Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Dry eye disease (DED) is an umbrella term comprising a variety of clinical manifestations with differing underlying pathophysiologies.
In patients presenting with DED, a comprehensive, systematic examination is needed; this should include medical history, symptom assessment, ocular surface evaluation, and vital dye staining.
Point-of-care tests and imaging technologies complement the clinical examination and help to subtype DED, stratify severity, and tailor treatment.
Available point-of-care tests include quantification of tear osmolality and qualitative detection of ocular surface inflammation via matrix metalloprotease 9.
A variety of imaging technologies can be used to assess components of the ocular surface, including noninvasive tear break-up time, bulbar hyperemia, meibomian gland anatomy, and corneal irregularity.
Corneal esthesiometry, assessed prior to instillation of topical anesthetic drops, provides information about the functional health of the corneal nerves.
Confocal microscopy can be used to assess the density and morphology of the corneal nerves.
I would like to acknowledge Amy Kloosterboer for her help preparing this chapter.
The tear film has many important roles, including providing a refractive surface for light entering the eye, maintaining the health of the ocular surface, and aiding in repair after an insult or injury. The tear film is a bilayer structure composed of a mucoaqueous phase, which is in contact with the epithelium, and an overlying lipid layer. The mucin component is in closest contact with the epithelium and is produced by various cells on the ocular surface (epithelial and goblet cells). At least 20 mucin (MUC) genes have been identified, and mucins are classified into two categories: secretory mucins (gel-forming and non–gel forming) and transmembrane mucins. The most commonly expressed gel-forming mucin on the ocular surface is MUC5AC. The aqueous component of tears, produced by the main and accessory lacrimal glands, contains water, electrolytes, proteins (including lactoferrin and lysozyme), sugars, and other water-soluble substances. The lipid layer is mostly composed of meibum, produced by the meibomian glands, which are housed within the tarsal plate. Meibum is composed of synthesized lipids, membrane-derived lipids, and bacterial degradation products (e.g., free fatty acids). Nonpolar lipids (wax and sterol esters) constitute the largest portion of meibum (77%) and act as a lubricant and barrier to water. Polar lipids (phospholipids) constitute a lower percentage (8%) and act as surfactants to help spread the lipid layer across the ocular surface and also to provide structural support to the nonpolar lipids. The lipid layer functions to stabilize the tear film, retard evaporative tear loss, and act as a barrier from outside contamination. Proper eyelid position and function, a smooth conjunctival surface, and a healthy epithelial layer are also needed to support proper mixing and stability of the tear film components. In addition, intact and healthy corneal and conjunctival nerves are needed to maintain epithelial cell integrity and provide correct information to the brain and lacrimal functional unit.
Dry eye disease (DED) can occur when any of the entities mentioned are damaged or abnormal. The Tear Film and Ocular Surface Society defined DED as “a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.” Implicit in this definition is that DED is an umbrella term for a variety of potential clinical manifestations driven by a number of etiologic factors. DED has traditionally been broken down into aqueous tear deficiency (ATD), evaporative deficiency, and mixed categories. More recently, another recognized aspect of DED is nerve abnormalities, which can lead to painful DED symptoms both with and without the presence of tear deficits. Many intrinsic and extrinsic factors can influence tear film health, including hormonal status, medication use, anatomy, comorbid diseases, and environmental exposures (pollution, prolonged computer use). Given this complexity, this chapter summarizes a systematic approach in the evaluation of DED and reviews available point-of-care tests and imaging technologies that can aid in the diagnosis and stratification of disease.
Ophthalmologists rely on patients’ symptoms to diagnose and subtype DED, assess its severity, and determine its impact on quality of life. Standardized questionnaires that record and quantify symptoms are practical tools for the diagnosis and management of DED. , DED symptoms can include a wide range of dysesthesias—often characterized as “dryness,” “irritation,” “burning,” “tenderness,” and “aching,” to name a few—as well as visual complaints such as blurred or fluctuating vision, tearing, and/or contact lens intolerance. Exacerbating conditions can include changes in environmental conditions such as air travel, changes in humidity, and exposure to wind and light as well as activities inducing reduced blink rates such as reading and computer use. A comprehensive ocular history should investigate prior ocular surgery (e.g., refractive surgery, surgery involving the eyelid), prior facial palsies, use of topical medications, and contact lens use and hygiene. A comprehensive medical history should explore dermatologic diseases, systemic inflammatory diseases, neurologic conditions, chronic pain conditions, sleep disorders (e.g., sleep apnea and treatment with continuous positive airway pressure), atopic disease, prior malignancies requiring radiation of the orbit, hormonal changes, chronic viral infections, trauma, mental health disorders, and use of systemic medications (e.g., antihistamines, antidepressants). It can be difficult to address all aspects of a patient’s history in a short office visit; therefore physicians often utilize questionnaires to help in this endeavor.
Several validated questionnaires can be used in the clinical setting to assess DED, the most common of which is the Ocular Surface Disease Index (OSDI). It consists of 12 questions that evaluate the presence of ocular symptoms, the timing and frequency of symptoms over the preceding week when the patient was performing vision-related activities, and the occurrence of symptoms with certain environmental triggers. Each question has a five-category Likert-type response option, ranging from “none of the time” to “all the time.” An option of “not applicable” is available for patients who did not perform the activities or experience any of the vision-related functions and environmental conditions outlined. The questionnaire requires approximately 5 minutes to complete and is scored from 0 to 100 points, with DED symptoms categorized as normal (0–12), mild (13–22), moderate (23–32), or severe (33–100). , Other validated questionnaires include the Dry Eye Questionnaire 5 and the Standard Patient Evaluation of Eye Dryness (SPEED). , Although none of the questionnaires encompass all aspects of DED symptoms, they are nonetheless helpful in highlighting whether DED symptoms are pain related, vision related, or both, and they also quantify symptom severity. Of note, a challenge is that a DED symptom report can often conflict with the clinician’s observed signs.
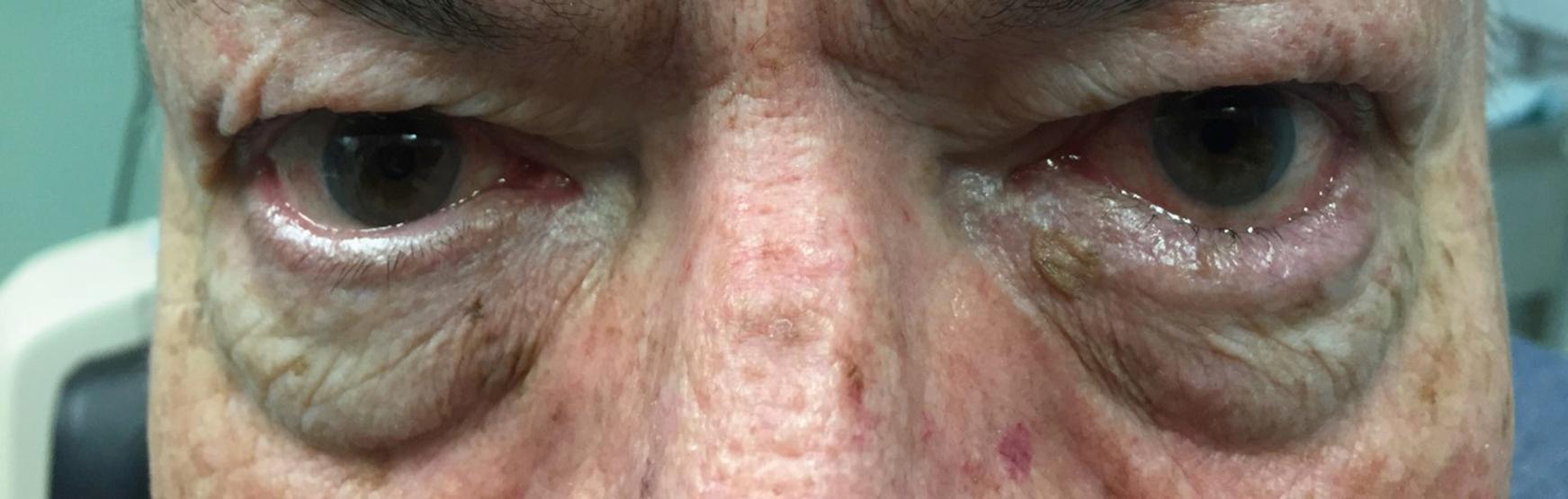
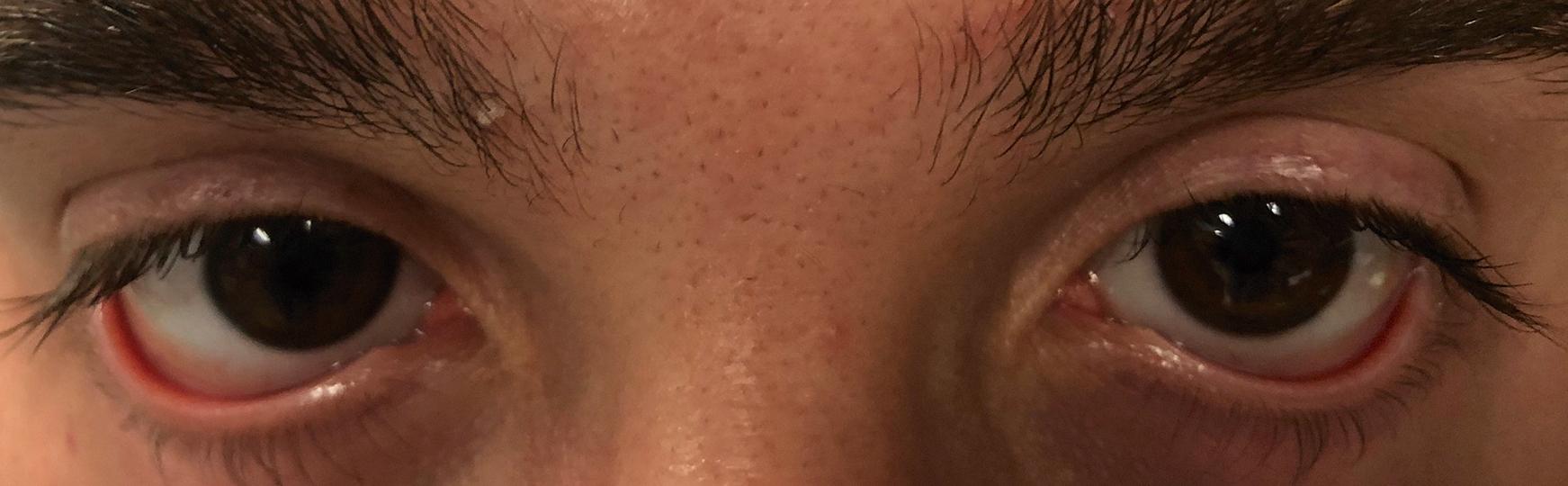
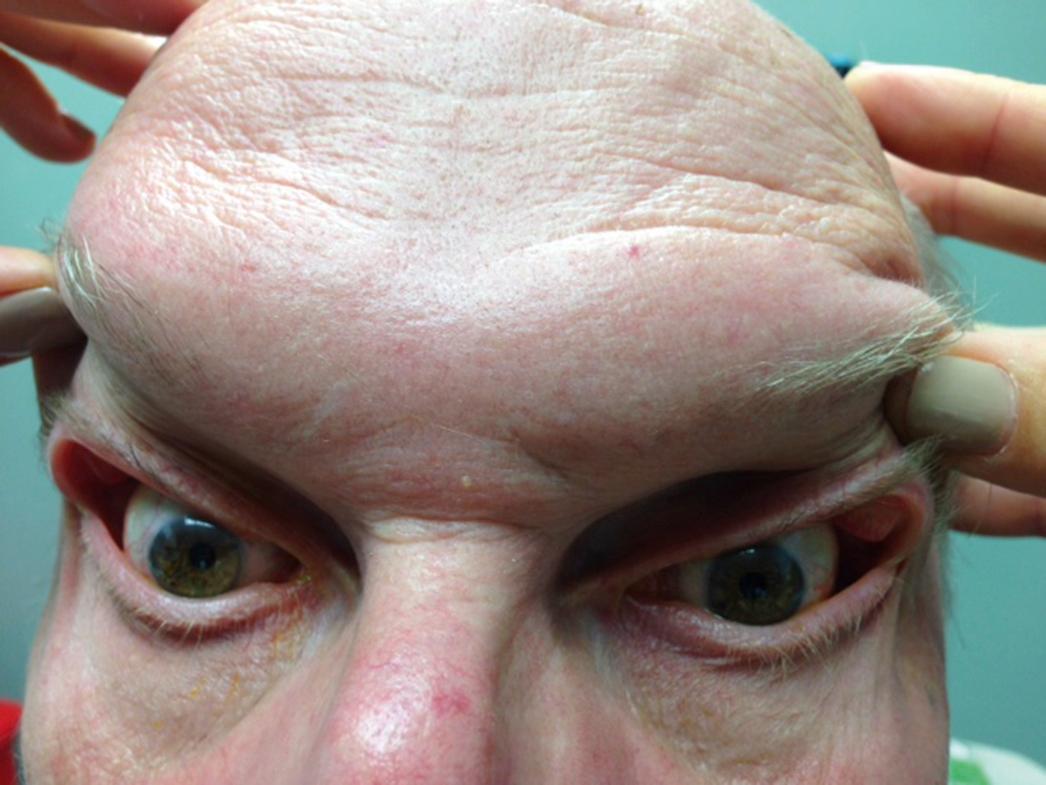
The DED examination should begin as soon as the physician enters the room. His or her attention should be drawn to the patient’s face with a focus on the periorbital skin, looking for signs of rosacea (often seen in individuals with meibomian gland dysfunction [MGD]) or seborrheic dermatitis (often seen in individuals with anterior blepharitis). Furthermore, the examiner should note blink abnormalities. Individuals with neurologic diseases, such as Parkinson disease, often have a reduced blink rate, which can exacerbate DED. Eyelid anatomy should then be examined with a focus on eyelid position. The eyelids should nicely approximate the ocular surface, and no scleral show should be noted. Scleral show above the limbus suggests underlying thyroid eye disease, whereas scleral show below the limbus suggests a component of exposure. Next, function should be examined by asking the patient to close the eyes softly (looking for lagophthalmos) and forcefully (looking for spastic entropion [ Fig. 8.1 ] and/or upper eyelid override). In addition, the presence of an intact Bell reflex can be evaluated by asking the patient to close the eyes while the examiner holds the upper eyelid and observes the cornea. Interestingly, about 5% of the normal population has an absent or deficient Bell reflex. Finally, eyelid laxity can be evaluated by pulling down on the lower eyelid and up on the eyebrows. Failure of the lower eyelid to “snap back” and oppose the globe is an indication of lower eyelid laxity ( Fig. 8.2 ). Flipping of the upper eyelids when pulling on the eyelash skin indicates the presence of upper eyelid laxity ( Fig. 8.3 ). Eyelid laxity has been associated with sleep apnea, and a referral for evaluation is needed in individuals with appropriate risk factors (e.g., snoring, excessive daytime sleepiness). Any eyelid abnormality—including ectropion, entropion, and lagophthalmos—can interfere with normal tear film dynamics and thus underlie or be a component of DED.
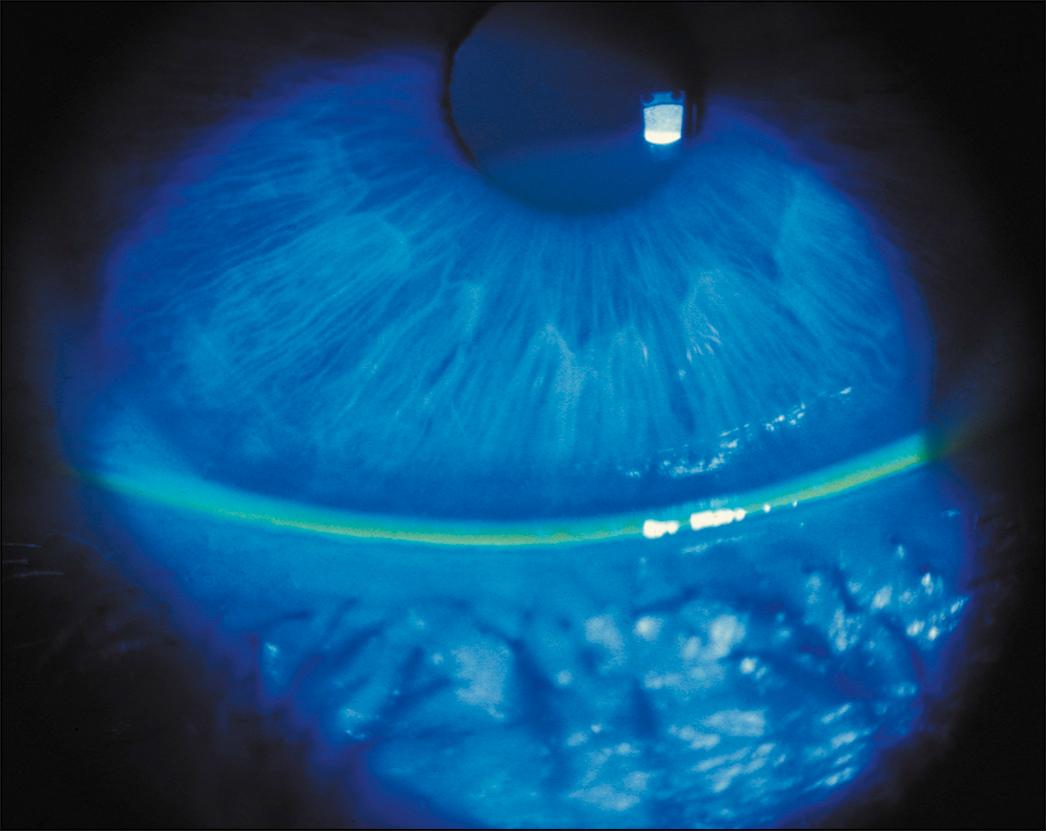
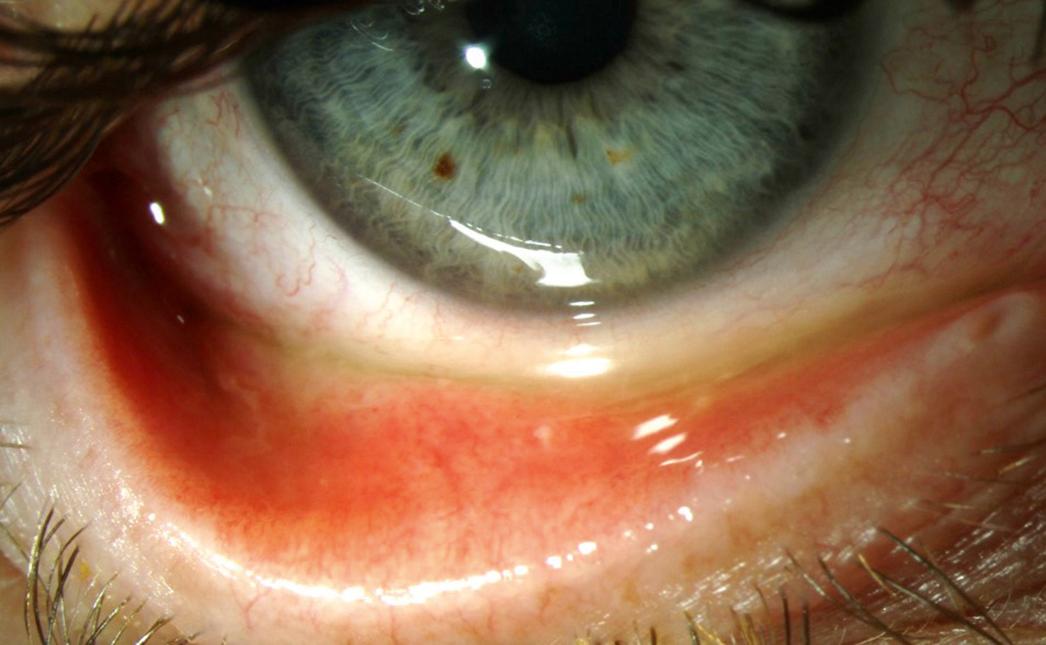
Next, a slit lamp examination should be performed to systematically examine the ocular surface, starting with the lid margin and looking for signs of anterior blepharitis, trichiasis, telangiectasias, and/or meibomian gland plugging. Next, the presence and height of the tear meniscus should be noted. The tear meniscus is the line of tears just above the lower lid ( Fig. 8.4 ). It is normally about 0.5 mm in width and has a concave upper aspect. A thin or discontinuous strip can be seen in individuals with ATD. Fluorescein staining can be used to visualize this, but care must be taken not to flood the surface by overwetting the fluorescein strip. Instead, the strip should be barely moistened. The presence of conjunctivochalasis should also be evaluated for and is seen as a replacement of the tear meniscus with folds of inferior conjunctivae. This can occur focally (in the medial, central, and/or temporal aspects of the tear meniscus) or can occur diffusely ( Fig. 8.5 ).

The lower eyelid should then be pulled down and the palpebral conjunctiva examined to evaluate for papillae, follicles, and/or scarring. Mild papillae can be seen with many DED sub-types ( Fig. 8.6 ), but diffuse papillae suggest a comorbid issue such as allergy. Scarring of the conjunctivae can likewise be due to a variety of insults (e.g., adenoviral conjunctivitis), but the presence of such an insult can also suggest that an immune-bullous disorder, such as mucus membrane pemphigoid, underlies the DED. As such, a biopsy is warranted in these cases to evaluate for diseases comorbid with DED. The quality of the tears should also be examined, with specific attention to the presence of debris in the tear film, which suggests an unhealthy tear composition. Finally, the bulbar conjunctivae should be examined for injection and/or anatomic abnormalities such as pterygium, pingueculum, or elevated bleb, all of which can have a negative effect on tear health.
Tear film instability can be detected in many individuals with an unhealthy/abnormal tear film. After the components of the tear film have been mixed by the action of the lids, a meta-stable tear film is established. Over time (usually 10–30 seconds), the tear film thins, leading to the development of randomly distributed dry spots in the precorneal tear film ( Fig. 8.7 ). The interval between the last complete blink and the appearance of the first random dry spot is defined as the tear break-up time (TBUT). This is generally measured by applying fluorescein to the bulbar conjunctiva. A wide slit lamp beam with the cobalt blue filter is used to scan the cornea after the patient has first been instructed to blink several times and then not to blink. The appearance of the first dry spot in the fluorescein-stained precorneal tear film is measured using a timer. This is repeated several times and averaged. Values less than 10 seconds are considered marginal and values less than 5 seconds are considered abnormal. Of note, the amount of applied fluorescein can influence TBUT results; consequently noninvasive (without fluorescein) imaging techniques have been developed (e.g., as included in the Keratograph 5M [OCULUS]). The two measures do correlate with one another, and in one study, average noninvasive TBUT had better between-visit agreement compared with fluorescein-assisted TBUT.
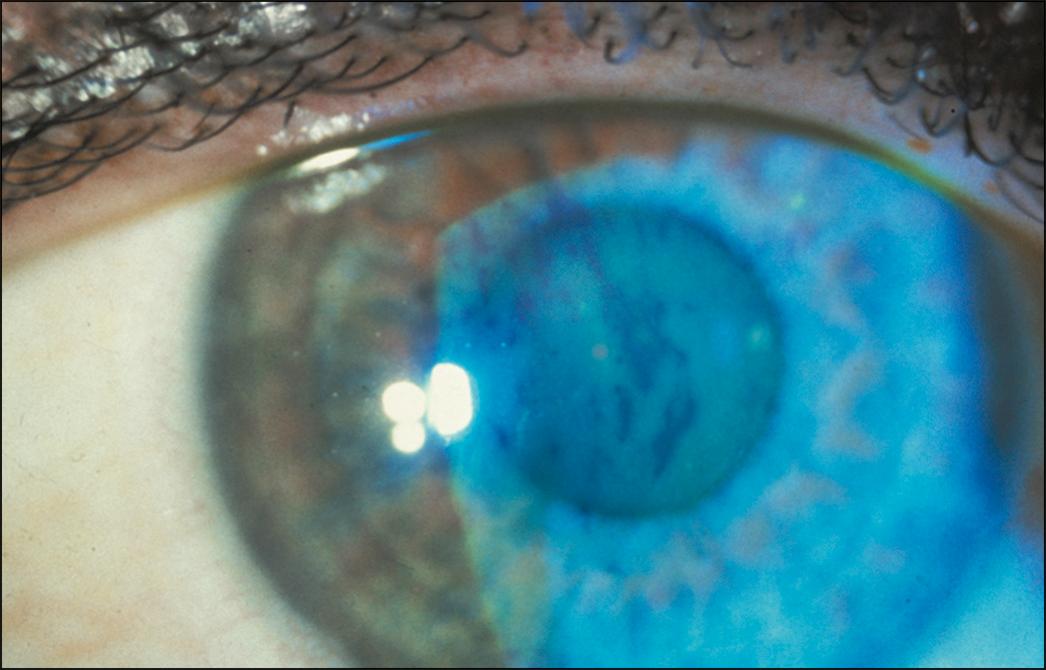
Abnormal TBUT values can be seen in a variety of DED subtypes, including ATD, evaporative deficiency, and in the setting of environmental triggers (e.g., computer use). Furthermore, the tear film will break up rapidly over an underlying epithelial irregularity such as a Salzmann nodule; thus decreased values are not necessarily indicative of an underlying tear film disorder. Furthermore, it is imperative not to consider TBUT values in isolation but to also consider blink rate, as a healthy epithelial surface can generally be maintained if the time between blinks is faster than the TBUT.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here