Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Introduction to [CR] , The Evaluation and Management of Thyroid Nodules.
Thyroid nodules are common and may be found in up to two-thirds of the population. By definition, thyroid nodules are discrete lesions contained within, yet radiologically distinct from, the parenchyma of the thyroid gland. A substantial increase in both thyroid nodule detection and thyroid cancer detection has occurred over the past three decades; this is largely attributable to increases in health care access/utilization and the use of medical imaging. Despite increased detection of thyroid nodules and malignancies, thyroid cancer mortality has remained low and virtually unchanged.
The initial workup of thyroid nodules includes clinical history, physical examination, assessment of risk factors, thyroid function tests, and neck ultrasound. Subsequent cytologic evaluation by fine needle aspiration (FNA) is guided largely by size, sonographic features, and consideration of other risk factors. Definitive surgical management is recommended based on either symptomatology or high-risk FNA classification. Diagnostic surgery is required in selected cases where cytology is indeterminate; molecular testing is assuming a greater role in guiding such cases. Although many aspects of workup and management of thyroid nodules are well described, there continues to be a high degree of variation in care.
The challenge clinicians face, as increasingly more nodules are being identified, is to determine which nodules are clinically significant, specifically those which will require intervention due to high risk of malignancy or large size with compressive symptoms. Overall, the vast majority (about 90%) of thyroid nodules will not pose any risk to the patient and will not require intervention. Herein, we discuss the evaluation and management of thyroid nodules with a focus on recent literature and updated practice recommendations.
Thyroid nodule evaluation begins with history and physical examination focused on the thyroid and cervical neck. In general, risk factors for nodules include increased age, female sex, obesity, and iodine deficiency. Nodules may be identified at differing rates depending on survey method (e.g., ultrasound versus palpation). Therefore thresholds to perform these elements or extensions of physical examination should be considered based on symptomatology or risk of malignancy. Malignancy occurs in approximately 8% to 15% of cases, and risk is similarly influenced by clinical variables, such as sex, age, and the presence of unique symptoms or examination findings. Elements of patient history that are concerning for thyroid malignancy include history of childhood irradiation to the head and neck, exposure to radioactive fallout, family history of thyroid cancer in a first-degree relative, or family history of hereditary syndromes associated with thyroid cancer (e.g., multiple endocrine neoplasia type 2 [MEN 2] association with hereditary medullary thyroid cancer [MTC]).
The strongest risk factors for differentiated thyroid cancer (DTC) are family history and prior head and neck radiation exposure. Family history of DTC is a known risk factor for thyroid malignancy. Epidemiologic evidence shows that 5% to 10% of DTCs have a familial occurrence; however, it is often unclear whether diagnosis of DTC in patients with a positive family history represents familial versus sporadic disease. Pedigrees showing one to two family members with thyroid cancer are not uncommon; evidence shows that the chance of nonmedullary DTC being sporadic is much lower when three or more family members are affected (< 6% sporadic), compared with two or fewer (62% to 69%). Syndromes associated with DTC in a first-degree relative include phosphatase and tensin homolog (PTEN) hamartoma tumor syndrome (i.e., Cowden’s disease), familial adenomatous polyposis (FAP), and Carney complex.
Thyroid exposure to ionizing radiation during childhood imparts significant risk for malignancy, because the thyroid is among the most radiosensitive organs. Importantly, radiation exposure increases thyroid cancer risk predominantly when the exposure occurs during childhood. Risk is most significant when exposure occurs at < 5 years of age, and no increased risk is observed when exposure occurs beyond 20 years of age. Further, radiation-induced cancers often harbor mutations that convey more aggressive clinical behavior, including a high prevalence of RET/PTC (reaaranged during transfection/papillary thyroid carcinoma) chromosomal rearrangement. Annual thyroid examination by palpation (though not routine ultrasound) has been advocated for survivors of childhood cancer previously treated with neck radiation.
The Chernobyl nuclear power station explosion in 1986 is a notable example of widespread radiation exposure, and the effects of this exposure on thyroid cancer risk have been well studied. It is estimated that this exposure was responsible for > 4000 new cases of thyroid cancer between 1986 and 2002 in Belarus, Russia, and Ukraine. A nearly 150-fold increase in incidence between 1986 and 1996 has been recorded in the most heavily-contaminated regions of Belarus (0.085 to 12.6 per 100,000 children/year). A similar trend was seen after the Fukushima Daiichi nuclear power plant accident in 2011.
Increasing prevalence of nodular disease is observed as patient age increases. In a large prospective cohort analysis of more than 6000 patients with more than 12,000 thyroid nodules, Kwong et al. found that the average number of thyroid nodules increased with age (1.6% annual increased risk for multinodularity); however, there was a lower risk of malignancy in newly-identified nodules with advancing age (2.2% annual decrease in relative risk of malignancy between 20 to 60 years of age). Despite the latter finding, older patients were also observed to have higher-risk histologic phenotypes. Although older patients are at increased risk for high-risk cancer, the number of individuals with high-risk cancer is low; older patients with nodular thyroid disease and additional comorbidities, such as other cancers and coronary artery disease, are more likely to die of their comorbidities.
The incidence of thyroid cancer is highest in patients age 65 and older. The balance of treatment risks and benefits is shifted in favor of more conservative care in the older patient population. Advanced age is more associated with higher rates of thyroid-specific surgical complications than historically reported in literature, with hypocalcemia and vocal fold paralysis observed in up to 13.6% and 7.1% to 9.5% of patients > 65 years, respectively. The increased risk of thyroid cancer recurrence and disease-specific mortality in older patients argues in favor of surgical management, and it is reflected in more advanced staging schema for patients ≥ 55 years in the updated American Joint Committee on Cancer (AJCC) 8th edition staging system. However, limited life expectancy, significant comorbidities, and risk of surgical complications argue in favor of more conservative treatment or observation. Therefore advanced age is an important factor when considering surgical intervention for thyroid nodules.
Thyroid cancer is three times more common in women, but men evaluated for thyroid nodules are more likely to have a thyroid malignancy. In a retrospective study of nearly 2000 nodule patients with more than 3500 thyroid nodules, the rate of thyroid cancer in men was nearly double that of women. The biological mechanism for this increased risk is unclear; however, this trend is, in part, explained by the much higher incidence of thyroid nodules identified in women overall.
When present, symptoms of thyroid nodules are related to location, size, and compression of nearby structures. Common symptoms associated with large or enlarging thyroid nodules include difficult or painful swallowing (dysphagia, odynophagia as seen in nodules in a posterior location with compression of esophagus), foreign body sensation in the throat (globus as seen with large central nodules), dyspnea, hoarseness or other voice complaints, and pain. A very rapidly enlarging nodule is also concerning for hemorrhagic nodule, high-risk malignancy such as anaplastic cancer, thyroid lymphoma, or infection.
Rapid growth of a thyroid nodule, the presence of firm neck adenopathy, or nodule fixation to surrounding structures are all associated with a higher risk of malignancy. Characterization of nodules and/or lymph nodes on examination by neck palpation is limited to rough size estimates and gross localization (e.g., right versus left lobe, superior versus inferior pole), and tactile features (e.g., firm versus soft, mobile versus fixed). Nodules that are small or posteriorly located may not be appreciated on examination. Thus thyroid/neck ultrasound is the gold standard for nodule characterization and provides additional data to drive risk stratification.
Serum thyrotropin measurement of thyroid stimulating hormone (TSH) should be included in the initial workup of known or suspected thyroid nodules ( Figure 10.1 ). Suppressed TSH may be indicative of hyperfunctioning nodules, which are associated with an exceedingly low risk of malignancy. Radionucleotide scan should be performed to identify hyperfunctioning nodules when serum TSH is low and nodules are present; this may distinguish between a solitary hot nodule, toxic multinodular goiter, or scintigraphically cold nodule(s) concurrent with Graves’ disease.
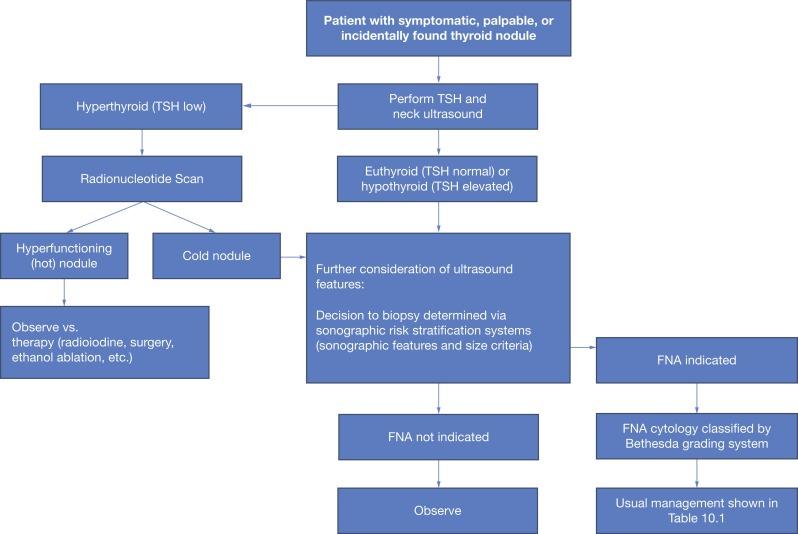
Thyroglobulin is commonly used to monitor thyroid cancer patients treated with thyroidectomy for recurrence; however, measurement of baseline thyroglobulin is not recommended as part of the workup of thyroid nodules. Likewise, calcitonin is a serum marker useful in the diagnosis and surveillance of MTC of parafollicular C cell origin; this is not routinely evaluated for thyroid nodule workup in the absence of high clinical suspicion for MTC. When there is suspicion for MTC, serum calcitonin levels > 100 pg/mL are suggestive of MTC, with higher levels being more suggestive of metastatic disease. Additional laboratory tests, such as serum calcium or 25-OH vitamin D may be included in the preoperative evaluation; however, such tests do not aid in the characterization of thyroid nodules.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here