Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Hearing is one of the most important senses. In combination with vision and the ability to speak, it contributes, in a significant way, to the quality of life. In our daily routine, we unconsciously sort out meaningful sounds from background noise, localize the source of sounds, and react (many times in a reflex mode) to unexpected sounds. About 12% of people in the general population experience a diminution or loss of hearing during their lifetime, which in some cases may represent a significant disability.
The auditory apparatus is adapted for receiving sound waves at the tympanic membrane and transmitting auditory signals to the central nervous system. Injury to elements of the peripheral apparatus, such as the ear ossicles, may result in conductive deafness. Alternatively, damage to the cochlea or the cochlear portion of the eighth cranial nerve may result in sensorineural ( nerve ) deafness. When central auditory pathways are injured, the apparent hearing dysfunction ( central deafness ) is usually combined with other signs and symptoms. Central lesions seldom result in complete deafness in one ear. These three types of hearing losses are considered in more detail when we discuss the external and middle ear, the cochlea and cochlear nerve, and the central auditory pathways, respectively. To understand the neurophysiologic and audiologic methods used in assessing peripheral and central auditory disorders, it is essential to understand the structure and function of the cochlea and central auditory pathways.
Complex sounds are mixtures of pure tones that are either harmonically related, thus having pitch, or that are randomly related and therefore called noise. The cochlear apparatus is designed to analyze sounds by separating complex waveforms into their individual frequency components.
The frequency of audible sounds is measured in cycles per second, or hertz (Hz). A simple sine wave ( Fig. 21.1 ) depicts the cyclic increase and decrease in the compression of air molecules that constitutes a pure tone. The time interval between two peaks is the period, the distance traveled is the wavelength, and the number of cycles per second is the frequency. The intensity is the peak-to-trough amplitude of force at the eardrum.

The normal frequency range for human hearing is commonly described as between 20 and 20,000 Hz. Most human speech takes place in the range of 100 to 8000 Hz, and the most sensitive part of the range is between 1000 and 4000 Hz. Exposure to loud noise can result in selective hearing loss for certain frequencies, and normal aging may reduce the range.
The hearing apparatus is exquisitely sensitive to sound intensity (perceived as loudness) over an enormous dynamic range. Intensity is related to a measure of sound pressure level at the tympanic membrane and is usually expressed on a logarithmic scale in units called decibels (dB). A sound that is 10 times more intense than a just audible sound is said to have a 10-dB sound level, and a sound one million times greater, 60-dB sound pressure level. Normal conversational levels of sound are about 50 dB. In our industrial society, we are exposed routinely to noise in the 85- to 100-dB range, such as city traffic, lawn mowers, vacuum cleaners, and even a portable music player’s headphones. Other common examples of noises above 120 dB that may cause varying degrees of discomfort include rock concerts, thunderclaps, firecrackers, and jet engines.
The brain derives the location of a sound by computing differences in the shape, timing, and intensity of the waveforms that reach each ear. The path of the sound is affected by the distance to the ears and by obstacles such as the head ( Fig. 21.1 ). Thus interaural time and intensity differences are related to the angle between the direction in which the head is pointing and the direction of the sound source. Interaural time differences are more important for localizing low-frequency sounds, whereas interaural intensity differences are more important for localizing high-frequency sounds.
Sound waves are captured by the external ear ( pinna ) and channeled through the external auditory meatus to the tympanic membrane ( Fig. 21.2 A ). Resonance features of the pinna and meatus enhance some frequencies more than others in a direction-dependent fashion. For example, sounds coming toward the back of the head are baffled compared with those coming toward the side of the head. Monaural ( single-ear ) localization depends on such cues, and accuracy in localizing sound is impaired by damage to the pinna.
The middle ear or tympanic cavity is an air-filled space in the temporal bone that is interposed between the tympanic membrane and the inner ear structures ( Fig. 21.2 A ). Sounds are transmitted across the space from the tympanic membrane to the fluid-filled inner ear by a chain of three bony ossicles: the malleus, incus, and stapes. On one end of this chain, the arm of the malleus is attached to the tympanic membrane, and at the other end, the footplate of the stapes fits into the oval window at the interface with the fluid-filled vestibule of the inner ear. The three bones act as levers to reduce the magnitude of movements of the tympanic membrane while increasing their force at the oval window. In this way, air pressure waves striking the tympanic membrane result in plunger-like movements of the stapes against the oval window that have the necessary force to produce fluid pressure waves in the cochlea.

The mechanical stiffness of the ossicle chain acts to compensate for the difference in impedance between air and fluid environments (a function called impedance matching ) so that there is optimal transfer of energy between the two media. The stiffness of the ossicle chain can also be modified by two muscles of the middle ear, the tensor tympani and stapedius muscles (middle ear reflex). Diseases such as otosclerosis and otitis media result in conductive hearing loss by affecting the efficiency of the ossicle movement. Otosclerosis, the cause of middle ear conductive hearing loss in about half of cases, may be an inherited disease and is characterized by tissue overgrowth and resultant fixation of the stapes in the oval window. Otitis media is an inflammation of the middle ear and may be accompanied by the accumulation of pus or exudate. In addition, fractures of the temporal bone with direct damage to the ossicles, or indirect damage by bleeding into the middle ear, may result in a conduction deafness.
A conductive deafness is a deficit related to an obstructed, or altered, transmission of sound to the tympanic membrane or through the ossicle chain of the middle ear. For example, damage to the pinna results in a failure of sound waves to be properly conducted to the auditory meatus. In addition, infection involving the auditory canal ( otitis externa, sometimes called swimmer’s ear ), inflammation of or trauma to the tympanic membrane, and even the excessive accumulation of cerumen (wax) in the auditory canal are other causes of conduction deafness. The deficit experienced by the patient may range from decreased hearing to total deafness in the affected ear. Depending on the cause, conduction deafness may resolve with medication or by removal of the obstruction.
The cochlea is the coiled portion of the inner ear and is named for its similarity to a conch shell ( Fig. 21.2 ). Its main elements include a labyrinth of fluid-filled canals, specialized sensory epithelium of the organ of Corti, and neurons of the spiral ganglion with their peripheral and central axonal branches.
The bony cochlea encases a fluid-filled labyrinth with a vestibule common to both the cochlear and vestibular osseous labyrinth, and spiraling fluid-filled canals ( Fig. 21.2 B ). It surrounds the membranous labyrinth of the cochlea. The central bony core around which the canals spiral is the modiolus. The canals of the osseous and membranous labyrinth of the cochlea spiral two- and two-third turns from base to apex over a length of 34 mm. In cross section, the osseous and membranous labyrinth comprise three canals. Uncoiled, the outer canals of the osseous labyrinth resemble a U-shaped tube and thus essentially are one canal. Scala vestibuli, the upper chamber in cross section, begins at the vestibule of the inner ear opposite the oval window and spirals from its base to the apex of the coiled cochlea (blue line in Fig. 21.2 B ). Scala tympani, the lower chamber in cross section, is continuous with the upper chamber at a hair pin curve, the helicotrema, at the apex of the cochlea. It returns to the base of the cochlea, where the round window separates it from the middle ear (tympanic) cavity (red line in Fig. 21.2 B ). The fluid with which the vestibule and scala vestibuli and tympani are filled is perilymph.
The middle canal of the cochlea is the cochlear duct or scala media ( Fig. 21.2 B ). It comprises the membranous labyrinth and at its base is connected by ductus reuniens to the saccule of the vestibular membranous labyrinth. In cross section, the scala media is pie shaped ( Fig. 21.3 ). Its upper boundary, the Reissner membrane, separates it from the scala vestibuli. The basilar membrane, extending from the spiral osseous lamina of the modiolus (as from threads of a screw) to the spiral ligament at the outer wall of the canal, is the lower boundary separating the scala media from scala tympani below. Stria vascularis ( Fig. 21.3 ) forms the outer wall of the scala media. The endolymph, which fills the cochlear duct, is elaborated by the cells and rich capillary bed of the stria vascularis.
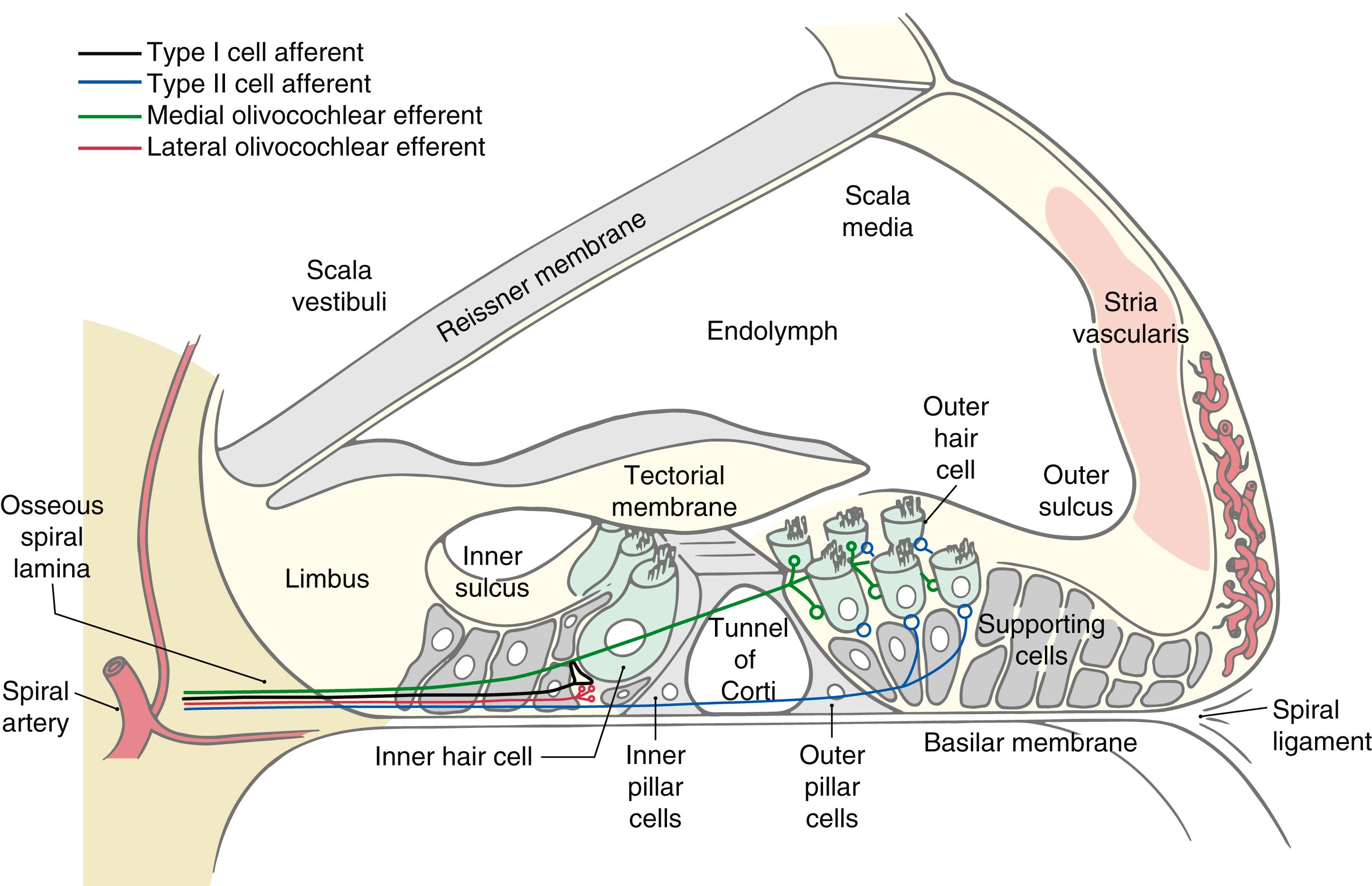
The organ of Corti is the specialized sensory epithelium resting on the basilar membrane in the cochlear duct ( Fig. 21.3 ). It is composed of inner and outer hair cells, supporting cells, and the tectorial membrane. The inner hair cells are separated from the outer hair cells by the tunnel of Corti ( Fig. 21.3 ). This tunnel is formed by the filamentous arches of the inner and outer pillar cells and is filled with fluid similar to perilymph.
Inner hair cells form a single line spiraling from base to apex, and the outer hair cells form three parallel lines that follow the same course ( Fig. 21.4 ). Once damaged, human hair cells do not regenerate. Research has not yet found a way to augment this. It is uncertain how many of the inner (about 3500) or outer (about 12,000) hair cells must be lost to disease, trauma, or aging before a just-noticeable sensorineural hearing loss ensues. Projecting from the apical surface of each hair cell is a hair bundle consisting of 50 to 150 stereocilia arranged in curving rows ( Fig. 21.4 ). Each hair bundle is polarized so that the longest stereocilia are on the outer border ( Fig. 21.4 ), and the rows of stereocilia are linked by filamentous material at their tips.
The tectorial membrane is a gelatinous arm that extends outward over the sensory epithelium from the limbus of the osseous spiral lamina ( Fig. 21.4 ). The taller stereocilia in each hair bundle are in contact with or embedded in the tectorial membrane. Consequently, movement of the basilar membrane and the organ of Corti will bend the stereocilia against the tectorial membrane and cause a graded depolarization of the hair cells.
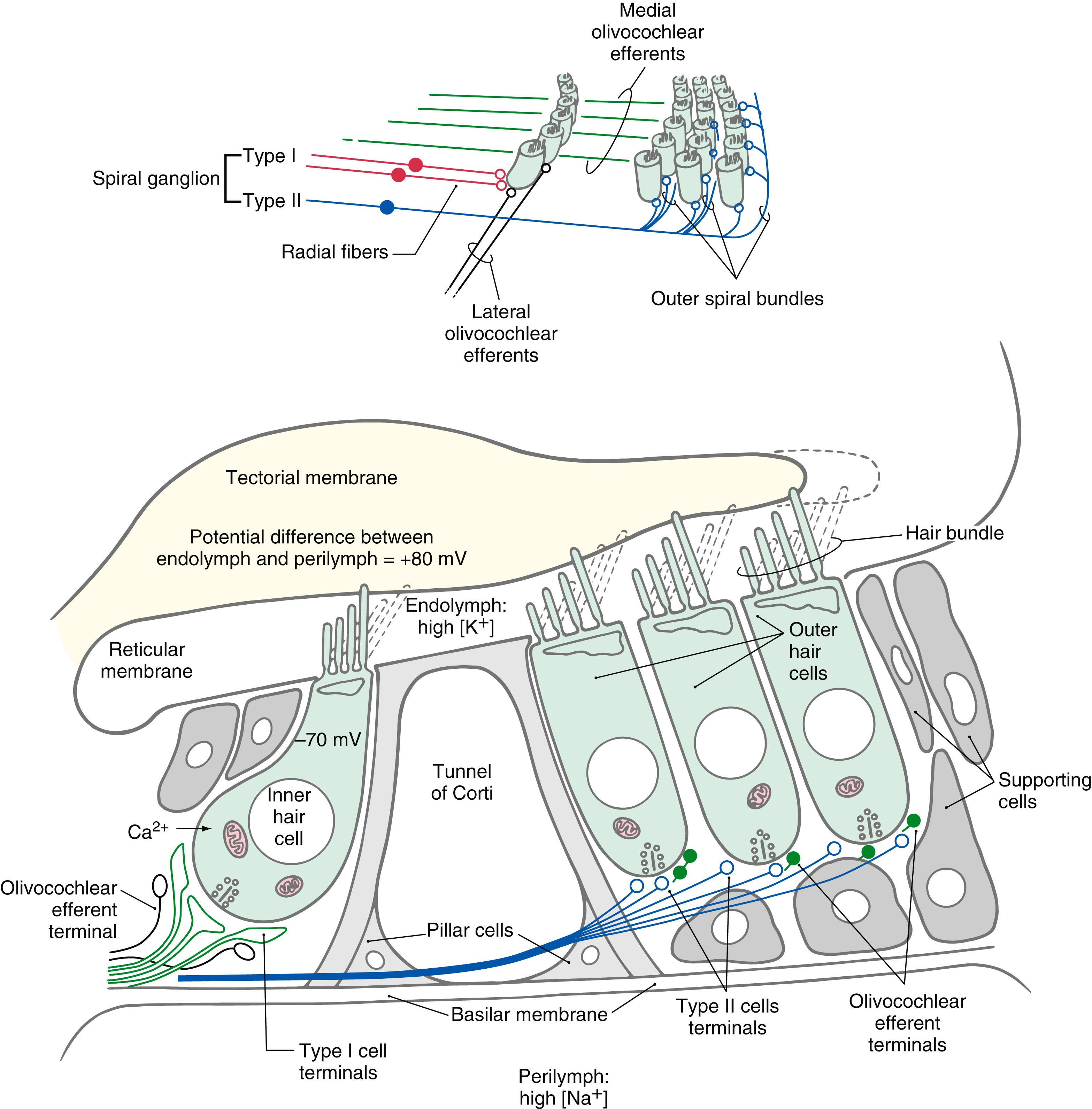
The hollow of the modiolus of the bony labyrinth houses the spiral ganglion ( Figs. 21.2 and 21.3 ). At the edge of the osseous spiral laminae of the modiolus, the peripheral processes of the bipolar ganglion cells lose their myelin and pass through perforations to the basilar membrane, where they synapse on the base of the inner and outer hair cells ( Fig. 21.4 ). The central processes of the spiral ganglion cells form the cochlear portion of the vestibulocochlear nerve ( cranial nerve VIII ). Efferent fibers to the cochlea either spiral along the inner part of the basilar membrane to synapse on inner hair cells or travel radially across the tunnel of Corti to contact outer hair cells ( Fig. 21.4 ).
Inner hair cells are extremely sensitive transducers that convert the mechanical force applied to the hair bundle into an electrical signal ( Fig. 21.4 ). At their apical surface and hair bundle, inner hair cells are bathed in endolymph. Endolymph, like intracellular fluid, has a high concentration of potassium and low concentration of sodium ions. At the basilar membrane inner hair cells oppose the perilymph-filled scala tympani. In contrast to endolymph, perilymph, like cerebrospinal fluid, has a high concentration of sodium and low concentration of potassium ions. As indicated in Fig. 21.4 , the potential difference between the endolymph and the perilymph is +80 mV. This endolymphatic potential appears to be due to the selective secretion and absorption of ions by the stria vascularis. At the same time, ion pumps in the hair cell membrane produce a resting intracellular potential of about −70 mV ( Fig. 21.4 ).
As the basilar membrane moves up in response to fluid movement in the scala tympani, the taller stereocilia are displaced against the tectorial membrane. This causes ion channels at the tips of the stereocilia to open, allowing potassium flow along the electrical gradient to depolarize the cell ( Fig. 21.4 ). The large potential difference between the endolymph and the hair cell interior creates a force of 150 mV that drives potassium into the cell and that increases the range of the cell’s graded electrical response to mechanical displacement. Damage to the stria vascularis results in loss of the endolymphatic potential and failure of mechanoelectrical transduction.
When a hair cell depolarizes, voltage-gated calcium channels at the base of the cell open, and the resulting influx of calcium causes synaptic vesicles to fuse to the cell membrane and to release a neurotransmitter into the synaptic cleft between the hair cell and the cochlear nerve fibers ( Fig. 21.4 ). The transmitter causes depolarization of the afferent fiber, and an action potential is transmitted along the cochlear nerve fiber.
The stimulus-related changes in the electrical potential between the perilymph and the hair cells can be recorded anywhere in the cochlea. Electrocochleography measures patterns of fluctuation of electrical potentials in the cochlea to monitor effects of inner ear fluid changes or of surgery on cochlear function.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here