Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
T-cell lymphomas are a highly heterogeneous group of diseases sharing common origin from mature T-lymphocytes. Heterogeneity is appreciated on biologic and clinical grounds, both in terms of presentation (leukemic, nodal, extranodal, and cutaneous forms) and clinical behavior (aggressive versus indolent). Historically, peripheral T-cell lymphomas (PTCL) have always been separated by cutaneous T-cell lymphomas (CTCL), mostly because the former has a nodal and aggressive presentation, while the latter display relatively indolent (although progressive in most of the times) course.
Treatment approaches are mostly inadequate for the treatment of highly aggressive disease subtypes. New molecules hitting specific biologic targets have emerged as opportunities in patients with relapsed disease, yet with very short response durations. On the other hand, combinations of newer agents with conventional chemotherapy in untreated patients or strategies within chemo-free combination is an active field of research.
All the most frequent and clinically relevant leukemic T-cell lymphomas, PTCL, and CTCL will be treated in detail in the following sections of this chapter, with a focus on their epidemiology, morphology, biopathology, clinical manifestations, and prognosis. Current treatment approaches and future directions in the treatment of these diseases are discussed separately for leukemic T-cell lymphomas, PTCL, and CTCL at the end of the corresponding sections of the chapter.
In 2017, the classification of hematologic and lymphoid neoplasm was updated, and the World Health Organization (WHO) now recognizes 29 established and provisional entities of mature T-cell non-Hodgkin lymphomas ( Table 89.1 ). Provisional entities are lymphoma categories for which scientific data are not fully validated by large studies, thus forthcoming additional biologic elements are required to confirm—or not—these entities as established varieties.
|
|
* Entities in bold type are explained in the text. Provisional entities are listed in italics.
This complex classification underscores the complexity and the great heterogeneity of these neoplasms, as it emerges from the advances in the molecular partitioning of these diseases. On the other hand, a more appropriate classification—based on morphological, immunophenotypical, clinical, and molecular grounds—is aimed at better stratifying patients in terms of prognosis and outcomes, at guiding physicians’ decisions before and throughout treatment, and at providing new insights into potentially more effective therapies or drug combinations (see box on Advances in Mature T-Cell Lymphoma Classification ).
The significant advances occurred in the classification of both nodal and extranodal T-cell and natural killer-cell neoplasms have led to revisions: many of these changes are the result of genomic studies using approaches to examine the gene expression profile and the genetic landscape of these neoplasms. This box highlights the innovations with greater impact on clinical and therapeutic grounds. More details are found in the text.
Peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS) . Subsets are recognized based on phenotype and molecular abnormalities as that may have clinical implications. Stratification however is mostly not a part of routine practice at present.
Nodal T-cell lymphomas with T FH phenotype . This is an umbrella category that encompasses the spectrum of nodal lymphomas with a T FH phenotype. It includes AITL, follicular T-cell lymphoma, and other nodal PTCL with a T FH phenotype and it serves to highlight the specific clinical and pathologic differences among these subtypes. Overlapping recurrent molecular and cytogenetic abnormalities are shared by these entities.
Anaplastic large-cell lymphoma (ALCL), ALK − . It is now a regarded as a definite entity that includes cytogenetic subsets with possible prognostic implications (e.g., rearrangements at IRF4/DUSP22 locus).
Breast implant–associated anaplastic large cell lymphoma (BIA-ALCL) . New provisional entity distinguished from other ALK-ALCL; noninvasive disease associated with excellent outcome.
Enteropathy-associated T-cell lymphoma (EATL) . This denomination is only to be used for cases formerly known as type I EATL. It is typically associated with celiac disease.
Monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL) . Formerly type II EATL, it is segregated from type I EATL and it is given a new definition due to its distinctive nature and lack of association with celiac disease.
Lymphomatoid papulosis . New subtypes are described with similar clinical behavior but atypical histologic/immunophenotypic features.
T-cell lymphomas are mature T-cell neoplasms, given that the postulated cell of origin is always a mature, post-thymic T-lymphocyte; this means that all mature T-cell lymphomas originate from a T cell born in the bone marrow that has already undergone T-cell receptor (TCR) gene rearrangement and has completed its pre-antigenic maturation ( Fig. 89.1 ). Mature T cells consist of α/β TCR (more than 90%) and γ/δ TCR lymphocytes: the former derive from precursor T cells that colonize the thymus, whereas the latter may originate from thymic or extrathymic tissues. The final maturation step of T-lymphocytes into either CD4 + or CD8 + T cells is determined by the kinetics and strength of TCR signals and the specific milieu of cytokines that dictates the selective expression of transcription factors.
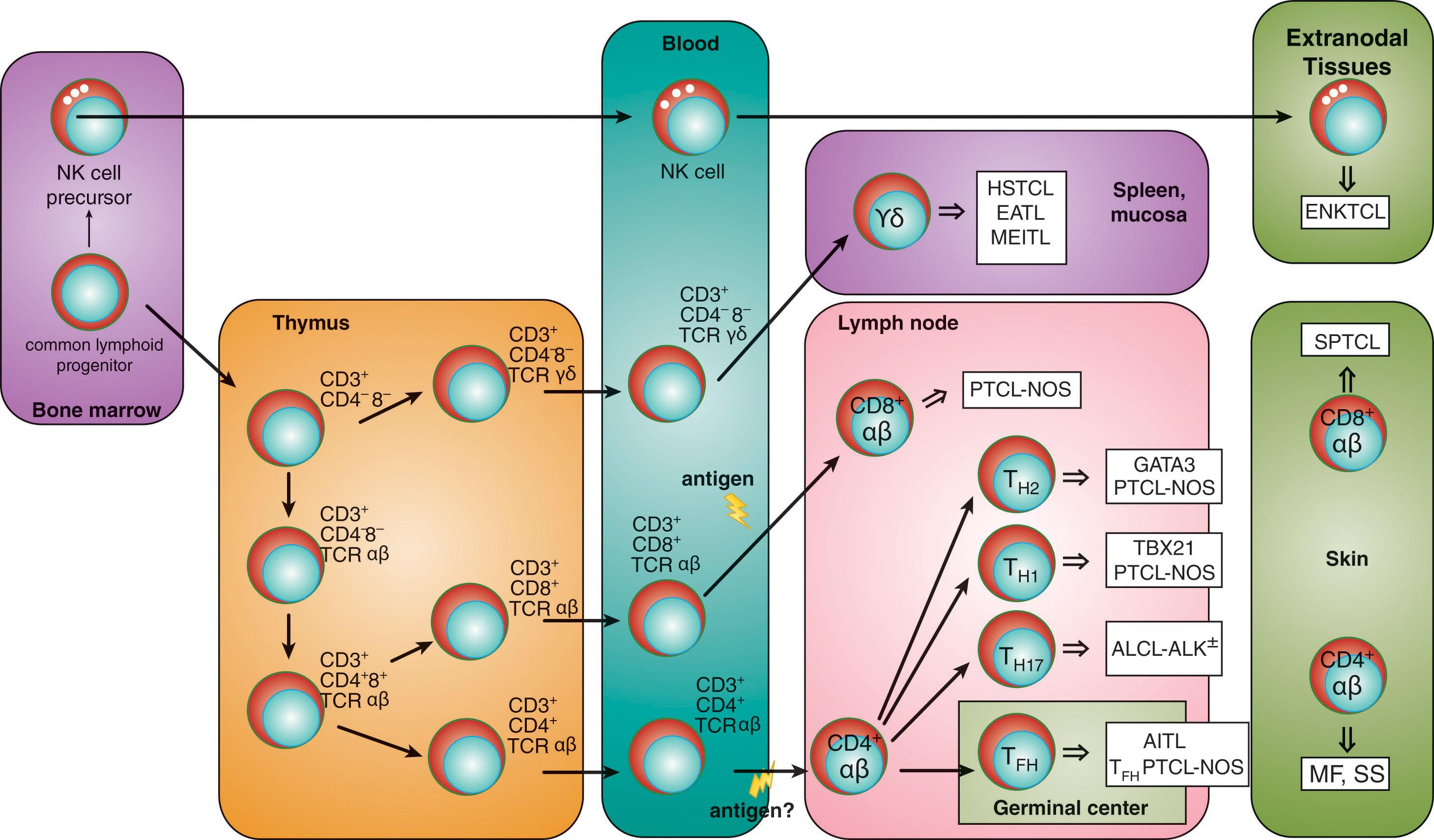
Under the heading mature T-cell lymphomas , four broad groups of diseases can be distinguished according to their presentation ( Table 89.2 ): leukemic, nodal, extranodal, and cutaneous. This partition has limitations, however, as in many instances nodal diseases may display a concomitant extranodal involvement, a cutaneous presentation, or a peripheral blood leukemic dissemination.
Nodal Forms : Among the nodal forms, peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS), angioimmunoblastic T-cell lymphoma (AITL), and anaplastic large-cell lymphoma (ALCL) and its two variants (distinguished according to the expression of the anaplastic lymphoma kinase [ALK]) are the most relevant entities. PTCL-NOS includes a heterogeneous group of neoplasms derived either from mature CD4 + or CD8 + α/β TCR lymphocytes and does not fit into any of the specifically defined entities. AITL originates from T follicular helper (T FH ) cells, a subset of T-lymphocytes that derive from naïve T cells homing to the germinal center of the lymph node and differentiating under the pressure of a strong TCR signal through the interaction with dendritic cells, cytokines, and B-lymphocytes. T FH cells are putatively the normal counterpart of follicular T-cell lymphoma and PTCL with T FH phenotype. ALCL-ALK + has been suggested to derive from thymocytes, whereas the origin of ALCL-ALK − is still debated.
Extranodal Forms : Among the extranodal forms, NK/T-cell lymphoma nasal type (ENKL), subcutaneous panniculitis-like T-cell lymphoma (SPLTCL), hepatosplenic T-cell lymphoma (HSTCL), and primary intestinal T-cell lymphomas—which in turn are distinguished into enteropathy-associated T-cell lymphoma (EATL) and monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL)—are the most clinically relevant subtypes. All nodal PTCL and most extranodal PTCL display an aggressive behavior, as they usually present with systemic symptoms and may cause a rapid clinical decay if not promptly treated. ENKL derives from the transformation of NK cells or cytotoxic innate cells, probably under the oncogenic pressure of the Epstein-Barr virus (EBV). HSTCL, EATL, and MEITL may derive from either innate cells (NK/T cells or γ/δ TCR CD3 + lymphocytes), adaptive cells (CD4 + or CD8 + α/β TCR lymphocytes), or γ/δ TCR CD3 + intestinal intraepithelial lymphocytes.
Cutaneous Forms : Mycosis fungoides (MF) and primary cutaneous CD30 + T-cell lymphoproliferative disorders are cutaneous diseases with a prolonged natural history and treatment may be unnecessary in the first stages of the disease. MF, however, invariably and progressively evolves into more aggressive stages, requiring an adequate treatment, and it is ultimately fatal. Sézary syndrome (SS) can be considered as the leukemic phase of MF, since the tumor arises in an extranodal organ—i.e., the skin—but also displays a characteristic leukemic picture: this is an aggressive form of CTCL, requiring specific treatment and characterized by a dismal prognosis. MF, SS, and CD30 + T-cell lymphoproliferative disorders derive from CD4 + T-lymphocytes with a specific tropism for the skin.
Leukemic Forms : These are mainly represented by T-cell prolymphocytic leukemia (T-PLL), T-cell large granular lymphocytic leukemia (T-LGL), aggressive natural killer (NK)-cell leukemia, and adult T-cell leukemia/lymphoma (ATLL). These diseases always arise in the bone marrow and then evolve with a peripheral blood leukemic presentation. T-PLL is discussed in more detail in this chapter.
|
|
|
|
|
|
|
|
T-PLL is a rare, post-thymic, mature T-cell neoplasm, accounting for 2% of mature lymphoid leukemias. It generally displays an aggressive clinical course and only a minority of patients are asymptomatic at presentation. Disease progression occurs rapidly, even in asymptomatic patients. Older adults are generally affected (median age of 61 years), with a male predominance.
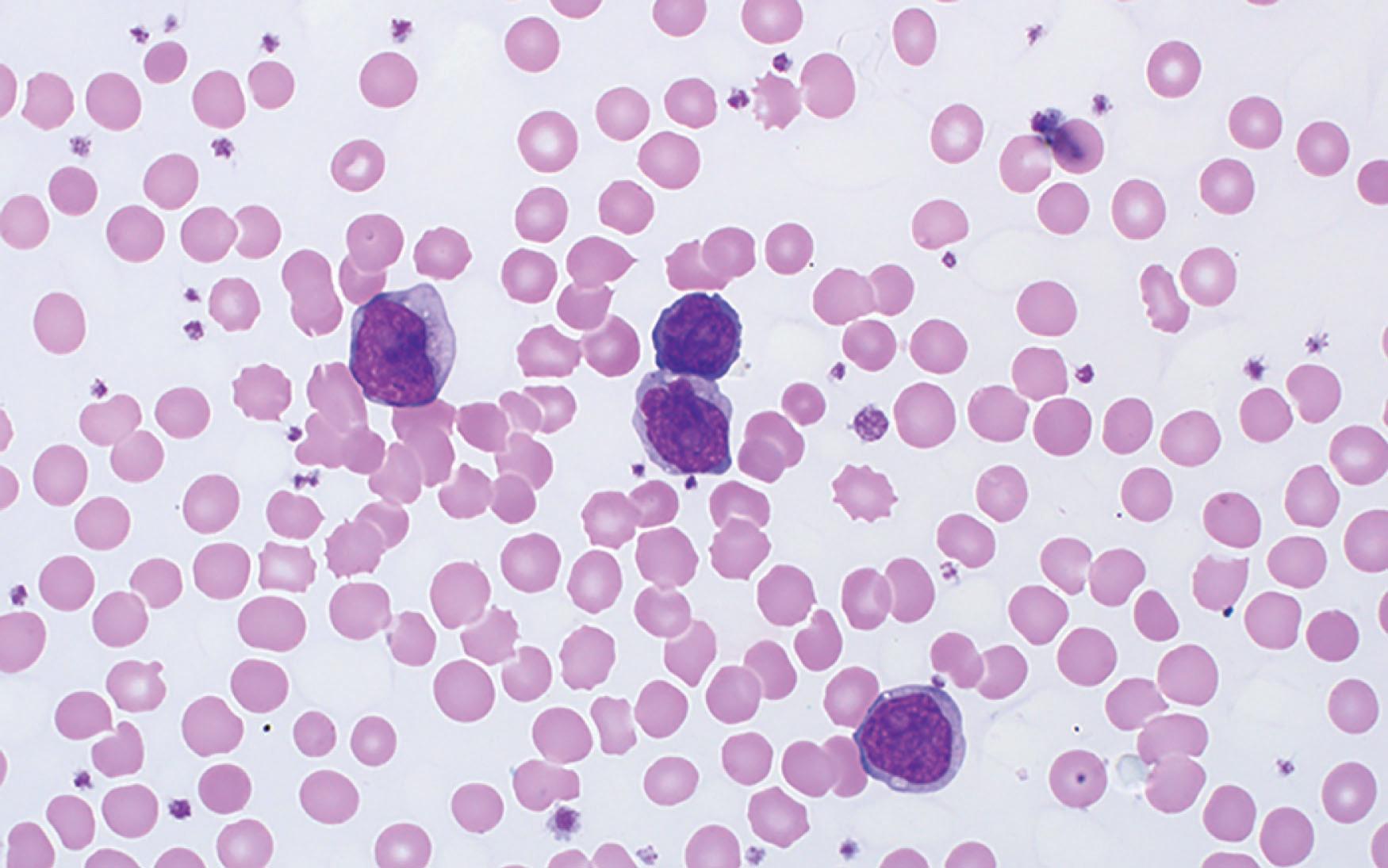
Prolymphocytes are medium-sized cells, displaying a high nuclear-cytoplasmic ratio, with round, oval, irregular, or convoluted nuclei and a single prominent nucleolus. Their cytoplasm is agranular, frequently with blebs. In up to 20% of cases, prolymphocytes may resemble small lymphocytes or show a cerebriform nuclear appearance ( Fig. 89.2 ). Bone marrow is constantly infiltrated, with a diffuse and interstitial pattern, as well as the spleen, which shows an expansion of both red and white pulp.
In virtually all cases, the Xq28 ( MTCP1 locus) or 14q32.1 ( TCL1 and TCL1b loci) regions are involved in translocations or inversions with TCRA/D at 14q11: this determines the activation of all the three genes and their consequent overexpression, all being implied in T-PLL leukemogenesis by conferring resistance to cell death and determining cell growth arrest. Other complex karyotype alterations may be detected, above all chromosome 8 unbalanced rearrangements, like trisomy, monosomy, t(8;8)(p12;q11), or other translocations with another chromosomal partner. Mutations of the ATM gene at 11q21–q23 are also demonstrated in virtually all sporadic cases of T-PLL, reinforcing the association of T-PLL with ataxia-telangiectasia, regarded as a predisposing condition.
Splenomegaly, seen in almost two thirds of patients, and marked peripheral blood lymphocytosis (usually much higher than 100,000/mmc), with circulating prolymphocytes, are distinctive clinical aspects. Hepatomegaly and lymphadenopathies are seen in up to half of patients, as well as skin nodules, maculopapular rash, or erythroderma. Peripheral edema (periorbital or conjunctival), pleuroperitoneal effusions, or central nervous system involvement may also be present, especially during disease progression. Anemia and thrombocytopenia are usually documented, along with lactate dehydrogenase (LDH) elevation.
It is established on both morphological and immunophenotyping tests. Tissue histology, however, is not mandatory for diagnosis. Immunophenotyping by flow cytometry demonstrates a positivity for CD7 (strong), CD5, and CD2, with lack of expression of terminal deoxynucleotidyl transferase (TdT) and CD1a. Most cases are CD4 + and CD8 − (60%), but the phenotype may also be CD4 + /CD8 + (15% to 25% of the cases) or CD4 − /CD8 + (10% to 15% of the cases). CD52 is expressed at high density. TCR α/β genes are rearranged in most of the cases, and just in a minority of instances a γ/δ rearrangement is detected.
Table 89.3 displays the requirements to establish the diagnosis of T-PLL: the diagnosis is confirmed when all the three major criteria are met or if the first two major criteria and one minor criterion are met.
| Major Criteria | Minor Criteria |
|
|
T-cell neoplasms with leukemic presentation other than T-PLL can be ruled out by morphology evaluation and flow cytometry assays. The most relevant diseases that can resemble T-PLL are represented by: T-cell acute lymphoblastic leukemia (T-ALL), leukemic dissemination of PTCL, T-LGL, SS, and ATLL. Table 89.4 summarizes the criteria to accomplish a correct differential diagnosis.
| Disease | Morphology | Immunophenotype |
|---|---|---|
| T-PLL | High nuclear-cytoplasmic ratio, round or oval and irregular or convoluted nuclei, single prominent nucleolus, agranular cytoplasm, frequently with blebs | TCL1 + (>90%), CD3 + (>80%), CD4 + (60%), CD5 + (100%), CD7 + (>90%), CD8 + (15%), CD4 + CD8 + (25%) |
| T-ALL | Blasts with high nuclear-cytoplasmic ratio, rather condensed chromatin, inconspicuous nuclei, scarce agranular cytoplasm | TdT + , CD1a + |
| Leukemic PTCL | Highly pleomorphic mature lymphocytes | TCL1 − |
| T-LGL | Round eccentric nucleus with dense chromatin, abundant pale cytoplasm with numerous azurophilic granules | CD8 + , CD57 + , CD16 + |
| SS | Convoluted (cerebriform) nucleus with condensed chromatin and sometimes evident nucleoli, agranular cytoplasm, sometimes with a ring of vacuoles | CD7 − , CD4 + , CD25 + |
| ATLL | Highly pleomorphic mature lymphocytes, sometimes with a characteristic lobated nucleus (flower cells) | CD4 + , CD25 + , HTLV1 + |
Since the disease is usually symptomatic, clinically aggressive, and rapidly progressive, a prompt initiation of a systemic treatment is required. On the other hand, 20% to 30% of patients demonstrate an initially stable or slowly progressive disease (“inactive” T-PLL). In these cases, a period of close observation is recommended. However, almost all “inactive” T-PLL turn into “active” (symptomatic) disease within 1 to 2 years.
Symptomatic T-PLL is defined as (at least one of the following): presence of constitutional symptoms (fatigue, unintentional weight loss of >10% of normal body weight within 6 months, drenching night sweats or fever >38°C without signs of infection); symptomatic bone marrow failure (hemoglobin <10 g/dL and/or platelet count <100,000/mmc); rapidly enlarging lymph nodes, spleen, and liver (>50% enlargement within 2 months, or diameter doubling within 6 months, or symptomatic organomegaly); increasing lymphocytosis (>50% within 2 months when lymphocytes are >30,000/mmc or doubling time shorter than 6 months); extranodal involvement (organ infiltration, peritoneal or pleural effusion, central nervous system involvement).
Intravenous alemtuzumab, a humanized anti-CD52 antibody, is the first-line treatment of choice, yielding up to an 80% of complete response (CR) in treatment-naïve patients. Of note, this treatment is frequently complicated by cytomegalovirus (CMV) reactivations, sometimes requiring adequate antiviral treatment. Thus, CMV-DNA monitoring during the courses is strongly recommended. Prophylaxis for Pneumocystis jirovecii is also indicated.
Although prolonged responses to alemtuzumab have been described, all patients have a tendency to relapse; therefore, a consolidation with an allogeneic stem cell transplantation in first remission may offer a chance for a better long-term survival, when feasible. Autologous stem-cell transplantation may also be beneficial for patients, but it does not result in a cure. Retreatment with alemtuzumab at disease relapse is feasible if remission duration is longer than 6 months. However, the emergence of a CD52 − phenotype clearly indicates a mechanism of resistance to alemtuzumab. Purine-analogues, such as pentostatin, cladribine, nelarabine, or fludarabine, may be a good treatment alternative, with some evidence of activity in T-PLL. Pentostatin, in particular, is the agent of choice when suboptimal responses to alemtuzumab are seen.
Experiences with targeted agents, like the combination of venetoclax and ibrutinib, are now ongoing. Reports exist on salvage treatment with JAK inhibitors in patients with multi-treated T-PLL harboring JAK mutations.
Treatment response criteria are outlined in Table 89.5 : both clinical and laboratory parameters need to be considered to define the depth of the response. A general recommendation on adequate timing to assess a response cannot be made, as response kinetics appear different depending on the agents used (chemotherapy versus antibodies).
| Group and Parameter | Complete Response (All Criteria to Be Met) | Partial Response (≥2 Criteria in A and ≥1 in B) | Stable Disease (All Criteria to Be Met) | Progression (≥1 Criterion in A or B to Be Met) |
|
|
|
|
|
|
|
|
|
|
Response to alemtuzumab is the main outcome predictor: non-responders have a median survival of only 4 months. Five-year overall survival (OS) is nearly 21%, with high white cell counts, older age, and short lymphocyte doubling time being adverse prognostic factors. Reports exist on the possible development of EBV-driven non-Hodgkin lymphomas in patients receiving alemtuzumab for T-PLL: an aggressive clinical course and rapid development of nodal lesions in an otherwise responding T-PLL patient always deserve attention (with imaging studies and nodal biopsy).
This section reviews some general aspects regarding the epidemiology of PTCL and the shared mechanisms underlying the lymphomagenesis of these entities. Specific disease subtypes are reviewed especially in terms of their biopathology, clinical peculiarities, and prognostic tools. First-line treatment approaches are discussed at the end, with a particular focus on the management of some specific subtypes, bearing in mind that the vast heterogeneity of these diseases, coupled with their rarity, has always hampered the design and development of adequately powered clinical studies able to provide strong treatment evidence. Recommended strategies for the treatment of relapsed and refractory disease, along with a focus on newly available single agents and innovative combinations, is also provided.
The most common entity is PTCL-NOS, which includes a heterogenous group of mature T-cell lymphomas representing up to a third of all PTCL. AITL is the second most common subtype, accounting for 15% to 30% of all PTCL, according to different case series. ALCL represents nearly 15% of all PTCL. Among extranodal entities, ENKL accounts for nearly 10% of all PTCL, followed by primary intestinal T-cell lymphomas (5% to 6% of all PTCL), HSTCL (2% of all PTCL), and SPLTCL (<2%). Some lymphomas display a peculiar geographical distribution: the broad category of PTCL-NOS is uniformly distributed in Western countries, where it represents the most frequent category. Similarly, AITL is more frequently diagnosed in Europe, although incidence varies across regions. It should be noted, however, that many cases previously characterized as PTCL-NOS can be reclassified into more recent 2017 WHO-recognized categories. Such update of classification may affect the real epidemiology of AITL and PTCL-NOS. Part of PTCL-NOS cases display a T FH phenotype, and thus require to be more correctly classified as T FH -derived PTCL. ENKL is mostly observed in Asia and Central-South America and shows higher prevalence among Hispanic people, American Indians, Asians, and Pacific Islanders (with a frequency of 15% among all PTCL). Likewise, ALCL seems to be more frequent in Hispanic people and American Indians than in Black or non-Hispanic people.
A family history of hematologic disorders, genetic background, viral infections, occupational and environmental exposure, dietary intake, immune-mediated diseases, and persistent immunosuppression have all been considered relevant risk factors for the development of PTCL.
As it happens with all lymphoid neoplasms, the accumulation of genomic defects over time leads to an altered balance between genomically aberrant lymphocytes and normal host cells, ultimately determining the neoplastic transformation of the tissue and the clinical manifestation of the disease. Multiple mechanisms have been described as responsible for the T-cell transformation into an overt neoplasm. Given the wide heterogeneity of PTCL, not all the following mechanisms apply to each disease category, although some shared pathogenetic steps may be identified; most PTCL, in fact, demonstrate intrinsic defects which end up with the deregulation of multiple signaling pathways. Six shared mechanisms are briefly described in this paragraph; peculiar genomic or epigenomic changes specifically correlated with well-defined disease entities are instead described separately.
Role of the TCR signaling . Some PTCL acquire defects that uncouple the TCR signaling from the TCR engagement. Somatic mutations in co-stimulatory molecules (such as CD28, as seen in AITL) or downstream components of the signaling cascade (PI3K-AKT signaling, NFκB signaling), structural alterations (as it happens with ITK-SYK fusion protein in PTCL-NOS and with ALK-chimeric proteins in ALCL-ALK + ) or loss of TCR-negative regulators (like the phosphatase DUSP22, which works as an onco-suppressor) all contribute to the uncontrolled TCR activation, fostering a neoplastic phenotype.
Epigenomic changes . Epigenetic dysregulation is a hallmark of several PTCL, mainly those arising from T FH cells, where TET2 , IDH2 , and DMT3A mutations are characteristically detected. Epigenetic alterations lead to a deregulated expression of genes that promote T FH differentiation and sustain a transformed phenotype synergistically with additional defects. Moreover, they contribute to an increased self-renewal capacity of stem cells and hematopoietic progenitors and alter their terminal differentiation. Of note, mutations of TET2 and DNMT3A are also detected in hematopoietic progenitors of ageing people and in clonal hematopoiesis processes, putting those individuals at greater risk of neoplasm, mainly myeloproliferative diseases. Importantly, patients with TET2 or DNMT3A -mutated AITL who later developed a myelodysplastic or myeloproliferative syndrome showed the same mutation in their hematopoietic stem cells and in the neoplastic myeloid cells as well, thus leading to postulate that these mutations constitute a pre-malignant status that precedes the malignant transformation of both myeloid and lymphoid cells.
Deregulated activation of the JAK-STAT pathway . The JAK-STAT pathway controls cell growth arrest and apoptosis and its constitutive activation results in cells that are independent of (or at least less dependent on) extracellular signals. Together with the loss of negative regulators (e.g., due to mutations of PTPN2 or PTPN6 -encoded phosphatases), cells become insensitive to inhibitory signals, thus being prone to a malignant transformation. The JAK-STAT pathway is commonly deregulated in PTCL and mutations in the JAK and STAT system in T-cell neoplasms have been detected in the SH2 domain of STAT3 and STAT5 and in the pseudokinase domain of JAK1 and 3. This has been particularly observed in ALCL, PTCL-NOS, CTCL, ENKL, and rarer extranodal entities.
Metabolic dysregulation . Metabolic reprogramming of cancer cells also contributes to tumorigenesis and to the maintenance of the tumor itself: high glucose uptake, high glycolytic activity, nucleotide, and membrane phospholipids synthesis promote proliferation and sustain PTCL cells under stress conditions. These mechanisms have been particularly highlighted in ALCL, where they seem to be correlated to the activity of ALK-fusion proteins along with the constitutive activation of certain regulatory proteins (e.g., STAT3).
Microenvironmental interactions . Neoplastic cells in PTCL highly depend on their microenvironment: TCR activation and secretion of cytokines through paracrine and autocrine stimulations (e.g., IL-4, IL-5, IL-13, and VEGF) are of great importance for tumor progression and maintenance and explain the majority of systemic symptoms patients affected by PTCL complain of. Moreover, the successful growth and survival of neoplastic cells is modulated by pro-tumorigenic signals from surrounding elements (e.g., functional recruitment of surrounding suppressor regulatory T reg cells operated by PTCL-NOS and ALCL) and by decreased immunogenicity due to loss of HLA class I protein or via the interaction between programmed death-1 (PD-1) and its ligand PD-L1, the latter being highly expressed on the surface of ALCL-ALK + and ENKL cells.
EBV-mediated oncogenesis . Apart from B cells, EBV may infect an early precursor lymphoid cell which ultimately can differentiate into a B, T, or NK cell. A spectrum of EBV + T-cell lymphoproliferative neoplasms is now recognized, ranging from aggressive ENKL to more indolent cutaneous lymphoproliferative diseases. The pathogenesis of these disease entities and how EBV + T and NK cells progress and ultimately escape from immunosurveillance is not entirely clear. It can be postulated that in ENKL EBV + T and NK cells derive from the clonal expansion of a pre-malignant EBV-infected normal T or NK cell, which likely occurs in a unique genomic background (this may be an explanation of the much higher incidence of this lymphoma in Asia).
The diagnosis of PTCL is always established on the biopsy of the involved tissue, which is mainly represented by a lymph node. However, virtually any extranodal sites may also be the site of biopsy: liver, small intestine, and skin are among the mostly involved extralymphatic tissues. The review of all slides and of formalin-fixed paraffin embedded tissue by a pathologist with expertise in T-cell lymphoma is always encouraged. Generally, diagnostic accuracy is very good for ALCL-ALK + , but agreement is lost in case of other lymphoma subtypes, with a rate of concordance of about 75% for the most common subtype, PTCL-NOS.
Molecular studies may be helpful under certain circumstances to clarify or refine the diagnosis: at present, however, no molecular markers specifically dictate or guide treatment decisions. The sole demonstration of T-cell clonality through the assessment of TCR rearrangement alone is not sufficient for diagnosis, as this may be seen with reactive and inflammatory processes.
The clinical approach to the patient is described in the box on Clinical Approach to a Patient Affected by PTCL and Staging Recommendations .
Accurate past medical history: concomitant diseases (e.g., celiac disease or concomitant hematological neoplasms); occupational exposure.
Recent medical history, particularly focused on lymphoma-related symptoms (recent weight loss, fever, night sweats).
Performance status assessment.
Physical examination, which must include the evaluation of Waldeyer’s ring, nasopharynx, node-bearing areas, liver and spleen, thoracic auscultation, and a full skin inspection.
Laboratory: complete blood count with differential counts (including the examination of a peripheral blood smear, when indicated), liver and renal function tests, lactate dehydrogenase. Reticulocytes and bilirubin (complete and fractionated) are useful markers in case of suspect hemolysis, which may be associated with some PTCL cases (mainly AITL). Direct Coombs test is also necessary to establish a diagnosis of autoimmune hemolytic anemia.
Staging procedures: computed tomography (CT) scan of neck, chest, abdomen, and pelvis with intravenous contrast and unilateral bone marrow trephine biopsy. 18 F-fluorodeoxyglucose positron emission tomography (PET) scan is not mandatory but may be helpful (see box on The Role of PET Scan as a Staging Tool in Peripheral T-Cell Lymphomas ).
Like nodal B-cell lymphoma, PTCL are staged according to the Ann Arbor staging system. Computed tomography (CT) scan of neck, chest, abdomen, and pelvis with contrast and a bone marrow trephine biopsy are requested to accomplish disease staging. The role of positron emission tomography (PET) during staging and response assessment is briefly reviewed in the box on The Role of PET Scan as a Staging Tool in Peripheral T-Cell Lymphomas .
18 F-fluorodeoxyglucose (FDG) PET scan is not mandatory for disease staging, although it has proven to be helpful in detecting FDG-avid nodal or extranodal lesions that can be missed by a CT scan evaluation.
PET scan may change the disease stage in no more than 5% of patients at diagnosis as compared to CT. This change generally does not translate into treatment modification or intensification.
PET positivity found at the end of induction treatment and in patients who have received autologous stem cell transplantation is a strong predictor of reduced survival.
Maximum standard uptake values in patients with PTCL are lower (and much more heterogeneous) than in aggressive B-cell counterparts, and usually less pronounced for extranodal lesions than for lymph node localizations of the disease.
PTCL-NOS remains a category of biologically and clinically heterogeneous diseases which cannot be further classified into any other of the existing entities defined by the current WHO classification. As a consequence, this disease is heterogeneous, as it displays a broad cytological spectrum and a multiplicity of molecular aspects. Gene expression profile studies have shown that many of these cases resemble ALCL, adult T-cell leukemia/lymphoma, AITL, or ENKL; in some cases, molecular markers help define a peculiar T FH origin.
PTCL-NOS is the most common subtype, accounting for at least 25% to 30% of PTCL. Its prevalence is geographically variable, given that it is equally spread across North America and Europe (34% of PTCL cases), and slightly less diffused in Asia, accounting for 22% of PTCL cases.
The normal architecture of the lymph node appears effaced and neoplastic elements show a variable morphology, ranging from small cells with irregular nuclei to large cells with prominent nucleoli and mitotic figures. An inflammatory background is often present including small reactive lymphocytes, eosinophils, plasma cells, and epithelioid histiocytes. Epithelioid histiocytes are particularly numerous in the lymphoepithelioid variant (also termed Lennert lymphoma). Neoplastic cells stain positively for CD2, CD3, CD5, and CD7, although CD5 and/or CD7 may be frequently lost. The expression of CD4 and CD8 is also variable. The ALK protein is always negative and given that CD30 can be expressed in more than 75% of the neoplastic cells, the differential diagnosis with an ALCL-ALK − constitutes a diagnostic challenge. The proliferation index according to Ki-67 is usually high and rates exceeding 70% are associated with a worse prognosis ( Fig. 89.3 ).
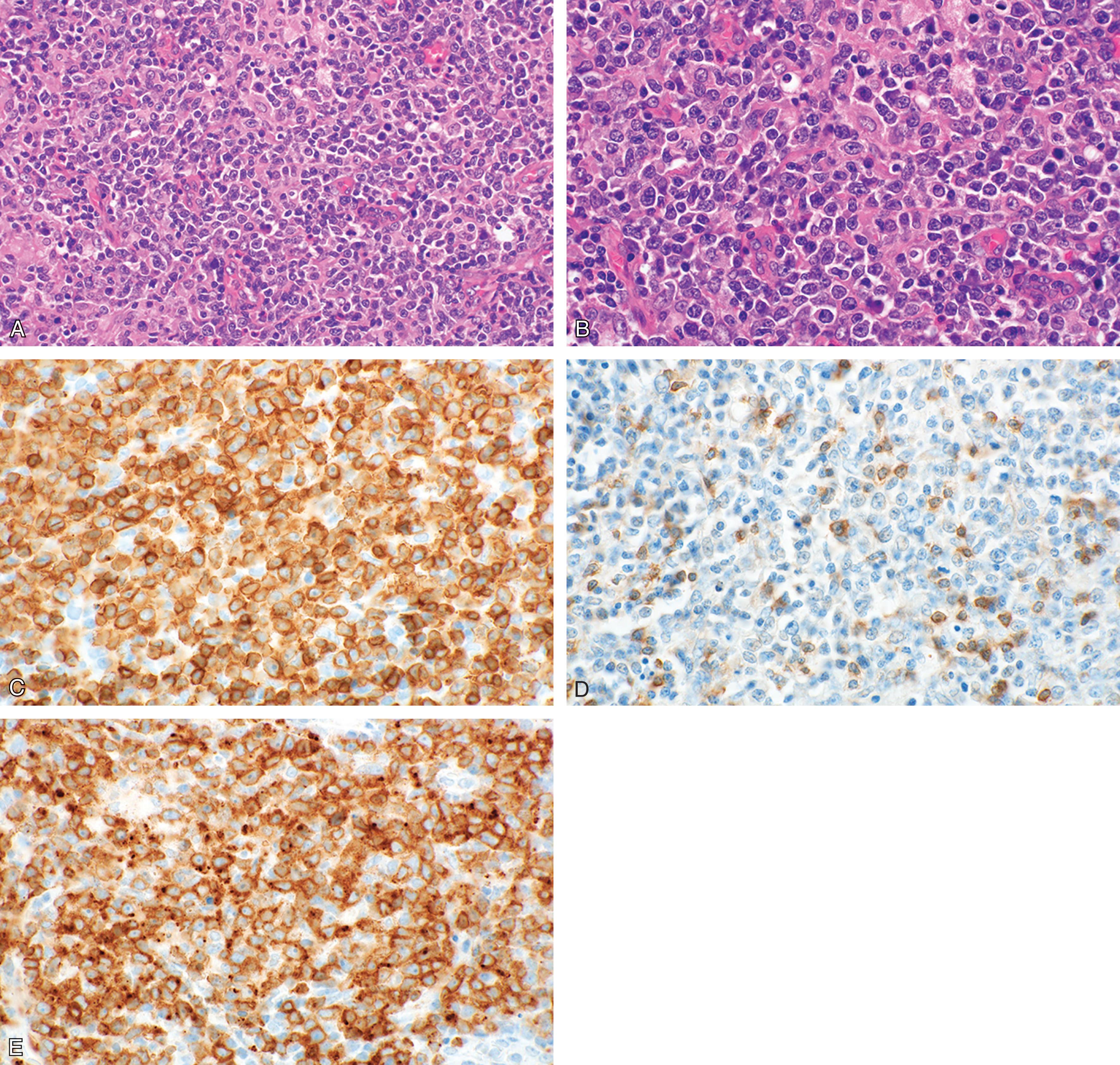
Rare cases of PTCL-NOS express the cytotoxic granule-associated proteins (perforin, granzyme-B, and TIA-1), and this specific pattern has been observed in the lymphoepithelioid variant.
Significant improvements have been made in delineating specific biological and prognostic subgroups within the PTCL-NOS spectrum: in particular, two major molecular clusters have recently been delineated: one subgroup shows a high expression of GATA3 and an enrichment of gene signatures related to cell proliferation ( MYC ), mammalian target of rapamycin (mTOR), and β-catenin; the other significantly expresses TBX21 ( T-bet ) and is enriched of interferon (IFN)- γ and NFκB-induced gene signatures. GATA3 regulates T-helper 2 cell differentiation, whereas TBX21 polarizes cells to a T-helper 1 and cytotoxic T-cell phenotype. This distinction has an important prognostic correlate, as GATA3 expression confers a poor prognosis, with a 5-year OS of 19%. On the contrary, cases with a TBX21 signature display more favorable outcomes, and may display a 5-year OS of 38%.
Recurrent genetic abnormalities of TET2 , IDH2 , DNMT3A , RHOA , and CD28 mutations may be observed in cases of PTCL-NOS that manifest a T FH phenotype, as it happens with AITL: for this reason, the former follicular variant of PTCL-NOS has been moved to the new T FH lymphoma category in the most recent (2017) WHO classification.
Adult patients are mostly affected, as median age at presentation is 60 years. Children can be affected, albeit rarely. The disease shows predilection for males, with a male-to-female ratio of 2:1. Nodal involvement is the most relevant clinical feature at diagnosis, although any organ can be involved, including bone marrow, liver, spleen, gastrointestinal tract, and skin (the latter being the most widely involved, 16% of the cases). Synchronous nodal and extranodal involvement is not infrequent. Nearly 70% of cases present with advanced stage (III-IV according to the Ann Arbor staging system); lymphoma-related symptoms, LDH elevation and poor performance status may be documented in half of the patients.
The International Prognostic Index (IPI), widely applied in aggressive B-cell lymphomas, retains its association with treatment outcomes in T-cell neoplasms: patients with low score (0 to 1 prognostic factors) have a 5-year OS of 50%, in contrast with those with high score (4 to 5 prognostic factors), whose 5-year OS is only 11%. Newer scores have been specifically built up for PTCL-NOS patients: the Prognostic Index for PTCL-NOS (PIT) and the modified PIT (m-PIT): they share age (>60 years), Eastern Cooperative Oncology Group (ECOG) performance status (>1) and LDH elevation with IPI; PIT also takes into account bone marrow infiltration, while m-PIT integrates the expression of the proliferation-associated protein Ki-67. A fourth prognostic index derived from the International T-cell Lymphoma Project (ITCLP) indicates age (>60 years), ECOG (>1), and thrombocytopenia (<150,000/mmc) as the three most relevant parameters for prognostic stratification ( Table 89.6 ). Importantly, all these prognostic scores have been validated in subpopulations treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or at least an anthracycline-containing regimen. The four scores divide PTCL-NOS patients in 3 (m-PIT, ITCLP) or 4 (IPI, PIT) prognostic categories: the lower-risk group, whatever score is used (score 0 to 1 for IPI and m-PIT, score 0 for PIT and ITCLP) emerges as a clearly separated category and shows better outcomes than all the other risk groups ( Table 89.7 .)
| IPI | PIT | m-PIT | ITCLP | |
| Age (>60 years) | • | • | • | • |
| ECOG (>1) | • | • | • | • |
| LDH (elevated values) | • | • | • | |
| Ann Arbor stage (III-IV) | • | |||
| Extranodal involvement (≥2 sites) | • | |||
| Bone marrow involvement | • | |||
| Platelet count (<150,000/mmc) | • | |||
| Ki-67 (≥80%) | • |
| Disease | Score | 5-year OS (%) | 5-year FFS (%) | ||
| Low Score | High Score | Low Score | High Score | ||
| PTCL-NOS | IPI | 50 | 11 | 36 | 9 |
| PIT | 50 | 11 | 34 | 8 | |
| AITL | IPI | 56 | 25 | 34 | 16 |
| PIAI | 44 | 24 | 28 | 15 | |
|
|
|
|
|
|
|
|
|
|
|
|
| EATL | ( ** ) | 20 | 4–17 | ||
| HSTCL | ( ** ) | 0–43 | 0–20 | ||
| SPTCL | ( ** ) | 60 | 30–40 | ||
* “Low score” means score 0 to 1 for IPI, score 0 (group 1) for PIT, and score 0 to 1 for PIA. “High score” means score 4 to 5 for IPI, score 3 to 4 (group 4) for PIT, and score ≥2 for PIA.
** IPI (or any other prognostic score) not relevant for prognostic stratification of patients.
AITL is a rare disease, which accounts for only 1% to 2% of non-Hodgkin lymphomas and 15% to 20% of PTCL. Disease incidence is higher in Europe (29% of all cases of PTCL), followed by Asia (18%) and North America (16%); the reasons for this heterogeneity in different parts of the world is largely unexplained.
AITL is a T FH -derived neoplasm that originates from follicular helper T-lymphocytes: these cells are normally present in lymphoid follicles and are mainly implicated in the germinal center reaction in close collaboration with germinal center B-lymphocytes. Given that a number of recurrent mutations and a characteristic gene signature characterize a significant proportion of AITL cases, as well as some cases of PTCL-NOS, which manifest a T FH phenotype, the 2017 revision of the WHO classification unifies under a common heading AITL, follicular T-cell lymphoma and nodal PTCL with T FH phenotype.
AITL is a disease of the elderly, with a median age of 65 years at presentation, with no gender predilection.
The architecture of the affected lymph node is effaced by a T-cell infiltrate of polymorphous lymphocytes that extends beyond the nodal capsule and characteristically spares the subcapsular sinus, which appears open and dilated. Regressed follicles may be appreciated, especially in earlier stages of the disease, indicating that the disease arises in association with germinal centers, extending to extrafollicular regions as it progresses. Neoplastic cells are admixed with reactive small lymphocytes, eosinophils, plasma cells, and an abundant amount of follicular dendritic cells, which represent the accompanying non-neoplastic populations. Scattered large CD20 + immunoblastic cells, usually staining positively for EBV-encoded RNA (EBNA), are found in most cases. The prominent proliferation of high endothelial venules with a tendency to arborization is a characteristic feature of AITL. T-cell-associated antigens are demonstrated by immunohistochemistry, although neoplastic cells may show aberrant phenotypes: CD3, CD4, CD10, PD-1, and sometimes BCL6 are the most frequently encountered antigens, whereas CD5 and CD7 are frequently defective ( Fig. 89.4 ).
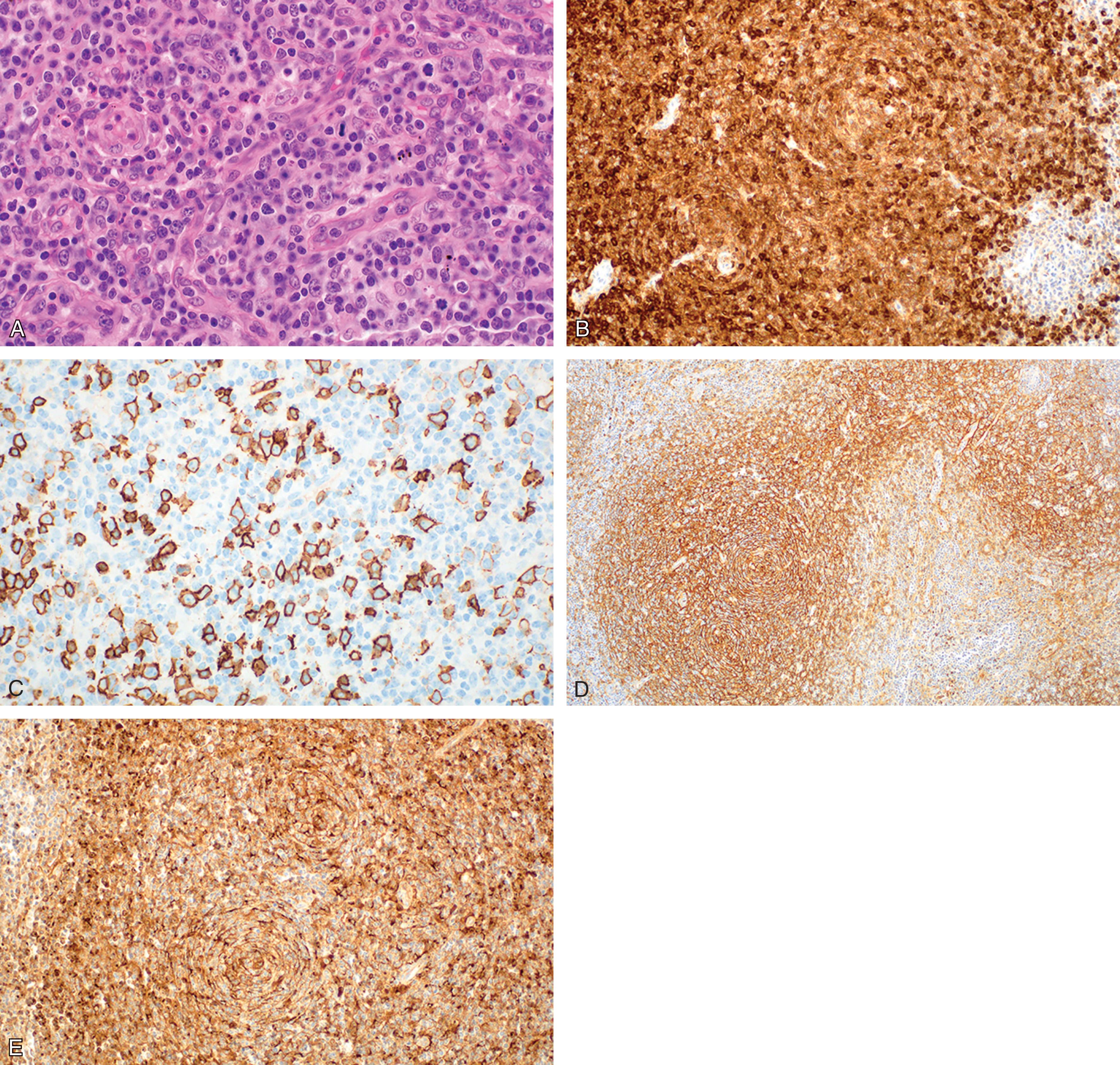
Several genes overexpressed in AITL (like CD200 , PDCD1 , CD40L , NFATC1 , LIF ) have been reported as overexpressed in T FH compared to other T-cell subsets, thus supporting the AITL derivation from T FH cells. The detection of at least three T FH -associated molecules by immunohistochemistry (CD10, BCL6, PD-1, CXCL13, CCR5, SAP, ICOS), which is a surrogate for gene expression profiling, is considered sufficient to suggest the derivation of a given T-cell neoplasm from T FH . This is generally true for AITL, but also for a proportion of PTCL-NOS, as said. The cellular derivation of AITL from T FH cells provides an explanation to the peculiar pathological and biological features of the disease, like the expansion of B-lymphocytes, the intimate association with germinal centers in early disease stages, the associated immune deficiency and the deregulated immunoinflammatory response that characterizes its clinical course.
The AITL mutational landscape is complex: TET2 , IDH2 , DNMT3A , and RHOA are among the most relevant mutated genes, possibly with a role in lymphomagenesis. Mutations of TET2 are the most prevalent among AITL (half to three quarters of the cases) and generally correlate with advanced-stage disease, high IPI, thrombocytopenia, elevated LDH, and shortened survival. TET2 and DNMT3A mutations seem to occur at an early stage of hematopoietic differentiation and may represent early events of lymphomagenesis. Mutations of IDH2 at R172 are also characteristic of AITL and tend to co-occur with TET2 mutations: they define a unique subgroup of AITL with distinct gene expression, and IDH2 / TET2 double-mutant cases display a significantly more polarized T FH phenotype. Somatic RHOA mutations have also been described in nearly 70% of AITL, constantly co-occurring with TET2 and very often with DNMT3A mutations: RHOA is activated downstream of TCR engagement in mature T-lymphocytes, and when mutated it shows an impaired GTP-ase function that also inhibits its wild-type form. The coexistence of these mutations occurring at different times of a multistep model of lymphomagenesis, the co-operation of the aberrant encoded proteins, along with other mutations affecting genes related to TCR signaling that deregulate its activation may co-operate in the pathogenesis of AITL and other T FH -derived PTCL.
This disease behaves aggressively: at least 70% of patients display systemic symptoms at onset, including fever and weight loss, and the disease presents with advanced stage in about 75% of cases. Generalized lymphadenopathy along with a fluctuating skin rash (due to cutaneous dissemination) constitute a distinctive clinical presentation. Hepato-splenomegaly may be found in one third of cases and the bone marrow is infiltrated in nearly half of patients. Peripheral edema, pleural or peritoneal effusion, joint pain, or cold agglutinin disease can be associated phenomena. Autoimmune cytopenia(s)—above all Coombs-positive autoimmune hemolytic anemia—are also a common feature of the disease: they often respond to lymphoma-directed therapies, and their evolution parallels the one of the lymphoma itself.
A specific laboratory abnormality associated with AITL is the detection of a polyclonal hypergammaglobulinemia in 30% to 50% of cases, sometimes with the presence of a monoclonal serum immunoglobulin. Hypergammaglobulinemia is probably related to the fact that the tumor cells are derived from T cells with a specific B-cell helper capacity. This also explains the frequent detection of autoimmune markers, such as rheumatoid factor, circulating immunocomplexes, and anti-smooth muscle antibodies.
According to retrospective series, when patients are treated front-line with anthracycline-containing combination chemotherapy, the 5-year OS for those with an IPI score of 0 to 1 is 56% and 25% for patients with a score of 4 to 5. An alternative prognostic model based on age (>60 years), performance status (>2), extranodal site involvement (≥2 sites), presence of B-symptoms, and platelet count (<150,000/mmc) has been recently proposed (prognostic index for AITL, PIAI) and was more predictive of OS than IPI in patients with lower (score 0 to 1) and higher (score 3 to 5) risk. Anemia (hemoglobin <12 g/dL), mediastinal lymphadenopathy, white blood cell count (>10,000/mmc), and immunoglobulin A concentration (>400 mg/dL) (see Table 89.4 ) have also shown a significant impact on prognosis.
ALCL should be conceived as a group of T-cell non-Hodgkin lymphomas with unifying pathological characteristics although displaying heterogeneous clinical and genetic features. The current WHO classification includes four distinct ALCL entities. Two of them are of nodal origin and are distinguished by the expression (or not) of the ALK protein into an ALCL-ALK + and an ALCL-ALK − , the latter being no longer conceived as a provisional subcategory, but rather defined as a specific disease entity. This distinction is of importance, as the two entities display a heterogeneous clinical behavior, a different prognosis when treated frontline with conventional therapy, and are managed differently. The third entity has an exquisitely cutaneous presentation, and thus it is termed primary cutaneous ALCL (pcALCL): for this reason, it is discussed in the cutaneous T-cell lymphoma section, among the primary cutaneous CD30 + T-cell lymphoproliferative disorders. The last entity is inherently associated with breast prostheses and therefore termed breast implant-associated (BIA) ALCL, nowadays considered as a new provisional category.
ALCL represents nearly 15% of all PTCL and accounts for approximately 2% of all adult non-Hodgkin lymphomas and 10% to 30% of childhood non-Hodgkin lymphomas. Taken as a whole, it shows a bimodal age of presentation with a peak in children and young adults and a second peak around the age of 60. More precisely, ALCL-ALK + is mostly diagnosed in the first three decades of life; on the other hand, ALCL-ALK − usually affects adults, with a median age of 54 to 61 years. BIA-ALCL typically presents approximately one decade after implant placement, with a median age of 52.5 years.
ALCL is histologically characterized by the presence of sheets of hallmark cells : these are large anaplastic cells with round to kidney-shaped nucleus, central nucleolus, and variably basophilic cytoplasm. Different morphological variants of ALCL-ALK + are recognized: the common type (diffuse growth and sinus invasion), the lympho-histiocytic type (with hallmark cells hidden within a reactive lympho-histiocytic background), the small cell type (the neoplastic cells are mainly of small size), and the Hodgkin-like type (characterized by nodular growth and fibrosis which suggest a nodular sclerosis variant of a classical Hodgkin lymphoma). All morphologic variants can be represented in the same lymph node. ALCL-ALK − more commonly presents with a common type pattern of growth.
The phenotype is often aberrant, lacking the common leukocyte antigen (CD45) and some or all the T-cell associated markers (CD3, CD2, CD5 e CD7) in up to 30% of cases. ALCL are more frequently of CD4 origin, but CD8 + or CD4 − /CD8 − cases can occur. The most peculiar immunophenotypic feature is the wide and strong expression of the lymphoid activation molecule CD30. Additionally, ALCL-ALK + shows a nuclear positivity for the ALK protein ( Fig. 89.5 ).
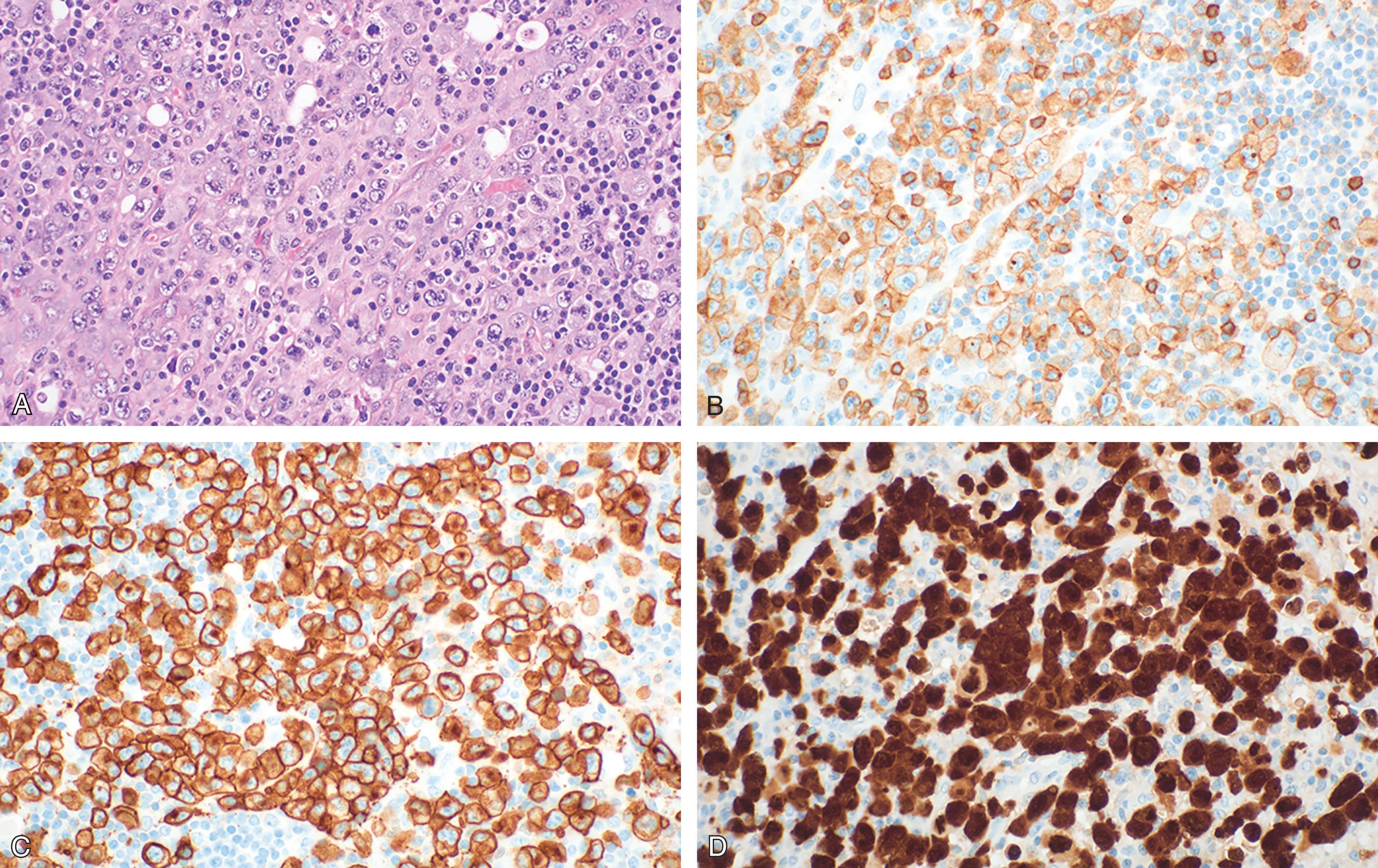
ALCL-ALK + harbors the t(2;5)(p23;q35) translocation, which juxtaposes the ALK gene to the NPM (nucleophosmin) gene on chromosome 5. The ALK gene encodes for a tyrosine-kinase receptor belonging to the insulin receptor superfamily, which is usually silent in normal lymphoid cells and absent in normal (but few cerebral) cells. The NPM protein is expressed at a nuclear level in normal cells and acts as a shuttle protein from the nucleus to the cytoplasm. This translocation produces an ALK-NPM fusion gene capable of encoding for a novel chimerical aberrant protein absent in normal tissues. The NPM side of the newly produced molecule resides both in the nucleus and the cytoplasm, where it can be detected with specific commercially available antibody. Translocation variants have been described, which always involve the ALK gene on chromosome 2. The aberrant NPM-ALK protein, as well as the fusion proteins resulting from variant translocations, constitutively activate downstream effectors, including phospholipase Cγ, PI3K-AKT, Ras-ERK, JAK3-STAT3, and the Sonic hedgehog signaling pathways, all of which lead to prolonged survival and proliferative advantage of the neoplastic cells.
The molecular features of ALCL-ALK − are far less known. Approximately 30% of cases harbor rearrangements of DUSP22 as a consequence of the t(6;7) translocation: this alteration identifies a subset of patients with more favorable outcomes, closely resembling ALCL-ALK + , as the 5-year OS approximates 90%. Conversely, rearrangements of the TP63 gene, marked by inv(3) and identified in 8% of the cases, are associated with very poor outcomes, as the 5-year OS is only 17%. Other genetic aberrations, including PDRM1/BLIMP1 gene inactivation and the 17p13/ TP53 genetic loss, define a poor prognostic clinical entity.
BIA-ALCL does not harbor recurrent translocations found in other ALCL-ALK − ; conversely, activation of STAT3 is common and recurrent mutations of STAT3 , JAK1 , or SOCS1 have been described. There is a close association of this disease with textured rather than smooth breast implants; implant surface texturing may promote bacterial proliferation, with lymphocyte activation and chronic inflammation due to a bacterial biofilm infection around the implant. The associated chronic T-cell stimulation could promote lymphomagenesis.
Clinical presentation of ALK − and ALK + subtypes is similar. About 70% of patients with ALCL present with advanced-stage disease at diagnosis. In one third of patients, peripheral or abdominal nodal disease is associated with bone marrow infiltration or extranodal involvement, more commonly at the skeleton, lungs, and liver. Advanced-stage disease is documented in at least 60% of cases, and lymphoma-related symptoms—mainly fever—can be often encountered at onset.
BIA-ALCL first presents as a seroma fluid that accumulates between the implant and the surrounding fibrous capsule. More than 90% of the cases are localized at the time of diagnosis (stage I–II), sometimes with regional adenopathy, thus suggesting that surgical capsulectomy may be curative. Alternatively, the disease may develop as a growing mass, eventually with distant dissemination. The clinical course varies widely from apparent spontaneous resolution to systemic treatment-resistant disease and death.
Outcomes are highly variable depending on the specific disease subset: this indicates why a clear cut among subtypes is necessary. The 5-year OS rate ranges between 70% and 86% for ALCL-ALK + and between 30% and 49% for ALCL-ALK − . The 5-year OS of ALCL-ALK + however gets poorer in patients with advanced age and more advanced disease stage at presentation (see Table 89.7 ). BIA-ALCL displays a good prognosis if properly managed, with a 5-year OS rate of 89%.
HSTCL is an aggressive subtype of extranodal lymphoma, accounting for less than 1% of all non-Hodgkin lymphomas and less than 5% of all PTCL. A strong association with immunosuppressive treatments, such as those related with solid organ transplantation, as well as with some immune-compromised states, as it happens with pregnancy, inflammatory bowel disease, and other systemic autoimmune disorders, has been noted.
HSTCL results from a proliferation of non-activated cytotoxic T cells, usually monomorphic and medium-sized, that exhibit a unique sinusoidal pattern of infiltration of the splenic red pulp, liver, and bone marrow. For this reason, diagnosis is usually confirmed on liver and bone marrow biopsy, and more rarely upon splenectomy. Neoplastic cells generally express CD2, CD3, CD7, CD16, and CD56, and lack CD4, CD5, CD8, and CD57. Positivity for TIA-1 and granzyme-M is usually documented, but cells tend to be negative for perforin and granzyme-B. The majority (80%) of cases express the γ/δ dimer of the TCR (for this reason, the disease was previously known as hepatosplenic γ/δ T-cell lymphoma, a term now abandoned), but importantly the remaining 20% of cases express an α/β dimer.
Given the rarity of HSTCL, precise mechanisms underlying pathogenesis and disease evolution remain unclear. The most frequent known genetic abnormalities in HSTCL are isochromosome 7q and trisomy 8 but the role of somatic mutations and other genomic alterations in HSTCL has yet to be defined. Mutations in genes like SETD2 , INO80 , STAT5B , SMARCA2 , TET3 , and PIK3CD are frequently encountered. Importantly, it has been demonstrated that disruptions of SETD2 mediate the increased cellular proliferation rate in HSTCL. On the other hand, mutations seen in other T-cell lymphomas and involving genes like RHOA , CD28 , and CCR4 are characteristically absent or occur at much lower frequency.
HSTCL demonstrates a predilection for young males. Median age at presentation is 34 years, although epidemiology data are rather scarce. Most patients present with B-symptoms and organomegaly. Massive splenomegaly may be encountered in nearly 80% of cases; nodal involvement is not a characteristic feature, being reported in less than 25% of the cases. Hepatic involvement is the rule, but despite parenchymal infiltration elevated transaminases may only be seen in 50% of cases. Pancytopenia is common, as the bone marrow is often infiltrated. Peripheral blood lymphocytosis is uncommon and when it occurs it may mimic acute leukemia.
The present treatments are largely unsatisfactory, and this disease displays a severe prognosis, with a 5-year OS of 7% and progression-free survival (PFS) of 0%. Prolonged survival intervals have been anecdotally reported after an induction with alemtuzumab or cladribine or after allogeneic stem cell transplantation.
Primary intestinal T-cell lymphomas are neoplasms with an aggressive behavior that comprise approximately 20% of all intestinal lymphomas and less than 10% of all PTCL. EATL and MEITL are regarded as the most common entities and together they encompass more than 90% of all cases. Since EATL and MEITL have overlapping clinical and pathologic features, they were considered disease variants in the prior WHO classification and termed EATL types I and II, respectively. Biomolecular studies performed over the last decade have revealed biological differences that required their distinct categorization in the revised 2017 edition of the WHO classification. Both entities are described in this paragraph, but their features are presented separately.
EATL is a neoplasm derived from intraepithelial lymphocytes that develops in individuals with celiac disease, a common immune-mediated gluten-sensitive enteropathy with an incidence of nearly 1% in Western populations. This lymphoma accounts for slightly less than 5% of all gastrointestinal lymphomas and 5% of PTCL, but it is acknowledged as the most common primary intestinal T-cell lymphoma in Western countries, accounting for 66% to 80% of all cases, with a median age at onset of 64 years.
EATL is more frequently observed in Europe and the United States where the celiac risk alleles HLA DQA1*0501 and DQB1*0201 are more common, compared to Eastern regions. Homozygosity for HLA-DQ2.5 (encoded by HLA DQA1*0501 and DQB1*0201), poor adherence to a gluten-free diet, and older age are all risk factors for the development of this disease.
The histopathologic features of EATL are highly variable, either among different patients or within a single case. The neoplastic lymphocytes morphologically vary between highly polymorphic elements to a more monomorphic appearance, sometimes resembling immunoblastic elements. In some cases, a mixed inflammatory infiltrate composed of histiocytes, eosinophils, and plasma cells may obscure the malignant cells. The histiocytic granulomatous aggregates may lead to the mistaken impression of Crohn disease. The neoplastic cells frequently spread within the epithelial compartment thus migrating adjacently to or distantly from the initial tumor mass. Portions of small intestine away from the tumor mass frequently show typical histopathologic features of celiac disease; importantly, however, mucosal changes are more prominent proximally and tend to improve distally.
EATL typically expresses CD103, CD2, cytoplasmic CD3, CD7, TIA-1, perforin, and granzyme-B, and lack CD4, CD5, and CD56 expression. Most cases lack surface CD3 and TCR. The Ki-67 proliferation index is generally higher than 50%.
Virtually all EATL cases have clonal TCR rearrangements, mainly of β and γ chain genes, with an association to HLA DQA1*0501 and DQB1*0201, as said. Genome-wide copy number analyses have revealed recurrent gains of chromosomes 9q, 7q, 1q, 5q, and losses of 16q, 8p, 13q, 9p, but a clear identification of the altered genes remains largely unknown. Recurrent genetic mutations have been detected in members of the JAK-STAT pathway ( JAK1, JAK3, STAT3 STAT5B, SOCS1 ), MAPK pathway ( BRAF , KRAS , NRAS ), and epigenetic modifiers ( TET2 , SETD2, YLPM1 ); mutations in DNA damage response and repair and tumor suppressor genes ( TP53 , BRIP1 , TERT , BCL11b , DAPK3 , PRDM1 ) have also been reported.
EATL may be associated with abdominal discomfort and pain at onset, along with fatigue, anorexia, diarrhea, nausea and vomiting, and possibly B-symptoms, especially weight loss. Symptoms may be present for weeks to years prior to the diagnosis of EATL or may arise acutely in individuals without known celiac disease. Likewise, reappearance of malabsorption in a patient with a history of celiac disease favorably responding to a gluten-free diet or, alternatively, the sudden onset of gluten-insensitive severe malabsorption in an otherwise healthy individual can be key symptoms of disease at its onset. More than half of the patients with EATL (58%) have no prior diagnosis of celiac disease and in these cases celiac disease is diagnosed either concurrently with EATL or afterward.
Nearly half of the patients present with an acute abdomen secondary to intestinal perforation and hemorrhage, because of disease penetration into the intestinal wall. The jejunum is the mostly affected site, although any segment can be involved. When multiple segments of the small intestine have been involved, it is likely that the disease has disseminated to nearby and distant organs (mesenteric nodes, liver, spleen, lungs, and bone marrow).
In some celiac disease patients, development of EATL is preceded by a precursor disease state known as refractory celiac disease (RCD): this is defined as the persistence—or recurrence—of severe gastrointestinal symptoms and villous atrophy despite adherence to a gluten-free diet for at least 12 months, after excluding any secondary etiologies for the symptoms. RCD has been categorized into two subtypes (RCD-I and RCD-II): RCD-I represents a polyclonal expansion of intraepithelial lymphocytes that display a normal immunophenotype, whereas RCD-II (also known as intraepithelial T-cell lymphoma or cryptic EATL), is a clonal proliferation of immunophenotypically abnormal intraepithelial lymphocytes. Both RCD-I and RCD-II patients experience recurrent or persistent episodes of malabsorption, but the severity of malnutrition in RCD-II is usually more profound than in RCD-I. Also, the risk of developing EATL is different between the two subpopulations, being 3% to 14% within 5 years in patients with RCD-I compared to nearly 50% in RCD-II.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here