Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In the 1920s, Frederick Banting and Charles Best isolated insulin and made possible the treatment and survival of millions of diabetics. This helped initiate the era of modern medicine, in which the empiricism of the 18th century and its practice in the 19th century came to fruition in the wondrous cures of the 20th century.
This discovery was made possible by early research into pancreatic function that focused on the difference in hormonal signalling between the acini, the cells that secrete digestive enzymes, and those in the islets of Langerhans.
Islets have an endocrine function, secreting hormones into the bloodstream to act at distant target sites.
Acini have an exocrine function, secreting enzymes out of the body and into the lumen of the intestines.
The major pancreatic endocrine hormones, insulin and glucagon, serve to regulate the body’s metabolism of carbohydrates, lipids, and proteins.
By controlling the construction and breakdown of these energy-containing organic molecules, they ensure a constant supply of energy to cells regardless of fluctuations in dietary intake.
Insulin promotes storage of energy-laden molecules, while glucagon promotes the consumption of energy stores.
The pancreas lies in the posterior abdomen behind the peritoneum (i.e., retroperitoneal). This organ typically weighs 80 to 100 g in volume; it extends from the curvature of the duodenum to the medial edge of the spleen ( Fig. 28.1 ). (For more detail on the exocrine functions of the pancreas, see Ch. 24, Ch. 25 ).

As previously mentioned, the endocrine pancreas is responsible for hormone secretion ( Fig. 28.2 ).
This organ is organized histologically into islets of Langerhans, which are roughly spherical clusters of cells that account for 1% to 2% of the total weight of the pancreas.
Islets are buried amid the pancreatic acinar cells (acini), which make up the exocrine pancreas.
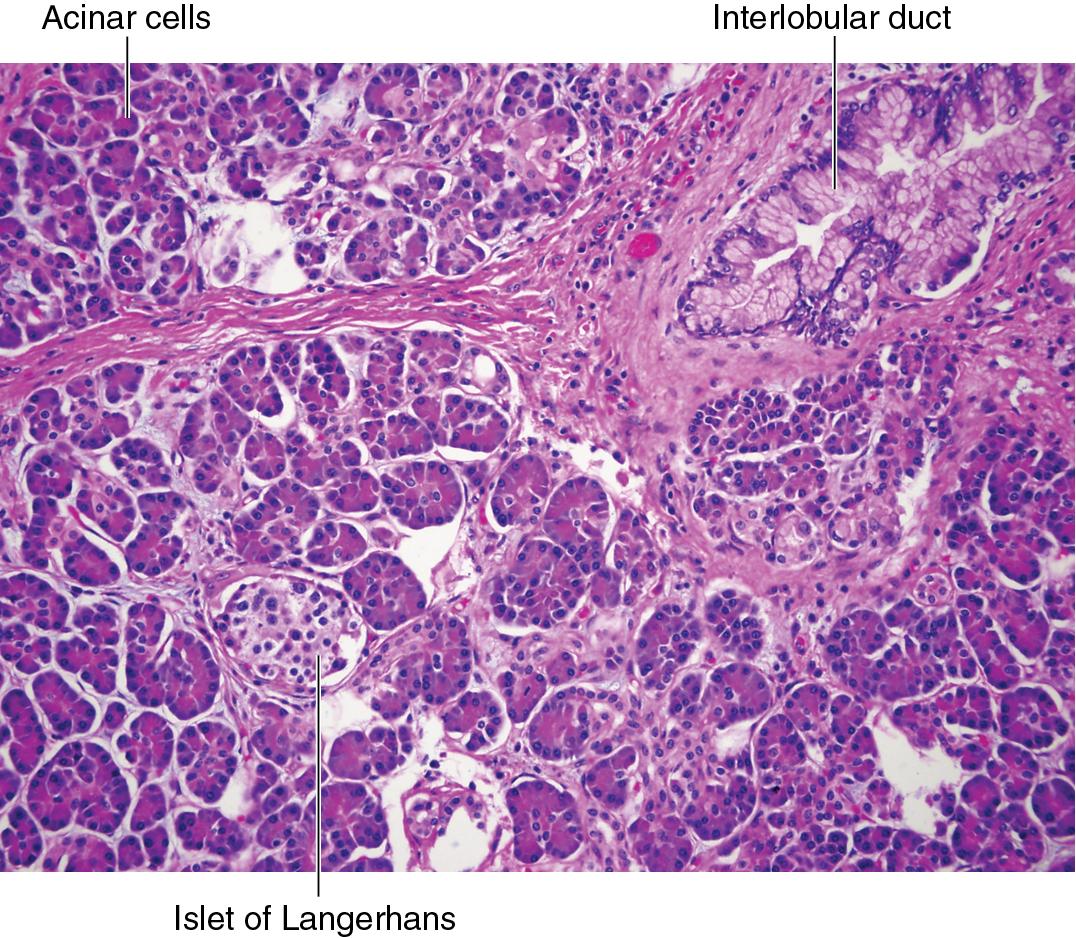
The islets of Langerhans consist primarily of beta cells and alpha cells.
Beta cells synthesize and secrete the peptide hormone insulin.
Alpha cells make and secrete the peptide hormone glucagon.
Others cell types include:
Delta cells, which synthesize somatostatin
PP cells, which make pancreatic polypeptide
D1 cells, which secrete vasoactive intestinal peptide (VIP)
Enterochromaffin cells, which secrete serotonin
Because the major products of the endocrine pancreas—insulin and glucagon—are peptide hormones, the organelles involved in protein synthesis and secretion play important roles in the islet cells.
Insulin ( Fig. 28.3 )
The intracellular production of an insulin molecule begins with a large peptide precursor called preproinsulin, which is cleaved into proinsulin and then insulin.
The final product of this intracellular processing—the active hormone insulin—consists of two protein chains, known as A and B, which are 21 and 30 amino acids in length, respectively, bridged by a connecting C chain of 31 amino acids.

Glucagon
Synthesized in alpha cells in a precursor form called preproglucagon, which is then processed to proglucagon, which is then processed to form glucagon.
The active hormone glucagon is a 29-amino-acid, single-chain polypeptide.
Although diffusely expressed, insulin receptor is most highly expressed in three organ systems:
Liver
Muscle
Adipose tissue
The insulin receptor is a transmembrane protein complex with four subunits.
Insulin binds to the extracellular domain of the receptor.
This activates the cytosolic domain, which is a tyrosine kinase.
Glucagon, on the other hand, binds to transmembrane receptors on hepatocyte and triggers the adenylate cyclase/cyclic adenosine monophosphate (cAMP) pathway. (See the online appendix to Ch. 1 on signal transduction for a fuller explanation of tyrosine kinase and cAMP mechanisms.)
Insulin and glucagon, the major hormones produced by the endocrine pancreas, govern the metabolism, storage, and release of energy-storing molecules. These molecules include:
Carbohydrates
Lipids
Proteins
Energy is stored in their bonds that can be liberated by digestion and chemical breakdown in the body. Most body tissues can use any of these forms of fuel.
Glucose is the predominant molecule regulated by the pancreas, and, as such, the pancreas’ regulatory apparatus is tied most closely to the plasma glucose level. This is critical because glucose is the primary fuel source for the brain.
The brain contains no significant stores of glycogen (the polymerized storage form of glucose).
The brain is inaccessible to other sources of energy, like fatty acids, because the blood-brain barrier limits neuronal absorption of fatty acids from the bloodstream.
Thus decreased glucose in the plasma can have severe consequences for neurologic function.
To understand the effects of insulin and glucagon, it is prudent to discuss the differences between fed and fasting states (see Fast Fact 28.1 ).
Insulin means money in the bank (i.e., storage and synthesis).
Glucagon means money is gone (i.e., broken down and used to sustain vital processes).
Fed state
Postmeal, following digestion of carbohydrates and subsequent absorption of glucose into the bloodstream.
↑ plasma glucose.
↑ insulin production from the beta cells of the pancreas.
Insulin promotes anabolic (“synthesis and storage”) processes ( Fig. 28.4 ).
Uptake and storage of glucose (glycogenesis).
Uptake and storage of amino acids and triglycerides.

Fasting state
↓ plasma glucose.
↑ glucagon production from the alpha cells of the pancreas.
Glucagon promotes catabolic (“breakdown”) processes to supply the brain with glucose ( Fig. 28.5 ).
Breakdown of glycogen (glycogenolysis)
Synthesis of glucose (gluconeogenesis)
Synthesis of ketone bodies as alternate fuel sources

Although insulin acts fairly independently in the fed state, glucagon is supported by several other hormones in the promotion of fasted-state metabolism.
Glucocorticoids
Epinephrine
Growth hormone
Human chorionic somatomammotropin (in pregnancy)
Collectively, these hormones are known as the counterregulatory hormones because of their effect on fasting state metabolism.
Insulin decreases the plasma glucose level and drives fed metabolism.
Counterregulatory hormones increase the plasma glucose level and drive fasted metabolism.
The primary trigger for insulin secretion from beta cells is elevated plasma glucose level. Beta cells detect the increased glucose level through the intracellular enzyme glucokinase.
When the plasma glucose level rises, glucose diffuses down its concentration gradient through the glucose transporter (GLUT)1 and GLUT2 transporters into the beta cells.
Glucokinase, which is very sensitive to changes in the glucose concentration at physiologic levels, proceeds to phosphorylate the glucose.
This acts to trap glucose inside the cell so that it may undergo glycolysis.
After glycolysis, increased concentration of adenosine triphosphate (ATP) inhibits ATP-sensitive K + channels on the beta cell.
This leads to depolarization of the cell.
Depolarization triggers voltage-sensitive Ca 2 + channels, leading Ca 2 + to flood into the cell and further depolarize the beta cell.
Increased Ca 2 + concentrations eventually trigger exocytosis of insulin granules ( Fig. 28.6 ).

The set point at which glucokinase triggers insulin release is a plasma glucose concentration of 5 mmol/L (90 mg/dL).
By this sensing mechanism, the postprandial (“after meal”) spike in plasma glucose stimulates the beta cells of the pancreas and results in a biphasic increase in plasma insulin levels ( Fig. 28.7 ). The constitution of the meal will determine the exact amount of insulin secreted.
Within 3 to 5 minutes of beta-cell stimulation, the insulin concentration rapidly rises in the bloodstream to as much as 10 times the basal level.
This occurs because of immediate release of stored insulin from cytoplasmic granules.
Over the next 15 to 20 minutes, a second peak in insulin concentration gradually occurs.
This represents both the further release of stored insulin and the release of newly synthesized insulin (see Clinical Correlation Box 28.1 ).

Diabetic patients will inject differing amounts of insulin before a meal depending on the carbohydrate content (a process known to the diabetic as carbohydrate counting).
Beyond elevated plasma glucose, other stimuli of insulin secretion include:
Increased plasma amino acids
Increased plasma fatty acids
Acetylcholine (i.e., increased parasympathetic tone)
In addition, endocrine signals from the gut called incretins are also known to promote insulin production and secretion in response to a meal.
GIP , gastroinhibitory polypeptide
GLP-1 , glucagon-like polypeptide-1
In contrast, insulin secretion is inhibited by:
Low plasma glucose
Norepinephrine and epinephrine (i.e., increased sympathetic tone)
The autonomic nervous system is thought to have separate glucose-sensing capabilities, through which it controls insulin secretion in parallel with the direct effect of glucose on the beta cells. Specifically, glucose-sensing neurons in the hypothalamus detect low blood sugar levels and stimulate the sympathetic nervous system (see Clinical Correlation Box 28.2 ).
In diabetic patients, increased sympathetic tone produces the warning signals of hypoglycemia, such as sweating, tachycardia, and restlessness. Fortunately, these warning signs occur before the glucose level is low enough to cause changes in consciousness. However, long-term diabetics, even with well-controlled blood sugar levels, may lose their sensitivity to hypoglycemia, and the loss of warning signals may result in episodes of neurologic dysfunction as a first sign of hypoglycemia.
As insulin mediates metabolism in the fed state, its functions are generally anabolic (i.e., promoting the synthesis and storage of molecules rather than consumption). However, insulin also promotes the use of glucose by stimulating glycolysis, the breakdown of glucose into constituents that may enter into the citric acid cycle or undergo anaerobic energy extraction.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here