Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Secondary inflammation of the peritoneum frequently presents with the patient in extremis requiring a procedural intervention and is commonly referred to as “surgical peritonitis.” Primary peritonitis or SBP has a distinct pathophysiology and is discussed in Chapter 93 .
This chapter discusses the disease processes that affect the peritoneum, mesentery, omentum, and diaphragm.
The peritoneum is the largest serous membrane of the human body, with an estimated surface area of 1.8 m 2 , which is almost the same area as the skin (or total body surface area). The embryologic development of the peritoneum occurs in the gastrulation phase, where the 3 layers of the embryo—the ectoderm, mesoderm, and endoderm—further differentiate into the visceral plate mesoderm and the parietal plate mesoderm. The parietal peritoneum secretes serous fluid, whereas the visceral peritoneum invests organs and the mesentery that suspends the gut tube from the abdominal wall, thus providing a pathway for vessels, nerves, and lymphatics. The peritoneal sac, as it is often referred to, is sealed in men and open to the exterior via the ostia of the fallopian tubes in women.
The parietal peritoneum in a completely developed fetus covers the internal surfaces of the abdominal wall including the diaphragms and the pelvis, whereas the visceral peritoneum invests all intraperitoneal organs (such as small intestine, gall bladder, stomach) and the anterior surface of retroperitoneal organs (pancreas, kidneys, ascending and descending colon). Remarkably, the understanding of the anatomy of the peritoneum, omentum, and mesentery is evolving. Meyers identified numerous ligaments and 2 mesenteries including the coronary, gastrohepatic, hepatic, duodenal, falciform, gastrocolic, duodenocolic, gastrosplenic, splenorenal, and phrenicocolic ligaments and the transverse mesocolon and the small bowel mesentery. Although important in understanding and predicting spread of disease and inflammation that manifests in clinically relevant situations, this is an oversimplification of the anatomy. For instance, patients with perforated peptic ulcer disease may present with RLQ pain due to dependent drainage along the right paracolic gutter. Prior to imaging techniques, patients would be placed in semi-recumbent positions (Fowler position) in order to encourage dependent accumulation of infected fluid with the goal that the eventually encapsulated pelvic abscess could be drained transrectally.
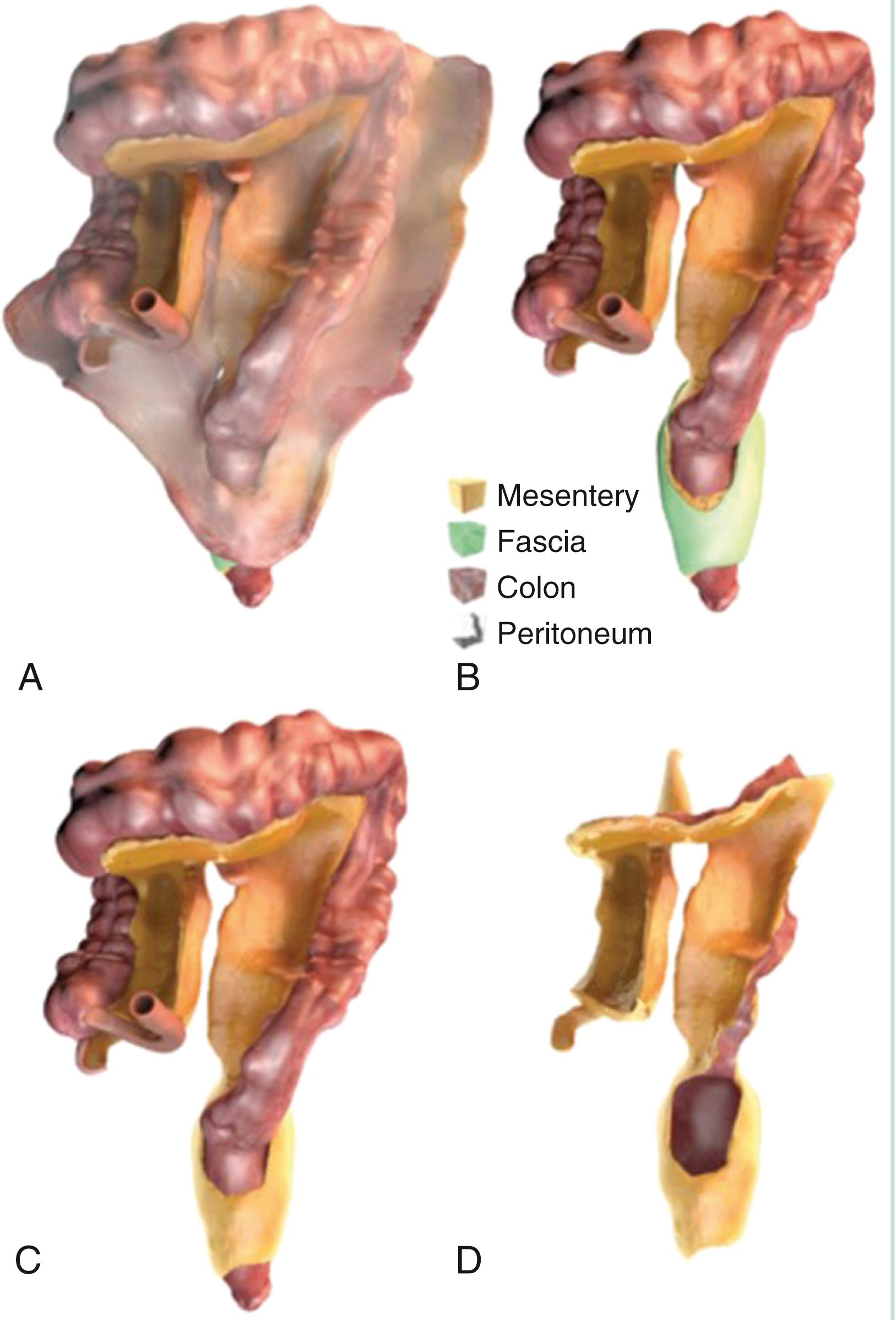
Current understanding of the mesentery and the parietal peritoneum suggest that the mesentery distal to the duodenojejunal flexure is a contiguous and extra-retroperitoneal organ. The right and left mesocolic regions and the mesosigmoid are flattened against the abdominal wall, held in place by Toldt fascia. It has been suggested that the intestine and mesentery are contiguous from diaphragm to pelvic floor and that the mesogastrium and mesoduodenum are indeed contiguous with the mesentery. This description of the mesentery is broken into 6 flexures: duodenojejunal, ileocecal, hepatic, splenic, between descending and sigmoid, and sigmoid and rectum. The peritoneal reflections, although contiguous, are variably named as Jackson membrane, anterior reflection, pouch of Douglas, and the lateral peritoneal reflection ( Fig. 39.1 ). This understanding is translating into “total mesocolic or mesorectal” excisions for oncologic purposes.
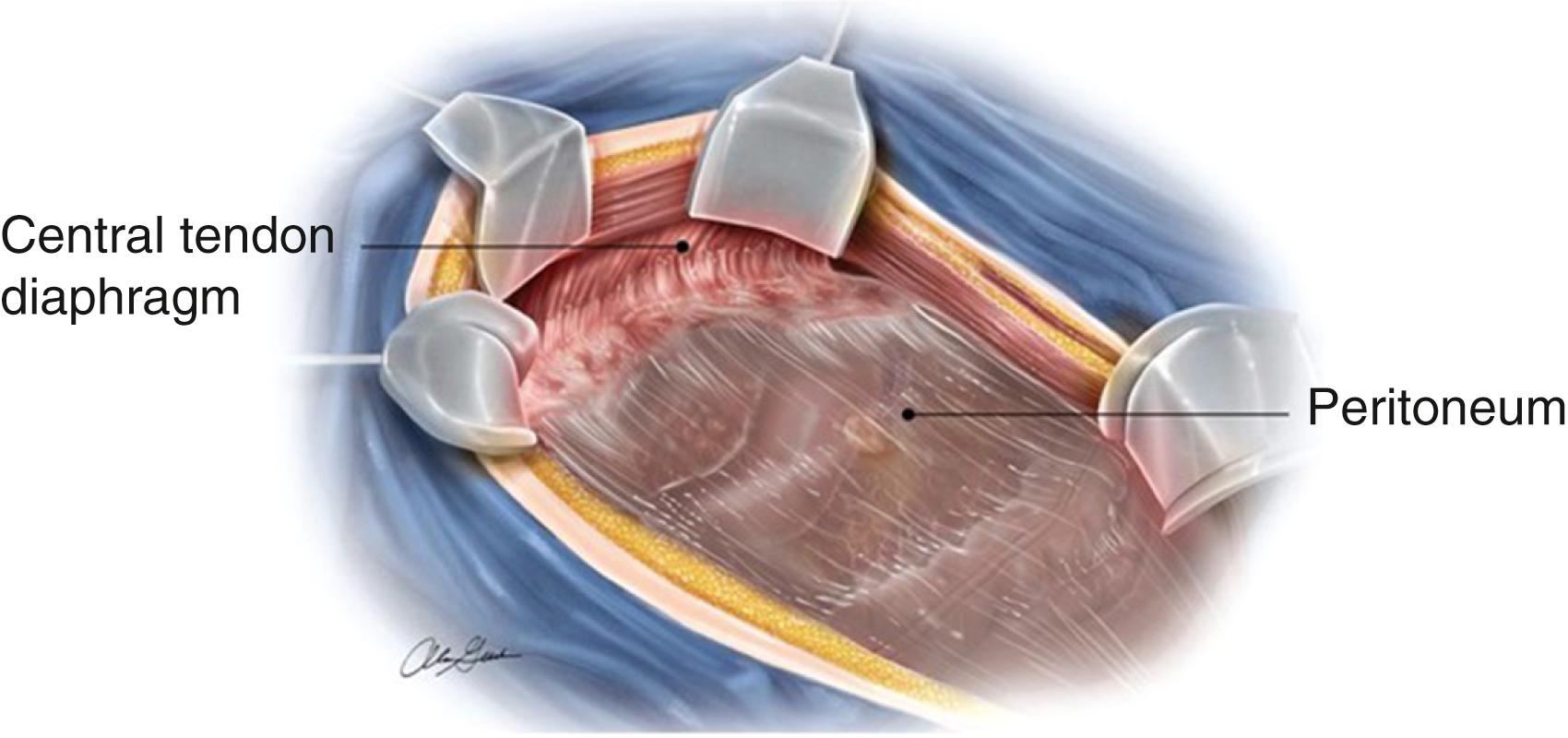
The greater omentum is an intraperitoneal organ derived from the greater curvature of the stomach and spleen, draping across the transverse colon, which separates the abdomen into the greater and lesser sacs. The lesser omentum attaches the lesser curvature of the stomach to the liver and is also referred to as the gastrohepatic omentum. The right edge of the lesser omentum is also known as the hepatoduodenal ligament, and the opening posterior to this (the epiploic foramen of Winslow) is the only connection between the lesser and greater sacs.
The word peritoneum is derived from the Greek peri- , meaning “around” and tonos, meaning “a stretching around.” The visceral and parietal peritoneum have a similar structure and consist of 3 layers: the mesothelium, the basal lamina, and the submesothelial stroma. The mesothelium is formed by a monolayer of cuboidal mesothelial cells approximately 25 μm in diameter. Mesothelial cells possess both epithelial and mesenchymal characteristics and peritoneal pathology can lead to epithelial-mesenchymal transitions. The peritoneum contains stomata, which are direct portals to the lymphatic system. These are abundantly present on the diaphragm. At the apical surface of the peritoneum are numerous microvilli and occasional cilia in which lamellar bodies are embedded. These release surfactant that allows a friction-free state. On top of the microvilli and lamellar bodies, a glycocalyx is present consisting of proteoglycans and glycosaminoglycans. These are responsible for intercellular contact, inflammatory regulation, tissue remodeling, and possibly transport. Mesothelial cells are joined by well-defined intercellular junctional complexes, including tight junctions, adherens junctions, gap junctions, and desmosomes that establish and maintain the semipermeable barrier for fluid, solutes, and particles.
The visceral peritoneum is supplied by the splanchnic blood vessels, and the parietal peritoneum by intercostal, subcostal, lumbar, and iliac vessels. The venous blood from the visceral peritoneum returns via the portal vein, whereas the parietal peritoneum drains via the inferior vena cava. The visceral peritoneum is supplied by nonsomatic nerves, whereas the parietal peritoneum is supplied by somatic nerves. Therefore, visceral pain is poorly localized, diffuse, and vague (see Chapter 11 ). Visceral pain is caused by stretching, distention, torsion, and twisting. The visceral peritoneum does not produce pain when it is cut or burned. When visceral pain fibers of midgut structures are stimulated, a vague periumbilical discomfort results because the visceral pain fibers enter the spinal cord at the same level as the T10 dermatome somatic fibers (see Chapters 11 and 12 ). This sensation is, therefore, experienced as discomfort in a dermatomal distribution. Likewise, visceral stimulation from foregut structures produces epigastric (T8 distribution) discomfort, and visceral stimulation in the hindgut produces suprapubic (T12) discomfort. Parietal (somatic) pain fibers are activated by such stimuli as cutting, burning, and inflammation. This type of pain is sharply localized. A good example of this process is acute appendicitis. Early in the disease process the patient experiences periumbilical discomfort secondary to distention of the appendiceal lumen, and this progresses to localized RLQ pain and tenderness as the inflammation becomes transmural and involves the parietal peritoneum.
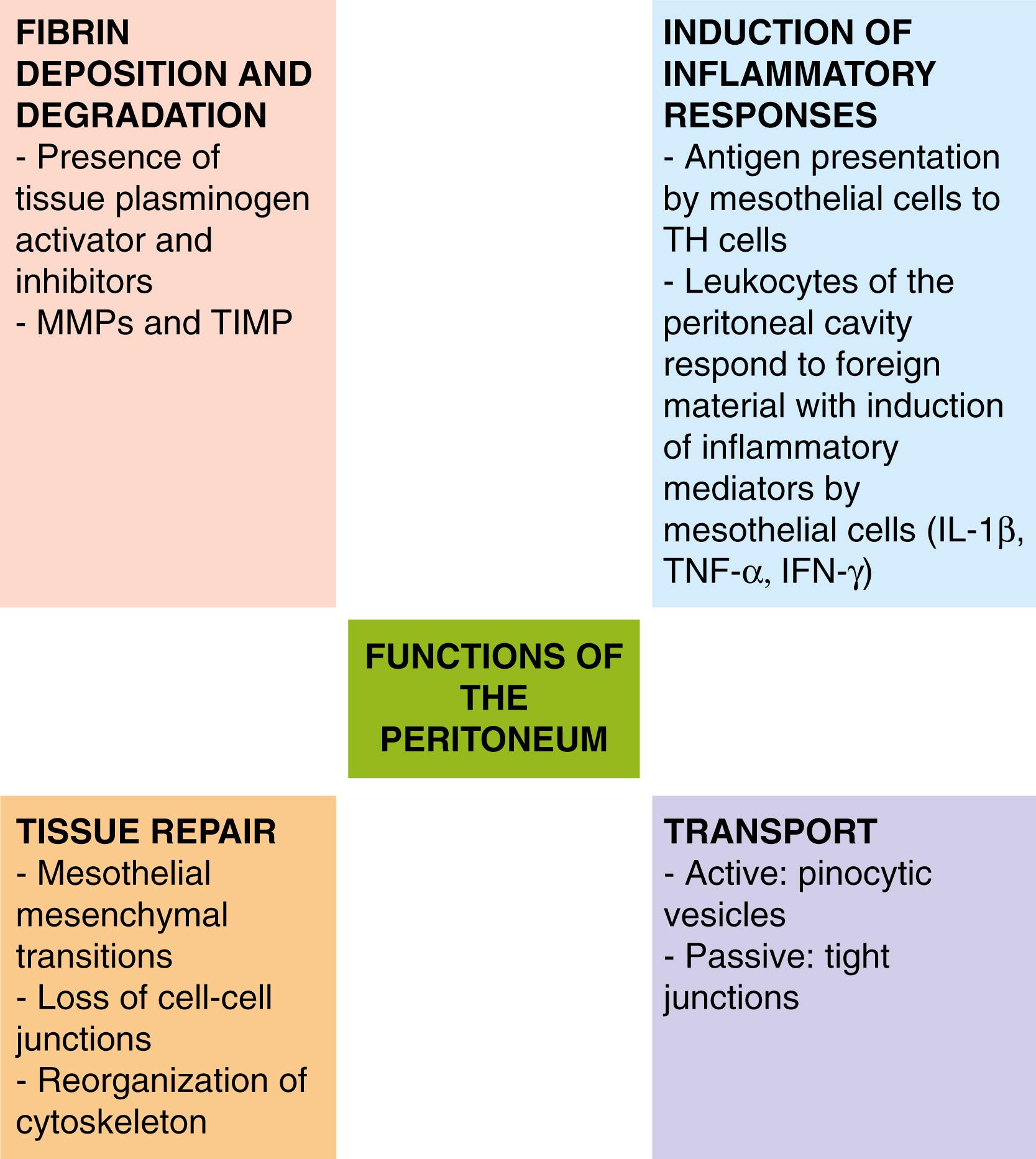
The mesothelial cell maintains homeostasis of the peritoneal cavity and synthesizes the matrix proteins on the basal surface that maintain the architecture of the peritoneal membrane ( Fig. 39.2 ). The peritoneum can regenerate after injury or surgery. The peritoneum can also cause fibrosis by exerting epithelial-mesenchymal transitions. In addition, these cells also can promote degradation of fibrin by converting plasminogen to plasmin, thereby activating tissue plasminogen activator. In animal models of abdominal wall hernias repaired with composite mesh grafts, a functional neoperitoneum covers the graft in 7 to 14 days.
Under conditions of inflammation, the mesothelial cell initiates and regulates the inflammatory response by synthesis of cytokines, chemokines, and growth factors. The peritoneal mesothelial cell is capable of phagocytosis and can serve as an antigen-presenting cell.
Finally, in health and in inflammatory conditions, the mesothelial cell facilitates transport of fluids, solutes, and particulate matter across the peritoneal membrane. Fluid and solute movement are governed by convection and diffusion. Particles are absorbed from the peritoneal cavity by 2 different anatomic routes. Particles smaller than 2 kd may be absorbed through peritoneal venous pores and are directed to the portal circulation. Particles larger than 3 kd are absorbed through peritoneal lymphatics, entering the lymphatic thoracic duct and from there the systemic circulation. This last route of absorption plays an important role in controlling abdominal infections because it has a huge capacity for absorption. The anatomic structure of these large channels between the peritoneal cavity and the diaphragmatic vessels and the negative pressure of the thorax during inspiration make this mechanism extremely effective in the removal of bacteria and cells. The large surface area and semi-permeability of the peritoneal membrane can be exploited therapeutically in peritoneal dialysis ( Fig. 39.3 ).
Secondary (surgical) peritonitis is a result of an inflammatory process in the peritoneal cavity secondary to inflammation, perforation, or gangrene of an intra-abdominal or retroperitoneal structure. Surgical intervention is usually required to treat these processes. However, in certain situations such as perforated peptic ulcer, nonoperative treatment may also be successful (see Chapter 53 ). Antibiotics play only an adjunctive role in severe intra-abdominal infection. If untreated, secondary peritonitis will, in most cases, lead to septic shock and death. It is common medical parlance to equate nonoperative management and “conservative” therapy; however, the reverse is more often the case for secondary peritonitis (that is, operative intervention is in fact the “conservative” approach).
The diagnosis of secondary peritonitis is based on history, physical examination, imaging studies, and operative exploration. History and physical examination are very important in secondary peritonitis, and a good history and physical examination can often reduce or eliminate the need for further studies. Secondary peritonitis has numerous causes. Some of the more common causes of secondary peritonitis include perforated peptic ulcer disease, appendicitis, diverticulitis, acute cholecystitis, pancreatitis, and postsurgical complications. Other sources of inflammation such as autoimmune serositis (e.g., SLE), endometriosis, and malignancies cause peritoneal inflammation but rarely cause clinical peritonitis.
Other nonbacterial causes of peritonitis include leakage of blood into the peritoneal cavity due to rupture of a tubal pregnancy, ovarian cyst, or aneurysmal vessel. Blood is highly irritating to the peritoneum and may cause abdominal pain (hemorrhagic peritonitis) similar to that found in septic peritonitis. Bile leakage into the peritoneal cavity also can cause signs and symptoms of peritonitis, especially when there is also bacterial contamination of the bilious contents. However, sterile bile in the abdomen can be surprisingly asymptomatic. Large bilomas may have minimal symptoms.
Bacteria can reach the peritoneal cavity by a variety of pathologic processes: transmural inflammation with luminal obstruction (see Chapter 123 ), perforation of the GI tract, bacterial translocation (see Chapter 58 ), and intestinal ischemia (see Chapter 118 ). The initial inoculum of bacteria is determined by the normal flora in the involved portion of the GI tract (see Chapter 3 ).
Although the flora of the gut, especially of the large bowel, is diverse and extensive, the number of types of organisms rapidly decreases after leakage of gut contents into the peritoneal cavity. Aerobes such as Escherichia coli and enterococci and anaerobes such as Bacteroides fragilis and Clostridium organisms predominate. A study of infections associated with ruptured colonic diverticulitis reported anaerobes in only 15% of cases, aerobic bacteria in only 11%, and mixed aerobic and anaerobic flora in 74%; cultures from peritoneal abscesses detected anaerobic bacteria alone in 18%, aerobes alone in 5%, and mixed aerobic and anaerobic flora in 77%. In addition to bacteria, fungi in intra-abdominal infection are more frequently recognized and may have clinical significance. For example, a positive fungal culture is quite common in perforated PUD and may adversely affect outcome.
On the basis of an animal model of monomicrobial and polymicrobial peritonitis with various combinations of bacteria, it is apparent that E. coli is the organism most often responsible for death from this form of iatrogenic peritonitis, at least in part because of its ability to cause bacteremia, and that combinations of anaerobes and facultative organisms lead to abscess formation. Other adjuvant substances, such as devitalized tissue, mucus, bile, hemoglobin, and barium, can act synergistically with microorganisms to increase mortality in surgical peritonitis through their ability to interfere with phagocytosis and killing of bacteria. These considerations form the basis for the treatment of surgical peritonitis, which is described later.
The peritoneal cavity possesses several lines of defense against bacterial infection ( Box 39.1 ). Peritonitis results when these defenses are overwhelmed.
Peritoneal clearance of bacteria through the diaphragm via the thoracic duct
Microvilli of the mesothelial cell
ICAM-1 (CD 54) and VCAM-1 (CD 106)
Neutrophil recruitment through omental high endothelial venules
Macrophages (with glutamate metabolic bursts)
Neutrophils
Opsonins
Complement C3b
Immunoglobulin G
Fibronectin
Mast cell-derived leukotrienes
Fibrin trapping of bacteria
Formation of fibrinous adhesions
Omental loculation of foci of inflammation
SBP (see Chapter 93 )
Chronic ambulatory peritoneal dialysis
Mycobacterium tuberculosis
AIDS associated
Chlamydia trachomatis
Neisseria gonorrhoeae (Fitz-Hugh-Curtis syndrome)
Rare causes
Polyarteritis nodosa
SLE
Primary Sjogren syndrome
Familial Mediterranean fever
Once bacteria enter the peritoneal cavity, clearance of the offending microorganisms begins immediately. Within 6 minutes of intraperitoneal inoculation of bacteria in dogs, bacteria can be cultured in thoracic lymph, indicating passage of organisms through the diaphragm. Twelve minutes later, bacteremia may be evident. This clearance mechanism is probably important in survival because blockade of the thoracic duct in an animal model of peritonitis decreases bacteremic episodes but increases mortality and induces liver necrosis. This appears to be directly related to the amount of endotoxin to which the liver is exposed. Decades before it was known that the diaphragm was the predominant site of clearance of bacteria, Fowler, in 1900, proposed his head-up, pelvis-down position for prevention of absorption of toxins from infected peritoneal cavities. In the pre-antibiotic era, documentation of the delayed clearance of bacteria from experiments in infected dogs in the head-down position confirmed the wisdom of this positioning for patients with peritonitis.
Clinical history and careful physical examination are the key factors in making a timely diagnosis of surgical peritonitis. In general, the sooner the diagnosis is made, the better the prognosis. Abdominal pain is the hallmark of peritonitis. The exact details of the onset of pain can be helpful in drawing attention to the affected organ (see Chapter 11 ). The pain’s character, location, area of radiation, change over time, and provocative and palliative factors are key pieces of information in assisting with the diagnosis. Peritoneal inflammation is typically associated with ileus, and therefore nausea and vomiting are common symptoms.
The ability of the clinician to elicit an accurate history of abdominal pain and peritoneal signs is limited in patients with neurologic and immunologic compromise. The pain of peritonitis can be reduced or even absent in older adult patients. Infants and children may be incapable of furnishing any history or cooperating with the physical examination. Notoriously difficult patients to assess for secondary peritonitis include emergency room patients under the influence of alcohol or illicit drugs, trauma patients with central nervous system or spinal cord injuries, and sedated and ventilated ICU patients. Analgesics typically will not relieve the findings of peritonitis on physical examination, but may relieve some discomfort. Diabetic patients may have deficits in both neurologic and immune function. Patients receiving immunosuppressive and anti-inflammatory drugs, such as glucocorticoids and chemotherapeutic drugs, may have blunted perception of pain and minimal signs of peritoneal irritation. Patients with cirrhosis and ascites may show no pain during episodes of SBP unless the parietal peritoneum becomes involved with the inflammatory process (see Chapter 93 ).
On examination, the patient with surgical peritonitis usually prefers to remain immobile because any movement acutely worsens the pain. Fever of 100°F or higher is typical, as is tachycardia, which may be in part secondary to pain. Hypotension is usually a late finding in sepsis. Fever is a fundamental endogenous mechanism to help fight infection. In fact, the increase in body temperature that is usually found during bacterial infections, including peritonitis, seems to be essential for optimal host defense against bacteria. The absence of hepatic dullness to percussion suggests the presence of free air in the peritoneal cavity. Exquisite tenderness to percussion should lead to very gentle palpation. Overly vigorous palpation of a very tender abdomen may cause patients such pain that they are subsequently unable to cooperate for the remainder of the examination.
Palpation should begin farthest from the area that the patient identifies as the source of the most pain. Palpation of a truly board-like abdomen is so impressive to the examiner that it cannot be forgotten. Lesser degrees of rigidity must be compared with this extreme end of the spectrum. Voluntary guarding in the presence of mild tenderness may be misinterpreted as rigidity by the inexperienced examiner if the patient is anxious and palpation too vigorous. It is usually not necessary to check for rebound tenderness to palpation if rebound tenderness is noted during auscultation or percussion. Often, the presence of rebound tenderness can be inferred if the patient’s pain is exacerbated when the bed or stretcher is jarred.
Peritoneal signs signify inflammation of the parietal peritoneum secondary to an intra-abdominal process. Peritoneal signs include rebound tenderness, involuntary guarding, and extreme tenderness on palpation. Peritonitis can be diffuse, such as that associated with perforated ulcer, or localized, such as in sigmoid colonic diverticulitis confined to the LLQ. Significant septic processes may be confined to the pelvis by overlying bowel and omentum, with a resulting absence of peritoneal signs in the anterior abdominal wall. Therefore, careful rectal and pelvic exams are essential in order to detect pelvic peritonitis. The presence of iliopsoas and obturator signs (described in Chapter 120 ) can be helpful in detecting retroperitoneal or pelvic inflammation and abscesses.
Repeated physical examination by the same examiner may reveal evidence of progressive peritoneal irritation. The evolution of the physical exam over time provides additional information for diagnosis and evaluation of response to initial nonoperative therapy. This, together with laboratory tests and imaging procedures described later, may indicate the need for surgical intervention.
The most common and widely available laboratory test that is abnormal in otherwise immunocompetent patients with peritonitis is an increased WBC count with left shift. The presence of circulating juvenile forms (i.e., bands) reflects an increasing demand for new white cells from the bone marrow. A low WBC count during a bacterial infection, associated at times with gram-negative septicemia, may indicate the presence of an exhausted bone marrow and carries a poorer prognosis. In addition, metabolic acidosis, hemoconcentration, and prerenal azotemia may be present.
Free air may be evident on upright chest radiograph or on upright or decubitus abdominal films, but the finding of pneumoperitoneum by radiography has limited sensitivity in gut perforation. The absence of free air should not delay surgical intervention in an otherwise appropriate clinical setting. US can be helpful in demonstrating abscesses, bile duct dilation, and large fluid collections but is usually not the best first-line choice for diagnostic imaging. CT of the abdomen and pelvis, generally with both oral (and occasionally rectal) and IV contrast, is increasingly preferred as the most sensitive and specific imaging modality for acute abdominal pain. Multidetector CT scanners are capable of imaging the entire abdomen and pelvis in a single breath-hold. The axial images are of extremely high resolution and can be reconstructed in coronal, sagittal, and 3-dimensional sets of images. CT is much more sensitive than plain films for the detection of free air, and with multidetector CT it is possible to visualize the actual site of perforation. Although CT images are increasingly accurate and the images compelling, they should not delay surgical consultation, resuscitation, and operation in a patient with suspected peritonitis.
The diagnosis of surgical peritonitis is suspected on the basis of history, physical examination, and laboratory and imaging tests and is confirmed at laparotomy or laparoscopy when purulent fibrinous peritonitis is found. In those patients whose history and physical examinations are unreliable, CT and peritoneal lavage are valuable in confirming the diagnosis of surgical peritonitis. CT is less invasive, but if not available, or if a patient is too hemodynamically unstable to undergo scanning, peritoneal lavage is an effective alternative that can be rapidly performed. Peritoneal lavage involves insertion under sterile conditions of a catheter into the peritoneal cavity and infusing 1 L of normal saline. If the effluent contains more than 500 WBCs/mm 3 , effluent amylase or bilirubin levels greater than the corresponding serum value, or bacteria are visible on Gram stain, there is approximately a 90% likelihood of surgical peritonitis. Surgery is usually indicated in this setting. Finally, diagnostic laparoscopy is highly accurate in the diagnosis of surgical peritonitis, and many of the underlying diseases can be dealt with laparoscopically, avoiding the need for laparotomy.
Two principles in the management of secondary peritonitis cannot be overemphasized. First, not all patients with peritonitis require surgery. For example, a patient with localized LLQ peritonitis secondary to sigmoid colonic diverticulitis can be managed with bowel rest and IV antibiotics alone. Another patient with the same clinical presentation and findings of a diverticular abscess on CT scan can be successfully treated with antibiotics and percutaneous drainage (see Chapter 29 ). The second principle is that the absence of peritonitis does not exclude the possibility of surgical emergency. The classic example of this clinical situation is early acute mesenteric ischemia with abdominal pain out of proportion to physical examination findings (see Chapter 11 ). Likewise, a complete mechanical SBO generally requires emergency operation before the development of peritoneal signs that indicate progression to perforation or vascular compromise (see Chapter 123 ).
For most cases of secondary peritonitis, fluid resuscitation and antibiotic therapy followed by urgent laparotomy or laparoscopy are the mainstays of treatment. The patient should be aggressively fluid resuscitated to treat intravascular volume depletion secondary to movement of fluid out of the vascular space. Fluid resuscitation (bolus of 30 mL/kg) is guided by frequent monitoring of physiologic parameters in an intensive care setting, including blood pressure (by arterial line if shock is present), heart rate, central venous pressure, mixed venous oxygen saturation, and urine output. Hematocrit, WBC, electrolytes, glucose, creatinine, and blood gases should also be monitored. Hypovolemia, hypotension, metabolic acidosis, hypoxia, and hemoconcentration from loss of plasma into the peritoneal cavity are expected. Vasopressor therapy should be initiated only after adequate volume resuscitation has failed to correct hypotension and hypoperfusion. Measurement of serum lactate levels to guide resuscitative efforts has been included in the new update for surviving sepsis guidelines. Glucocorticoids that were previously empirically administered are restricted to patients who fail to respond to fluid and vasopressor therapy. Surgical intervention for source control should be pursued as soon as the patient is hemodynamically stable for operation.
Antibiotic therapy is required before, during, and after surgical intervention . The type of bacteria causing secondary peritonitis depends in part on the normal flora of the part of the GI tract that is the source of sepsis and in part on the clinical setting. Two recent sets of guidelines for the management of complicated intra-abdominal infections recommend broader antimicrobial therapy for hospital-acquired infections than in community-acquired infections. In community-acquired peritonitis, susceptible gram-negative bacilli, strict anaerobic bacteria, and enterococci are typically found. In health care–associated infections, the flora may have been altered by previous antibiotic exposure and previous disease, with more antibiotic-resistant organisms present. In general, antibiotics directed against the most likely pathogens should be chosen. For example, colonic processes require coverage for gram-negative aerobes and anaerobes. In animal models, antibiotics directed against gram-negative enteric aerobic organisms minimize mortality, and drugs effective against anaerobes prevent abscess formation. It has been shown that there is synergy between aerobic and anaerobic bacteria in experimental models of peritonitis. The coverage of all potential organisms is not necessary. The flora of surgical peritonitis simplifies with time, even before initiation of antibiotics. Killing certain key species may change the microenvironment sufficiently to prevent growth and allow killing of other flora. If a Candida species is cultured from the peritoneal cavity, this organism should be treated if the patient is in septic shock, in an immunocompromised state, or in a hospital-acquired setting. On the other hand, hemodynamically stable immunocompetent patients with secondary peritonitis in a community setting do not need treatment for Candida. Examination of short course antibiotics (4 ± 1 days) versus antibiotics until resolution of fever/leukocytosis (approximately 8 days) after source control suggested equivalence in the STOP-IT trial.
A variety of antibiotic regimens have been proposed using the following classes of antibiotics alone or in combination: second-generation cephalosporins, third-generation cephalosporins, broad-spectrum beta-lactams, fluoroquinolones and metronidazole, and aminoglycosides with clindamycin or metronidazole. Many controlled trials of antibiotic regimens show equivalency. For example, it has been shown that monotherapy with a broad-spectrum beta-lactam is as effective as combination therapy with a beta-lactam and an aminoglycoside. Data-supported guidelines regarding optimal treatment have been hampered by suboptimal study design and nonuniform efficacy criteria in the controlled trials that have been performed. A Cochrane review of 40 randomized trials involving 16 different regimens showed no difference in mortality. The specific antibiotics chosen should take into account other considerations such as the avoidance of toxicities, the sensitivity profile of cultured organisms, the ease and route of administration, the risk of emergence of antibiotic resistance, and cost. The availability of broad-spectrum antibiotics, including beta-lactams, fluoroquinolones, and third- and fourth-generation cephalosporins, makes it unnecessary to use aminoglycosides with their potential nephrotoxicity in patients with compromised renal function.
The failure to clear secondary peritonitis after an appropriate course of antibiotic therapy or the recurrence of peritonitis is termed tertiary peritonitis. Nosocomial infections occurring in patients after long periods of hospitalization may include infections with multiresistant Pseudomonas , Enterobacter , Enterococcus , Staphylococcus , and Candida species. The development of multiple organ dysfunction syndrome after an initial operation should prompt an aggressive search for inadequate source control and for abscesses, involving repeat CT, percutaneous or operative drainage of abscesses, and culture of persistent fluid collections, in addition to antimicrobial therapy.
Antibiotics help treat or prevent fatal bacteremia but do not cure most patients with surgical peritonitis unless operative intervention is also undertaken. Neither free leakage of gut contents nor large abscesses can be sterilized by antibiotics alone in the absence of drainage. Surgical intervention should occur as soon as possible after the patient has been stabilized and resuscitated and antibiotics have been given. Laparotomy remains the gold standard for definitive diagnosis and mainstay of therapy in surgical peritonitis. However, a recent review confirms the success of an increasing number of laparoscopic procedures for some forms of peritonitis. With either laparoscopic or conventional open operations, the aims of surgical treatment are source control, peritoneal decontamination, and prevention of recurrent infection.
Repeat laparotomy after temporary abdominal closure may be useful when control of the source of infection is not possible at the initial operation. Surgical re-exploration may be undertaken for the following reasons: (1) tenuous control of the source of infection; (2) reassessment of bowel viability; (3) inadequate or poor drainage; (4) hemodynamic instability; (5) infected pancreatic necrosis or diffuse fecal peritonitis at the initial operation; (6) reassessment of a tenuous anastomosis; and (7) the development of intra-abdominal hypertension (abdominal compartment syndrome). Abdominal compartment syndrome, which is described in more detail in Chapter 11 , develops when the closure of the abdomen at either the level of the fascia or skin causes intra-abdominal pressure to rise to a degree that impairs respiratory, hepatic, and/or renal function.
Preoperative and postoperative fluid and nutritional support are crucial to prompt wound healing and survival. Peritonitis has been compared with a 50% total body surface area burn, and even a calorie intake of 3000 to 4000 kcal/day may not achieve positive nitrogen balance. Inability to achieve positive nitrogen balance may, however, be secondary to accelerated proteolysis, and negative nitrogen balance associated with pathologic proteolysis cannot be reversed by any amount of caloric intake. This proteolysis may only be thwarted with treatment of the septic process and recovery of the patient. The enteral route of nutrition is preferred over parenteral (see Chapter 6 ). Placement of a feeding jejunostomy tube at the initial operation is therefore prudent in these critically ill patients.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here