proliferation and to sculpt or refine developing tissue structures, select cells must be eliminated from developing tissues. To this end, loss of cell contact with the ECM leads to a specialized apoptosis termed anoikis during development and cellular differentiation. , Tissue-specific ECM components also regulate the transcription of genes associated with specialized differentiated functions, including alkaline phosphatase expression in osteoblasts, albumin production in hepatocytes, and intermediate filament protein expression in keratinocytes. , The critical role of the ECM during heart morphogenesis is apparent by the dependence of precardiac cells’ directional movement on a gradient of fibronectin, a matrix protein involved in the active migration of cells across the substratum. Efficient specification to cardiomyocytes is also directly dependent on cell attachment strength and matrix compliance. These observations are supported by the identification of ECM-responsive transcription factors and cis elements within gene promoters. , Moreover, stem cell maintenance, self-renewal, and cell fate determination in adult stem cell populations depend on the ECM. For example, matrix-mediated changes in cell adhesion of hematopoietic stem cells in their microenvironment allow for the self-renewal and subsequent differentiation of these multipotent progenitors into blood and other cell types. Therefore, precise cell-matrix interactions act as an important biologic switch that dictates stem cell differentiation or mobilization at specific tissue sites during development and maintenance of adult stem cell populations.
Mapping and identifying gene mutations that lead to heritable connective tissue disorders along with generating animal models in which ECM genes have been mutated or ablated have been successfully used to ascertain the functions of individual ECM proteins within specific tissues. Many of the diseases resulting from ECM gene mutations are due to the defective structural integrity of specific tissues. Mutations in collagen type VII, collagen type XVII, and laminin can cause the skin-blistering disease epidermolysis bullosa. , Mutations in type I collagen genes cause osteogenesis imperfecta, and mutations in both collagen I and tenascin-X genes can cause Ehlers-Danlos syndrome. In addition to gene defects that alter mechanical properties of the ECM, mutations in the fibrillin-1 gene that cause Marfan syndrome appear to increase transforming growth factor (TGF)-β signaling, leading to a cellular disease phenotype.
Although mutations in ECM genes can produce heritable disorders, animal studies suggest that many structural ECM glycoproteins are essential for embryonic or fetal development. As a result, mutations in these genes often cause lethality early in development, complicating the study of gene function in vivo. Inactivation of the fibronectin gene in mice results in embryonic death due to mesodermal, neural tube, and vascular developmental defects and has been shown to be required for normal gastrulation. More targeted studies have provided data on the role of fibronectin in several developmental processes. Injection of inhibitory peptides or antibodies into post-gastrulation embryos prevents fibronectin-cellular interactions and disrupts neural crest migration. In contrast with fibronectin, knockout (KO) of the tenascin-C gene in mice results in viable and fertile adults, suggesting that other ECM proteins may be able to compensate for tenascin-C deficiency. Consistent with this notion, experiments using isolated adult hypertensive pulmonary arteries, in which tenascin-C expression has been suppressed, indicate that osteopontin substitutes for tenascin-C in promoting smooth muscle cell proliferation.
Mutations in ECM component genes may also cause adult-onset phenotypes. Some of these phenotypes may result from abnormalities not visible at birth, and many of these animals have not been analyzed in enough detail to rule out developmental defects. For example, tenascin-C gene-KO mice suffer from several neurologic defects, including hyperactivity, poor sensorimotor coordination, clinging, and freezing behavior, as well as poor performance in passive avoidance tests. Other defects in tenascin-C gene-KO animals have subsequently been detected, including reduced corneal wound healing and hematopoiesis. , Tenascin-C gene-KO mice also exhibit less severe inflammation in an arthritis model, suggesting that ECM components participate in the inflammatory response.
An alternative approach to genetic manipulation for elucidating the role of matrix proteins during various cellular processes is exposure to agents that perturb protein-cell interactions. Such agents can include small molecules or protein-specific antibodies that will interfere with the matrix protein function. Fibronectin-binding antibody or synthetic peptides have demonstrated the importance of fibronectin-cell interactions during cell migration and normal heart development in the chick. Genetic studies have also been useful in revealing unexpected functions for certain matrix proteins. For example, ablation of the elastin gene was predicted to cause structural defects in the three-dimensional structure of blood vessels. Elastin-null animals, however, die within days of birth as a result of obstructive arterial disease characterized by proliferation of the subendothelial smooth muscle. Thus elastin exerts an unexpected growth-inhibitory role during normal vascular morphogenesis. Elastin haplo-insufficient adult mice are hypertensive, also as a result of abnormal vascular development and remodeling. Results from ECM-mutant animals therefore demonstrate the important roles ECM components play in both normal development and response to injury.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here