Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
With the advent of early, better tolerated, less toxic, antiretroviral therapy (ART), including fixed-dose combinations and single-tablet regimens, the life expectancy among people with HIV has come closer to that of the uninfected population. However, many complications may arise that are either unique to HIV infection or occur at a younger age or with greater frequency than in HIV-negative individuals. To some degree, these complications may be related to the high prevalence of traditional risk factors for systemic diseases in people with HIV, e.g., substance use, poor dietary habits, or smoking. The chronic inflammatory state and immune activation caused by HIV infection, even when fully suppressed, also may play a large role in the noninfectious systemic complications seen in people living with HIV.
In most resource-rich countries, including the United States, more than 50% of people living with HIV are now ages 50 years or older. Many comorbidities are more common in the HIV population at any given age, and aging-related syndromes, such as frailty and falls, may also occur at earlier ages than in the general population ( Chapter 24 ).
Multimorbidity—having two or more chronic conditions—is common in older people with HIV. The higher prevalence of potentially confounding factors such as smoking, alcohol and substance use, depression, hepatitis C virus infection, poverty, and unstable housing further complicates the attribution of multimorbidity and aging-related syndromes to HIV infection per se.
HIV enters the central nervous system (CNS) early during infection, and aseptic meningitis may be a presenting sign of primary infection ( Chapter 355 ). The presence of HIV in the cerebrospinal fluid (CSF) causes a pro-inflammatory state that is ameliorated but not fully resolved by combination antiretroviral therapy. People living with HIV may develop complications in any part of the neurologic axis, and the incidence of some of these manifestations may be increasing as a function of the increased survival and aging of people with HIV.
Diffuse white matter changes occur more commonly in people living with HIV, become more extensive the longer the duration of HIV infection, and are associated with neurocognitive deficits. In the absence of antiretroviral therapy, up to 20 to 30% of patients develop HIV-associated dementia, which is characterized by memory deficits, psychomotor slowing, and personality changes. With effective antiretroviral therapy, the incidence is less than 5%, but a milder form of cognitive dysfunction, termed HIV-associated neurocognitive disorder , affects about 40% of people living with HIV. No effective treatment is available.
Efavirenz, a non-nucleoside reverse transcriptase inhibitor (NNRTI), has been commonly associated with neuropsychiatric side effects such as sleep disorder, depression, and seizure. Immune reconstitution inflammatory syndrome (IRIS; Chapter 358 ) is described with a variety of opportunistic infections shortly after the start of antiretroviral therapy. HIV-associated neurocognitive disorder IRIS may present as worsening of a preexisting neurocognitive disorder or may be the cause of new-onset cognitive dysfunction.
Peripheral neuropathy ( Chapter 388 ) is the most common neurologic condition affecting people living with HIV. Distal symmetrical peripheral polyneuropathy is seen in about 30 to 70% of people living with HIV. Peripheral neuropathy may be either an indirect effect of HIV infection or a side effect of antiretroviral therapy. Patients typically present with numbness, burning pain, and paresthesias in a glove-and-stocking distribution. The diagnosis is typically made clinically, based on symptoms, absent ankle reflexes, and diminished vibratory and pinprick sensation in the feet. Nerve conduction studies and electromyography (EMG) are reserved for cases with atypical presentations.
Less commonly, patients may develop mononeuritis multiplex ( Chapter 388 ). In the early stages of HIV infection, mononeuritis multiplex is typically due to HIV-related inflammation, whereas with more advanced immunosuppression, it is important to exclude opportunistic infections, notably cytomegalovirus.
Patients with acute HIV infection may present with unilateral or bilateral facial nerve palsy (Bell palsy; Chapter 388 ). Other manifestations include sensorineural hearing loss and foot or wrist drop.
Another rare complication is an inflammatory demyelinating polyneuropathy, which may present acutely as a rapidly progressive ascending, symmetrical flaccid paralysis similar to Guillain-Barré syndrome. Patients have areflexia and weakness but do not have sensory loss. They may also have autonomic dysfunction and even develop respiratory failure. Symptoms peak at about 4 weeks with recovery shortly thereafter.
Acute inflammatory demyelinating polyneuropathy typically develops early during HIV infection and may occur at the time of seroconversion. The CSF may have a mild lymphocytic pleocytosis (10 to 50 cells/μL) and a slightly elevated protein level, in distinction to Guillain-Barré syndrome, in which CSF analysis will typically show an elevated protein but no pleocytosis. Chronic inflammatory demyelinating polyneuropathy has a relapsing and remitting course over more than 8 weeks.
In cases of antiretroviral toxic neuropathy, removal of the offending agent may result in improvement. Otherwise, treatment is the standard approach to peripheral neuropathy ( Chapter 388 ).
Autonomic neuropathy is typically seen with advanced AIDS and may be asymptomatic or a cause of orthostatic hypotension, impotence, and abnormalities in sweating. Resting tachycardia may be seen in asymptomatic individuals. After eliminating other treatable conditions, such as cardiomyopathy and adrenal insufficiency, treatment is similar to the non-HIV patient ( Chapter 386 ).
Vacuolar myelopathy may occur at any stage of HIV infection but is more common with advanced disease, when it can present as a slowly progressive (over weeks to months) spastic paraparesis with loss of vibration and position sense similar to that seen with B 12 deficiency ( Chapter 150 ). The pathogenesis of HIV-associated myelopathy is unknown. On pathologic examination, demyelination of the dorsal and dorsolateral columns is seen with prominent vacuoles in the myelin sheaths. Virus is not thought to invade the spinal cord directly, so the changes are considered to be related to inflammatory cytokines produced by macrophages. HIV-associated myelopathy is a diagnosis of exclusion, and it is important to look for other, potentially treatable causes.
The NRTI zidovudine (AZT) has been associated with a toxic myopathy in 0.4% of patients receiving the drug. HIV-associated polymyositis is a rare condition, with an incidence of less than 1%, that can be seen at any stage of HIV infection. It presents with slowly progressive, symmetrical proximal muscle weakness similar to autoimmune polymyositis ( Chapter 248 ).
Other than discontinuing any offending drugs, the treatment is the same as in non-HIV-infected patients ( Chapters 369 and 389 ).
Despite antiretroviral therapy, the risk of stroke is increased by an estimated 20 to 80% in people living with HIV, even after adjusting for traditional risk factors such as diabetes, hyperlipidemia, smoking, and hypertension. Increased immune activation and chronic inflammation are likely causes.
Both acute kidney injury (AKI) and chronic kidney disease (CKD) occur more commonly in people living with HIV ( Fig. 359-1 ). AKI is most commonly a result of systemic infection, sepsis, or medication-related adverse effects. CKD, which is more common in Black patients, may be related to HIV infection itself, medications used to treat HIV, or comorbid conditions, such as diabetes mellitus, hepatitis B, hepatitis C, and hypertension.
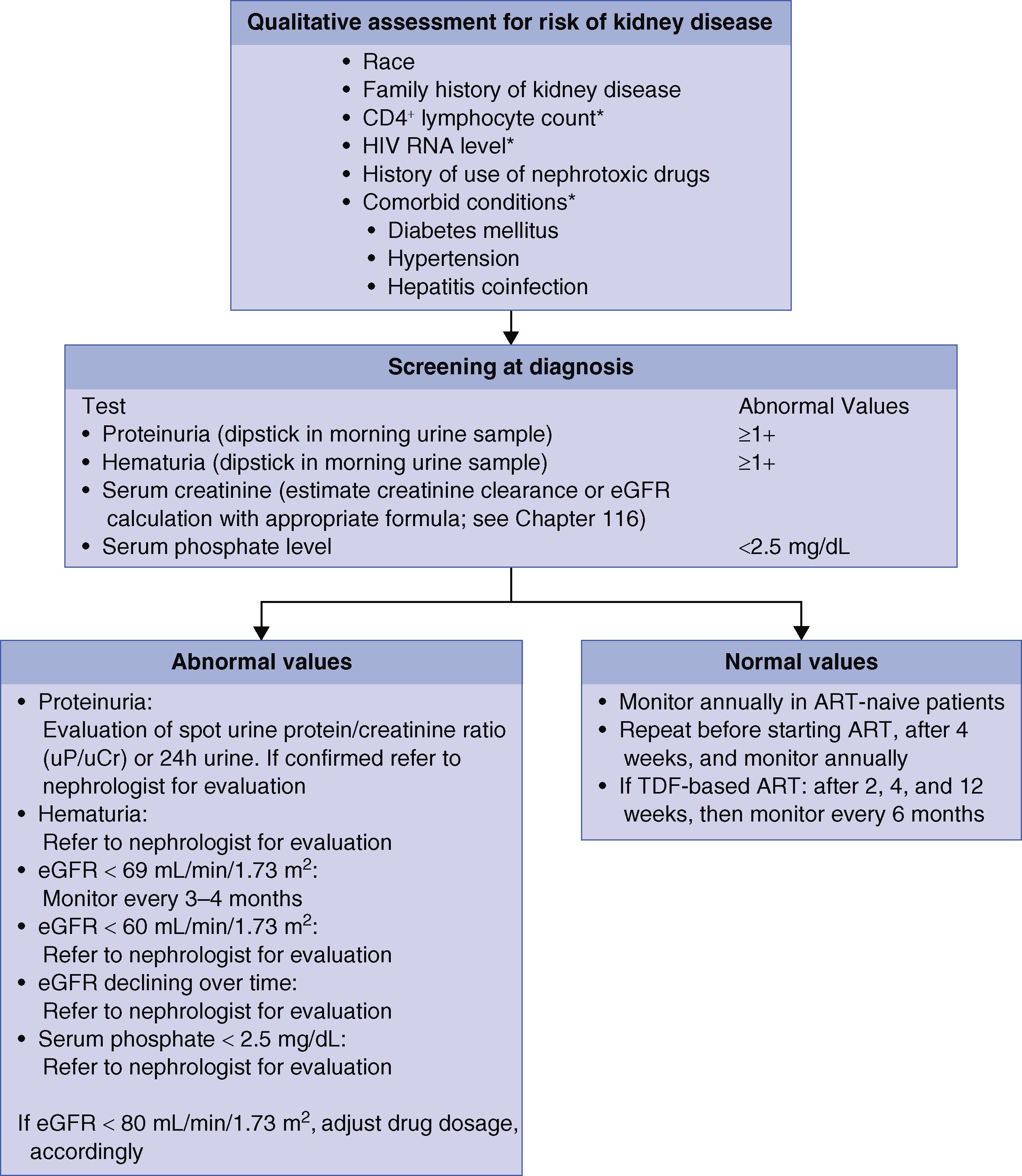
HIV-associated nephropathy ( Chapter 107 ) typically presents with nephrotic-range proteinuria. Before antiretroviral therapy was available, it almost always progressed rapidly to end-stage renal disease. In contrast to nephrotic-range proteinuria from other causes, patients typically do not have peripheral edema, likely because of a concomitant salt-wasting component. It occurs predominately in individuals of African origin, related to an APOL1 gene mutation that is protective against African trypanosomiasis ( Chapter 317 ), and is more commonly seen with advanced AIDS. The risk for HIV-associated nephropathy has decreased by 60% in the antiretroviral therapy era. Other than antiretroviral therapy, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers are useful, and corticosteroids may be helpful for persistent disease (see Chapter 107 ).
HIV-associated immune complex renal disease refers to a heterogeneous group of conditions characterized by immunoglobulin G (IgG) or immune complex deposition. Immune complex glomerulonephropathies, IgA nephropathy, and lupus-like glomerulonephritis all fall under the umbrella of HIV-associated immune complex renal disease. The renal manifestations depend on the location and extent of the glomerular deposits. Proteinuria may be nephrotic in range. Hematuria, decreased GFR, and low serum levels of complement are also often seen.
HIV is also associated with tubular interstitial nephropathy, which is often associated with the use of tenofovir disoproxil fumarate (the pro-drug of tenofovir), and with poorly controlled HIV ( Chapter 108 ). The diagnosis of tenofovir nephrotoxicity is based on clinical presentation and requires renal biopsy only if the presentation is atypical. If nephrotoxicity is suspected, prompt discontinuation of the drug is critical to prevent potentially irreversible damage. Another tenofovir pro-drug, tenofovir alafenamide, is less nephrotoxic.
The thrombotic microangiopathies, which include thrombotic thrombocytopenic purpura and atypical hemolytic-uremic syndrome, occur with increased frequency in people living with HIV but present similarly to HIV-uninfected individuals. Indinavir and atazanavir are associated with crystalluria and frank nephrolithiasis ( Chapter 111 ), with the likelihood increasing with longer durations of therapy.
Rilpivirine, dolutegravir, bictegravir, and the pharmacokinetic enhancer cobicistat may artifactually raise creatinine levels by interfering with the tubular secretion of creatinine. The change is on the order of 0.14 mg/dL. However, glomerular filtration is not diminished, and measurement of a cystatin C level can clarify whether renal damage has actually occurred.
People who are living with HIV and develop end-stage renal disease are candidates for either peritoneal or hemodialysis.
NRTIs, other than abacavir, are not tightly protein bound and may be removed by dialysis, so they are administered after dialysis. Integrase inhibitors, NNRTIs, and protease inhibitors are not typically removed by dialysis.
People living with HIV also should be considered for transplantation evaluation because both graft survival and mortality compare favorably to HIV-uninfected individuals. Transplanting HIV-infected donor kidneys into recipients with HIV is safe and effective.
Any hematopoietic cell line may be decreased in the setting of infection with HIV. HIV can directly infect hematopoietic progenitor cells. In addition, the bone marrow milieu is affected by the inflammatory cytokines engendered by immune dysregulation. Cytopenias may also result from bone marrow infiltration from malignancy or opportunistic infection in the setting of advanced AIDS. Multicentric Castleman disease (see later) is associated with profound cytopenias, high fevers, and lymphadenopathy. Nutritional deficiencies, in particular B 12 , folate, and iron, are more common in advanced disease, especially in resource-poor settings. Not surprisingly, cytopenias tend to increase in incidence with more advanced immunosuppression and improve after the institution of antiretroviral therapy.
Zidovudine frequently caused a macrocytic anemia as well as neutropenia. However, it is not associated with thrombocytopenia and instead is beneficial in the treatment of HIV-associated thrombocytopenia.
The highest incidence of anemia is in people who are not yet on antiretroviral therapy or have symptomatic AIDS. The prevalence of anemia has decreased from 20 to 25% in the pre-antiretroviral therapy era to 4% in patients on antiretroviral therapy. Isolated anemia may result from antibody-mediated or drug-related hemolysis, infections, and bone marrow infiltration ( Table 359-1 ).
| ETIOLOGY | EVALUATION | TREATMENT |
|---|---|---|
| Uncontrolled HIV infection | HIV viral load | Antiretroviral therapy |
| Chronic inflammation | Exclude other etiologies as below | Erythropoiesis-stimulating agents for refractory anemia |
| Nutritional | B 12 , folate, iron studies | Supplementation |
| Endoscopy as indicated if low iron level | Treatment of underlying condition | |
| Medication related | Review medication list:
|
Change medication where possible, support with erythropoiesis-stimulating agents |
| Thrombotic microangiopathy | Peripheral smear for schistocytes, ADAMTS13 level | Plasmapheresis for TTP (see Chapter 158 ) Eculizumab for atypical HUS (see Chapter 158 ) |
| Hemolytic anemia | LDH, reticulocyte count, haptoglobin, indirect bilirubin | |
|
Coombs test | Corticosteroids |
|
Dapsone, primaquine (G6PD deficiency) | Discontinue dapsone, primaquine Reduce ribavirin dose |
| Parvovirus B19 | Parvovirus B19 PCR | Intravenous immune globulin (see Chapter 342 ) |
| Bone marrow biopsy (pronormoblasts) | ||
| Bone marrow infiltration | Histoplasma urine/blood antigen | Treatment of underlying etiology |
| Blood culture for MAC | ||
| LDH for lymphoma | ||
| Bone marrow biopsy if noninvasive tests are unrevealing |
Although about 15% of people living with HIV may have a positive direct Coombs test, clinically significant autoimmune hemolytic anemia ( Chapter 146 ) is rare. Individuals who are glucose-6-phosphate dehydrogenase (G6PD) deficient ( Chapter 147 ) are at risk for hemolysis with medications such as dapsone and primaquine that are used in people living with HIV.
Malaria ( Chapter 316 ) is an important cause of anemia in coinfected patients in sub-Saharan Africa. Infection with parvovirus B19 ( Chapter 342 ) can cause severe anemia, including aplastic crisis or pure red cell aplasia, but is readily treatable with IVIG.
HIV infection is associated with a decrease in endogenous granulocytic colony-stimulating factor (G-CSF), with a resultant decrease in granulocytic and macrophage progenitor cells. The risk for bacterial infection is increased with an absolute neutrophil count of less than 1000 cells/µL and, in particular, if less than 500 cells/µ. It is reasonable to consider the use of granulocyte colony-stimulating factor ( Chapter 153 ) in patients who have profound neutropenia (<200 cells/µL) despite removal of the offending agent.
Thrombocytopenia was common before the availability of effective antiretroviral therapy, likely caused by cross-reactions between the HIV envelope glycoprotein gp160/120 and the platelet glycoprotein IIb/IIIa. During the later stages of AIDS, megakaryocytes can be directly infected.
The treatment for HIV-associated thrombocytopenia is virologic suppression with antiretroviral therapy. For patients who do not respond, corticosteroids, IVIG, or anti-D immune globulin (if the patient is not splenectomized and is Rh positive) have all shown efficacy ( Chapter 158 ). Corticosteroids increase platelet counts in 40 to 80% of cases, but only 10 to 20% have long-term remission. IVIG is of short-term benefit, so it is typically reserved for use before invasive procedures or in the setting of acute bleeding. Thrombopoietin receptor analogues such as eltrombopag and romiplostim (see Chapter 158 ) have been used with some success in cases of refractory HIV-related thrombocytopenia. Because of concerns about the potential increased risk for thrombosis, it is important to use the lowest dose of these medications needed to obtain an acceptable platelet count. Surgical splenectomy and splenic irradiation have been used in particularly refractory cases.
People living with HIV have a prothrombotic tendency, likely related to their pro-inflammatory state and immune activation. Other factors include antiphospholipid antibodies, such as lupus anticoagulant, and acquired protein S and C deficiency ( Chapter 67 ). Up to about 60% of people living with HIV have anticardiolipin antibodies.
Systemic non-Hodgkin lymphoma, Kaposi sarcoma, and invasive cervical cancer are seen at substantially higher rates in people living with HIV than the general population and make up what historically have been called the AIDS-defining malignancies. Before the advent of antiretroviral therapy, the risk for Kaposi sarcoma was 2800-fold higher in people living with HIV relative to the general population, and the risks for non-Hodgkin lymphoma and cervical cancer were 10-fold and 3- to 4-fold higher, respectively.
These malignancies are all associated with specific oncogenic viral infections: Kaposi sarcoma with human herpesvirus-8 (also known as Kaposi sarcoma–associated herpesvirus), non-Hodgkin lymphoma with Epstein-Barr virus, and cervical cancer with human papillomavirus (HPV). The degree of immunosuppression and the resulting lack of immunologic control of the respective oncogenic viruses is the major explanation for the increased incidence of these malignancies. Other than cervical cancer, the incidence of the other AIDS-defining malignancies has decreased significantly since the introduction of antiretroviral therapy.
Most people living with HIV will be able to tolerate standard chemotherapy regimens under expert supervision. However, it is important to be mindful of the potential for drug-drug interactions in patients who are on HIV protease inhibitors or a regimen that contains the pharmacokinetic booster cobicistat.
Non-Hodgkin lymphoma is the most common AIDS-defining malignancy. The AIDS-defining non-Hodgkin lymphomas are mature B-cell lymphomas ( Chapter 171 ), including diffuse large B-cell lymphoma, primary CNS lymphoma, Burkitt lymphoma, primary effusion lymphoma, and plasmablastic lymphoma. Diffuse large B-cell lymphoma, which is the most common, and primary central nervous system lymphoma, which is limited to the brain and is seen in patients whose CD4 cell counts are less than 50 cells/μL, are closely associated with Epstein-Barr virus infection ( Chapter 348 ) and advanced immunosuppression. Burkitt lymphoma, which also is associated with Epstein-Barr virus in 25 to 40% of cases, may occur earlier in the course of HIV infection and is considered highly curable. Plasmablastic lymphoma is an Epstein-Barr virus–associated CD20-negative lymphoma that typically involves the oropharynx.
Primary effusion lymphoma ( Chapter 171 ), which is a rare B-cell non-Hodgkin lymphoma, comprises 2 to 4% of HIV-associated lymphomas and may also be associated with Epstein-Barr virus and/or human herpesvirus-8 infection. It presents as a malignant effusion involving the peritoneal, pleural, or pericardial spaces without evidence of a primary tumor mass. It is typically seen with advanced AIDS and has been associated with prior Kaposi sarcoma in about 25 to 70% of patients.
Kaposi sarcoma in the setting of HIV infection is seen primarily in men who have sex with men (MSM) and rarely in women or individuals who have intravenous drug use as a risk factor for HIV infection. Kaposi sarcoma typically occurs at CD4 cell counts of less than 200 cells/μL, although it can occur at higher CD4 cell counts. The most common manifestation is characteristic purplish lesions on the skin, which may appear as nodules or plaques (see Fig. 359-2 ). Kaposi sarcoma may affect mucosal surfaces as well, and oropharyngeal lesions are most often seen on the gingiva and hard palate. In dark-skinned individuals these lesions may be harder to identify and can be confused with dermatofibromas. Bacillary angiomatosis ( Chapter 291 ) also may be similar in appearance and require biopsy to be distinguished.
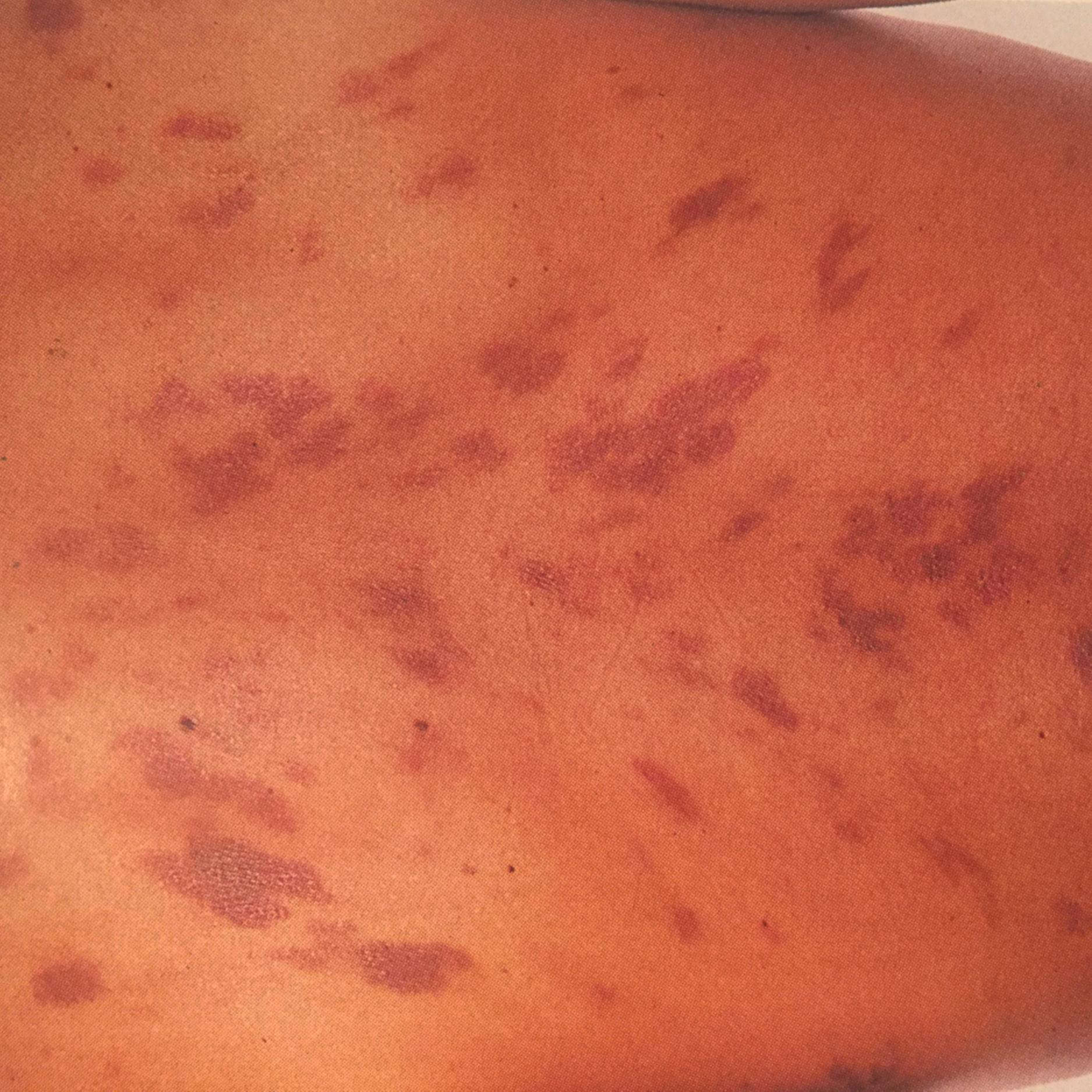
Most patients who have visceral involvement will have evidence of cutaneous disease, but it is possible to have isolated visceral disease. The most common sites are the lungs and the gastrointestinal tract. In the lungs, Kaposi sarcoma may present with parenchymal disease with or without associated effusions. Pleural effusions are characteristically bloody, carry a poor prognosis, and may require pleurodesis to control respiratory symptoms. Lesions similar to those seen on the skin may be seen on bronchoscopy but biopsy is not recommended due to their highly vascular nature. Endoscopy of the upper or lower gastrointestinal tract may also reveal the classical lesions of Kaposi sarcoma.
Antiretroviral therapy is the mainstay of treatment, and decreased serum human herpesvirus-8 viral loads have been documented after the start of antiretroviral therapy. Patients who have diffuse or disseminated disease or who do not respond to antiretroviral therapy may require chemotherapy with agents such as pegylated doxorubicin, liposomal daunorubicin, and paclitaxel under expert care.
When previously unrecognized Kaposi sarcoma or worsening of previously diagnosed disease occurs with the immune reconstitution inflammatory syndrome soon after starting antiretroviral therapy ( Chapter 358 ), corticosteroids should be avoided because it increases replication of human herpesvirus-8 and can worsen Kaposi sarcoma.
Multicentric Castleman disease ( Chapter 171 ), which is a lymphoproliferative disorder with a high risk for progression to lymphoma, is also associated with human herpesvirus-8 infection, and up to 70% of patients may have concomitant Kaposi sarcoma. Patients typically present with fever, weight loss, diffuse lymphadenopathy, and hepatosplenomegaly. Anemia and hypergammaglobulinemia are common laboratory findings. Infection with human herpesvirus-8 is universal in HIV-associated multicentric Castleman disease. The underlying basis for these symptoms is related to the high production of the inflammatory cytokine IL-6, which is upregulated by viral products. POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, M component, and skin changes) and TAFRO syndrome (thrombocytopenia, anasarca, fever, reticulin fibrosis, and organomegaly) have also been reported in conjunction with multicentric Castleman disease.
Multicentric Castleman disease in people living with HIV is thought to be associated with high rates of replication of lytic human herpesvirus-8, and these rates correlate with the severity of clinical disease (unlike the latent infection in Kaposi sarcoma). Treatment is with rituximab under expert supervision ( Chapter 171 ), with ganciclovir added if patients have low CD4 counts or concomitant Kaposi sarcoma. Etoposide is sometimes included for patients with more aggressive disease. Biologic agents that directly target IL-6 production are sometimes effective in HIV-negative patients but are not recommended in people with HIV.
Women with HIV infection are less able to clear infection with human papillomavirus ( Chapter 344 ) and are at a three- to four-fold increased risk for cervical cancer compared with HIV-uninfected women. This risk has not decreased despite the introduction of antiretroviral therapy. Cervical cancer ( Chapter 184 ) remains a particular problem in resource-limited settings where women do not have ready access to screening and early treatment and is a leading cause of cancer-related mortality in these countries.
The screening recommendations for women with HIV infection are slightly different from HIV-negative women. In particular, it is recommended that women with HIV continue screening after age 65.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here