Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This work was in part supported by grants from the National Institutes of Health (DK052230, DK093680, CA084197, and CA172113).
A typical eukaryotic cell cycle contains several distinct phases, which progress in an orderly fashion—a phase cannot commence without completion of the previous one. The four phases of the cell cycle are G 1 (G for gap), S (synthesis), G 2 , and M (mitosis) phases ( Fig. 8.1 ). The G 1 , S, and G 2 phases collectively make up the interphase. The DNA content of a cell in the G 1 phase is 2N (N is the number of chromosomes), also known as diploid, whereas the DNA content of a cell in the G 2 phase is 4N (tetraploid). The DNA content of a cell in the S phase varies between 2N and 4N, depending on the stage of replication. The M phase is in turn comprised of two processes: mitosis, in which the cell’s chromosomes are equally divided between the two daughter cells, and cytokinesis (or cell division), in which the cytoplasm of the cell divides in half to form two distinct daughter cells. Typically, the amount of time required for a single-cell cycle in actively proliferating human cells in culture is 24 hours. Of these, the M phase takes approximately 1 hour to complete and interphase takes up the remaining 23 hours.
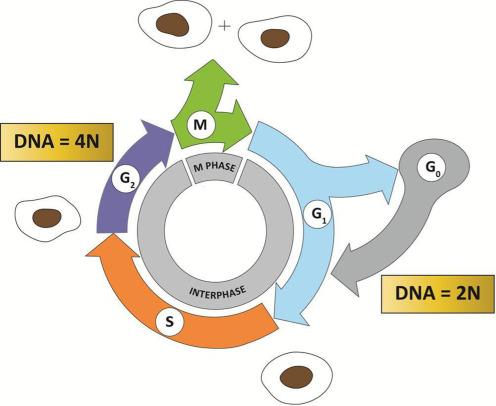
In addition to the four phases of the cell cycle listed above, one phase that lies outside the cell cycle is called the G 0 (0 for zero) phase. Cells in this phase are in the resting phase, which is often the result of their leaving the G 1 phase of the cell cycle. Typically, a cell enters G 0 phase if the environment is not conducive for the progression of the cell cycle, as in the event of deprivation of essential nutrients or growth factors, or if a cell has reached a fully differentiated state such as a hepatocytes or neuron. In these conditions, the cell is sometimes referred to as in a quiescent state. Additionally, a cell can enter the G 0 phase and become senescent due to DNA damage or telomere attrition. This is often an alternative to self-destruction of the damaged cell by apoptosis.
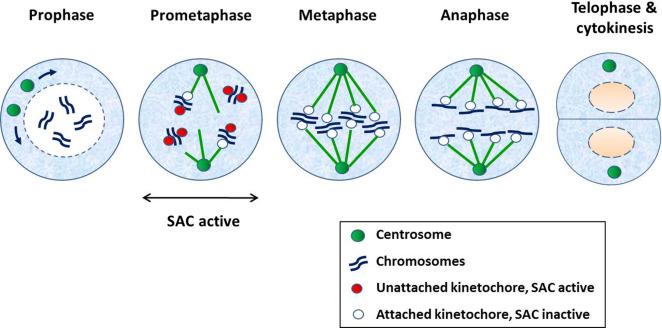
In the M phase, mitosis commences when DNA condenses into visible chromosomes, followed by the separation of the chromosomes into two identical sets. Cytokinesis is the last phase of mitosis when the two daughter cells separate, each with a nucleus and cytoplasmic organelles. Mitosis begins with nuclear membrane breakdown followed by condensation of the chromosomes and separation of the centrosomes (prophase). This is accompanied by the formation of mitotic spindles, which are attached on one end, the centrosomes, and the other end, kinetochore, a protein structure located at or near the centromeres of mitotic chromosomes (prometaphase). At this point, kinetochores that are unattached to the mitotic spindles generate a “wait” signal that delays the onset of anaphase until all chromosomes are properly attached and aligned. This signal is also called mitotic checkpoint or spindle assembly checkpoint (SAC), which is satisfied once all chromosomes are congregated at the equatorial plate (metaphase). This is followed by separation of the chromosomes to the opposite poles (anaphase) and formation of new nuclear membranes around the daughter nuclei and uncoiling of the chromosomes (telophase), eventually forming a cleavage furrow that leads to the formation of two daughter cells (cytokinesis). Fig. 8.2 illustrates the various stages of mitosis.
The organization of the cell cycle and its control system are highly conserved among eukaryotic organisms. Abundant information on how the cell cycle is regulated in vertebrates was derived from earlier studies in yeast. These studies demonstrate that both cell cycle progression and cell division are driven by the sequential activation, and subsequent inactivation, of two key classes of regulatory molecules, cyclins, and cyclin-dependent kinases (CDKs) Cyclins and CDKs form heterodimers with the former acting as the regulatory subunits and the latter catalytic subunits; CDKs are inactive in the absence of their cyclin partners. Specific pairs of cyclin-CDK dimers function in specific phases of the cell cycle ( Fig. 8.3 ). For example, the cyclin E-CDK2 complex becomes active late in the G 1 phase and is responsible for the transition from G 1 into S phase. When activated by a bound cyclin, CDKs phosphorylate a series of downstream target proteins, either activating or inactivating them, which then orchestrate the coordinated entry into the next phase of the cell cycle. The identities of the target proteins depend on the particular combination of cyclin-CDK complexes. While CDKs are constitutively expressed throughout the cell cycle, cyclins are synthesized (and destroyed) in specific stages of the cell cycle (hence the name cyclin), which is often dependent upon various signaling molecules. This cyclic nature of cyclin expression ensures that CDKs are activated and inactivated in a precise manner and safeguards the orderly progression of the cell cycle ( Fig. 8.4 ).
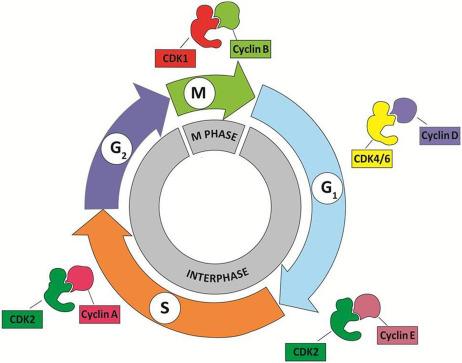
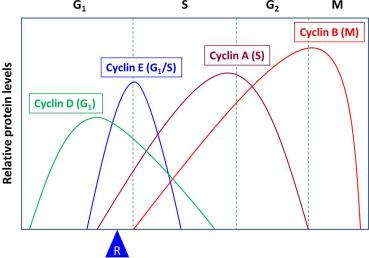
Cyclins were named because they undergo a cycle of synthesis and degradation in each cell cycle. Depending on the timing of their expression and functions in the cell cycle, cyclins are divided into four classes. Three of these classes, the G 1 /S cyclins, S cyclins, and M cyclins are directly involved in the control of cell cycle events. The fourth class, the G 1 cyclins, controls the entry into the cell cycle in response to extracellular growth factors or mitogens. In the G 1 phase, growth factors are necessary to initiate and maintain the proper transition to the S phase. Early in the G 1 phase, growth factors stimulate the synthesis of G 1 cyclins, represented by the cyclin D family of cyclins, which activates CDK4/6 to induce synthesis of downstream targets, one of which is cyclin E. The rise in cyclin E (a G 1 /S cyclin) levels and activity of its partner, CDK2, drive the cell past a restriction point (R in Fig. 8.4 ) in the cell cycle after which the cell is irreversibly committed to proceeding to DNA synthesis, even if the growth factors are withdrawn. Subsequently, the S cyclins (represented by cyclin A) and M cyclins (represented by cyclin B) are required for the initiation of DNA replication and entry into mitosis, respectively ( Fig. 8.4 ).
The G 1 cyclins are composed of the D-type cyclins that include cyclins D1, D2, and D3. Along with their partners, CDK4 and CDK6, G 1 cyclins act early in the G 1 phase of the cell cycle. The levels of G 1 cyclin are low in G 0 phase and increase progressively upon addition of growth factors or mitogens to the cells. The mechanisms by which mitogens or growth factors activate cyclin D1 are complex and occur at both transcriptional and posttranscriptional levels. At the transcriptional level, induction of cyclin D1 by growth factors is dependent on the RAS/RAF/mitogen- activated kinase (MEK)/extracellular signal-regulated kinase (ERK) signaling pathway. Once synthesized, cyclin D1 protein has a short half-life —its turnover being governed by ubiquitination and proteasomal degradation, which in turn are dependent on phosphorylation of cyclin D1 by glycogen synthase kinase-3β (GSK-3β). Growth factors prevent cyclin D1 degradation by inhibiting GSK-3β-dependent phosphorylation of cyclin D1 through the Ras/phosphatidylinositol-3-OH kinase (PI3K)/AKT pathway. It was subsequently demonstrated that growth factor induced cyclin D1 gene transcription by the MEK/ERK pathway and cyclin D1 protein stabilization by the PI3K/AKT pathway need to occur in a sequential manner with the former occurring early and the latter occurring late in the G 1 phase in order to drive the progression of the cell cycle.
One of the key targets of an activated cyclin D-CDK4/6 complex is the retinoblastoma (RB) protein. RB is one of three “pocket protein” family of cell cycle regulator proteins—the other two being p107 and p130—and has a major role in restraining the transition between G 1 and S phases of the cell cycle. In the absence of mitogenic stimuli, RB interacts with and inhibits the activity of the transcription factor E2F. As E2F-binding sites are present in the promoters of many genes required for cell cycle progression, the inhibition of E2F by RB prevents entry into the cell cycle. In addition to physically interacting with E2F, RB also recruits chromatin remodeling enzymes such as histone deacetylases (HDACs) that often serve as transcription corepressors. Thus, the binding of RB to E2F not only simply inhibits E2F activity, but the RB-E2F complex binds to promoters and actively represses transcription by blocking activity of the surrounding enhancers on the promoter.
The activity of RB is governed by phosphorylation catalyzed by CDKs. RB contains 16 potential phosphorylation sites by CDKs and oscillates between hypophosphorylated and hyperphosphorylated forms during the cell cycle. The form that inhibits E2F is the hypophosphorylated form. CDKs, in complex with their cyclin partners, phosphorylate the hypophosphorylated form of RB, leading to the hyperphosphorylated form. At least three different cyclin-CDK complexes are known to phosphorylate RB during the cell cycle-cyclin D-CDK4/6 acts early in G 1 ; cyclin E-CDK2 in late G 1 ; and cyclin A-CDK2 in the S phase. In this way, RB becomes sequentially phosphorylated in the cell cycle. It has been shown that successive phosphorylation of RB by cyclin D-CDK4/6 and cyclin E-CDK2 is necessary for the complete hyperphosphorylation of RB. The hyperphosphorylation of RB prevents its binding to E2F, thus releasing E2F from transcriptional repression. Once liberated from RB, E2F, along with its partner transcription factor DP, activate transcription of genes that are involved in nucleotide metabolism and DNA synthesis, thus allowing entry into the S phase . It is of interest to note that one of the target genes of an active E2F encodes cyclin E. It was also shown that phosphorylation of RB by cyclin D-CDK4/6 and cyclin E-CDK2 triggers sequential intramolecular interactions in RB that progressively block RB functions as cells move through G 1. The complete hyperphosphorylation of RB by cyclin D-CDK4/6 and cyclin E-CDK2 coincides with the R point ( Fig. 8.4 ), beyond which the cell is committed to completion of the cell cycle.
Cyclins E1 and E2 (collectively considered as cyclin E) are the G 1 /S cyclins. Transcription of the cyclin E gene is regulated by E2F, which, as described above, is activated due to cyclin D-CDK4/6-stimulated phosphorylation of RB. The amounts of cyclin E protein and its associated kinase (CDK2) activity are maximal in late G 1 and early S phases ( Fig. 8.4 ). Cyclin E-CDK2 completes RB phosphorylation in the G 1 phase and the transition from cyclin D-CDK4/6 to cyclin E-CDK2 accounts for the loss of dependency on growth factors. Cyclin E-CDK2 phosphorylates RB on different sites from cyclin D-CDK4/6, and these kinases may differentially impact the interaction between RB and E2Fs, HDACs, and other chromatin remodeling proteins. In contrast to cyclin D-CDK4/6, the functions of cyclin E-CDK2 are not limited to G 1 control. Thus, cyclin E-CDK2 phosphorylates other substrates that are more directly involved in the control of DNA replication, centrosome duplication, replication origin licensing and firing. The timing of cyclin E-CDK2 activation and its broader range of substrates suggest that cyclin E-CDK2 spans the interface between G 1 regulation and core cell cycle machinery during S phase.
In early S phase, cyclin E-CDK2 activity abruptly ceases as a consequence of cyclin E degradation ( Fig. 8.4 ). This is mediated by phosphorylation by both GSK-3β and CDK2, which target cyclin E for ubiquitination by the SCF Fbw7 E3 ligase, leading it to proteasomal degradation. Like cyclin D1, the involvement of GSK-3β, an enzyme that is inhibited by the PI3K/AKT signaling pathway, in regulating stability of cyclin E suggests that cyclin E can be influenced at least by one mitogen-dependent signaling cascade.
The S cyclins include both cyclins A1 and A2. While cyclin A1 is restricted to the germ cell lineages, cyclin A2 is ubiquitously expressed in all cell types. Low levels of cyclin A2 are first detected at the G 1 /S boundary. The levels then rise steadily as cells begin to replicate their DNA and do not decline until cyclin A is degraded in late G 2 ( Fig. 8.4 ). In S phase, cyclin A and its partner, CDK2, phosphorylate substrates that commence DNA replication from preformed replication initiation complexes. Cyclin A-CDK2 are also required to coordinate the end of the S phase with activation of the mitotic cyclin-CDKs.
During G 2 , A-type cyclins (the S cyclins) are degraded by ubiquitin-mediated proteolysis whereas B-type cyclins (the M cyclins) are actively synthesized ( Fig. 8.4 ). As a consequence, CDK1 (also known as Cdc2) binds to B-type cyclins—an association required for the commencement of mitosis. CDK1 preferentially binds to two main B-type cyclins, cyclins B1 and B2. In contrast, the third isoforms, cyclin B3, may have a function in the meiotic cell cycle. Cyclin B-CDK1 regulate events during both the G 2 /M transition and progression through mitosis. This is accomplished by the phosphorylation of over 70 proteins by the cyclin B-CDK1 complexes although the number of substrates could be much larger. Phosphorylation of target proteins leads to numerous events that include separation of centrosomes. condensation of chromosomes, breakdown of the nuclear lamina, and disassembly of the Golgi apparatus, among others. Finally, inactivation of the cyclin B-CDK1 complexes is required for proper exit from mitosis and this inactivation is achieved by the degradation of B-type cyclins by ubiquitin-mediated proteolysis that is regulated by the anaphase-promoting complex/cyclosome (APC/C).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here