Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The prospect of improving the debilitating symptoms of advanced pulmonary emphysema has long attracted the interest of thoracic surgeons. Previous surgical approaches such as costochondrectomy, phrenic crush, pneumoperitoneum, pleural abrasion, lung denervation, and thoracoplasty have largely fallen out of favor. Today, only three surgical procedures are offered for patients with severe emphysema refractory to intensive medical management: bullectomy, lung transplantation, and lung volume reduction surgery (LVRS). Bullectomy has roots dating to the early 1900s, when external drainage of the giant bulla was attempted to eliminate the space-occupying lesion by collapse rather than by resection. The general approach has evolved to include resection of the bulla with sparing of functional lung tissue. Lung transplantation was first successfully performed in 1963 by Hardy and colleagues, and after a long period of incremental progress, the operation became clinically feasible in the early 1980s, first as heart-lung transplantation and then as isolated lung transplantation. Today, emphysema is one of the most common diagnoses leading to lung transplantation. LVRS was first proposed by Brantigan and colleagues in conjunction with lung denervation, but it was discarded after the initial experience because of a 16% mortality rate. Observations about the physiologic behavior of emphysema patients during and after lung transplantation led to the reconsideration and modification of volume reduction by Cooper and associates.
The destruction of pulmonary parenchyma decreases the amount of functioning lung tissue where gas exchange can take place. As lung tissue is destroyed, the organ loses elastic recoil and expands in volume. This leads to the typical hyperexpanded chest seen in advanced emphysema patients with flattened diaphragms, widened intercostal spaces, and horizontal ribs. These anatomic changes result in the loss of normal biomechanics and bring about increased work of breathing and dyspnea. When destruction and expansion occur in a nonuniform manner, the most affected lung tissue can expand to crowd the relatively spared lung tissue to impair ventilation of the functioning lung. Obstruction in the small airways is caused by a combination of reversible bronchospasm and irreversible loss of elastic recoil by adjacent lung parenchyma. The suitability of a patient for any surgical treatment of emphysema depends in part on the relative contributions of lung destruction, lung compression, and small airways obstruction to the overall physiologic impairment.
Bullectomy, lung transplantation, and LVRS may be offered to patients who remain symptomatic despite optimal medical therapy. Initial medical treatment will include bronchodilators to treat any reversible component of airway obstruction and supplemental oxygen therapy. Smoking cessation is an absolute necessity and should be in effect for at least 6 months before surgical therapy is considered. Participation in pulmonary rehabilitation has been shown to relieve subjective dyspnea, to increase functional capabilities, and to improve subjective quality of life. All patients considered by the authors for surgical treatment of emphysema are enrolled in a supervised pulmonary rehabilitation program, and their subsequent consideration for surgery is based, in part, on their compliance and progress with rehabilitation.
Traditionally, giant bullous emphysema is characterized by a bulla or bullae that occupy approximately one third or more of the hemithorax. Most patients considered for surgery are symptomatic with dyspnea or spontaneous pneumothorax. Other rare symptoms include bleeding or infection within the confines of the bulla. Bullae treated expectantly with observation will typically demonstrate enlargement causing worsening dyspnea, although the rate of expansion is not well described. Factors making surgery less appealing include multiple smaller bullae, advanced emphysema in the nonbullous adjacent lung, and significant comorbidities. Bullectomy is performed relatively infrequently, as demonstrated by a systematic review several years ago that cited 22 individual publications on bullectomy during a 39-year period with a combined total of 476 patients.
The operative technique depends on the anatomic details of the bulla as well as the preferred approach of the surgeon. A well-demarcated bulla with a clear pedicle can be excised with a stapler by a muscle-sparing thoracotomy or a video-assisted thoracoscopic approach. Numerous bullae or bullae that merge indistinctly with the comparatively normal adjacent lung will require a large, stapled wedge resection placed to maximize resection of destroyed lung while minimizing resection of spared parenchyma. It is unusual for a formal lobectomy to be necessary, but when a lobe is almost completely destroyed and the fissures are complete, a lobectomy is an attractive option that might reduce the risk of a postoperative air leak and prolonged chest tube drainage. Many surgeons will combine a localized pleurectomy or a pleural tent with the bullectomy to help manage the pleural space and prevent a prolonged chest tube air leak.
The safety of bullectomy in well-selected patients is demonstrated by the 2.3% mortality reported by FitzGerald and colleagues more than 30 years ago. Our more recent results are similar, with a single death in 43 operations (2.3%). Properly selected patients will experience a perioperative increase in the first second forced expiratory volume (FEV 1 ), and subsequently there is a very low rate of respiratory failure or need for tracheostomy. Parenchymal air leaks are the most frequent single postoperative complication. Prolonged chest tube air leaks (more than 7 days) have been reported from 53% to 75% after bullectomy. Air leaks may be minimized intraoperatively with the surgeon's choice of buttressed stapled lines, pleural tent, pleurectomy, biological glues, or postoperatively with ambulatory Heimlich valves.
In a prospective study with 41 patients undergoing elective surgery for bullae, the 1-year mortality rate was 7.3%, and the overall 5-year mortality rate was 12.2%. When a subgroup analysis was performed, it was seen that all mortality cases were from patients whose bullae were surrounded by diffuse, generalized emphysema (rather than normal lung parenchyma). A small retrospective series in Iceland demonstrated no 30-day mortality, a 5-year survival rate of 100%, and an overall survival rate of 60% at 10 years. Our clinical series demonstrated a 5-year survival rate of 91%, with two late deaths attributed to pneumonia and one to pulmonary fibrosis.
In general, the freedom from long-term return of dyspnea is proportional to the quality of the remaining lung after bullectomy. Our experience demonstrated an improvement in the FEV 1 from 1.2 ± 0.6 liters preoperatively to 1.9 ± 0.9 liters at 6 months and 1 year postoperatively. The persistence of a measurable airflow obstruction after bullectomy underscores the presence of residual emphysema in the remaining lung, regardless of its seemingly normal appearance compared with the destroyed bullous regions. However, in a prospective series by Palla and colleagues, patients with and without underlying diffuse emphysema experienced a persistent FEV 1 benefit over 5 years of follow-up, with both groups reaching their maximum increase in FEV 1 2 years after surgery, followed by a gradual decline through postoperative year 5. For all patients in this series, a significant reduction (improvement) in the dyspnea score was seen immediately after surgery and only began to increase 5 years postoperatively. Despite the late progression in dyspnea, the improvement observed in dyspnea at 5 years remained clinically significant compared with preoperative values seen at presentation.
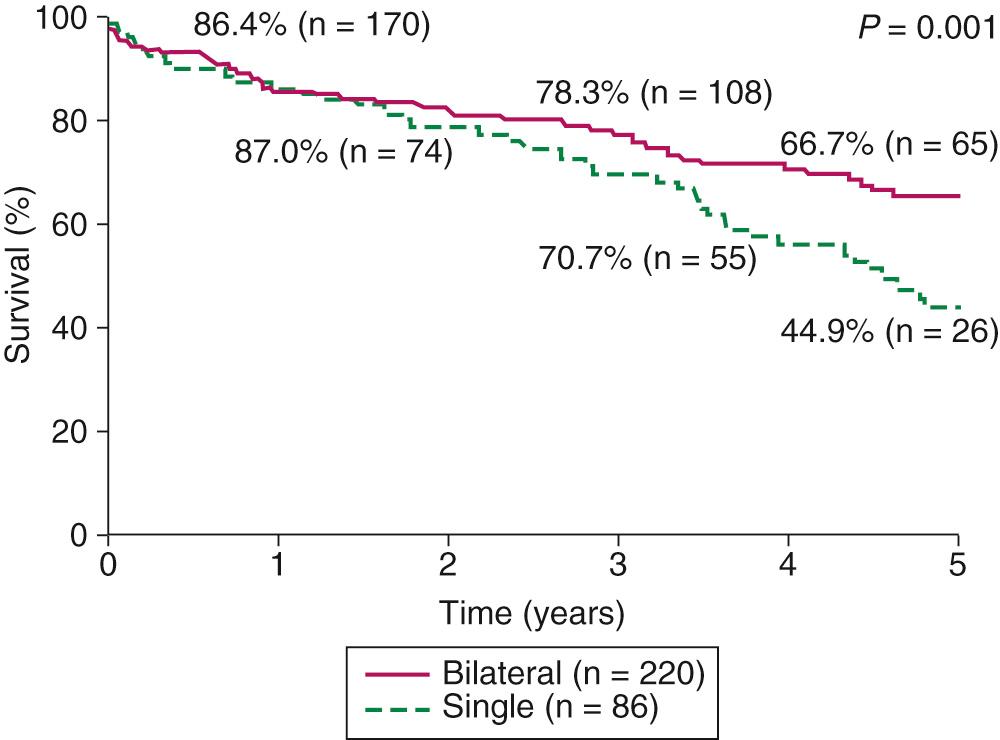
After the initial success with single-lung transplantation for emphysema was reported, and the development of techniques to allow safe, bilateral lung transplantation continued to progress, the application of lung transplantation for severe emphysema became increasingly common. From 1995 to 2012, chronic obstructive pulmonary disease (COPD) and emphysema were the most common indications for lung transplantation and comprised 33.5% of all lung transplants. During this period, three single-site studies found significantly higher 5-year survival rates for patients receiving bilateral as compared with single-lung transplantation, with no difference in 30-day mortality. A review of the International Society for Heart and Lung Transplantation (ISHLT) database's single and bilateral lung transplants (9883 cases) from 1987 to 2006 also confirmed a significant long-term survival advantage for patients receiving a bilateral lung transplant (6.41 years vs. 4.59 years; P < 0.0001), and a hazard ratio of 0.89 (0.80-0.97) after propensity-based matching to control for potential confounders between these patient groups. A retrospective analysis of 1656 lung transplant recipients who were 60 years or older showed no difference among 30-day, 1-year, or 5-year survival rates between those who received single versus bilateral lung transplantation. As an overall result of these findings, there has been an important shift in lung transplantation practice from a 27.5% bilateral rate in 1997 to 72.8% in 2011. Our previously published report included 306 patients, 86 of whom received a single-lung transplant and 220 of whom received a bilateral transplant. In contrast to earlier reports from our institution, the morbidity and mortality were comparable for the two groups, with an overall hospital mortality rate of 6.2%. There were no differences in hospital stay, intensive care unit stay, or duration of mechanical ventilation. There was, however, a difference in long-term survival; the 5-year survival rate ( Fig. 13-1 ) of the bilateral transplant recipients was 66.7%, whereas the survival rate of the single-lung recipients was 44.9%. The ISHLT database also demonstrates a significant long-term survival advantage for patients with α 1 -antitrypsin deficiency undergoing bilateral lung transplantation when compared with single-lung transplantation. Of note, among those patients undergoing single-lung transplantation, no difference in 30-day, 1-year, 3-year, or 5-year survival is observed based on right- or left-sided transplantation, and when such transplantations are carried out, they should be based on patient factors such as pretransplant perfusion scans.
The selection criteria for lung transplantation are chosen to identify patients with sufficient risk of death from lung disease to make the risks of the transplant operation worth bearing. In our experience, the mean supplemental oxygen requirement is slightly in excess of 4 liter/min. The typical profile of a COPD patient listed for lung transplant shows a post-bronchodilator FEV 1 of up to 20% (and frequently as low as 10% of predicted), resting PO 2 less than 55 mm Hg, significant pulmonary hypertension, hypercarbia (>55 mm Hg), life-threatening exacerbations, and severe functional limitations. Progressive elevation in P co 2 is frequently observed in patients, with several patients undergoing transplantation with the P co 2 in excess of 100 mm Hg. Although survival of emphysema patients has been notably difficult to predict, these patients typically exhibit an excellent survival rate on the waiting list compared with patients who have other lung diseases. In 2004, the BODE index (an acronym of the four factors that comprise the model: b ody mass index, airflow o bstruction, d yspnea, and e xercise capacity) was developed and found to be a better predictor of both all-cause mortality and respiratory-related mortality than FEV 1 . For example, the highest index quartile (BODE index 7 to 10) was associated with a mortality rate of 80% at 52 months and an overall median survival of approximately 3 years. As a result of studies using the BODE index to stratify mortality risk among patients with emphysema, the ISHLT implemented a BODE index exceeding 5 as indication for referral to a lung transplantation center and an index of 7 to 10 as an independent guideline for lung transplantation listing.
The implementation of the lung allocation score (LAS) in 2005 by the Organ Procurement and Transplantation Network altered the proportion of patients with emphysema undergoing lung transplantation after listing. Matches for donor lungs are now made on the basis of estimated mortality risk on the waiting list and estimated post-transplant survival probability. In a multicenter retrospective cohort study examining transplant characteristics before and after implementation of the LAS, a significant decrease in rates of lung transplantation for patients with emphysema was seen (from 45.9% of all transplant cases to 33.9%), with an increase in transplantation rates for patients with idiopathic pulmonary fibrosis (from 14.7% of cases to 24.6%). No differences in hospital mortality or 1-year survival rates were detected. Another analysis, of approximately 8000 patients listed for lung transplantation between 2002 to 2008, demonstrated that COPD patients had the lowest 6- and 12-month mortality rates on the waiting list pre-LAS and had a further significant decline in mortality rates after LAS implementation.
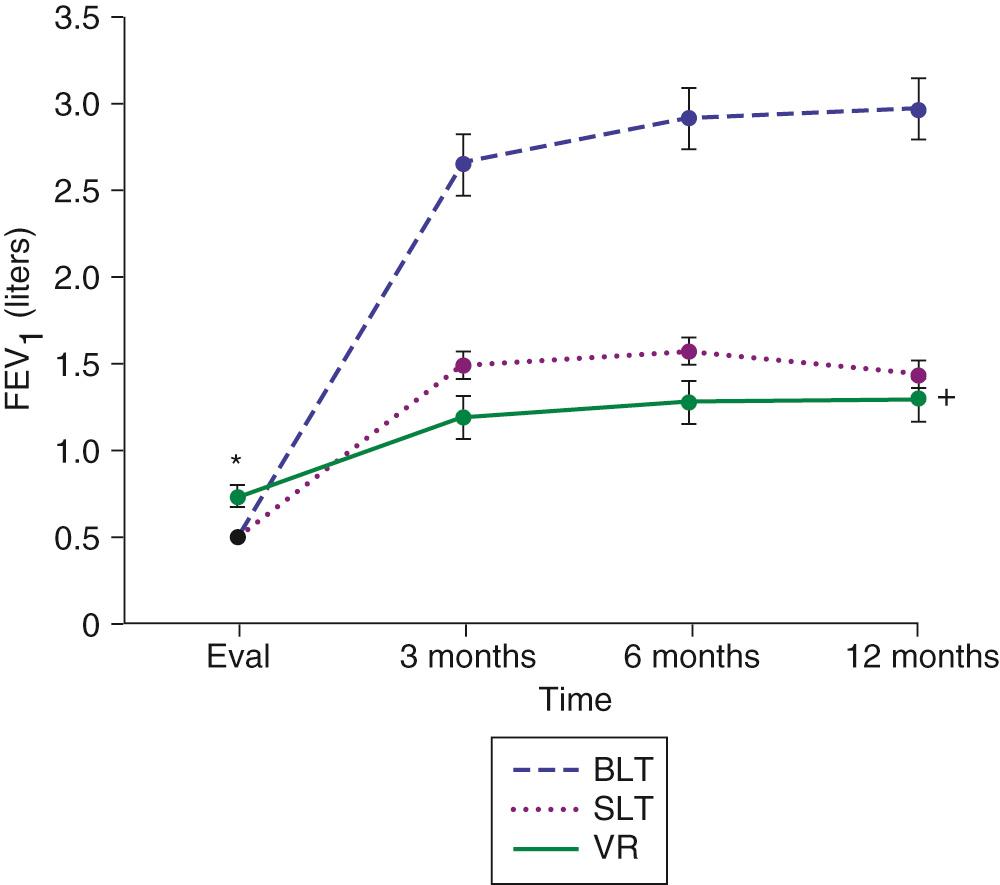
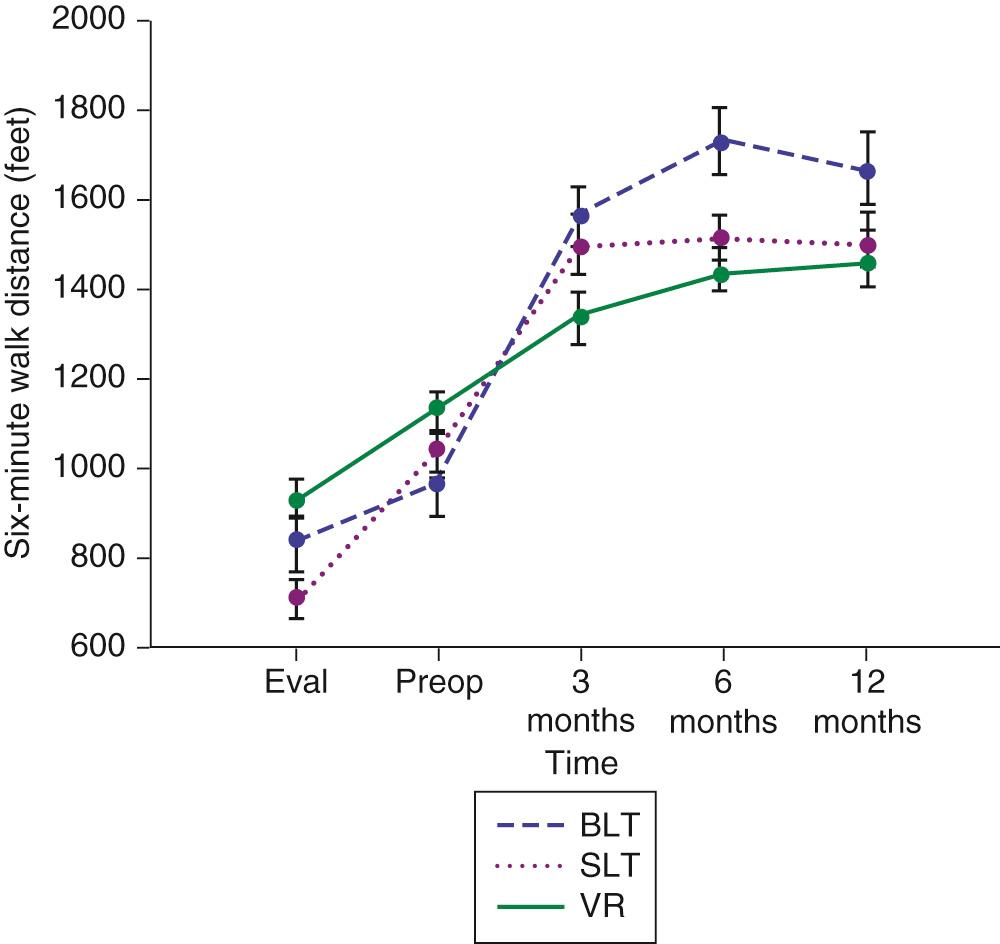
Initial and long-term function of patients with single or bilateral lung transplantation to treat emphysema shows a dramatic improvement in pulmonary function and exercise tolerance with elimination of the need for supplementary oxygen. Figure 13-2 demonstrates the magnitude of change in the FEV 1 as a result of single-lung transplantation, bilateral lung transplantation, and LVRS as reported by Gaissert and colleagues. Figure 13-3 shows a similar stratified analysis for exercise tolerance as measured by 6-minute walking distances. Although the improvement in both outcome measures is greatest for patients who underwent bilateral lung transplantation and least for those who underwent LVRS, the absolute differences in exercise tolerance are much less than those seen in pulmonary function. Improvements in quality-of-life measures have also been documented among lung transplant patients with a BODE index greater than 5 (with mean time of assessment 4 months after lung transplantation).
The disadvantages of lung transplantation are well known. First, the lack of available donor lungs can lead to wait times in excess of a year. Once lungs become available, the initial morbidity and mortality rates of lung transplantation are higher than those reported for lung volume reduction, with 30-day mortality variously described as 5% to 15% but typically closer to the lower end of that range. The presence of allograft lungs necessitates lifelong immunosuppression, incurring higher medical costs to the individual and society. It also predisposes the patient to increased risk of neoplasm (as well as increased growth rate of occult cancers with poor comparative survival versus non-immunosuppressed patients for any given stage) and infection compared with non-immunosuppressed patients. Finally, the risk for development of chronic lung allograft dysfunction (CLAD) increases as a function of time following transplantation, reaching 50% to 60% by 5 years. A retrospective study of 425 bilateral lung transplants from the ISHLT/United Network for Organ Sharing (UNOS) registry identified a survival difference when analyzed by age, with 30-day, 1-year, and 5-year survival rates for patients younger than 50 years old equal to 94.9%, 84.7%, and 68.2%, respectively, and patients 50 to 60 years old with rates of 93.0, 79.7, and 60.5%.
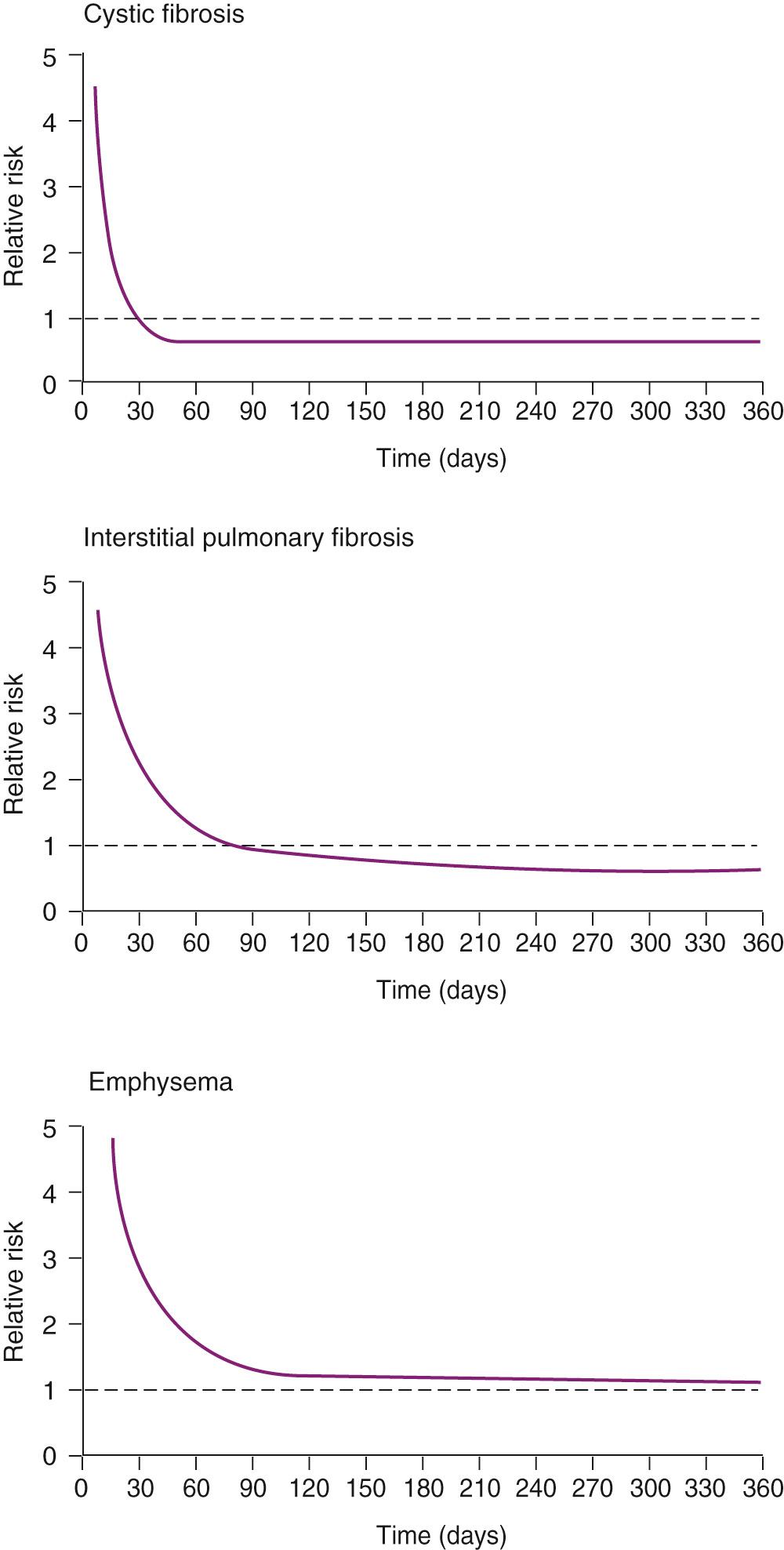
A controversial aspect of lung transplantation had been the question of whether it conveys a survival benefit to the patient with emphysema, with literature from the past decade divided. Of note, when patients transplanted for emphysema are stratified either by FEV 1 or BODE index, survival benefit with lung transplantation is seen for the most severe categories of each measurement. For example, in a numerical simulation based on a UNOS database of 8182 COPD patients on the waiting list for lung transplantation since 2004, it was calculated that 79% of patients with an FEV 1 less than 16% of the predicted value would gain an average of an additional year of life expectancy (in comparison to only 44.6% of listed patients gaining an additional year when not stratified by FEV 1 ). A survival benefit was also seen in a single-center retrospective analysis when COPD patients receiving lung transplantation were stratified by BODE index of 7 or higher. It is likely that the Lung Allocation System will optimize the life extension caused by lung transplantation by balancing the goals of transplanting the candidates with the lowest life expectancy on the wait list while selecting those most likely to survive and thrive postoperatively ( Fig. 13-4 ).
Our current approach to a bilateral sequential transplantation involves bilateral anterior thoracotomies in the fourth (or fifth) interspace. The internal mammary artery is ligated and divided bilaterally. The fourth rib is shingled anteriorly by resecting 1 cm of the costal cartilage at the sternal border. More mobility is obtained by dividing the intercostal muscle from within the pleural space back as far as the posterior axillary line. Should additional access to the thorax become necessary during the operation, the sternum can be divided transversely at the fourth interspace, and the entire chest is clamshelled open. The least functional lung, as determined by preoperative quantitative ventilation and perfusion scans, is resected and replaced first. Preliminary dissection of both lungs shortens the time that the first implanted lung is exposed to the entire cardiac output and thus lessens the likelihood of reperfusion edema.
The pulmonary arteries and veins are dissected beyond their primary bifurcations to preserve the length of the main trunks. The right pulmonary artery is usually transected by a vascular stapling device 1 cm beyond the ligated first branch to the right upper lobe. The left pulmonary artery is kept longer and transected between staple lines beyond the second branch to the left upper lobe. The vein branches are usually not stapled; rather, they are double ligated and divided at their secondary branch points to save length for the future recipient atrial cuff. The arterial and venous dissection and division are accomplished before the bronchial division to avoid prolonged contamination of the operative field by the open distal airway. The bronchus is transected between cartilaginous rings, and the posterior bundle of lymphatics and bronchial arteries is exposed to facilitate ligation and subsequent division. The pulmonary artery stump is mobilized centrally, then grasped with a clamp and retracted anteriorly to afford better access to the posterior bronchus. The pulmonary vein stumps are then grasped and retracted anteriorly and laterally to permit circumferential opening of the pericardium. With the pericardium freed, the vein stumps are then retracted and temporarily fixed anteriorly. This provides an excellent view of the bronchus, which is then mobilized well into the mediastinum and divided. Meticulous hemostasis in the mediastinum is achieved at this point, with the knowledge that reaching this portion of the operative field after implantation of the graft lung will be extremely difficult.
Meanwhile, the donor lung is prepared on the back table. The donor bronchus is divided immediately proximal to the upper lobe orifice. Care is taken to minimize dissection of the donor bronchus to preserve collateral flow through the peribronchial nodal tissue. The pulmonary artery and left atrial cuffs are freed from any pericardial attachments that may cause kinking after the anastomosis is completed.
Once the native lung is removed, the donor lung is placed into the chest and kept cold with iced saline and slush. We conduct the anastomoses from posterior to anterior in the following sequence: bronchus, artery, and atrium. The first stitches are 4-0 polydioxanone (PDS) sutures placed at the two corners of the bronchial anastomosis at the medial and lateral junction of the membranous and cartilaginous airways. These sutures are tied, and one end is used to join the donor and recipient membranous airways in a continuous suture. The cartilaginous rings of the donor and recipient are joined with interrupted figure-of-eight 4-0 PDS sutures.
The second anastomosis is the pulmonary artery. The pulmonary artery (which has already been extensively and circumferentially dissected into the mediastinum) is clamped centrally, with care taken to avoid including the Swan-Ganz catheter in the jaws of the Satinsky clamp. The vascular staple line is resected at a location that matches the size of the donor and recipient artery. A larger recipient artery can be divided beyond the first ligated branch to match a smaller donor artery; a smaller recipient artery is usually divided proximal to the first branch, or through it, to maximize circumference. The donor pulmonary artery is trimmed to an appropriate length, and the anastomosis is created with running 5-0 Prolene suture. This anastomosis must be made with precise, small suture bites to avoid any anastomotic stricture.
The atrial anastomosis is performed last. A Satinsky clamp is placed centrally on the atrium. Placement of this clamp too centrally can reduce venous drainage from the contralateral lung and decrease cardiac output, whereas placement of the clamp too peripherally will compromise the recipient atrial cuff. The ties are then cut off the recipient vein stumps, and the bridge of atrium between vein stumps is divided to create the atrial cuff. The anastomosis is performed with 4-0 Prolene suture, and the last few sutures are left intentionally loose to allow flushing and de-airing of the graft and the recipient atrium. For this maneuver, the lung is partially inflated, and the pulmonary artery clamp is loosened momentarily. The lung is flushed to force out the residual pulmonary perfusate solution. The pulmonary artery clamp is then reapplied, and the atrial clamp is opened momentarily to completely de-air the atrium. The atrial sutures are then secured and the clamps removed. All suture lines are then checked for hemostasis as ventilation and perfusion are restored.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here