Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter discusses common presentations of surgical emergencies in the newborn. Frequently encountered scenarios are presented as a descriptive case followed by a discussion and explanation of the disease process and treatment options. Necrotizing enterocolitis (NEC) is the most commonly encountered abdominal emergency in the premature newborn and is discussed in further detail in a separate section of this book.
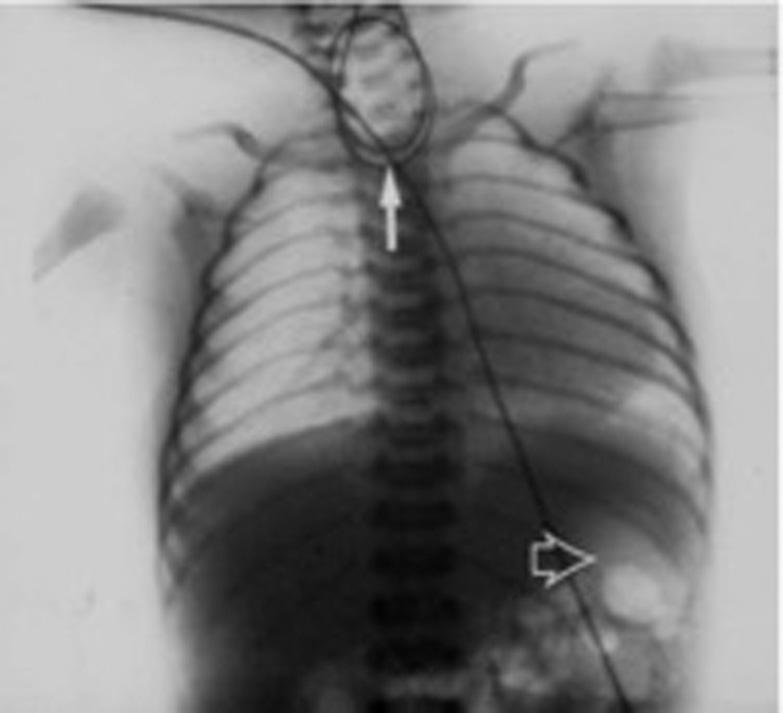
Within a few hours of birth, a newborn full-term male is noted to have excessive salivation. Upon feeding, he experiences choking and gagging. A feeding tube is attempted but cannot pass beyond 10 cm. The mother was found to have polyhydramnios during pregnancy, which was otherwise uncomplicated. A chest x-ray reveals the orogastric tube coiled in the proximal esophagus ( Fig. 21.1 ).
Upon diagnosis, what is the next appropriate step?
Immediate intubation for airway protection
Nothing by mouth (NPO) with initiation of total parenteral nutrition (TPN)
Replace orogastric tube under endoscopic guidance for abdominal decompression
Repeat physical examination
Which of the following prenatal findings may be associated with this diagnosis?
Abnormal cardiac anatomy
Abnormal renal anatomy
Abnormal volume of amniotic fluid
Absence of gastric bubble
All of the above
Based on the information given and the chest x-ray shown, what is the most likely diagnosis?
Isolated esophageal atresia
Esophageal stenosis
Esophageal atresia and distal Tracheo-esophageal fistula
Esophageal atresia and proximal TEF
Which of the following is crucial to obtain before surgery?
Echocardiogram
Repeat plain abdominal films
Renal sonogram
Formal upper GI barium contrast study
D
E: EA/TEF is associated with other anomalies in 50% of cases, most commonly cardiac (25%), genitourinary (20%), and gastrointestinal (20%). Many of these anomalies can be suspected antenatally.
C: Presence of air in bowel confirms distal fistula.
A: An echocardiogram is useful to determine side of aortic arch, which may influence surgical approach. It also details presence of congenital heart defects, which is crucial in surgical and anesthetic planning.
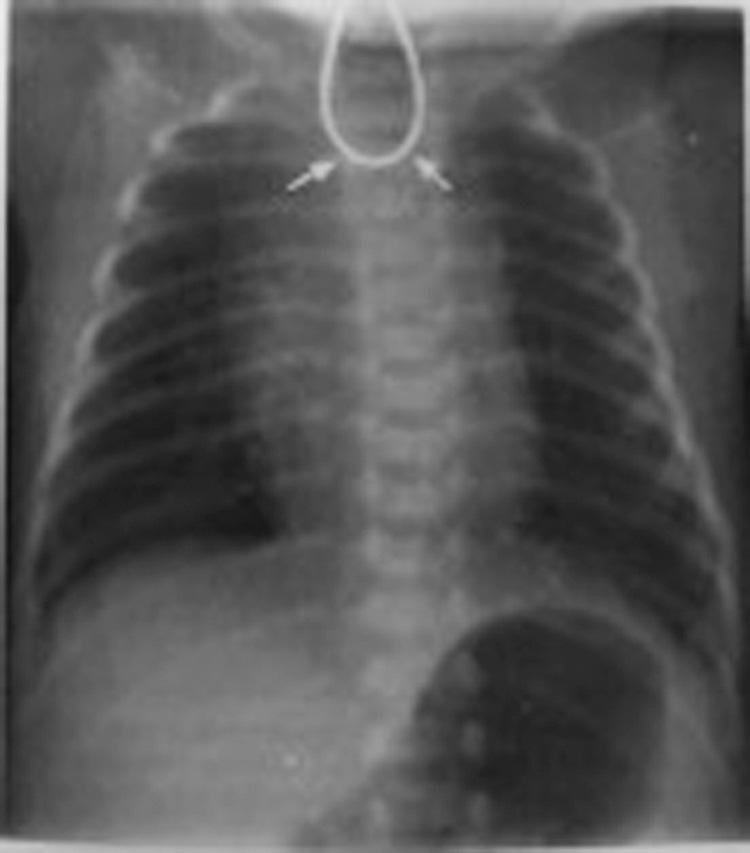
The incidence of congenital tracheoesophageal malformations is about 1 in 2500 to 3000 births. Infants with esophageal atresia and TEF typically become symptomatic within the first few hours of life. Prenatal ultrasonography can suggest EA/TEF by demonstrating a small or absent stomach bubble in association with maternal polyhydramnios, but definitive prenatal diagnosis occurs less than 50% of the time ( ). After birth, a child with EA presents with excessive salivation, mucus coming out of the mouth or nose, and noisy breathing with episodes of choking or cyanosis. Inability to pass a nasogastric or orogastric feeding tube strongly suggests esophageal atresia. An anterior-posterior and lateral x-Ray (“babygram”) that includes the neck, chest, and abdomen reveal the tube coiling within the proximal esophageal pouch ( Fig. 21.2 ). An x-ray is crucial for many reasons:
Presence of abdominal gas confirms the diagnosis of esophageal atresia with distal fistula, which is present in 85% of cases. A gasless abdomen, conversely, suggests a pure or isolated EA, which may require a staged or delayed surgical approach.
Vertebral and other structural anomalies can be identified.
Cardiac malformations can be detected.
Abdominal gas pattern can differentiate esophageal atresia from duodenal atresia.
Lungs can be assessed for other conditions and pneumonia ( ).
Once the diagnosis is made, a physical examination is necessary to identify associated anomalies. EA/TEF may be part of the VACTERL association (vertebral, anal, cardiac, tracheoesophageal, renal, and limb) so it is important to survey for these abnormalities. Spinal and renal ultrasounds are indicated, but an echocardiogram is the most important and is time sensitive as it is useful to determine location of aortic arch, which influences the surgical approach. The presence of congenital heart defects affects outcome as well.
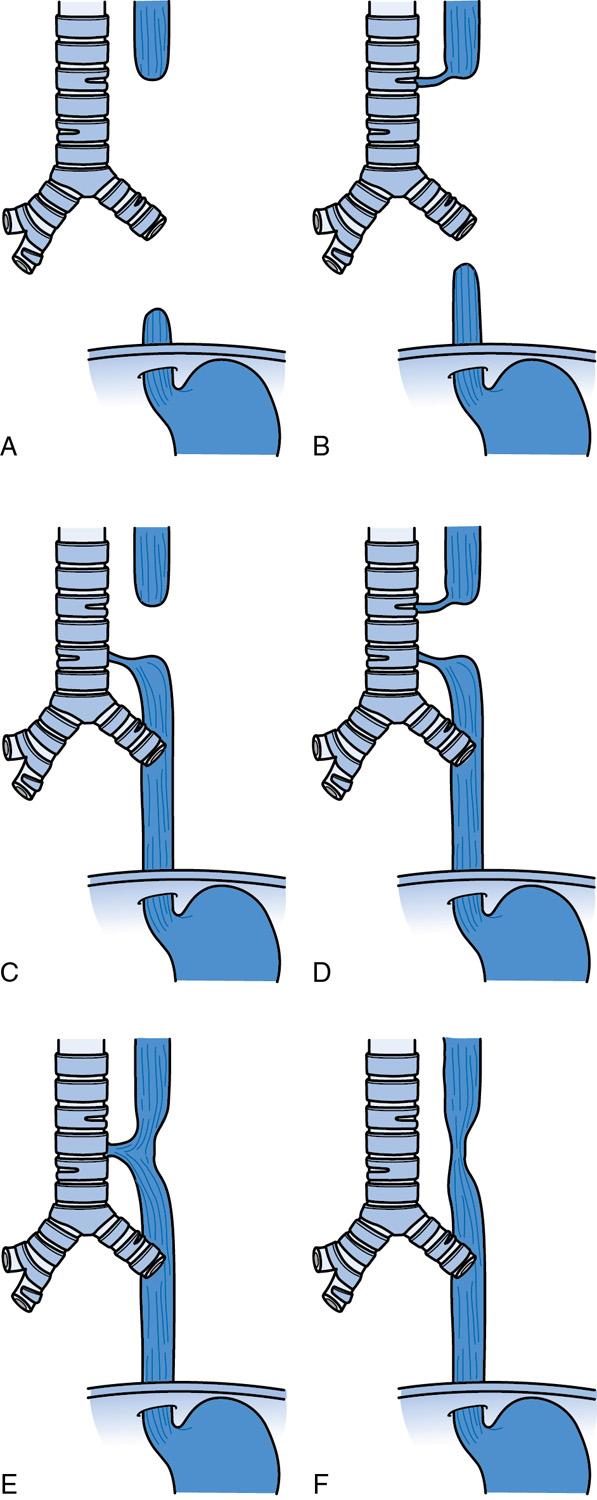
There are five types of tracheoesophageal anomalies that are described with type C, or EA with distal TEF, being the most common ( Fig. 21.3 ). An infant that has choking and coughing with feedings and repeated pneumonia, especially of the right upper lobe, suggests an isolated H type anomaly ( ). These patients need further imaging, such as a contrast esophagram to establish the diagnosis.
The surgical approach to tracheoesophageal anomalies depends on the type of anomaly and the status of the newborn. The initial treatment in these infants involves preventing aspiration and pneumonitis. The Replogle is positioned to continuously aspirate saliva and the infant is positioned upright to minimize reflux and aspiration. H-2 blockers and broad spectrum antibiotics are initiated empirically. Intubation is typically avoided, as positive pressure ventilation may cause gastric dilation and worsening respiratory distress secondary to abdominal distension, as much of the tidal volume will enter the stomach via the fistula if present. When intubation is necessary, such as in preterm infants with respiratory distress and EA/TEF, emergent intervention to ligate the fistula is employed and is frequently lifesaving ( ).
Operative repair of EA/TEF involves an esophageal anastomosis and closure of the TEF through a right thoracotomy or thoracoscopy. Overall survival is 85% to 95%, but those associated with other major anomalies, especially cardiac, have a poorer prognosis. Very low birthweight neonates (<1500 g) pose a major risk factor for surgery as well. Postoperative complications are common but manageable. Leaks are not infrequent (15%) and can typically be treated nonoperatively. Most leaks seal spontaneously. Strictures can be treated with balloon dilatation.
A newborn term male born without complications is tachypneic, grunting, cyanotic, and pale. On examination, you notice a scaphoid abdomen ( Fig. 21.4 ), absent breath sounds on the left, and decreased breath sounds on the right.
What is the next step in management?
Initiate empiric antibiotics for concern for aspiration
Intubation and placement of a nasogastric tube
Obtain echocardiogram
Placement of a left thoracostomy tube
What is the most appropriate initial diagnostic test?
Echocardiogram to assess for congenital heart defect
Chest and abdomen plain film
Ultrasound of the chest
Chest computed tomography (CT)
Upon manipulation of the infant, the preductal saturation reads 88% with postductal saturation of 65%. Fi o 2 on the ventilator is increased to 100%. The next step in management is:
Surgical consultation for urgent operative repair
Extracorporeal membrane oxygenation (ECMO)
Muscle paralysis to aid in adequate ventilation
Gentle mechanical ventilation without paralysis
B
B
D
Congenital diaphragmatic hernia (CDH) is a relatively common cause of neonatal respiratory distress, occurring in 1 in 2000 to 4000 births, with posterolateral defects accounting for 85% to 90% of occurrences ( ); 80% to 90% of CDH occur on the left side. Diagnosis is typically made on prenatal ultrasound, which aids in predicting outcome by estimating the severity of pulmonary hypoplasia and allowing for a planned delivery at an appropriate center. At birth, respiratory symptoms are determined by the degree of pulmonary hypoplasia and reactive pulmonary hypertension ( ). The severity of pulmonary hypoplasia depends on the duration and timing of visceral herniation into the chest. The most severely affected develop respiratory distress at birth, while a majority declare themselves within the first 24 hours of life. Classically, the infants have a scaphoid abdomen with an asymmetrically distended chest, and chest radiography reveals intestines in the chest cavity ( Fig. 21.5 ).
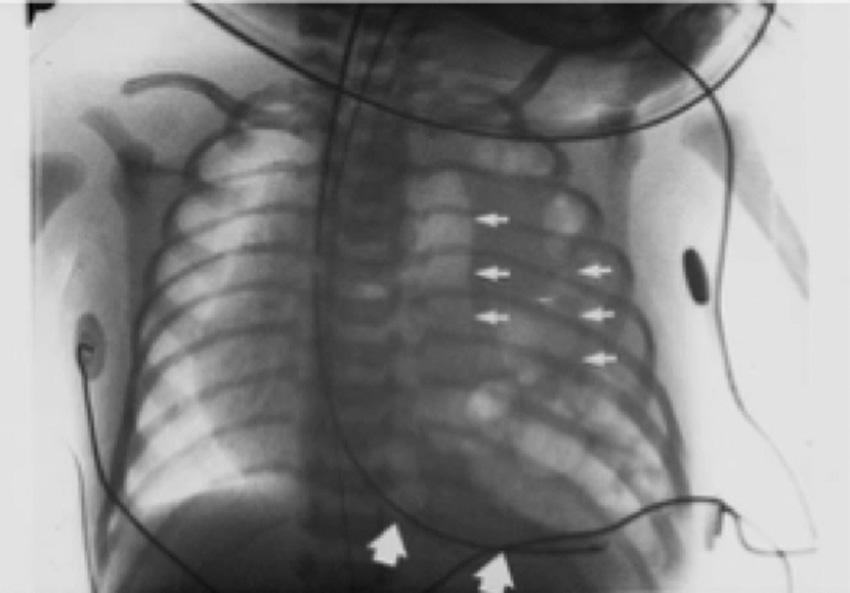
Lung hypoplasia is most severe on the ipsilateral side of the hernia but occurs on both sides due to mass effect exerted by intrathoracic viscera. Alveoli in the lungs of infants with CDH are immature, and gas exchange is limited by fewer bronchial divisions, immature alveoli, and surfactant deficiency. The pulmonary vasculature has increased muscularization, which contributes to pulmonary hypertension. With pulmonary hypertension, deoxygenated blood is shunted into the systemic circulation via a foramen ovale and the patent ductus arteriosus, leading to a gradient in the preductal and postductal pulse oximetry ( ). Some infants require a pulmonary vasodilators, although nitric oxide may be contraindicated when there is left ventricular dysfunction. Those that are refractory to medical treatment may require ECMO as a bridge to lung maturation and normalization of pulmonary arterial pressure ( ).
All newborns with CDH require operative intervention. Surgery does not immediately lead to reexpansion of the hypoplastic lung and actually often worsens respiratory status in the short term. As such, treatment protocols have evolved from emergency surgery at birth to stabilization of the infant and a semiselective repair. Optimal timing of repair is not known with certainty but is typically delayed once there is evidence of recovery or resolution of pulmonary hypertension. Monitoring of pre- and postductal oxygen saturations is useful in this regard. Immediate interventions at birth include respiratory support and decompression of the gastrointestinal tract.
You are contacted by a community hospital pediatrician where a 32-week gestation, 1900 g male newborn has a large amount of intestine outside the baby’s abdomen. He describes the bowel as thick, stiff, and leathery ( Fig. 21.6 ). He cannot visualize the size of the defect under the loops of exposed intestine. The only other notable finding on physical examination is undescended testes.
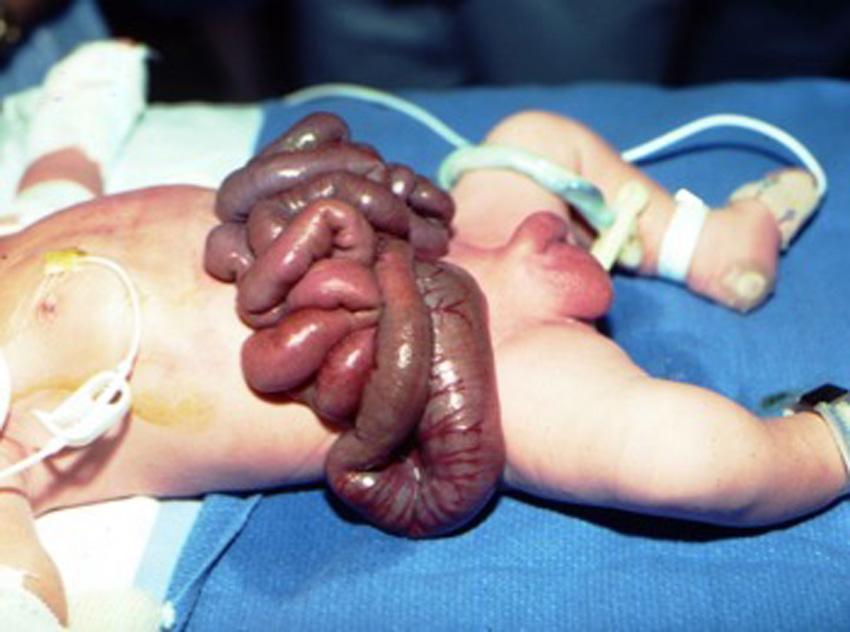
Based on the description given to you from the referring hospital, what kind of abdominal wall defect is described?
Ruptured omphalocele
Omphalocele
Gastroschisis
Pentalogy of Cantrell
Epigastric hernia
What is the next step in management?
Immediate transfer for surgical evaluation
Placement of nasogastric tube
Placement of moistened gauze dressing and a bowel bag
Attempt immediate reduction to prevent further damage to the exposed viscera
B and C
Gastroschisis is associated with cardiovascular defects such as tetralogy of Fallot, atrial septal defect (ASD), and ventricular septal defect (VSD)
True
False
The child arrives at your hospital and is being considered for primary closure of the defect by the surgery team. What is the appropriate preparation to ensure optimization for surgery?
Endotracheal intubation
Secure IV access for fluid resuscitation
Ensuring a warm environment
Identifying bowel congestion and kinking of the mesentery
All of the above
In the postoperative period, the infant is found to have decreasing urine output, worsening abdominal distension and firmness, and new lower extremity edema. What is the next step in management?
Fluid challenge
Lasix bolus to relieve urinary retention postop
Reopening of the abdomen
Abdominal ultrasound to rule of IVC clot
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here