Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Globally cardiovascular disease (CVD) is the leading cause of death, accounting for greater than 17.3 million deaths per year in 2013.
The mechanisms involved in acute coronary thrombosis are well established and include of plaque rupture, erosion, and less frequently, calcific nodules.
The correlation of coronary angiography, coronary computed tomography angiography and intracoronary imaging with histopathology created the concept of the thin-cap fibroatheroma (TCFA).
Morphologic features alone are not enough to identify a TCFA that is likely to rupture. Most of them stabilize, only the minority rupture, and most of the rupture events are totally asymptomatic.
Acute coronary event risk as a simple cause-and-effect principle centered on high-risk or vulnerable plaques has shifted to a complex model involving plaque burden, systemic disease, and inflammation.
Reducing plaque burden and inflammation are effective measurements that lead to disease stabilization and plaque regression.
Globally cardiovascular disease (CVD) is certainly the leading cause of death, accounting for greater than 17.3 million deaths per year in 2013. This number that is expected to grow to greater than 23.6 million by 2030. Deaths attributable to ischemic heart disease increased by an estimated 41% from 1990 to 2013. Just in the United States alone, an estimated 16.5 million people have coronary heart disease (CHD), with approximately 1 million developing either new or recurrent events.
Coronary atherosclerosis is a condition that remains asymptomatic over decades. Therefore, the natural history of the disease offers an opportunity for early diagnosis and therapy. Prevention of the transition from asymptomatic disease to acute coronary thrombosis may reduce mortality. In fact, the incidence of ST-segment elevation myocardial infarction (STEMI) in the United States and Europe has reduced 25% in the last 8 years.
The mechanisms involved in acute coronary thrombosis are well established and include plaque rupture, erosion, and less frequently, calcific nodules. The correlation of coronary angiography, coronary computed tomography angiography (CCTA), and intracoronary imaging with histopathology created the concept of the thin-cap fibroatheroma (TCFA). The enthusiasm associated with this concept was the focus of several years of research and even clinical trials. However, the dynamic nature of this disease proved the hypothesis wrong. Morphologic features alone are not enough to identify a TCFA that is likely to rupture. Many stabilize and become thick cap fibroatheromas (ThCFA). Only the minority rupture. However, most of the rupture events are totally asymptomatic. Therefore, conceptualizing acute coronary event risk as a simple cause-and-effect principle centered on high-risk plaques has shifted to a complex model involving plaque burden, systemic disease, and inflammation. Most importantly, control of these factors will lead to disease stabilization and plaque regression.
This comprehensive review will cover a wide spectrum of the disease, from atherogenesis to plaque regression. The chapter is divided in five major topics as follows:
Atherosclerotic lesion classification and mechanisms of plaque progression
Vascular remodeling and thin-cap fibroatheroma
The myth of the “vulnerable plaque” and the transition to atherosclerotic disease burden
Intracoronary imaging
Mechanisms of plaque regression
Although primarily devoted to interventional cardiologists, the first two topics (pathophysiology) are also written for clinicians interested in the field. They summarize two decades of effort in understanding this fascinating but simultaneously fierce disease.
The human arteries have three layers; the innermost layer, called tunica intima , consists of a single layer of endothelium, basal lamina, subendothelial layer, and internal lamina. The middle layer, the tunica media, consists of layers of smooth muscle cells (SMCs) and extracellular matrix, also known as elastic lamellas . The outer layer is a rather thin layer of connective tissue, vasa vasorum, lymphatic vessels, and nerve fibers, and it is called the tunica adventitia .
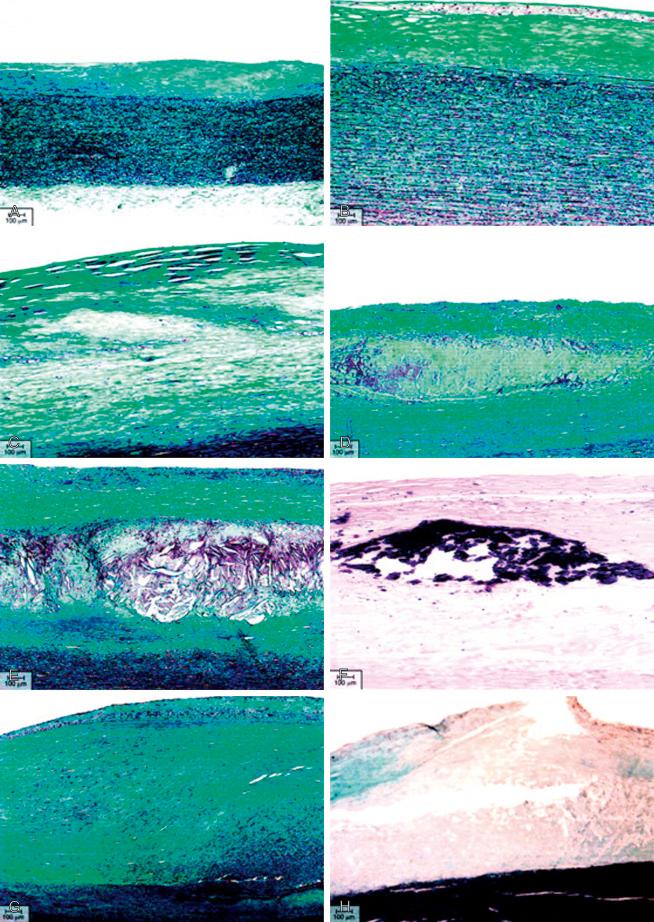
The primary event that triggers lesion formation is an abnormal accumulation of atherogenic plasma derived lipoproteins in the arterial intima. This abnormal accumulation of lipoproteins initiates a pathological cell reaction involving oxidation, inflammation, and neovascularization that result in the lesion formation. In the 1990s the American Heart Association (AHA) published a series of reports from the committee on vascular lesions of the council on arteriosclerosis lead by Herbert C. Stary MD; they proposed a practical classification of the atherosclerotic lesions according to their histological composition and structure ( Fig. 66.1 , Table 66.1 ).
Type I lesions are microscopically detectable lipid accumulations in the intima with a small group of macrophage containing lipid droplets (macrophage foam cells); this happens particularly in regions of intima with adaptive thickening or atherosclerosis prone regions.
Type II lesions consist of fatty streaks or spots in the intima with adjacent layers of foam cells and T lymphocytes.
Type III lesions or intermediate lesions are the preatheroma lesions. They are characterized by accumulation of extracellular lipid pools that lie just below layers of macrophages and macrophage foam cells. These lipid pools replace intercellular matrix proteoglycans and fibers and drive SMCs apart.
Type IV lesions, or atheroma, consist of dense, well-defined accumulations of extracellular lipid in the intima (lipid core), microscopical macrophages, macrophage foam cells, and lymphocyte, concentrated in the lipid core formation.
Type V lesions often lead to an increase of the vessel external boundary without any significant narrow of the lumen (positive remodeling). When a prominent fibrous connective tissue has formed around the lipid core, the lesions are considered type V lesions. If the fibrous connective tissue is part of the lipid core, the lesion is called fibroatheroma or type Va lesion. When the lipid core or other regions of the lesion are calcified, it is referred as type Vb (also called type VII lesion in a later review). A type V lesion without a lipid core or minimal lipid accumulation is called fibrotic or type Vc lesions (also called type VIII lesion in a later review).
Type VI lesions are a complex disease, including plaque rupture, erosion, or intraplaque hemorrhage.
| Type of Lesion | Composition | |
|---|---|---|
| Type I | Initial lesion with macrophage foam cells | Clinically silent, growth mainly from lipid accumulation, earliest onset as soon as first decade of life |
| Type II | Fatty streak, intracellular lipid accumulation in multiple foam cell layers | |
| Type III | Preatheroma: intra and extra cellular lipid accumulation | |
| Type IV | Atheroma: formation of the lipid core | Clinically silent or symptomatic, type IV lesions growth mainly from lipid accumulation, type V and VI growth from accelerate smooth muscle increase, collagen increase, hematoma and thrombosis. |
| Type Va | Fibroatheroma: lipid cored and fibrotic layers | |
| Type Vb (Type VII) | Calcific plaque: lipid core, fibrotic layers and calcium deposition | |
| Type Vc (Type VIII) | Fibrotic plaque: fibrous layers of connective tissue without significant lipid accumulation | |
| Type VI | Complicate lesion: type IV or V plaques with disruption of the surface, hemorrhage-hematoma or thrombosis |
Oxidized low-density lipoprotein (oxLDL) cholesterol is avidly taken up by macrophages via scavenger receptors, producing a cytoplasm overloaded with lipid droplets. As the inflow of oxLDL continues, it leads to extracellular lipid accumulation in the matrix of the plaque and cell death. Distribution of oxLDL plays a pivotal role in plaque growth according to mathematical models based on physical laws. Similarly, preclinical experimentation has demonstrated how oxLDL isoforms are associated with TCFA, depending on the predominant type of molecule and their induction of macrophage apoptosis. The necrotic core is formed by apoptosis of lipid-laden macrophages, foam cells, and erythrocytes. Active collagen dissolution by metalloproteinases contributes to core expansion, which plays a major role in plaque rupture.
In the aorta, the necrotic core area corresponds to 40% of the total plaque area in the TCFAs and up to 50% in rupture plaques. However, other studies of coronary arteries show necrotic areas of as little as 24% and 34% in TCFAs and ruptured plaques, respectively. Core composition may influence the propensity for plaque rupture and thrombosis. An increased ratio of free cholesterol to esterified cholesterol with oxidized cholesterol increases the likelihood of thrombosis by interacting with the oxLDL (lectin-like) receptor 1 (OLR1), a cell surface receptor for oxLDL, and enhancing the expression of tissue factor.
Finally, oxLDL triggers an inflammatory response that activates hypoxia inducible factor alpha (HIF-1α) in monocytes. HIF-1α is a key transcriptional regulator responding to hypoxia and activating genes, which promote angiogenesis, among them vascular endothelial growth factor (VEGF). This proangiogenic effect of oxLDL provides the link between hyperlipidemia, inflammation, and angiogenesis in atherosclerosis.
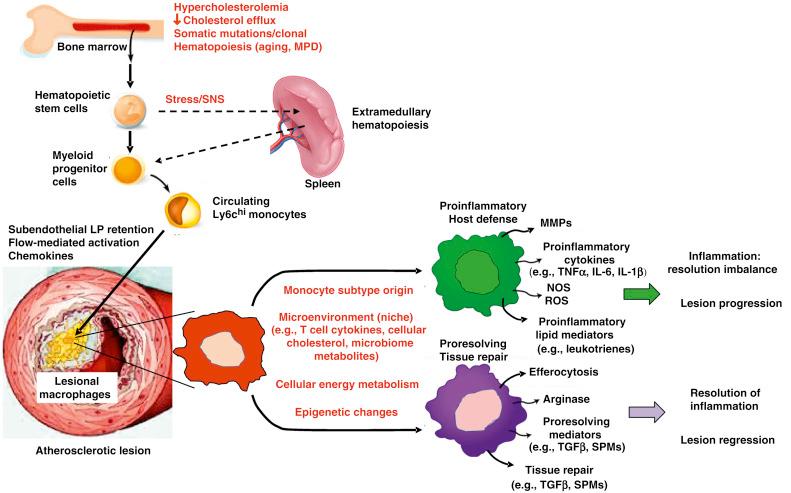
Once the inflammatory response is trigger by the lipoproteins, especially oxLDL, it provokes entry of bone-marrow derived monocytes into the intima. Then, monocytes differentiate into macrophages, which are the major inflammatory cells involved in the progression of atherosclerosis.
Initially, macrophages were classified as proinflammatory/proatherogenic (M1), and antiinflammatory/antiatherogenic (M2). Nevertheless, recent evidence suggests that the microenvironment may be fundamental in directing the cell into morphologically and functionally distinct phenotypes. It is the complex milieu of atherosclerosis that determines macrophage differentiation.
In progressing lesions, macrophages take on a proinflammatory phenotype (previously called M1). This abnormal accumulation of inflammatory macrophages and dendritic cell activation is driven mainly by Interferon γ and other proinflammatory cytokines. Migration and activations of other immune cells may also contribute, including natural killer and T2 helper lymphocytes, platelets, mast cells. The proinflammatory macrophage releases proinflammatory cytokines (tissue necrosis factor α [TNF α], interleukin-1β [IL-1β], interleukin [IL]6, nitric oxide synthase [NOS], reactive oxygen species [ROS], leukotrienes, and matrix metalloproteinases [MMPs]), which leads to more inflammation, lesion growth, and tissue destruction.
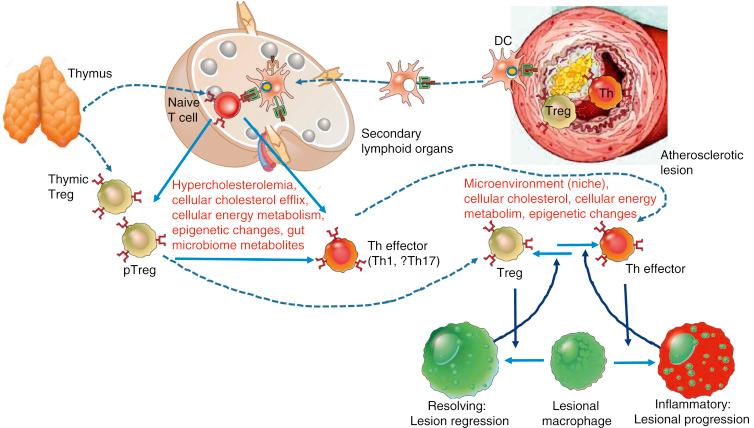
In normal circumstances, a postinflammatory response is carried out by cells that counterbalance the inflammatory process, promoting tissue repair. This postinflammatory response is driven mainly by regulatory T cells (Tregs). Tregs execute their antiinflammatory response by targeting naïve and effector CD4 + and CD8 + T cells. In addition, dendritic cells and macrophages may also contribute to this response via CD25. This action is mediated by competitive binding of IL-2 to on effector T cells and promoting antiinflammatory cytokines like IL-10.
Resolution of inflammation is probably the most important step to restore homeostasis and induce plaque stabilization. Antiinflammatory macrophages (previously called M2) are fundamental in this process. Resolving macrophages will promote tissue repair by efferocytosis, transforming growth factor β (TGFβ), IL-10, annexin A1, and inflammation-induced endogenous lipids, called specialized pro resolving meditators .
Efferocytosis, or the removal of short-lived apoptotic bodies from the atheroma, mediates resolution of inflammation. Efficient clearance of apoptotic cells prevents secondary or postapoptotic cellular necrosis. Efferocytosis triggers an antiinflammatory response through the induction of TGFβ, IL-10, and other antiinflammatory cytokines
Impaired removal of short-lived apoptotic bodies decreases macrophage efflux from the atheroma, leading to secondary necrosis, macrophage apoptosis, and the release of prothrombotic factors, inflammatory chemokines, and collagenases ( Fig. 66.2 ).
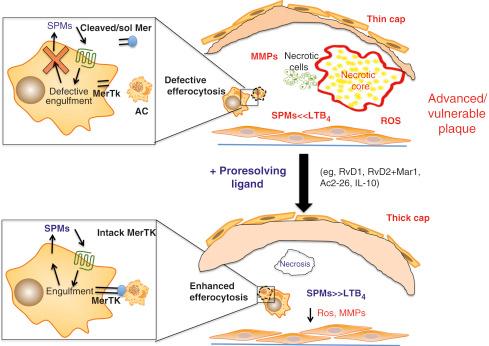
Defective efferocytosis is due to multiple factors mostly led by an excessive local proinflammatory environment, age, intracellular energy metabolism, gut microbiota, genetic, and epigenetic factors. Defective efferocytosis leads to lipid accumulation exceeding macrophage scavenger capacity, apoptosis, and secondary necrosis. MMPs release and tissue factor expression, triggering plaque rupture and atherothrombosis ( Figs. 66.3 and 66.4 ).
Atherosclerotic neovascularization evolves early in atherogenesis as a defense mechanism against hypoxia and oxLDL deposition in the tunica intima. In early and advanced disease, the neovessels may play a defensive role, allowing lipid removal from the plaque through the adventitia and leading to hypoxia resolution and plaque regression. However, in the absence of proper hypoxia resolution, chronic inflammation, oxidized lipids, and proteases will further promote angiogenesis.
Neovessels can serve as a pathway for leukocyte recruitment to high-risk areas of the plaque, including the cap and the shoulder. Expression of vascular cell adhesion molecule 1 (VCAM-1), intracellular adhesion molecule 1 (ICAM-1), and E-selectin is two- to threefold higher in neovessels compared with the arterial luminal endothelium, confirming the pivotal role of neovessels as a pathway for leukocyte recruitment in human coronary plaques.
Neovessels are characterized by a single layer of neo-endothelium. There is no tunica media or pericytes to stabilize these neovessels. Therefore, they are highly susceptible to injury by cytotoxic agents (e.g., oxidized lipids, oxidative stress).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here