Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Diseases of the aorta encompasses a broad range of topics far beyond the scope of a book chapter. However, we can provide an overview of common diseases and surgical issues involving the aorta, with the caveat that both our understanding of the pathophysiology of aortic disease as well as the medical and surgical management of aortic disease is constantly evolving. With the somewhat simplified acknowledgement that surgeons are either disease-oriented or procedure-oriented, this chapter aims to provide residents-in-training information regarding common disease processes and giving an overview of technical considerations. Systematic procedural descriptions are mostly avoided and information on available technology minimized, as both will change as part of the evolution in treatment.
A common theme in this chapter will be the difficulty in documenting the true incidence and prevalence of aortic disease. The reasons are many, patients may die of aortic processes (or other diseases) prior to diagnosis, and pathology involving the aorta is often only found incidentally during imaging for other issues. Aneurysmal disease is typically asymptomatic unless associated with rapid growth, aortitis, or rupture. The very definition of what constitutes an aneurysm may be up for debate. The first recognition of aortic dissection (AD) may occur at autopsy. Aortic disease may present as only one part of a systemic disease, and diagnosis made after preventative measures are no longer applicable.
Despite the late presentation of aortic disease, both surgical and medical therapeutic and management options are available. Operative therapy for patients previously deemed inoperable has become available with the introduction of endovascular approaches to the thoracic and abdominal aorta. Open surgery remains an important part of the surgeon’s armamentarium, and the merging of techniques with hybrid approaches has become common. Medical management also retains a role, the use of genetic testing has allowed earlier diagnosis and in some cases treatment with specific medical therapy, such as in the case of angiotensin II receptor blockers for the management of Loey-Dietz syndrome or beta-blockade for aneurysmal disease.
The embryologic formation of the aorta and its major branches begins in the third week of gestation and is a highly complex process of development that lends itself to the variants seen both structurally as well as histologically. Starting with paired right and left ventral and dorsal aortae, the two ventral pairs fuse to form the aortic sac and the dorsal pairs fuse to form the descending thoracoabdominal aorta. During the fourth and fifth weeks of development, the pharyngeal arches, or arch arteries, form, and as they develop in a cephalocaudal manner, six corresponding aortic arches form from the aortic sac, terminating on the dorsal aortae ( Fig. 62.1 ). While the embryologic aortic arches develop in a cephalocaudal fashion, they regress in the opposite order and are not necessarily present at the same time. The aortic arches begin to regress day 27 and by day 29 have completely disappeared with remnants contributing to the normal aorta.
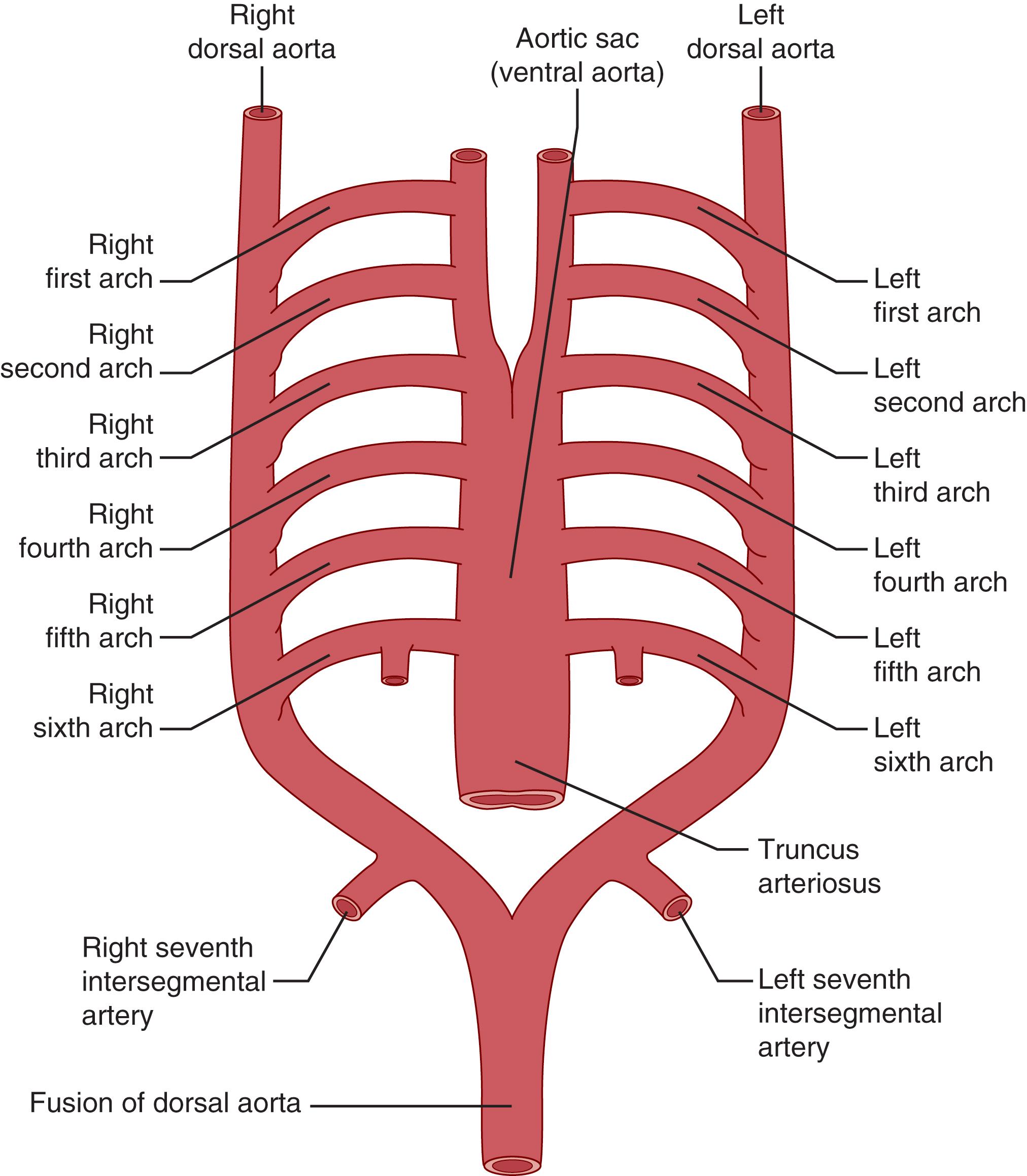
Edwards proposed a theoretical double aortic arch system to explain variants of the normal anatomy ( Fig. 62.2 ). Although the embryologic pharyngeal arch arteries appear and regress sequentially, Edwards’s proposal pictured the developing aorta as consisting of bilateral arches and ductus arteriosi encircling the trachea and esophagus. By considering the persistence and/or regression of a segment, this model can describe most variants and anomalies of the arch and aorta. For example, as seen in Fig. 62.2 , development of the normal left-sided aortic arch requires regression of segment 1, and a variant right-sided aortic arch will occur with regression of segment 4. Regression of segment 2 results in a left-sided arch with an aberrant right subclavian artery (SCA), and a right-sided arch with an aberrant left subclavian artery will occur with regression of segment 3.
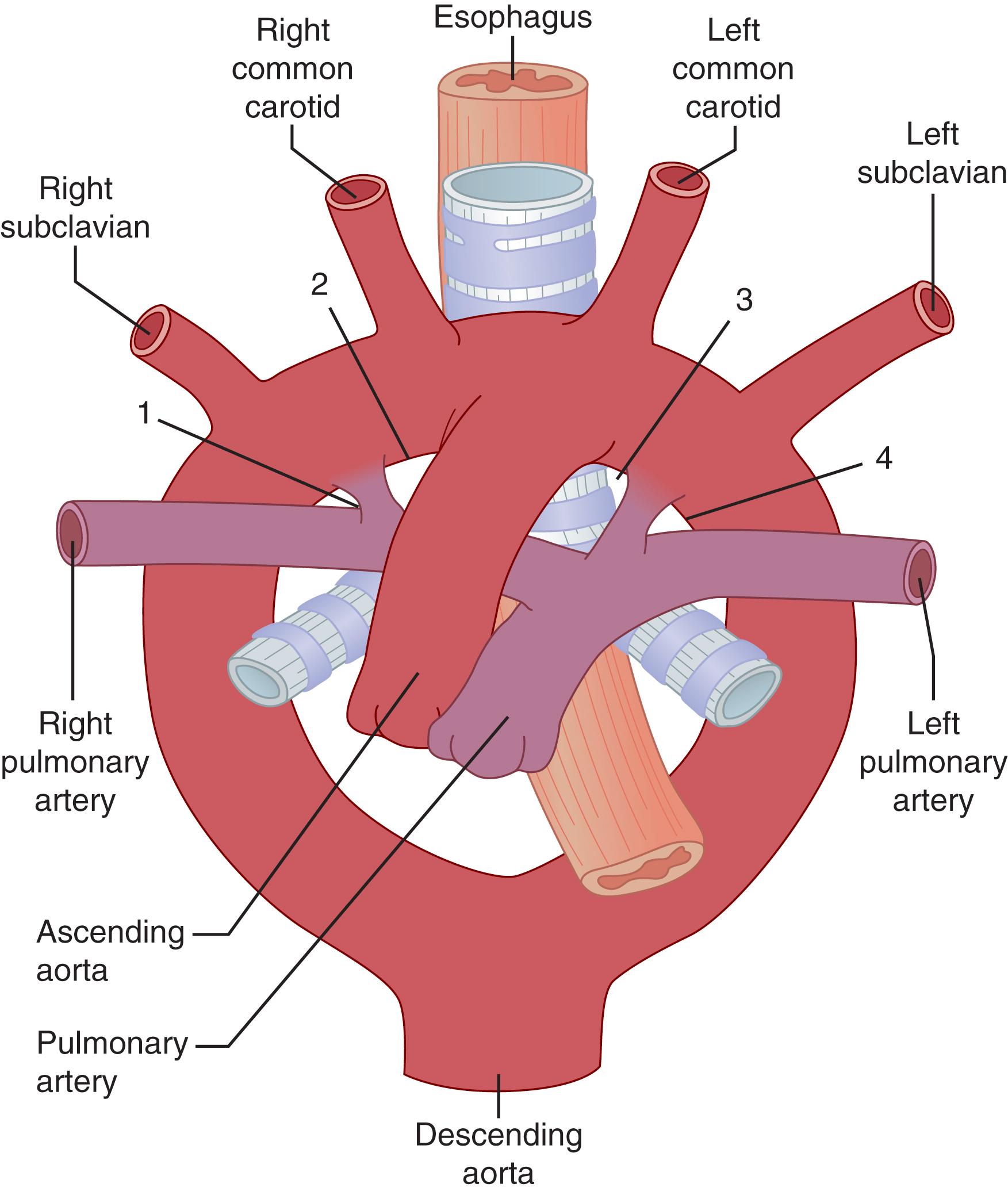
Anatomic descriptions of the aorta can be perplexing due to various overlapping nomenclature systems based on normal aorta as well as pathologic conditions. Well-defined landmarks are useful in subdividing the aorta into specific anatomic segments. Beginning at the aortic valve annulus and extending to the sinotubular junction is the aortic root, which traditionally includes the aortic valve. Embryologically, the root derives from the truncus arteriosus, the fused portion of the ventral aorta. Continuing from the sinotubular junction to either the take-off of the innominate artery or the pericardial reflection is the ascending aorta. Like the root, the ascending aorta originates embryologically from the ventral aorta. Distally, the ventral aorta during development bifurcates into two horns, the right becoming the brachiocephalic artery and the left the proximal portion of the aortic arch. The aortic arch starts at the terminal end of the ascending aorta and ends immediately distal to the left SCA. The remnant of the fourth embryologic aortic arch forms the portion of the aortic arch between the left common carotid and the left SCA. The descending thoracic aorta begins at the left SCA and extends to the aortic hiatus and transitions to the abdominal aorta, which then becomes the supra- and, subsequently, infrarenal aorta (the juxtarenal or pararenal tag is usually reserved for diseased segment terminology).
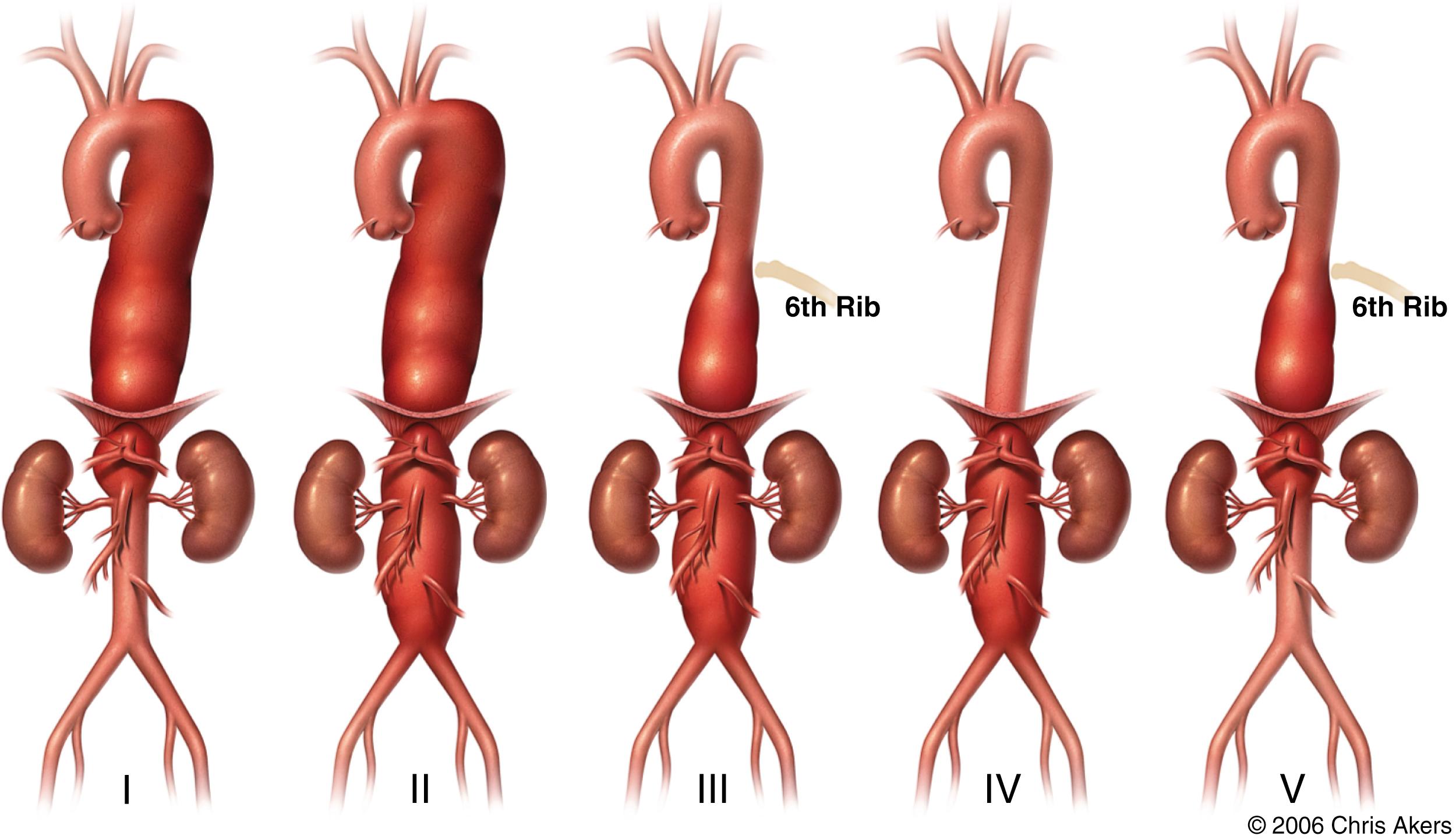
Aortic pathologic conditions have their own unique nomenclature. Aneurysmal disease is perhaps the easiest to describe since typically the label is simply the location (e.g., root aneurysm, arch aneurysm, etc.) with some exceptions. For example, Estrera and colleagues subdivide the descending thoracic aorta into three subtypes (A: proximal to the sixth rib, B: distal to the sixth rib, C: entire descending thoracic aorta) as this has implications on the risk of postoperative paraplegia ( Fig. 62.3 ). The Society for Vascular Surgery (SVS) divides the aorta into “zones” as it pertains to endograft attachment sites rather than to disease extent ( Fig. 62.4 ).
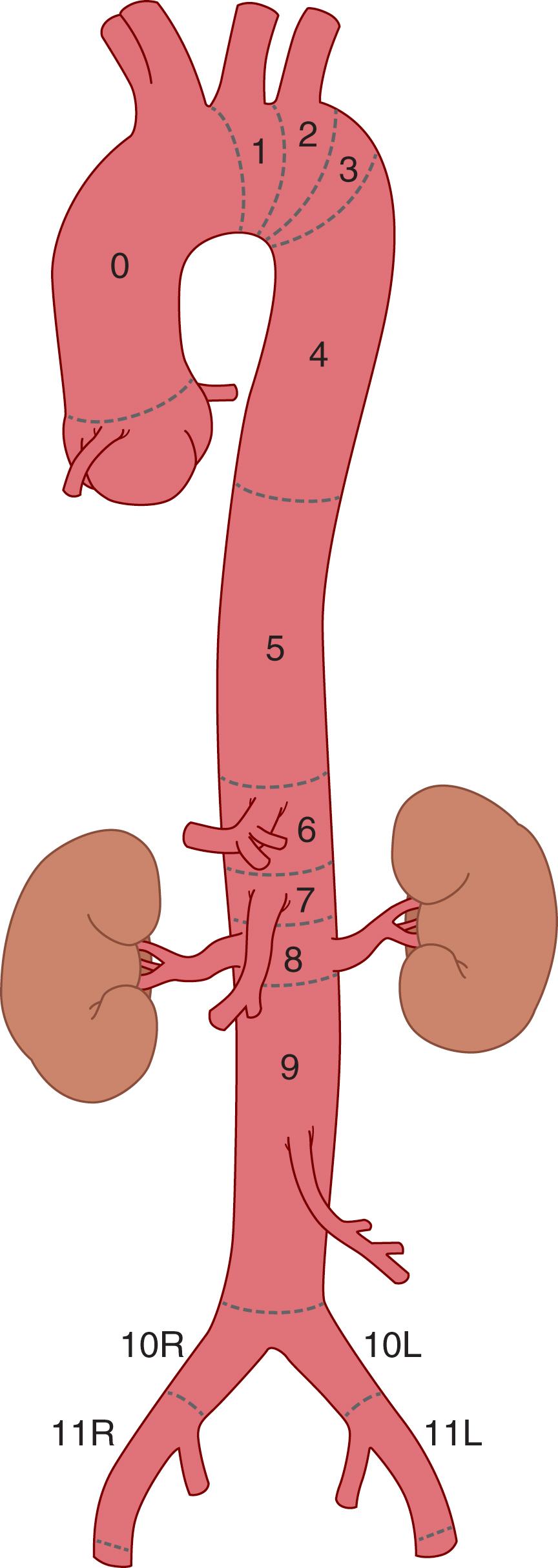
In the case of thoracoabdominal aortic aneurysms (TAAA), first catalogued and subsequently refined by Crawford and colleagues, , the original groupings attempted to describe the aneurysm in relationship to the extent of thoracic and abdominal involvement. The label “group” later evolved into “extent” or “type.” Extent I involved the descending thoracic aorta and abdominal aorta to the level of the celiac artery. Extent II involved the entire thoracic and abdominal aorta, and Extent III had “… lesser involvement of the thoracic aorta” and most of the abdominal aorta. Extent IV involved the entire abdominal aorta. In the original classification scheme there was also an Extent V, which involved the lower abdominal aorta and renal arteries. This classification scheme was refined to four groups, or extents, and later modified to five extents to account for aneurysms involving only a portion of the thoracic aorta with sparing of the infrarenal aorta ( Fig. 62.5 ). The Crawford classification system remains as an important tool in comparing procedural outcomes for TAAAs.
There are two major classification schemes for ADs ( Fig. 62.6 ). The DeBakey classification was the first attempt of distinguishing two clinically different scenarios (i.e., dissections limited to the descending aorta as compared to those involving the ascending aorta). When the ascending is involved, the dissection is either a Type I (involving the entire ascending, arch, and to some extent descending aorta) or a Type II (limited to the ascending aorta only). Dissection of the descending aorta alone is a DeBakey Type III with the further subdivisions of IIIa (tear only in the descending thoracic aorta) and IIIb (tear extending below the diaphragm). The Stanford classification scheme is limited to Type A, which encompasses dissections with any involvement of the ascending aorta, and Type B, which is for dissections distal to the left SCA. The Stanford scheme thus could, in a broad sense, distinguish between surgical management (Type A) and medical management (Type B), although there are exceptions, and with the ability for endovascular therapy of the descending aorta there is a shift of Type B’s away from medical management towards surgical intervention. The DeBakey scheme can also be separated into surgical (Types I and II) and nonsurgical (Type III), but in contrast to the Stanford classification, it also provides information on the extent of the disease process. By convention, the classical separation between an acute and chronic dissection occurs at the two-week mark, but a recent modification includes four time domains: hyperacute (<24 hours), acute (2–7 days), subacute (8–30 days) and chronic (>30 days).
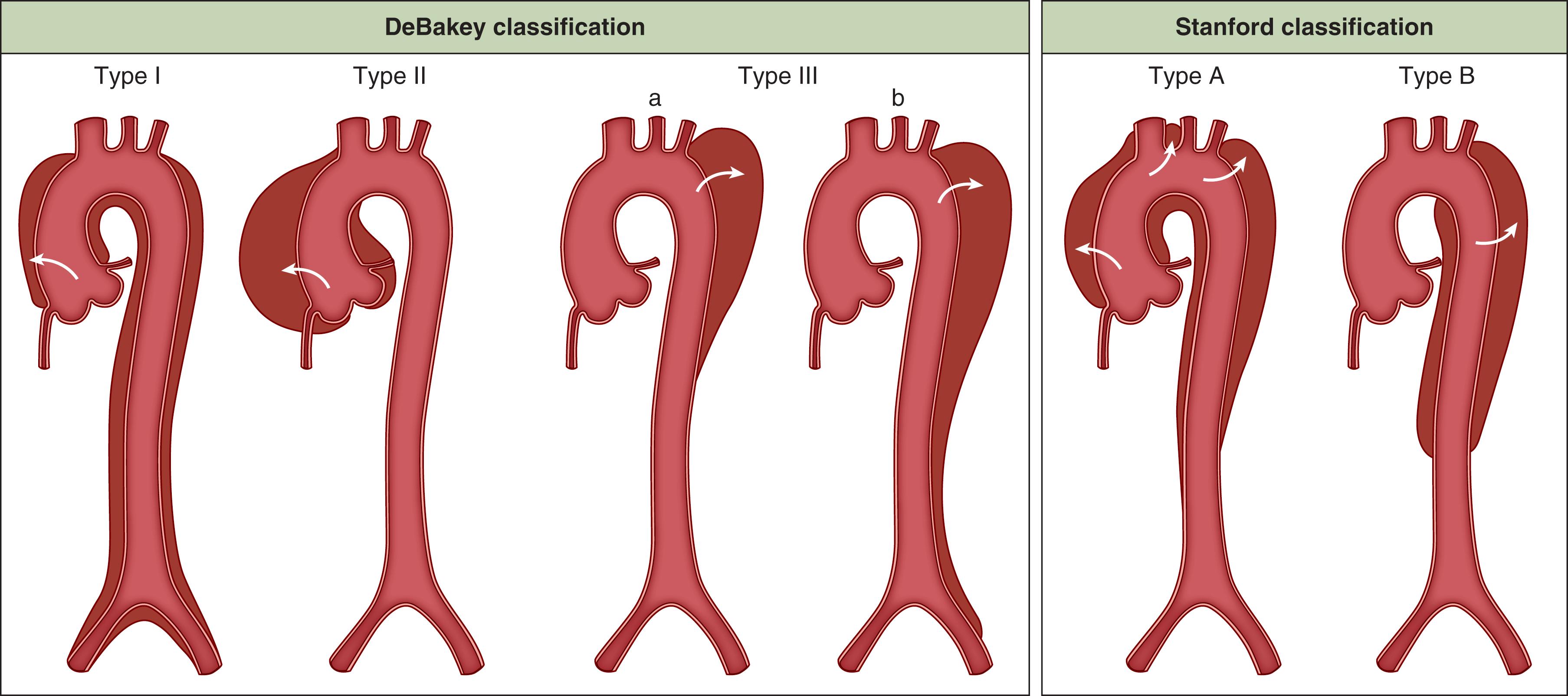
Both the DeBakey and Stanford classifications conveniently ignore dissections beginning or limited to the arch. Lansman and colleagues expanded on the Stanford classification to specify the site of intimal tear, and in this case, included arch dissections. In their series of 168 acute dissections, 139 patients had a Type A dissection (TAAD), and 30% of these had arch tears. There was a nonstatistically significant decrease in 10-year survival when an arch tear was present, although this did not affect hospital mortality. The Task Force on Aortic Dissection of the European Society of Cardiology proposed an entirely different classification based on anatomic presentation. The five classes are 1) classic AD with a distinct intimal flap separating the true and false lumen, 2) intramural hematoma (IMH), 3) subtle or discrete AD, 4) penetrating atherosclerotic aortic ulcer (PAU), and 5) iatrogenic or traumatic dissection. In this chapter, we consider traumatic dissection/transection, IMH, and PAU as separate, nondissection processes.
More recently, the Penn classification scheme has been introduced which combines the Stanford classification with the DeBakey dissection extent and a further distinction focused on clinical presentation and distal malperfusion. , The Penn classification was initially for TAAD. The four subgroups in the Penn classification are absence of branch vessel malperfusion or circulatory collapse (Penn class Aa), branch vessel malperfusion with ischemia (Penn class Ab), circulatory collapse with or without cardiac involvement (Penn class Ac), and finally both branch vessel malperfusion and circulatory collapse (Penn class Aabc). The rationale for this approach was an appreciation of the consequences of malperfusion in patient interventions, prognosis, and outcomes. Subsequently the classification was modified for Type B dissection to better differentiate complicated and uncomplicated Type B dissection. Similar to the TAAD classification, the four subgroups are absence of branch vessel malperfusion or circulatory collapse (Class A), branch vessel malperfusion with ischemia (Class B), circulatory collapse with or without cardiac involvement (Class C), and finally both branch vessel malperfusion and circulatory collapse (Class BC). Class A is subdivided into Type I (high risk for future aortic complications) and Type II (low risk), and Class C is subdivided into Type I (aortic rupture with hemorrhage outside the aortic wall) and Type II (threatened aortic rupture).
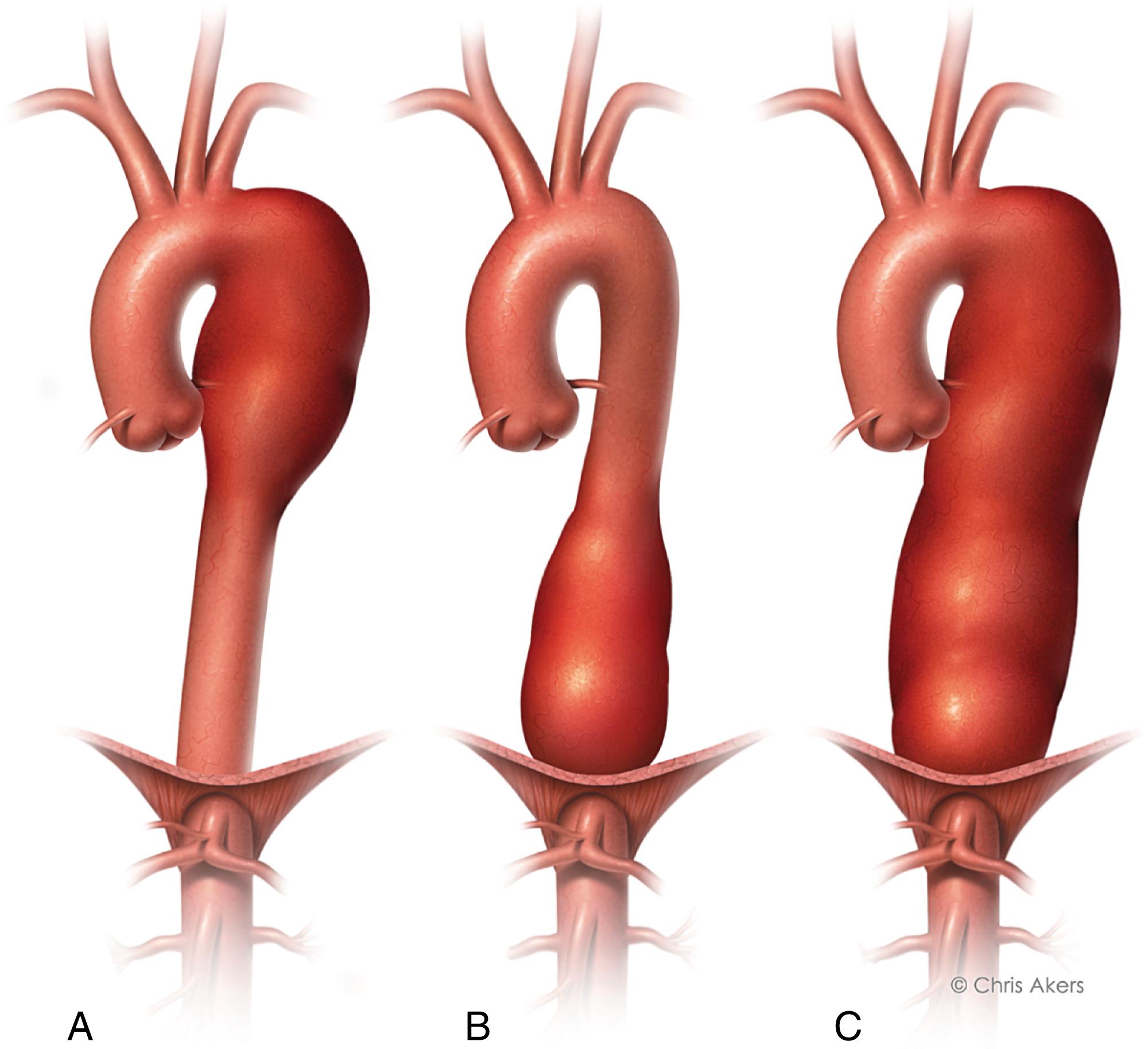
Aneurysms, typically defined as an increase in size of more than 50% above the normal arterial diameter, may occur anywhere along the aorta, from the aortic root to the bifurcation. As the mean aortic diameter differs between the ascending, descending, and infrarenal aorta, the absolute size criteria for aneurysm changes. Normal values will also vary due to methods of measurement, patient’s age, sex, and other factors. As an example based on a population-based magnetic resonance imaging (MRI) study of 70-year-olds, Wanhainnen and colleagues defined aneurysms of the ascending aorta as 4.7 cm in men and 4.2 cm in women, descending aorta as 3.7 cm in men and 3.3 cm in women, and infrarenal aorta as 3.0 cm in men and 2.7 cm in women. Anatomy or etiology may also be the basis for characterization of aneurysms. Anatomically, fusiform aneurysms exhibit smooth, circumferential dilatation across the entire vessel as opposed to saccular aneurysms, which appear as a focal outpouching of the arterial wall. Whereas true aneurysms involve all three layers of the vessel wall, false aneurysm or pseudoaneurysm describes a focal defect in the artery with an associated collection of blood contained by surrounding connective tissue. Pseudoaneurysms may be degenerative, infectious, or traumatic in etiology. These pseudoaneurysms may occur at sites of prior surgical anastomosis and represent anastomotic disruption. The majority of aneurysms addressed in this chapter are degenerative in nature. Less frequently, aneurysms may be associated with infection (mycotic aneurysms), inflammation, or autoimmune or connective tissue disease. These cases merit special consideration in their evaluation and management.
Aneurysmal enlargement of the aorta is associated with factors that result in weakening of the arterial wall and increased local hemodynamic forces. These may include heritable conditions, such as Marfan syndrome, familial thoracic aortic aneurysm and dissection, and vascular-type Ehlers-Danlos, as well as less well-defined entities that contribute to the significantly elevated incidence of aneurysm in patients with a family history of aneurysm. Factors that contribute to the degradation of extracellular matrix and reduction of elastin concentration are also associated with aneurysmal disease, and research in this area has focused on the role of matrix metalloproteinases, both their presence in the aneurysm specimens and for deficits of antiproteolytic enzymes that normally inhibit metalloproteinases. Ongoing avenues of investigation in this area also include the role of the immune response and hormone milieu. Finally, aneurysmal dilatation may also occur as a degenerative complication after AD.
As alluded to earlier, determining the incidence and prevalence of aneurysmal disease is difficult, in part because of the tendency for aneurysms to be asymptomatic and found incidentally during imaging for unrelated medical problems or symptoms. The incidence of abdominal aortic aneurysm (AAA), based on large screening studies, is estimated to range from 3% to 10%. A number of risk factors, in addition to genetic or familial disorders, for the development, expansion, and rupture of AAAs have been identified ( Table 62.1 ). Risk factors for development of an AAA include age, male gender, concurrent aneurysms, family history, tobacco use, hypertension, hyperlipidemia, and height. Female gender, black race, and diabetes appear to be protective. Recent studies have suggested that in first-world countries the incidence has been decreasing over the past two decades, most likely related to the decreased tobacco burden in those countries. Gender differences extend to the presentation, associations, and natural history of aneurysms. Men with AAA, for instance, are more likely to present with concurrent iliac or femoropopliteal aneurysms. Women are more likely to experience rupture and consistently demonstrate poorer outcomes after repair, perhaps because of a significantly higher incidence of challenging anatomy. With screening of an at-risk population, specifically for the 65- to 89-year-old demographic, the incidence of AAA is 5% to 7% with a male to female ratio of roughly four to one. Estimates of the prevalence of thoracic aortic aneurysms are 400 per 100,000 patients in 65-year-olds and 670 per 100,000 in 80-year olds, and in contrast to AAA, there does not appear to be a gender difference.
| Symptom | Risk Factors |
|---|---|
| AAA development | Tobacco use |
| Hypercholesterolemia | |
| Hypertension | |
| Male gender | |
| Family history (male predominance) | |
| AAA expansion | Advanced age |
| Severe cardiac disease | |
| Previous stroke | |
| Tobacco use | |
| Cardiac or renal transplantation | |
| AAA rupture | Female gender |
| ↓ FEV 1 | |
| Larger initial abdominal aortic diameter | |
| Higher mean blood pressure | |
| Current tobacco use (length of time smoking ≫ amount) | |
| Cardiac or renal transplantation | |
| Critical wall stress–wall strength relationship |
While aneurysms may be asymptomatic, an acutely enlarging or inflamed aorta may present with pain. Depending on the location of the aneurysm, other less common signs or symptoms may occur. For example, an enlarged ascending aorta or proximal arch may result in superior vena cava syndrome. An expanding proximal descending aorta can impinge on the left recurrent laryngeal nerve, leading to hoarseness (Ortner syndrome) or pulmonary embarrassment from extrinsic compression of the left main stem bronchus. Large AAAs may give a feeling of fullness or early satiety after small meals.
Chest x-ray (CXR) studies may detect incidentally thoracic aneurysm due to abnormalities in aortic contour, size, or calcifications, but CXR is not a good screening tool. A number of other noninvasive and invasive modalities are available for screening, case management, and surveillance. Ultrasound is an important tool in AAA management, as it is accurate, noninvasive, and cost-effective. Contrast-enhanced ultrasound is particularly useful in patients after endovascular aneurysm repair (EVAR) in detecting, localizing, and quantifying endoleaks. Echocardiography is ultrasonography when performed on the chest and its contents, and the noninvasive technique is transthoracic echocardiography (TTE). TTE is useful for measuring certain segments of the ascending aorta as well as assessing the degree of aortic valve insufficiency, if present. The invasive transesophageal echocardiogram (TEE) is capable of seeing the thoracic aorta from the aortic annulus to the celiac axis, with the exception of a short segment of the ascending aorta proximal to the innominate artery. This technique usually requires at least a modest level of sedation. Intravascular ultrasound (IVUS) is an additional modality for imaging of the aorta, and while it not typically utilized for diagnostic purposes, it is invaluable as an adjunct in endovascular procedures.
Computed tomography (CT) provides excellent imaging of the aorta, with greater reproducibility of diameter measurements than by ultrasound, and remains an important tool in the diagnosis, management, and surveillance of aortic disease. CT, particularly with the adjunctive use of iodinated contrast agents to perform CT angiography (CTA), provides a wealth of anatomic information; it detects vessel calcification, thrombus, and concurrent arterial occlusive disease and permits multiplanar and three-dimensional reconstruction and analysis for operative planning. Drawbacks include substantial radiation exposure, particularly in the setting of serial examinations, and the use of iodinated contrast media in a population with a high incidence of comorbid kidney disease.
MRI provides a reasonable alternative to CT for imaging of the aorta, with the benefits being the elimination of ionizing radiation and iodinated contrast. Spin-echo black blood and gradient echo sequences provide an intrinsic contrast between the blood flow and aortic wall to provide dimensional and geometric information. With the addition of intravenous gadolinium as a contrast agent, magnetic resonance angiography (MRA) provides rapid 3D imaging of the aorta without the need for electrocardiogram (ECG)-gating. The ability to acquire dynamic images throughout the cardiac cycle allows physiologic parameters such as estimates of wall shear stress to be determined, which may lead to clinical applications. Unlike CT, MRI does not demonstrate aortic wall calcification, which may be important in operative planning especially for endovascular approaches. MRI does not necessarily use iodinated contrast material but instead can utilize gadolinium, which has been associated with the development of nephrogenic systemic fibrosis in patients with low glomerular filtration rate. Additionally, a contraindication for MRI is the presence of incompatible metallic implants or foreign bodies. Mechanical valves, pacemakers, and implantable cardioversion devices currently marketed in the United States are MRI compatible. Newer ferrous-based contrast agents coupled with MRA may provide a viable alternative to vascular imaging when iodinated contrast or gadolinium is contraindicated.
Size and symptoms play a large role in determining the management of aortic aneurysms ( Table 62.2 ). For aneurysms that do not meet criteria or appropriateness for surgical intervention, medical management is a mainstay. Lifestyle modifications may be necessary, such as tobacco use cessation. Current smoking has been associated with significantly increasing the expansion rate of AAA (∼ 0.4 mm/yr). While moderate levels of exercise have a beneficial impact on patient’s cardiopulmonary health and progression of atherosclerosis, patients should avoid vigorous levels of activity and contact sports. Activities that cause a sudden spike in blood pressure may lead to aortic rupture or dissection in the presence of underlying aortic pathology. Blood pressure management, both in the reduction of systolic pressure as well as the pulse pressure (an indirect measure of dP/dt and wall stress), may require multiple medications. β-blockers, both selective and nonselective, are useful in accomplishing both goals.
| Location | Size Criteria | Comment |
|---|---|---|
| Ascending/root | ≥55 mm | All patients, including BAV |
| ≥50 mm | Patients with Marfan syndrome BAV with risk factors for dissection |
|
| ≥45 mm | Selected patients with Marfan syndrome | |
| >45 mm | When aortic valve is being intervened on | |
| >27.5 mm/m 2 | For patients with a small body size | |
| Arch | ≥55 mm | All patients |
| Any size | Signs or symptoms of local compression | |
| Descending | ≥55 mm | When TEVAR possible |
| ≥60 mm | Open repair (less for Marfan syndrome) | |
| Abdominal | ≥55 mm | All patients |
Prevention of aneurysmal dilatation or even regression would be an ideal consequence of medical therapy. This has been shown to be the case in the specific clinical scenario of the patient with Marfan syndrome treated with β-blockers, angiotensin II receptor blockers, and angiotensin-converting enzyme inhibitors. , None of these drugs has been shown to be effective in patients who do not have Marfan syndrome. HMG–coenzyme A reductase inhibitor (statin) therapy has been associated with reduced rates of AAA enlargement and is otherwise appropriate in a population with a high prevalence of concurrent atherosclerotic disease. Statin therapy improves survival following open and endovascular repair of AAAs and has been shown to decrease the incidence of major cardiovascular events (stroke, myocardial infarction, and death) for patients with the diagnosis of AAA. Finally, antiplatelet therapy using aspirin offers a secondary preventive benefit in this population.
Screening recommendations of aneurysms are informed by the sensitivity and specificity of ultrasound screening (or other imaging modality), the detection yield of screening based on various risk factor selection criteria, and cost. Screening for thoracic aneurysms is generally not done without a pretest high probability (e.g., suspicion of an aortic syndrome). The caveat is when an AAA is diagnosed it is a general recommendation that the thoracic aorta be screen to rule out metasynchronous disease. Current consensus guidelines recommend one-time screening of all men aged 65 years and older or men 55 years and older with a family history of AAA. In 2014, the U.S. Preventive Services Task Force (USPSTF) issued a more limited recommendation for one-time screening for AAA using ultrasonography of men between 65 and 75 years of age who have a personal smoking history and selective screening for nonsmokers. For women the recommendations remain controversial. The USPSTF concluded that there was insufficient evidence to recommend routine screening in women who smoke and recommended against routine screening in nonsmoking women. One issue that may have biased these results is the paucity of women in large screening trials. In one metaanalysis that looked at a combination of four studies with over 125,000 patients enrolled, less than 10,000 subjects were women. Payer policies regarding reimbursement may not track either of these recommendations. Medicare, for instance, because of the Screening Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act, reflects an intermediate approach in offering a screening benefit for men with a personal smoking history and men or women with a family history of AAA, although only as a part of the initial Welcome to Medicare physical examination. In a single-institution study of ruptured AAAs, only 17% of patients would have been eligible for screening.
Following the initial detection of a nonsurgical aneurysm, surveillance is necessary in addition to optimal medical management. Ideally, surveillance should be low-cost, high-sensitivity, and pose minimal harm to the patient. For the abdominal aorta, ultrasound follow-up is advisable. Those patients with a known AAA who do not have appropriate surveillance may have up to a sixfold increase in rate of rupture. The Society for Vascular Surgery Clinical Practice Council recommends the following screening intervals based on aneurysm size (maximum external aortic diameter) and associated risk of rupture:
<2.6 cm: no further screening recommended
2.6–2.9 cm: reexamination at 5 years
3–3.4 cm: reexamination at 3 years
3.5–4.4 cm: reexamination at 12 months
4.5–5.4 cm: reexamination at 6 months
These recommendations, as is the case for thoracic aortic aneurysms, stem from our understanding of growth rates of normal and abnormal aortas as well as published rates of rupture at given sizes. No large, prospective studies that compare surveillance intervals exist. For the abdominal aorta, while the SVS recommends no further screening for aneurysms less than 2.6 cm, others have suggested 3 cm as the cutoff. Countering this is the findings that a significant proportion of 65-year-old men (13.8%) with an initial aortic diameter of 2.6 to 2.9 cm developed aneurysms exceeding 5.5 cm at 10 years. Given current life expectancy projections, it is evident that a subset of patients deem “normal” at screening will go on to develop clinically significant aneurysms.
Surveillance of thoracic aortic aneurysms is more an art than science. With the exception of the aortic root, visible with TTE, the thoracic aorta generally requires CT or MRI contrast imaging. For the ascending, arch, and descending aortas, after the initial diagnosis a second study at 6 months should suffice to determine if there is measurable growth in the aortic diameter. This is followed with yearly scans until there is reasonable certainty of stability in size, at which time extending surveillance to a 2- to 3-year interval is tolerable. Conversion to MRI for surveillance avoids exposure of the patient to ionizing radiation.
Prevention of rupture of the growing aorta is the rationale for surveillance imaging, and risk models for rupture do exist. Juvonen and colleagues developed a model that included risk factors of age, presence of pain, chronic obstructive pulmonary disease, and maximal diameters of the thoracic and abdominal aorta. Based on this model, surgery was recommended when the calculated risk of rupture within one year exceeded the anticipated mortality risk of an elective procedure, even if the recommended aortic diameter for intervention was not reached.
Outcomes for surgical intervention of aneurysmal disease are dependent on location of the lesion, extent of disease, and technique of repair. Endovascular technology has had a dramatic impact on treatment options and outcomes, but open repair or hybrid approaches remain an important part of the armamentarium of the surgeon. For aneurysms involving the aortic root and ascending aorta, repaired electively, the mortality rate is approximately 5% for all patients and likely less when performed in high-volume centers. When aneurysms involve the arch, the mortality outcomes are similar to the ascending aorta, but there is an increase in morbidity, specifically with cerebrovascular injury.
With the descending thoracic aorta, the thoracic endovascular aorta repair (TEVAR) approach has become the standard of care for most aneurysmal disease. Since the first reported series by Dake and colleagues in 1994, the indications for TEVAR have expanded with a paucity of randomized controlled studies comparing TEVAR to open repair of descending thoracic aortic aneurysms. A metaanalysis of forty two nonrandomized studies with 5888 patients demonstrated a reduction of all-cause mortality and paraplegia, as well as a decrease in complications and length of stay. Beyond one year, there was no difference in mortality or surgical reintervention.
For treatment of TAAAs, no endovascular devices are currently approved for use in the United States, and open surgery is the mainstay of treatment. Recently reported surgical mortality has ranged from 2.3% to 7.5% in specialized centers and over 20% in “real-world” practice. A metaanalysis demonstrated a pooled mortality rate of 11.26% with Crawford Extent II having the highest risk of death. Pooled spinal cord ischemic rates (paraparesis and paraplegia) were estimated at 8.26%. Successful repair is reliant on a cohesive team approach, including cardiothoracic and/or vascular surgeons, cardiac anesthesiologists, trained nursing staff, and intensivists knowledgeable of the nuances and subtleties for caring for this patient population.
For infrarenal AAA, reported outcomes for open abdominal aortic repair vary from 1% to 4% for infrarenal repair performed in centers of excellence to 4% to 8% noted in statewide or nationwide databases. , Complications associated with open repair occur in 15% to 30% of patients. Both early mortality and complication rates are better with an endovascular approach to AAAs, although this may represent a selection bias. The United Kingdom EVAR trial investigators randomized 1252 patients with AAAs larger than 5.5 cm for elective open repair versus EVAR, and while the 30-day mortality rate was favorable for EVAR (1.8% vs. 4.3%, P = 0.02), this benefit was lost by the end of the study. Additionally, EVAR was more costly in part due to increased rates of graft-related complications and need for reintervention. A similar study by the Dutch Randomized Endovascular Aneurysm Management (DREAM) trial group found early advantages of EVAR to have disappeared by the two-year follow-up mark.
With currently approved and available technology, roughly 80% of nonemergent AAAs can be repaired with an endovascular approach. When considering an endovascular approach, accurate evaluation of anatomy is essential. This includes the neck of the aneurysm, which should be of sufficient length and without severe angulation, extensive mural thrombus, or calcification. The diameter of the proximal seal zone must not be too large to prevent a proximal seal. Access vessels (iliac and femoral arteries) should be of sufficient diameter to allow passage of appropriately sized sheaths.
As newer generation devices have become available, anatomic indications have expanded. AAAs with necks as short as 4 mm long can be treated with custom-made fenestrated stent-grafts or with suprarenal fixation and endoanchors. Many aortoiliac aneurysms can be treated with iliac-branched devices. Arteriotomy closure devices have allowed many aneurysms to be safely repaired with a totally percutaneous approach, and while postprocedure pseudoaneurysms are more common with arteriotomy closure devices, there are fewer seromas, wound dehiscences, and surgical site infections associated with the use of arteriotomy closure devices. On the horizon may include branched endografts to treat pararenal or TAAAs. As of yet, these devices are not available in the United States.
Postoperative evaluation of a successful EVAR includes serial CT scans and/or color duplex ultrasonography, looking for aortic sac diameter or volume, graft migration, and endoleaks. Surveillance imaging is generally recommended at 1 month, 6 months, 12 months, and yearly thereafter provided a stable exam. Because of the need for ongoing surveillance, patients who may be unwilling or unable to undergo postoperative imaging may not be appropriate candidates for EVAR.
When identified, endoleaks are classified as:
Type 1A (proximal seal zone)
Type 1B (distal seal zone)
Type 2 (retrograde flow from lumbar and/or inferior mesenteric arteries [IMAs])
Type 3 (component separation)
Type 4 (fabric porosity)
Type 5 (expanding aneurysm without demonstrable blood flow)
Type 1 and 3 endoleaks require repair. Type 2 endoleaks are common early after EVAR, with most resolving by 1 to 6 months. Management of persistent type 2 endoleaks is controversial, with most favoring observational management in the absence of sac growth. If sac growth occurs after EVAR with an otherwise stable type 2 endoleak, delayed type 1 or 3 endoleak may be the underlying culprit.
When anatomic considerations fall outside the “Instructions for Use” for endovascular devices, open repair remains the most acceptable treatment option. Additional potential considerations for open repair include younger patients, patients with connective tissue disorders, and, as discussed above, those who are unable or unwilling to participate in postoperative surveillance. For such patients whose comorbidity excludes open repair, novel approaches to endovascular repair have been described. These options may include snorkel/chimney EVAR, physician modified endografts, and use of investigational devices not currently available for clinical use in the United States. These options necessitate local expertise and may require federally approved Individual Device Exemption (IDE) prior to proceeding with these alternatives.
Ruptured AAAs deserve special attention. Approximately 15,000 annual deaths from ruptured AAAs occur in the United States. In ruptured AAAs where it would be ideal to treat with an endovascular approach, there has not been any statistical evidence of a short-term benefit of one approach versus another. In one European trial, comparison between open and EVAR showed no difference in operative mortality (39% vs. 35%, respectively) or 90-day mortality (42% vs. 40%). In the IMPROVE trial (The Immediate Management of the Patient with Ruptured Aneurysm: Open Versus Endovascular repair) there was no difference in 30- or 90-day mortality, but there was an advantage for EVAR at three years (42% vs. 54%); but once again this advantage was gone by 7 years. Interpreting these trials is challenging, as intention-to-treat and treatment received may not align, thereby biasing the conclusions. Post hoc analysis of these data suggest that those who were able to receive an endovascular repair enjoyed better outcomes, and therefore it is the recommendation of many to consider an “endovascular-first approach.”
Rapidly and effectively treating ruptured AAA requires trained personnel, access to endovascular inventory, and standard standardized protocols. Examples of such may include systems for rapid transport to an operating room with both open surgical and endovascular capability, minimal preoperative testing and imaging, and placement of an aortic occlusion balloon. , The latter should be placed prior to induction of anesthesia, as it may support blood pressure regardless of the type of repair offered. For patients amenable to EVAR for ruptured AAA, outcomes may be improved if general anesthesia is avoided altogether and the procedure performed under local anesthesia. ,
Of note, many patients with ruptured AAA present with significant comorbidities or severe acutely deteriorating clinical condition. For this reason over the past two to three decades, many scoring systems have been proposed to predict nonsurvivability and therefore consider comfort care instead of attempts at definitive repair. These scoring systems either were developed before the advent of EVAR or have not been validated with contemporary care. The University of Washington scoring system is one of the few that predicts futility rather than increased mortality risk and therefore may be more useful. A recent institutional study demonstrated that only a very small proportion of patients met futility criteria with this scoring system. Additionally, outcomes out-performed all predictive models, thereby raising concerns of the clinical usefulness of such scoring systems. As such, each patient should be judged individually to determine suitability for intervention and definitive repair.
With increasing specialization of vascular surgery and expansion of EVAR for ruptured AAA, many hospitals are no longer equipped to provide care, thereby necessitating the need for transfer of these critically ill patients. Utilization of transfer appears to be increasing, creating additional challenges for timely intervention. When available, local care may represent the best treatment; for although operative mortality is better for transferred patients, overall mortality is worse. This paradox is most likely explained by selection bias of those who are clinically stable to receive treatment once they arrive at the receiving hospital. In fact, approximately one in seven patients who are transferred do not receive treatment, which may be due to clinical deterioration, severe comorbidities that may not have been recognized at the sending hospital, or patient refusal. To address this, current guidelines as well as regional societies provide recommendations for transferring patients with ruptured AAA to optimize the transfer process and provide the greatest opportunity for definitive repair in transferred patients.
We consider AD, IMH, and PAU together because of the interplay between these three disease states, and each may be representative of the same disease within a spectrum and in fact may occur together. What separates the three, in part, is the level of involvement of the aortic wall. While aneurysmal disease involves all three layers of the aorta, PAU is a disease of the intima and media, and AD and IMH a disease of the media, with significant overlap between the three.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here