Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Polyurethane endotracheal tube (ETT) cuffs that have high-volume/low-pressure (HVLP) cuffs can conform to the irregular borders of the tracheal lumen and, therefore, are more effective at preventing microaspiration.
ETT placement has mechanical and physiologic consequences. Vigilant surveillance of skin hygiene, airway patency, cuff integrity, and ventilatory support is necessary to minimize injury and maximize support.
Evaluation of a cuff/air leak is a multifaceted endeavor requiring vigilance, diligence, and skill. An analysis of the potential causes by patient evaluation and airway assessment should provide the needed clinical information to determine the cause and direct a therapeutic intervention.
Once an ETT is in place, efforts must be aggressive and perpetual to decrease the risk of ventilator-associated pneumonia (VAP); deploying a multi-dimensional VAP bundle incorporating the use of specially designed ETTs and advanced nursing care regimens appears warranted. VAP appears to be more multifaceted than previously believed. Subglottic suctioning and biofilm management are just the beginning steps.
It is better to investigate any perceived ETT problem electively than to deal with its consequences after it becomes an acute emergency.
The landscape of ETT design, construction, and maintenance has changed and will continue to evolve over the next decade. Not all variations will prove effective, but improved patient care will take place.
The role of the endotracheal tube (ETT) in medicine is as invaluable as that of any other medical device created to date. The establishment of a definitive airway via the ETT in both elective and emergency situations has allowed for the delivery of immediate life-sustaining therapies during resuscitation, the maintenance of oxygenation and ventilation in prolonged illness, and the (temporary) delivery of inhaled anesthesia. This chapter begins with a brief history of the development of the ETT. It describes the various types of ETTs available along with their indications for use and respective limitations. It reviews basic airway anatomy with regard to ETT placement, proper positioning and stabilization of the ETT, and complications attributed to its use. Finally, it addresses respiratory care of the intubated and mechanically ventilated patient.
When ether was introduced in the 1840s, the performance of surgical procedures accelerated in number. General anesthesia was administered using devices that covered the patient’s mouth and nose. Aspiration as a complication of anesthesia administration was not well appreciated, and postoperative pneumonia was a common problem. Trendelenburg was credited with having designed the first inflatable cuff in 1869. It consisted of a thin rubber bag fitted over the end of a tracheostomy tube with the goal of providing a tight seal of the tracheal lumen to prevent aspiration under anesthesia.
In 1893, Eisenmenger first described the use of a cuffed tube coupled with the concept of a pilot balloon to monitor intracuff pressure. The precursor for the modern ETT was developed in 1917 by Magill and Rowbotham, who manufactured them from rubber for the purpose of administering anesthesia. In 1928, when Guedel and Waters added a protective cuff to prevent aspiration, the modern ETT was born. During the polio epidemic in the 1960s, the potential value of cuffed tubes for application of positive-pressure ventilation in respiratory failure was appreciated. Early ETTs made of red rubber had limitations in this application, however, such as increased stiffness with rising temperature and limited adhesive properties with different polymers, requiring the cuffs to be manufactured from the same polymer as the tube. These shortcomings led to the search for alternative materials.
In 1967, polyvinyl chloride (PVC) was popularized by Dr. S. A. Leader, and it has since been the material most used. One property that makes PVC attractive is that it provides stiffness to an ETT at room temperature to assist with intubation yet becomes more malleable as its temperature increases in vivo. Other beneficial properties include the ability to embed radiopaque stripes in the material to assist with positioning and recognition on a radiograph. The low cost of PVC and its compatibility with many different materials provides distinct advantages. It affords manufacturers the opportunity to exteriorize the inflation line and pilot balloon assembly to the PVC ETT with a variety of cuff materials.
The simple yet clever design of the 15-mm adapter allows for universality between ventilating devices such as a bag-mask ventilation system, anesthesia circuit, or ventilator circuit. The adapter fits ETTs as large as 12-mm internal diameter (ID) and as small as 3 mm ID, thereby providing further commonality among multiple ETTs and ventilating devices. Having one standard size also allows for interchange between devices made for tracheostomies or ETTs. The adapter is removable to allow for passage of intraluminal devices (e.g., a bronchoscope or suction catheter) or to allow passage of the ETT via a supraglottic airway (SGA). Adapter removal may facilitate the extraction of extensive biofilm accumulation or mucous plugs. Additionally, removal and reattachment of the adapter allows the clinician to resize (shorten) the ETT.
The Murphy eye, an elliptically shaped opening in the distal end of the ETT, is designed to provide an extra (secondary) portal for ventilation should the most distal lumen become blocked by bodily fluids, foreign bodies, or soft tissue. The most typical manifestations of this phenomenon occur when the distal lumen abuts the soft tissue of the tracheal tree or when secretion buildup occludes the distal opening. Despite its advantages and useful design features, its presence may allow an airway exchange catheter or bronchial blocker to travel astray with the potential for patient harm.
The cuffs on early ETTs were, like the tubes themselves, composed of rubber. Rubber ETT cuffs had limitations, such as the need for elevated inflation pressures (high-pressure, low-volume [HPLV]) to fill the cuff and occlude the airway surrounding the ETT. The cartilaginous U- or D-shaped trachea with a softer, flattened posterior wall is not properly shaped to be completely sealed with a round or ovoid cuff. HPLV cuffs inflate in a circular manner, thereby altering the structure of the trachea; the high pressure exerted on the tracheal wall, typically greater than 30 cm H 2 O, impairs capillary perfusion pressure and, thus, blood flow, which may result in mucosal ischemia. The use of HPLV cuffs has diminished significantly. The ETTs with HPLV cuffs used in current practice are primarily the silicone ETTs used with intubating laryngeal mask airways (LMAs). Caution should be exercised when using these ETTs for prolonged periods; given their inherent risk of tracheal mucosal damage, strong consideration should be given to exchanging the HPLV ETT, unless the time of intubation is projected to be brief.
The introduction of PVC-based cuffs has reduced this problem because of a thinner and more supple cuff wall, allowing the cuff to accommodate high volume under low pressure (HVLP), and thus providing an adequate seal with lower lateral wall pressures ( Fig. 44.1 ). , , The main value of the HVLP cuff is its ability to better conform to the irregular borders of the trachea. Polyurethane is thinner (10 μm vs 50 μm for PVC tubes) and more pliable with increased tensile strength, allowing for higher volumes, larger contact areas, and minimal mucosal pressures. Foam-based cuffs provide maximal conformation to the tracheal walls, but, unfortunately, they do little for the prevention of microaspiration.
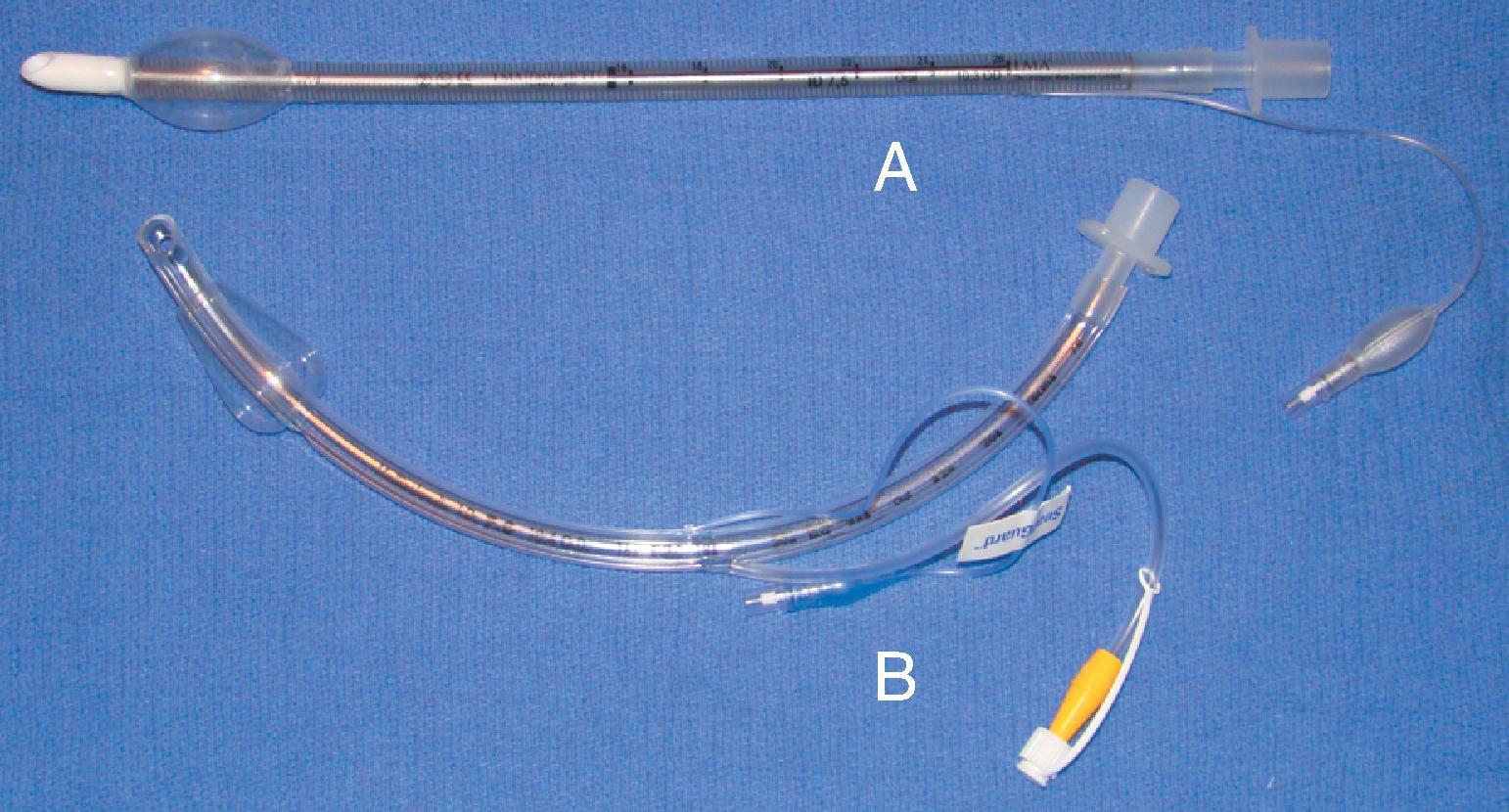
Remodeling of the cuff has been particularly driven by the desire to improve prevention of ventilator-associated pneumonia (VAP), and, as such, the shape of the cuff has also been altered. The Mallinckrodt TaperGuard Evac ETT (Medtronic, Minneapolis, MN) has a conical-shaped (tapered) cuff and has been demonstrated in randomized, controlled trials to reduce microaspiration by as much as 83% compared with traditional HVLP barrel-shaped cuffs ( Fig. 44.2 ). It is postulated that a barrel-shaped cuff tends to wrinkle and fold in an attempt to conform to the tracheal wall, creating small channels that allow for potential microaspiration of nasooropharyngeal secretions, debris, or gastroesophageal reflux-mediated aspiration, whereas the bulbous, conical shape of the TaperGuard Evac ETT may reduce wrinkling and thus decrease the incidence of microaspiration. A recent meta-analysis revealed that the use of tapered ETT cuffs alone did not reduce the incidence of VAP. Continued work in this area may lead to improved tracheal wall sealing capabilities at safe levels of pressure while minimizing potential pathways for the translocation of nasooral and gastroesophageal secretions, which is thought to be the primary etiologic pathway for VAP. An additional feature of the TaperGuard Evac ETT is subglottic drainage of secretions. This is accomplished by applying low wall suction to the separate dorsal suction lumen located just above the cuff via a separate suction line assembly. Utilization of this type of ETT is associated with a lowering of VAP incidence but does not clearly provide benefit regarding a reduced duration of mechanical ventilation, length of stay, antibiotic usage, or mortality in the intensive care unit (ICU).
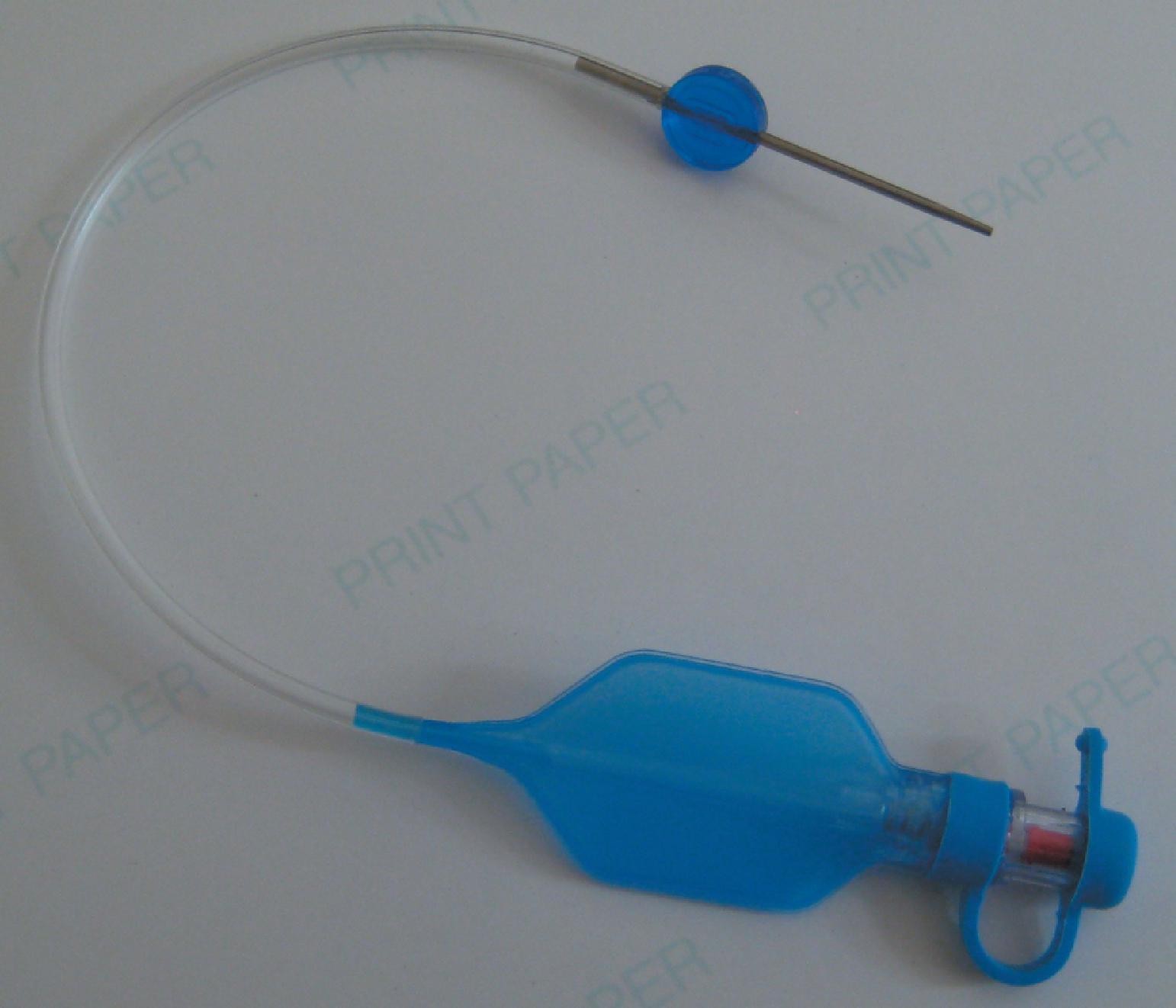
The pilot balloon of an ETT functions as an indirect volume gauge for the ETT cuff, relative to the amount of air located in the cuff (inflated or deflated). Manual assessment of the pilot balloon does not provide accurate information about the absolute volume insufflated or the pressure exerted on the tracheal mucosa except when the pilot balloon is firm (high pressure, e.g., >60 cm H 2 O) or when underpressurized or nearly collapsed (low pressure, e.g., <10 cm H 2 O). Use of a manometer is recommended for accurate cuff pressure assessment. When a pilot balloon assembly fails (balloon and/or tubing), options are generally limited to ETT exchange or bedside repair. Pilot balloon assembly failures have multiple causes: shearing along the ETT connection (usually because of contact with dentition), cracked inflation valves (from syringe manipulation or trauma), material aging, or pilot tubing laceration attributed to biting, among others. Simple techniques have been described to replace a pilot balloon in a variety of clinical situations using equipment readily available in the operating room (OR) or ICU setting. Needles or intravenous catheters with stopcocks or Luer connectors, epidural clamp connectors, and commercially available repair kits ( Fig. 44.3 ) provide reliable substitutions for an incompetent pilot balloon assembly. , The procedure for replacing an incompetent valve if a commercially available repair kit is not available is as follows: cut the inflation tube; insert a needle or intravenous catheter into the cut end (or affix the hub of an epidural catheter to the cut end); use a stopcock or Luer adaptor on the needle or catheter after insufflation; and evaluate the cuff pressure with a manometer. This option is best for the weaning patient in anticipation of extubation; otherwise, formal ETT exchange is recommended.
The purpose of the ETT has always been the same, and it has always had the same inherent problems. Technology continues to advance the standard ETT for improved function and decreased physiologic insult. Rather than compensate for the resistance produced by a rubber or PVC ETT, an ultra-thin polyurethane ETT that is reinforced with wire to resist collapsing and kinking is available. The wire reinforcement is unique in that it has an elastic shape memory to prevent deformation. The ID is increased without compromising the rigid shape of the ETT. The result is a tube with a resistance similar to that of the upper airway that is lighter, offers less airflow resistance, and, when compressed, forms an egg shape rather than an oval. Experimentally, this new design has been shown to decrease inspiratory and expiratory resistance by 60% each and the inspiratory, expiratory, and total work of breathing (WOB) by 70%, 47%, and 45%, respectively. , Unfortunately, the wire-reinforced ETT may undergo crimping or kinking due to acute bending or patient biting. Such extreme damage will not recoil, and the luminal diameter will not return toward its original caliber.
Use of the ETT continues to expand—no longer is it expected to be simply a conduit for ventilation. As the technology has advanced, the original ETT has steadily been outfitted with a host of successful innovations to improve patient care, whether for convenience or necessity. For example, modifications to the cuff to improve occlusion of the trachea in an effort to prevent microaspiration, coupled with an extra subglottic suctioning port (and other patient care maneuvers), have served to vastly reduce the incidence of VAP. , Another example is the modification of the surfaces of the ETT to minimize bacterial adhesion and thereby minimize biofilm accumulation. As for bells and whistles, there are ETTs with fiberoptic cameras at the distal tip, allowing for ease of placement and the possibility of continued intratracheal surveillance. Another example is the addition of multiple sensors, for so-called bioimpedance cardiography, that are capable of monitoring stroke volume variation, cardiac output (CO), systemic vascular resistance, and arterial pressures (because of the proximity of the ETT and the aorta) and thereby, at least theoretically, preventing the need for additional invasive technologies. Continued study of these modifications may provide justification to adapt these technologies to patient care, with the potential of limiting physiologic insult and reducing iatrogenic complications (e.g., from central line placement or radial arterial catheterization in the example of an ETT equipped for bioimpedance cardiography).
The placement of an ETT, whether oral, nasal, or translaryngeal, is unnatural. Certain physiologic changes occur that must be addressed, including those created by the ETT itself and those modified by its presence. The properties inherent to the ETT are relatively obvious: It causes a partial obstruction, resulting in a decrease in the normal airway circumference, and creates the potential for turbulent air flow patterns. Additionally, the narrowed conduit leads to higher pressures concurrent with lower flows because of the reduced airway diameter; higher pressures may lead to mucosal damage distally, particularly at points of turbulence or obstruction. The presence of an ETT is a trigger for the inflammatory cascade; despite its relatively hypoallergenic profile, it is still a recognizable foreign body and, as such, triggers well-defined host responses. Placement of the device, regardless of the care used in placing it, may result in mechanical trauma and therefore decreases the ability of the respiratory mucosa to protect itself. Finally, the ETT may cause airway alterations secondary to pressure injury, either caused by ETT translational pressures from resting against the mucosa or by turbulent flow patterns. Ventilatory strategies employed because of ETT placement may also lead to a triggered inflammatory response.
The body’s response to the ETT is also multifaceted, affecting mechanics, structure, and physiologic function. Loss of humidity and heat is the most obvious effect of replacing the regular mucosa with a foreign conduit. Bypassing the patient’s natural ability to warm and humidify the incoming air may lead to problems in the distal tracheobronchial tree. The delivery of dehumidified and cool gases may reduce ciliary function, thicken secretions, and increase mucous plugging. The normally motile respiratory cilia are essentially paralyzed and rendered dormant, leading to impaired secretion management. The body lacks its normal ability to move debris in a proximal (cranial) direction, and collection sites develop within the tracheobronchial tree, leading to multiple potential areas for infection. Additionally, these partially or completely occluded areas may result in lobar collapse and, consequently, a ventilation-perfusion mismatch. Obstructions caused by mucous plugging can also create an inability to completely exhale, leading to breath stacking and auto-positive end expiratory pressure (auto-PEEP) and possibly resultant barotrauma.
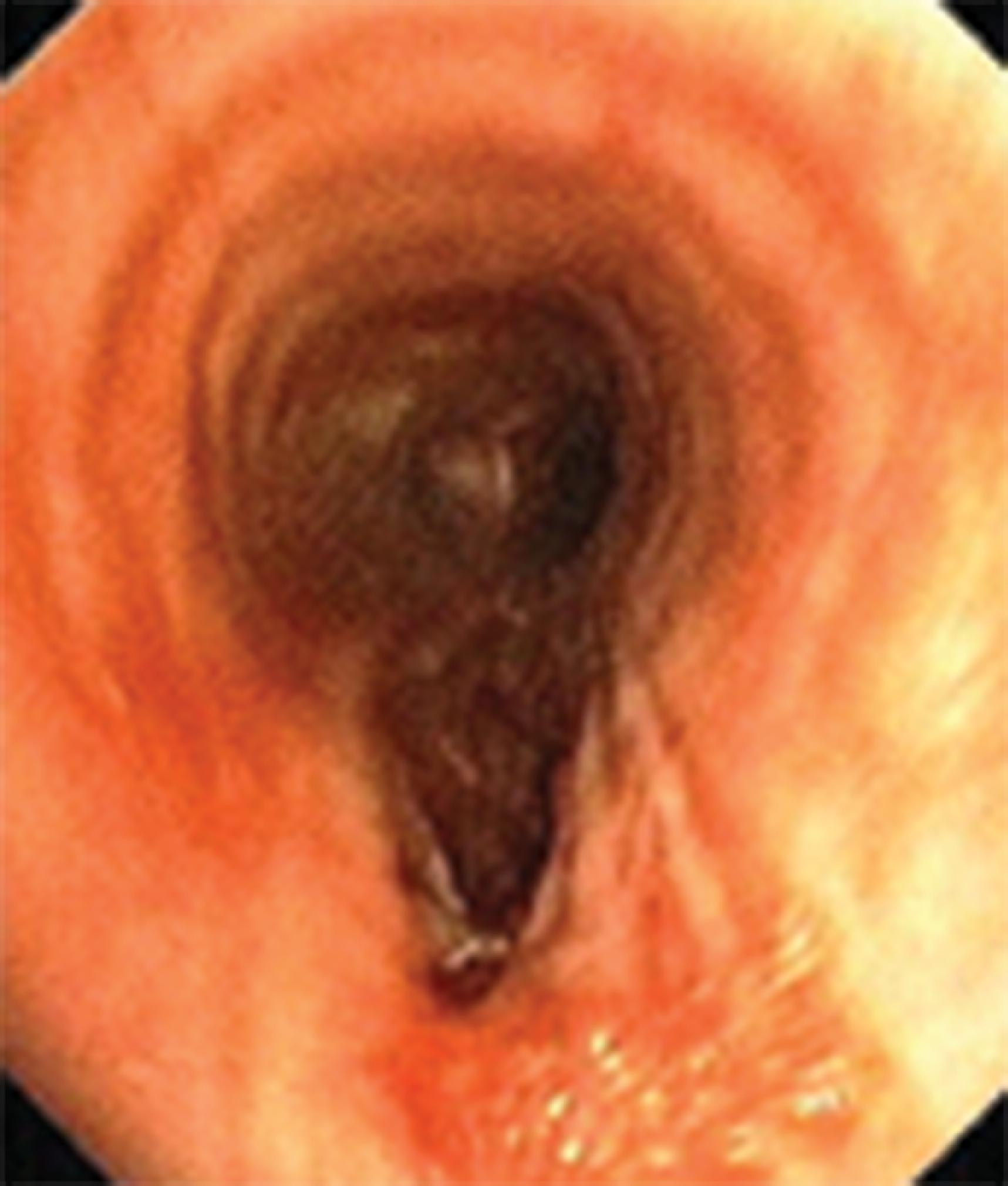
Complications associated with ETT placement can be grouped into three major subcategories: those that occur at intubation, those that occur with the ETT in situ, and postextubation sequelae. The problems associated with ETT placement are numerous and can be worsened because of emergency situations, inherent physiologic or anatomic complications leading to multiple attempts, the use of a variety of devices, operator inexperience, and patient-related anatomic factors. Problems at placement include dental and oral injury, maxillofacial damage, displacement of the arytenoid cartilages, vocal cord ulceration or dysfunction, airway perforation ( Fig. 44.4 ), autonomic hyperactivity, and, of course, failed intubation. Structural damage is unlikely to be repaired until the patient no longer requires intubation, unless the damage interferes with ventilation and oxygenation or leads to a life-threatening situation such as esophageal or tracheal rupture.
Problems that occur as a result of an in situ ETT include those caused by the ETT itself, such as aspiration; vocal cord paralysis or transient nerve palsy; ulceration and granuloma formation in the trachea or on the vocal cords ( Fig. 44.5 ); tracheal synechiae; subglottic stenosis ( Fig. 44.6 ); laryngeal webbing; tracheomalacia ( Fig. 44.7 ); tracheoesophageal, tracheoinnominate, or tracheocarotid fistula; and recurrent laryngeal or superior laryngeal nerve damage. Overpressurization of the ETT cuff contributes to these maladies, as can head and neck position and movement or forces from the ventilation circuit causing ETT angulation. Other complications are related to mechanical ventilation facilitated by the ETT and include aspiration, barotrauma (e.g., pneumothorax or pneumomediastinum), VAP, and ETT dislodgment.
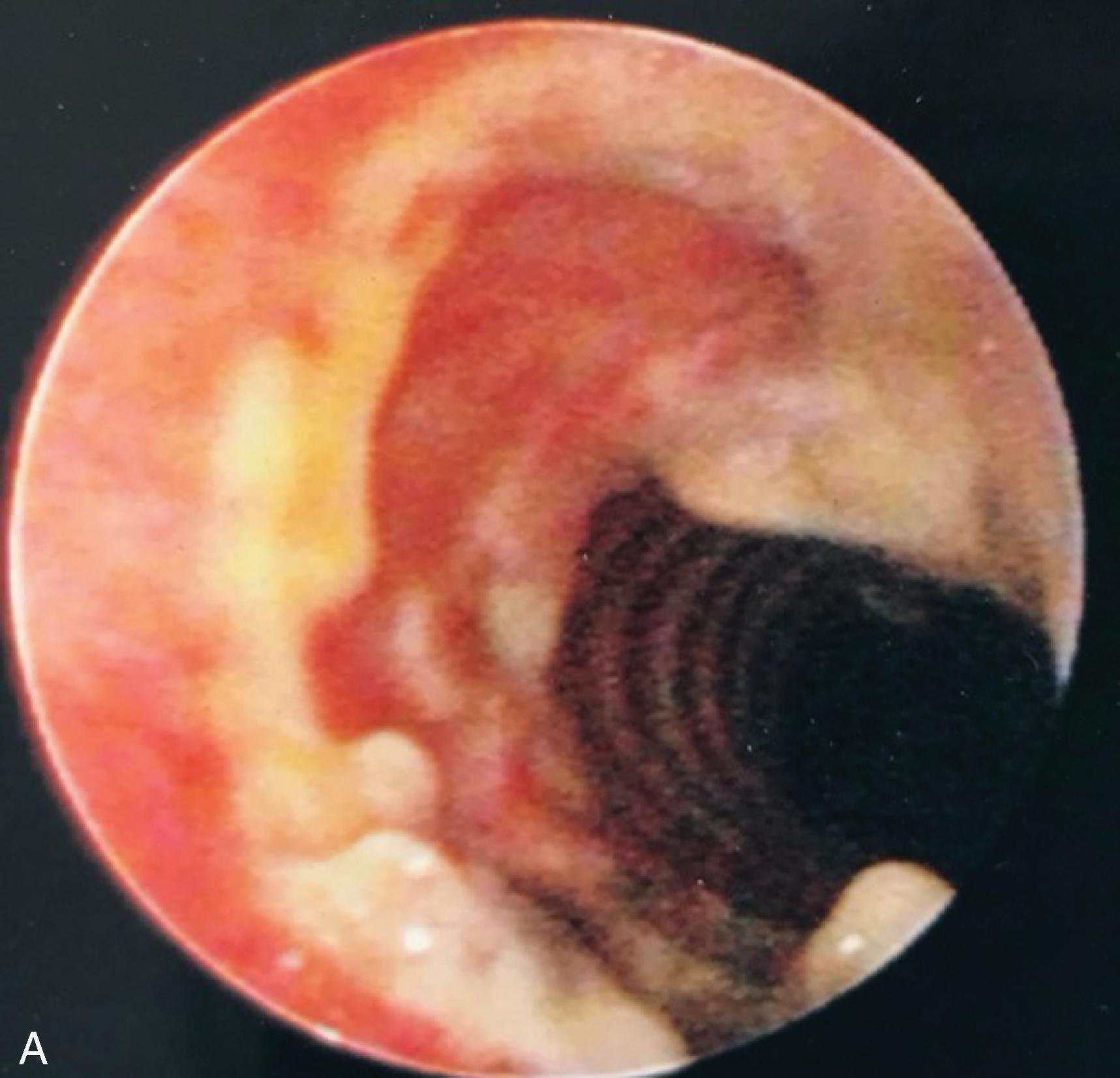
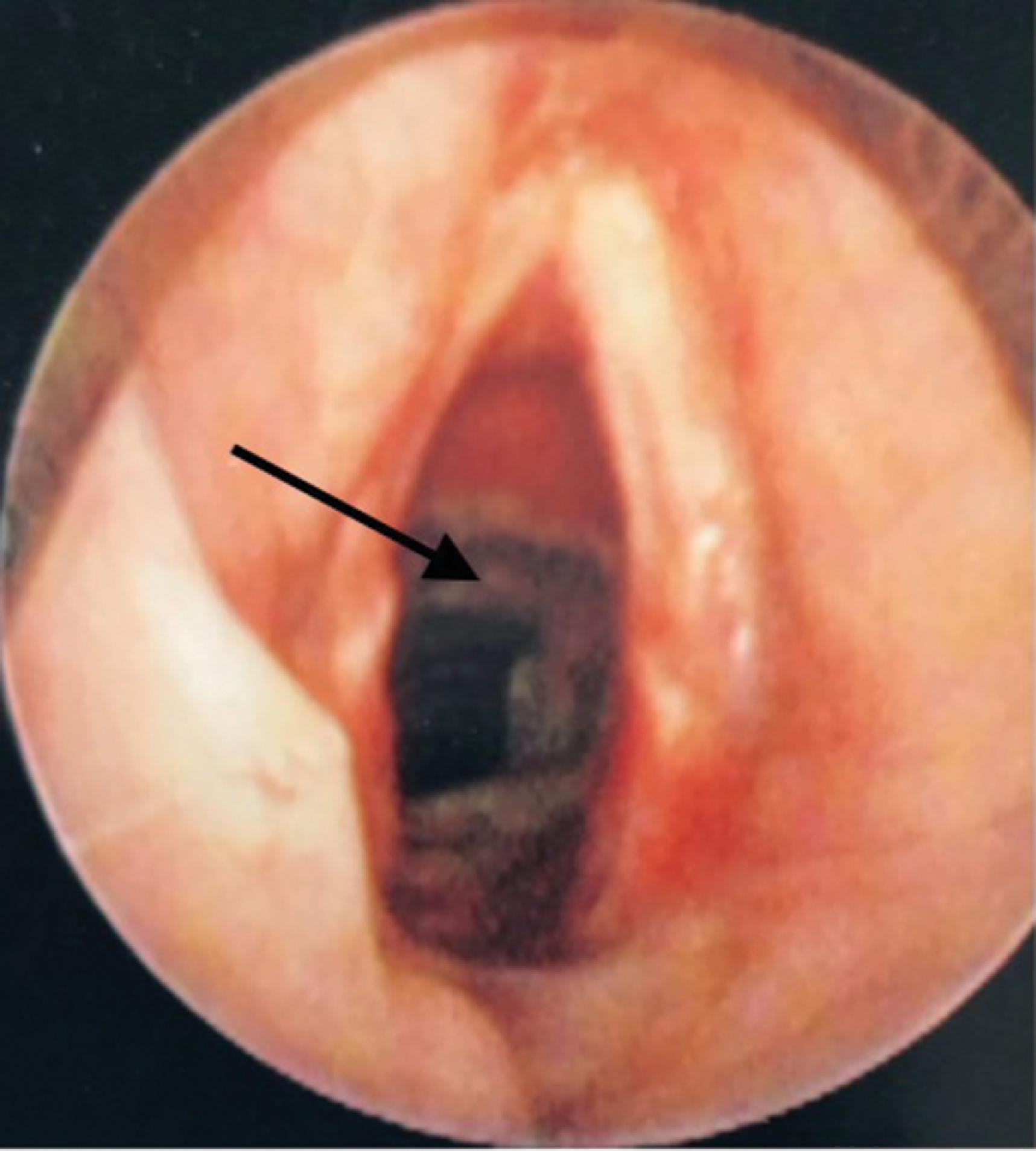
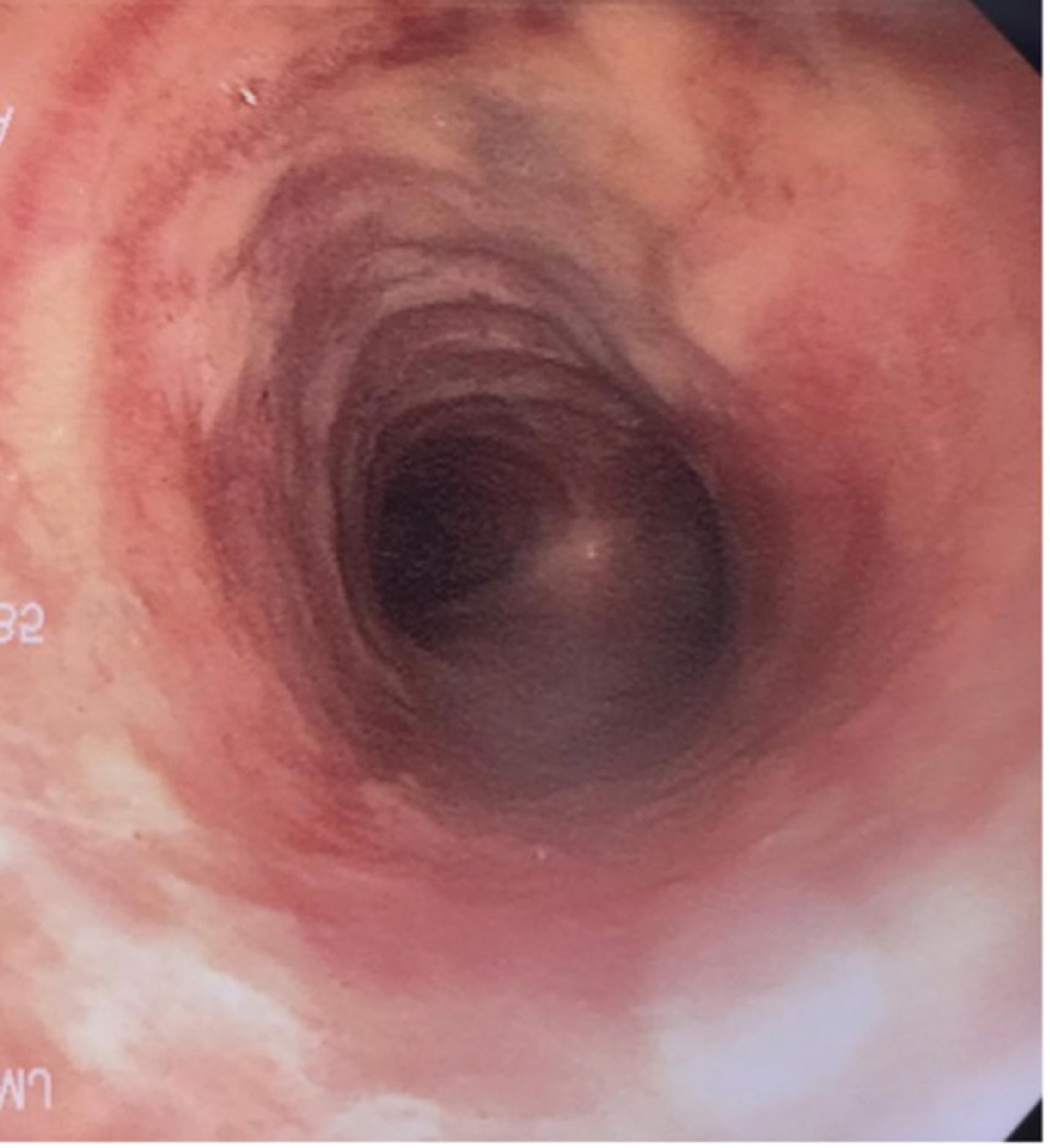
Finally, postextubation complications can lead to long-term morbidity or the urgent need for reintubation. Many of the postextubation culprits have already been encountered as complications of ETT placement or presence, particularly subglottic stenosis, vocal cord injury, and hoarseness. , Without a doubt, the most common postextubation issue is acute respiratory failure and the subsequent need for reintroducing invasive airway access.
Postextubation dysphagia is a common but often unrecognized issue in the critically ill patient requiring intubation for greater than 48 hours. Dysphagia associated with speech and swallow discoordination is linked to aspiration risk. Periglottic edema, ulceration, granulation tissue formation, scarring and vocal cord dysfunction may accompany intubation, particularly if extended in duration. Autopsy evaluation supports the nearly ubiquitous presence of laryngotracheal injury, to various degrees, in the majority of patients who experience prolonged tracheal intubation.
Tracheal stenosis, an abnormal narrowing of the tracheal lumen, is related to both tracheal intubation and creation of a surgical airway. Narrowing typically occurs at the site of the ETT cuff (or malpositioned ETT tip). Following mucosal denudation and injury, local inflammation and infection may lead to chondritis of the anterior and lateral tracheal walls. Vascular granulation tissue may develop at the injury site and impede airflow or contribute to hemorrhage during reintubation or ETT manipulation. Granulation tissue undergoes fibrosis and epithelialization followed by stenosis of the cartilaginous walls. Common risk factors include prolonged intubation, traumatic intubation, hypotension, sepsis, gastroesophageal reflux, advanced age, malnutrition, debility, inappropriately sized ETTs (too large or small), and excessive tracheal wall irritation from ETT motion.
Excessive cuff pressure, even for as short as 15 minutes, may initiate a cascade of compromised tracheal mucosal blood flow leading to ischemia or necrosis. Left unchecked, as in the ICU setting, webbed fibrotic changes, cartilaginous wall injury, and contracture may occur within 3 to 6 weeks. However, an even shorter duration overpressurization (e.g., in the OR) may contribute to dysphagia, altered phonation, coughing, blood-tinged sputum, and stridor. This may be worsened when accompanied by anterior tracheal wall damage from aggressive advancement of a styletted ETT with a hyperangled configuration. The basis of the ischemia is overpressurization of the cuff (>25–30 cm H 2 O) as it relates to the average mucosal capillary perfusion pressure (≈27 cm H 2 O). Ideally, cuff pressure should be maintained <25 cm H 2 O. HVLP cuff designs for both ETT and tracheostomy tubes have improved this undesirable consequence of airway control but not eliminated it. Head position and positive-pressure ventilation will alter the intracuff pressure as will N 2 O administration in the OR. Shearing forces from the ETT or its cuff may further aggravate injury. ETT cuff pressure is also directly related to peak inspiratory pressure during mechanical ventilation support. Even if cuff pressures are initially within recommended limits, increases in peak inspiratory pressure may result in an increase in cuff pressure.
The degree of local or circumferential contracture leading to tracheal luminal reduction varies widely and may remain clinically underappreciated and underreported in many patients. Dyspnea at rest or stridor may not be noted until the tracheal narrowing is <5 mm. Therapy for symptomatic tracheal narrowing is typically provided in a stepwise fashion based on the degree of narrowing and its response to one of many therapies. , Often, evaluation and diagnosis are performed with flexible scope assessment in the ICU, clinic, or office, and operative intervention may be indicated. Diagnostic and therapeutic rigid bronchoscopy alone or combined with serial tracheal dilatation may be successful. Moreover, stenting of the narrowed tracheal segment may be performed as a temporary intervention. If successful, the stent may be upsized or removed. If narrowing recurs, a permanent stent may be the best option. For patients with an acceptable medical/surgical history, surgical resection of the stenotic segment and tracheal reconstruction are considered the ideal management of postintubation tracheal stenosis since the development of safe surgical techniques. , As an alternative, the higher-risk, frail, or debilitated patient may be better candidate for serial dilatation with or without stenting.
Tracheomalacia, a weakening of the tracheal wall often accompanied by dilatation or compression, results from ischemic injury to the trachea following intubation or tracheostomy (see Fig. 44.7 ). Tracheal wall injury leading to chondritis may enhance destruction and necrosis of the supporting tracheal cartilage. Respiratory symptoms result from tracheal wall weakening and its collapse during expiration. Clinically, air trapping, failure to tolerate weaning of PEEP, limitation of expiratory airflow, and retained secretions may complicate patient care. FB may allow visualization of the expiratory collapse of the trachea. ,
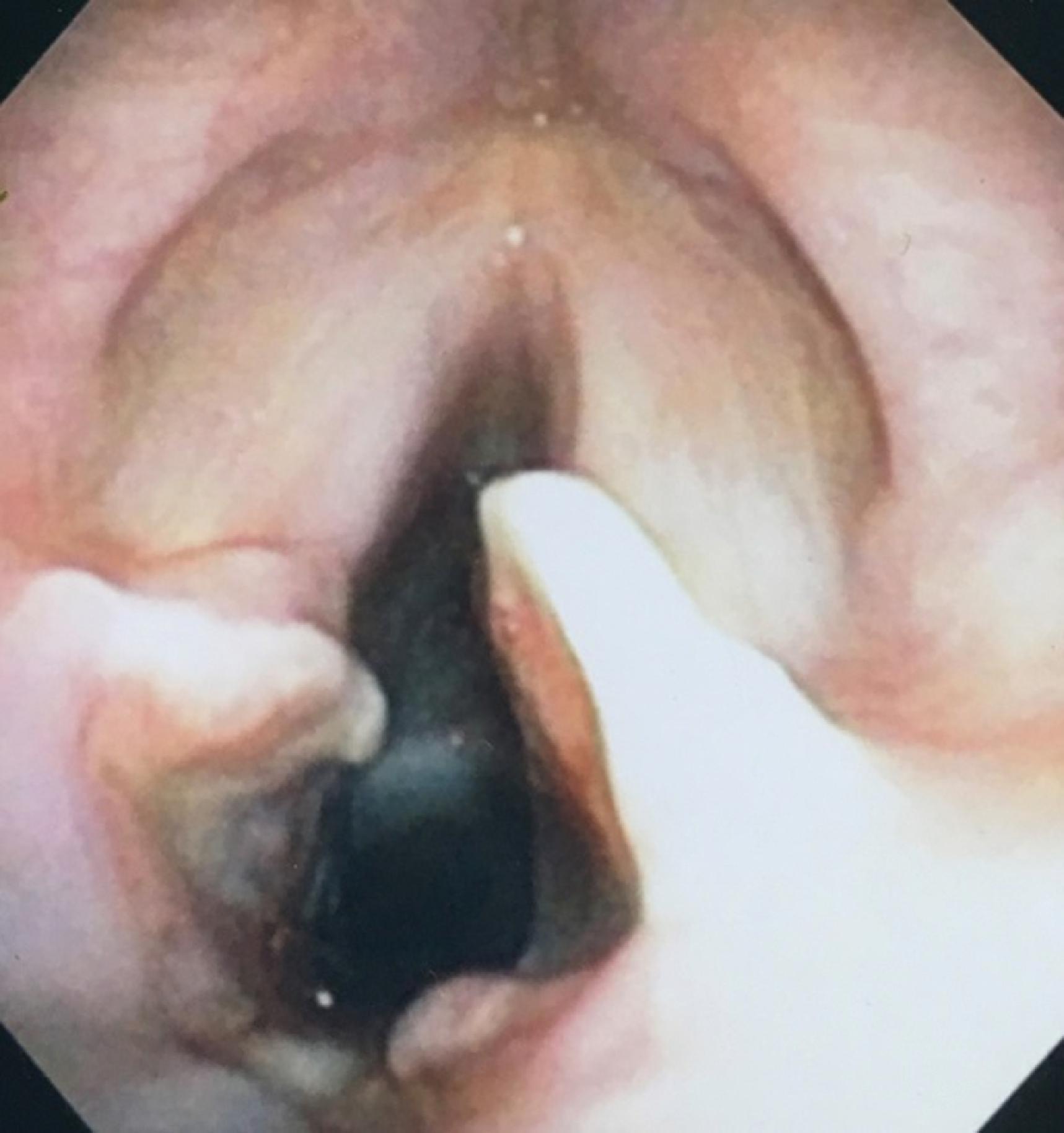
Intolerance of the extubated state has many confounding factors and etiologies. Marginal cardiopulmonary reserves or preexisting medical/surgical conditions combined with acute and chronic disease are prominent causes of extubation/decannulation failure. However, the presence of tracheal wall injury can be a hidden contributing factor to such intolerance that may not be appreciated until the patient undergoes reintubation or the tracheal stoma is recannulated. Even then, the formation of granulation tissue, luminal narrowing, scarring, tracheomalacia, or tracheal stenosis may not be appreciated until several extubation/decannulation failures prompt more detailed scrutiny of the airway. HVLP cuff designs have led to significant reduction, but not elimination, of tracheal wall injuries. Moreover, maintaining the cuff pressure in a safe zone (<25 cm H 2 O) has a prime role in decreasing the rate of ischemic injuries and postextubation stenosis. Unfortunately, the ETT itself acts as a foreign body exerting variable but persistent pressure on sensitive tissues. This contributes to periglottic edema and additional sites of laryngeal injury (e.g., true and false vocal cords, arytenoid cartilages, aryepiglottic folds, and posterior commissure) ( Fig. 44.8 ). , These, too, may contribute to postextubation respiratory distress, stridor, dysphagia, phonation difficulties, hoarseness, vocal cord dysfunction, and laryngeal incompetence. Laryngeal maladies include edema, ulceration, granulation tissue, and abnormal vocal cord mobility, paresis, or paralysis. Risk factors appear to be prolonged intubation duration, female gender, emergency intubation, difficulty with airway management, multiple attempts at intubation, self-extubation, and a smaller height:ETT diameter ratio. ,
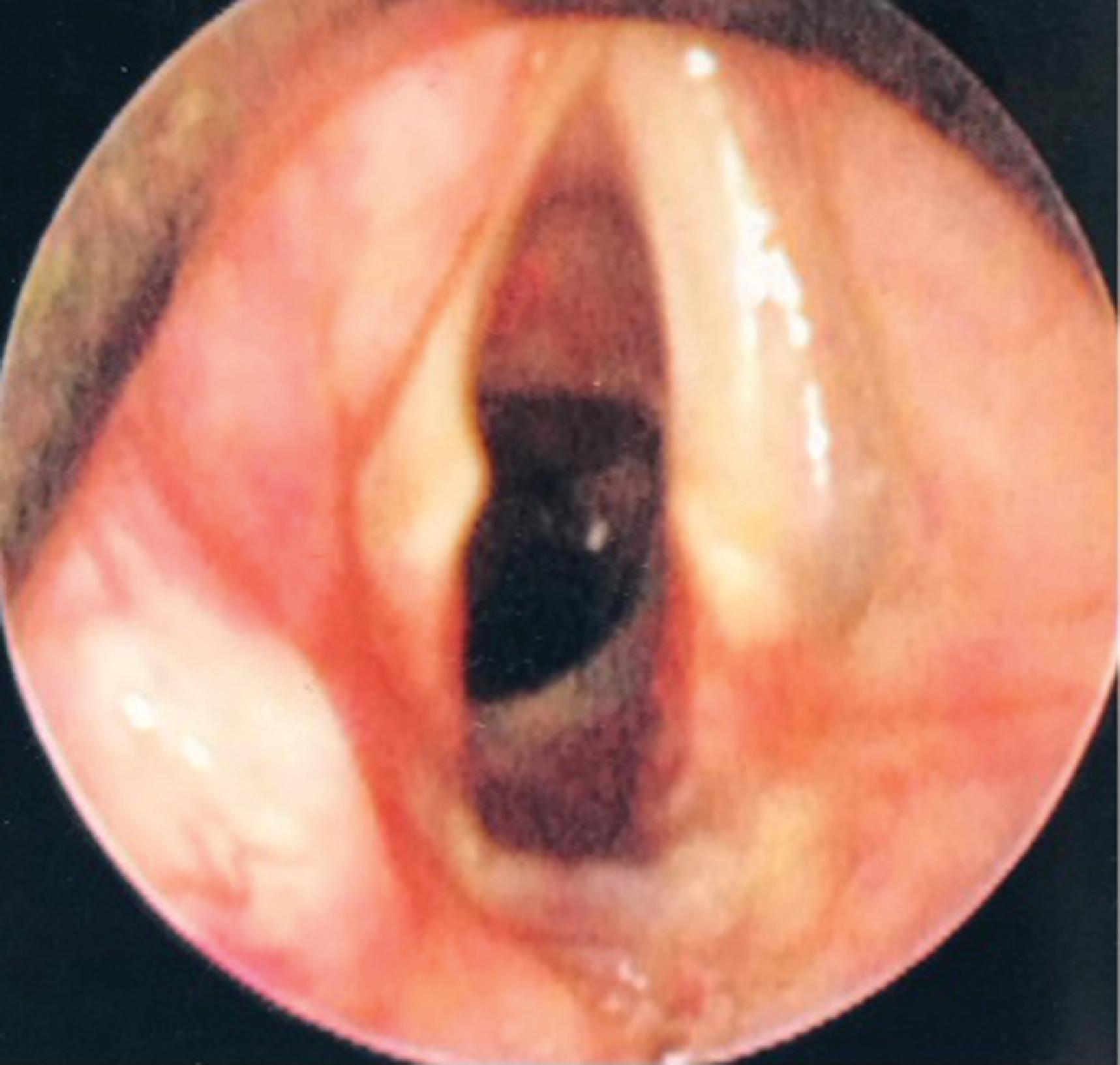
Unilateral or bilateral glottic scarring may lead to glottic stenosis. Arytenoid immobility due to scarring or dislocation may complicate prolonged or difficult intubation. Phonation difficulties, hoarseness, and impaired swallowing with an increase in aspiration risk postextubation may be rooted in such laryngeal injury. Air hunger and airway obstruction may be present in severe cases. If postextubation phonation difficulties or hoarseness persist, evaluation is encouraged. A delay in assessment may allow further scar tissue formation that could endanger or limit future therapy.
Indirect injury to the branches of the recurrent laryngeal nerve from a high-lying (subglottic), overpressurized ETT cuff compressing the internal nerve branch as it traverses the larynx at the level of the thyroid and cricoid cartilages is, fortunately, uncommon. Vocal cord paresis or paralysis may be based on similar factors that increase the risk for other laryngotracheal injuries (e.g., duration of intubation, cuff location and pressure, ETT size and curvature), as well as advancing age, vascular disease, hypotension, sepsis, and other potential low-perfusion states. Separating out individual causative factors may be difficult.
Given the variety of airway-related maladies that may afflict patients who have required tracheal intubation or placement of a surgical airway, careful evaluation with an elevated index of suspicion is warranted. Employing bronchoscopy, tracheoscopy, nasopharyngoscopy, or VAL at the bedside or the OR may be helpful. Defining and documenting any periglottic or tracheal pathology may shed light on weaning intolerance, failed decannulation, swallowing disorders, phonation difficulties, and tracheal pathology. Bedside video-assisted swallowing evaluations may provide valuable insight. Bedside flexible endoscopy may offer empiric evidence that the patient may benefit from a more thorough evaluation in the OR (e.g., rigid bronchoscopy or suspension laryngoscopy) with the opportunity for biopsy, excision, electrocautery, cryoablation, glottic injections, stenting, or dilatation of uncovered airway abnormalities. Ultra-high resolution computed tomography imaging or swallowing studies may render useful information. Spirometry, while limited in its application in the ICU setting, may provide evidence of obstruction and restriction of air flow.
It is imperative that patients receive assessment and therapy for postextubation disorders. Speech-language pathologists (SLPs) deliver clinical services for a broad range of disorders, including swallowing, speech, and voice disorders so prevalent in postintubation and tracheostomy patients. Moreover, stroke, traumatic brain injury, Parkinson disease, dementia, or instrumentation or trauma of the oropharynx, esophagus, and/or trachea may lead to brief, intermediate, and long-term consequences related to swallowing, speech, and voice integrity. The muscles and nerves that regulate speech, voice, and swallowing via the lips, tongue, pharynx, and larynx are intimately interlinked, thus providing coordinated choreography for swallowing, breathing, and phonation. Postintubation and posttracheostomy alterations deserve individualized screening tools, bedside swallowing evaluations, and assessment of hyolaryngeal movement and swallow coordination by hands-on midline palpation of the larynx. Intubation and tracheostomy placement may disrupt the essential biomechanical constructs of the three-way glottic closure mechanism directing foods and liquids to the esophageal portal (i.e., true and false vocal cord adduction and a downfolding epiglottis). Advanced diagnostic testing beyond a modified barium-swallow study is termed “instrumental studies” and is now the gold standard for assessment of such disorders. Instrumental testing may include pH manometry, ultrasonography, and fiberoptic endoscopic evaluation of swallowing (FEES) ( Fig. 44.9 ). Box 44.1 presents excerpts from SLP evaluations demonstrating their broad scope of practice in the postextubation and tracheostomy patient.
Patient A: 70-year-old female with diabetes mellitus, multiple hospitalizations and intubations for hypoxic respiratory failure (most recently discharged 5 days ago after intubation for pneumonia), on 2 to 3 L/min of nasal cannula oxygen at baseline. The patient presented to emergency department for right-sided back pain and hypoxia. Speech/language pathologist recommended an MBS to formally rule out silent aspiration vs. evaluate for reflux aspiration given history of gastroesophageal reflux disease. MBS revealed functional oropharyngeal swallow mechanism with no aspiration across consistencies. Oral phase characterized by intact bolus manipulation, cohesion, and transit across trials with no oral residue postswallow. Good bolus coordination observed across consistencies. Pharyngeal motor response was timely, consistently initiating at the level of the ramus. Intact hyolaryngeal elevation/excursion resulted in complete epiglottic inversion and laryngeal closure. The patient maintained adequate airway protection across trials with no airway compromise across consistencies. Functional base of tongue retraction and pharyngeal stripping wave present, with complete pharyngeal clearance and no stasis postswallow. Adequate bolus flow from the pharynx into proximal esophagus. Of note, upon transferring patient back to her bed, she had significant coughing with expectoration of thick secretions and barium-tinged contrast likely indicative of esophageal dysphagia and concern for reflux aspiration.
Patient B: The patient evaluated 4 hours postextubation (two emergency intubations, total of 7 days intubated). Seated upright in bed on 3 L/min of nasal cannula oxygen. Alert and oriented to person, place, and time. The patient was severely dysphonic and displayed a weak volitional cough. Clinical swallow evaluation was concerning for pharyngeal dysphagia (weak reflexive coughing observed with trials of ice chips and mildly thick liquids). Recommend continued NPO status except crushed meds and ice chips. Plan for FEES. FEES completed with patient alert and seated upright in bed. On 4 L/min of nasal cannula oxygen, pulse oximetry was 92% to 93% at baseline. Study demonstrated a functional oropharyngeal swallow. Adequate labial seal, bolus control, and mastication/manipulation with complete oral clearance. Timely swallow initiation with clear liquids only. Functional epiglottic inversion with normal-appearing whiteout. Functional pharyngeal stripping with minimal postswallow vallecular residue. Moderate amount of thin secretions pooled in pyriform sinuses. Significant glottic posterior gap from prolonged endotracheal tube presence (see Fig. 44.9 ). True vocal cords showed reduced movement bilaterally with posterior glottic gap visualized during phonation. High risk for aspiration due to periglottic edema and residual gap opening in posterior glottis. Abnormal airway protection with penetration and aspiration visualized. Of note, intermittent coughing was observed throughout the exam. The patient desaturated to 72% on pulse oximetry during consumption of ice chips and clears. Recommended initiation of a limited diet (ice chips only) and implementation of aspiration precautions. Consider otorhinolaryngologic consult to evaluate vocal cord dysfunction, glottic edema, and the glottic gap.
FEES, Fiberoptic endoscopic evaluation of swallowing; MBS, modified barium swallow; NPO, nothing by mouth ( nil per os )
In selecting an ETT, consideration must be given to the functional reason for placement, as well as patient-specific factors, such as body height, gender, airway integrity, airway pathology, and previous airway manipulation or instrumentation. Theoretically, short-term placement for anesthesia should be different from placement for prolonged support with mechanical ventilation or for fiberoptic bronchoscopy to aid therapy. Generally, the trachea of an adult female accepts a tube of 7.5- to 8.0-mm ID and that of a male accepts one of 8.5- to 9.0-mm ID, but typically a 7.0-mm ID ETT is used for females and an 8.0-mm ID tube is used for males, at least in the United States. It is also generally accepted that an ETT of at least 8.0-mm ID is needed for an adequate adult bronchoscopic investigation.
The physics of laminar gas flow through a conduit is described by the Hagen-Poiseuille equation, which reflects the relationship of resistance varying inversely with the fourth power of tube radius. Air flow through an ETT is often turbulent, which leads to increased airway resistance. The net effect on the increase in airway resistance with each millimeter decrease in ETT diameter is considerable, ranging from 25% to 100%. Airway resistance is affected by more than tube diameter; the presence of secretions within the tube, ETT kinking, and positioning of the head and neck may also increase the tendency for turbulent flow. The fundamental principle to be mindful of is that airway resistance induced by an ETT is inversely proportional to the tube size.
Airway resistance increases with decreasing ETT diameter whether because of internal occlusion, smaller size, or external compression. As airway resistance increases, WOB also increases. The increase in WOB associated with a 1-mm reduction in ETT diameter varies in accordance with tidal volume and respiratory rate at a given minute ventilation and can range from 34% to 154%. When ventilation is controlled, the increase in WOB related to ETT resistance is seldom of any consequence because it is overcome by ventilator adjustments. However, small-diameter tubes create greater difficulty for patients in weaning from ventilatory support because of the higher levels of resistance encountered when attempting to breathe spontaneously. , It has been suggested that an inability to spontaneously ventilate because of the increased WOB imposed by a 7.0-mm ETT might indicate that extubation will fail regardless of tube size. ,
Increased airway resistance associated with a smaller-diameter ETT may also be associated with inadvertent PEEP. Patients with high oxygen consumption, increased carbon dioxide production, or ventilation/perfusion relationships that produce high dead space ventilation (V. d) often require higher minute ventilations to achieve appropriate ventilation and oxygenation. The gas flows necessary to maintain such minute ventilation are also quite high, and the resistance imposed by a smaller-diameter ETT further prohibits the completion of expiration before initiation of the subsequent inspiration. This breath stacking results in air trapping and unwanted PEEP, magnifying the risk of mechanical ventilation as barotrauma and subsequent intrathoracic overpressure could result in circulatory compromise.
The restriction to gas flow through any ETT increases dramatically when devices, such as a suction catheter or bronchoscope, are placed in the lumen. The cross-sectional area of the tube is effectively reduced by an amount equal to the cross-sectional area of the device inserted into the tube. This limitation of gas flow has consequences for both the inspiratory and expiratory phases: inspiratory flow may be inadequate to maintain oxygenation and ventilation during the procedure, and obstructed expiratory flow may lead to overdistention of the lungs, resulting in barotrauma or circulatory compromise because of reduced venous return, particularly in the hypovolemic or hemodynamically compromised patient.
Whereas smaller diameter ETTs have disadvantages related to gas flow and airway resistance, larger tubes are more frequently associated with traumatic placement and damage to both the laryngeal structures and the tracheal mucosa. , , Larger ETTs are associated with a higher incidence of sore throat after general anesthesia compared with smaller diameter tubes, but this difference is relatively negligible with long-term intubation. With prolonged intubation, laryngeal trauma is more likely. Women and those of shorter stature, because of the inherently smaller size of their airway, are more susceptible to injury than men. ,
Laryngeal structures at particular risk for trauma are the arytenoid cartilages and the cricoid cartilage. Trauma results not only from the shape discrepancy between the round ETT and the angular, wedge-shaped glottic opening but also from direct contact and pressure on these structures and from repetitive tube movement, which leads to ulceration or erosion of the protective mucosa. Tracheal mucosal injury can also occur because of the irregular surfaces created by wrinkling and folding of the ETT cuff or the externalized pilot tube used to fill the ETT cuff. If the tracheal lumen is “overcrowded,” airway injury is more likely to occur when large tubes are used, and little cuff volume is required to seal the airway.
Modifications to the ETT that are made in the OR setting to accomplish specific surgeries are often developed in response to interference and access issues. The ability to work without disturbing the ETT has led to several variations of the ETT that can be placed safely and remove the risk of inadvertent advancement, dislodgment, kinking, or obstruction. ETTs used in remote locations also have airway access issues associated with tube kinking and partial occlusion, which typically are related to positioning problems and associated comorbidities. In part because of less stringent vigilance, unintended consequences of ETT use outside the OR may result in more drastic outcomes. In response to these dilemmas, a variety of tubes have been developed to maintain their shape and patency in locations where distortion might cause kinking and occlusion.
Rigid, preformed tubes such as those developed for long-term use in tracheostomy were known to maintain their patency despite the need for angulation. Preformed tubes have been developed for specific application in anesthesia practice as well. The Mallinckrodt Ring-Adair-Elwyn (RAE) tubes (Medtronic, Minneapolis, MN), both oral and nasal models ( Fig. 44.10 ), maintain a fixed contour similar to the average facial profile, allowing for head and neck surgery while minimizing surgical field interference. Their contour also reduces the risk of pressure injury to the posterior pharynx when repositioning is desired. The intraairway length is tied to the size of the ETT, with a relatively appropriate depth based on the average size of a patient for whom the tube might be selected. ,
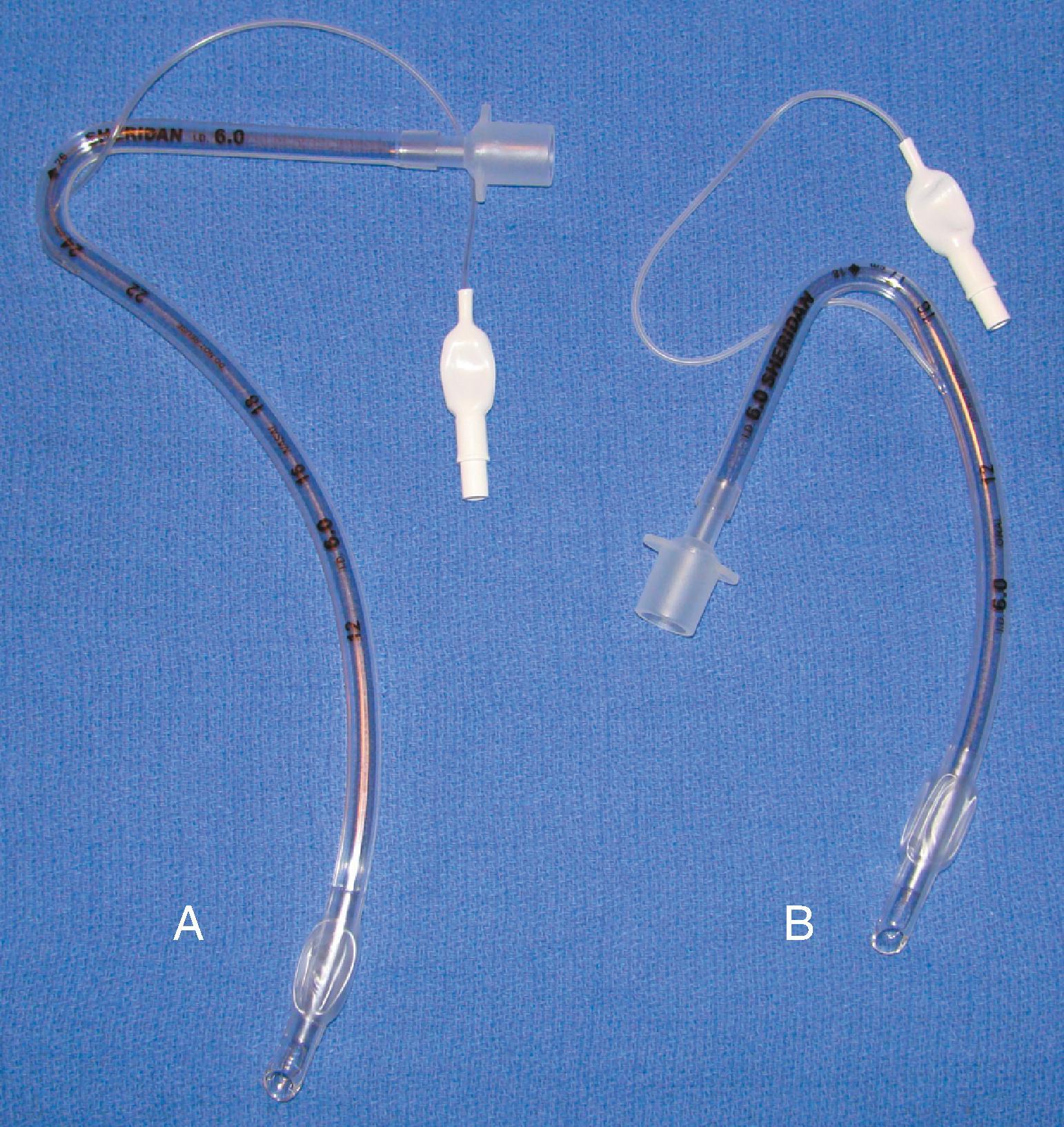
Wire-reinforced ETTs (also referred to as anode or armored tubes) have an embedded wire coil designed to minimize kinking even with exaggerated position-induced angulation. Wire-reinforced tubes are popular for use in head and neck surgery where remote airway access and the potential for kinking of the ETT are concerns. Placement of a wire-reinforced tube through a tracheostomy for procedures such as a laryngectomy is a common practice; it allows placement during surgical procedures such that the tube can be mobilized, or the circuit draped away from the field without a high risk of tube kinking. The other common use of a wire-reinforced tube is with the intubating laryngeal mask airway (ILMA). These tubes are designed to facilitate placement through the device and to be used for short periods of time. The HPLV cuff and the theoretical possibility of kinking and resultant airway obstruction make the long-term use of ILMA silicone ETTs risky.
The embedded wire concept of the reinforced ETT has also been developed for long-term tracheostomy use. Although the reinforced tracheostomy tube is not free of risks, one advantage is that its flexibility allows its length and intratracheal depth to be adjusted, which may be beneficial if tracheomalacia at the level of the cuff develops. These tracheostomy tubes are also popular for use in morbidly obese patients, in whom, because of the depth of tissue, preformed tracheostomy tubes may not have the shape required to fit an individual patient.
One major consequence of this type of reinforced ETT may occur when external pressure is applied to the wire-reinforced component (e.g., by patient biting). Once a compression threshold is reached, the luminal support provided by the wire may be compromised, and a permanent, irreparable indentation remains that can significantly impair ventilation and suctioning capabilities.
Progress in laser technology has advanced surgical capabilities, particularly for airway surgery. To protect patients and healthcare providers from laser-induced injury to eyes and airways, special precautions are required. Fire is the most serious danger associated with the use of lasers in the OR, especially when a laser is used in airway surgery. A major complication related to the use of lasers for laryngeal surgery is ignition of the ETT. The laser beam may ignite the tube by direct penetration or indirectly if burning tissue is inhaled into the tube. , , The ease of ignition is related to the ETT material, the concentration of oxygen in use, and any other adjunctive materials or gases that could support combustion. , Most ETTs are constructed of PVC, which is highly flammable. Ideally, PVC tubes should not be used for airways when a laser is employed. , ,
ETTs can be laser proofed or protected from the laser beam by wrapping them with reflective metal tape. Ideally, they should be constructed from noncombustible materials. In particular, the ETT cuff is vulnerable to puncture by the laser beam and should be filled with saline or water, which allows more energy to be absorbed before disruption. , A technique to enhance detection of a penetrated cuff is to place a dye indicator, such as methylene blue, into the solution that is instilled into the cuff. Any leakage will clearly mark the airway and alert the provider to the potential dangers. Protecting the tube from the laser beam by wrapping it with foil tape has proved effective (commercial devices are available) ( Fig. 44.11 ). Tubes made of materials, such as metal and silicone, and those with special double cuffs also reduce the risk of airway fires and injury during laser airway surgery. , (See Chapter 38 .)
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here