Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The treatment of atrial fibrillation (AF) with antiarrhythmic medications have been limited by modest efficacies, proarrhythmic side effects, and systemic toxicities. Conversely, rate-control strategies leave patients in AF and do not address the impaired hemodynamics or symptoms associated with this arrhythmia. This strategy may also render subsequent attempts at rhythm-control therapies less effective because patients may suffer irreversible atrial remodeling after a prolonged duration of AF. Both rate- and rhythm-control strategies necessitate the use of warfarin or direct oral anticoagulants, which carry a risk for major bleeding complications. These shortcomings encouraged the development of interventional options to treat AF, such as catheter and surgical ablation. Surgical treatment has been shown to be an effective option for patients with lone AF or patients with AF undergoing concomitant cardiac surgical procedures. The goal of surgical treatment is the elimination of AF, restoration of normal sinus rhythm, and restoration of normal atrial mechanical function.
Restoration of normal sinus rhythm (NSR) has several potential benefits over other strategies. These include improvements in atrial systolic function, which increases cardiac output and prevents the development of worsening symptoms in patients with congestive heart failure; a lower risk for stroke; the possibility for discontinuation of anticoagulation; and the potential benefit of reversing atrial structural and/or electrical remodeling. Although the strategy of pharmacologic rhythm control has never been shown to be superior to that of rate control, likely because of the aforementioned problems with drug efficacy and safety profiles, a follow-up analysis of outcomes from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial showed that the presence of NSR was associated with a significant 47% reduction in mortality. A meta-analysis of 10 studies including more than 7000 patients showed a lower rate of hospital readmission using a rate-control rather than rhythm-control strategy and identified a higher mortality with a rhythm-control strategy in patients younger than 65 years of age, possibly because of the proarrhythmic effects of common antiarrhythmic drugs (AADs; risk ratio [RR], 3.03; P < .0007). Given the significant prevalence and public health burden of AF, restoration of NSR with surgical or catheter ablation may be of great benefit to selected patients. In fact, we recently demonstrated a 20% survival benefit at 10 years for cardiac surgery patients with AF who underwent a concomitant procedure to treat the AF compared with those who did not have ablation.
The first effective interventional procedure for AF was introduced into clinical use by James Cox and colleagues in 1987 after extensive animal investigation at Washington University in St. Louis. This operation, known as the Cox-Maze procedure, was developed to provide a standardized anatomic approach that would be applicable to the treatment of AF in all patients. Although initial laboratory attempts failed to define the precise mechanisms of AF, this operation was designed to prevent AF by interrupting all potential macroreentrant circuits thought to be responsible for the maintenance of AF ( Fig. 135.1 ). The operation employed a pattern of biatrial incisions that allowed sinus impulse propagation from the sinoatrial (SA) node to the atrioventricular (AV) node and sufficient atrial myocardial depolarization to ensure adequate contraction. More recent basic science studies have suggested that it is effective in preventing the sustainability of AF by reducing the critical mass of tissue. The Cox-Maze procedure was successful in restoring sinus rhythm and AV synchrony, thereby significantly reducing the risk for thromboembolism and hemodynamic compromise from the arrhythmia. , It also allowed for the preservation of atrial transport function in most patients. ,
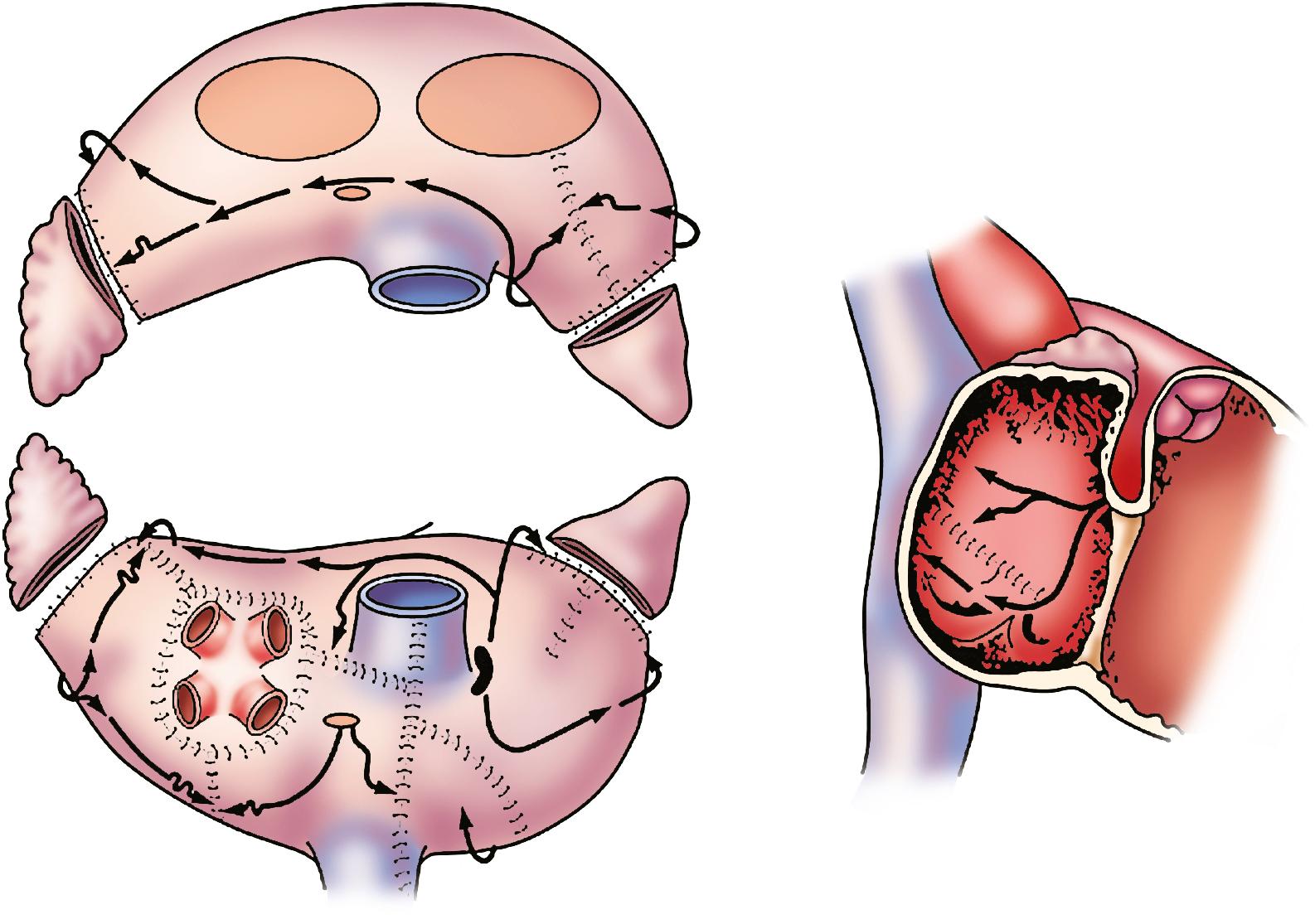
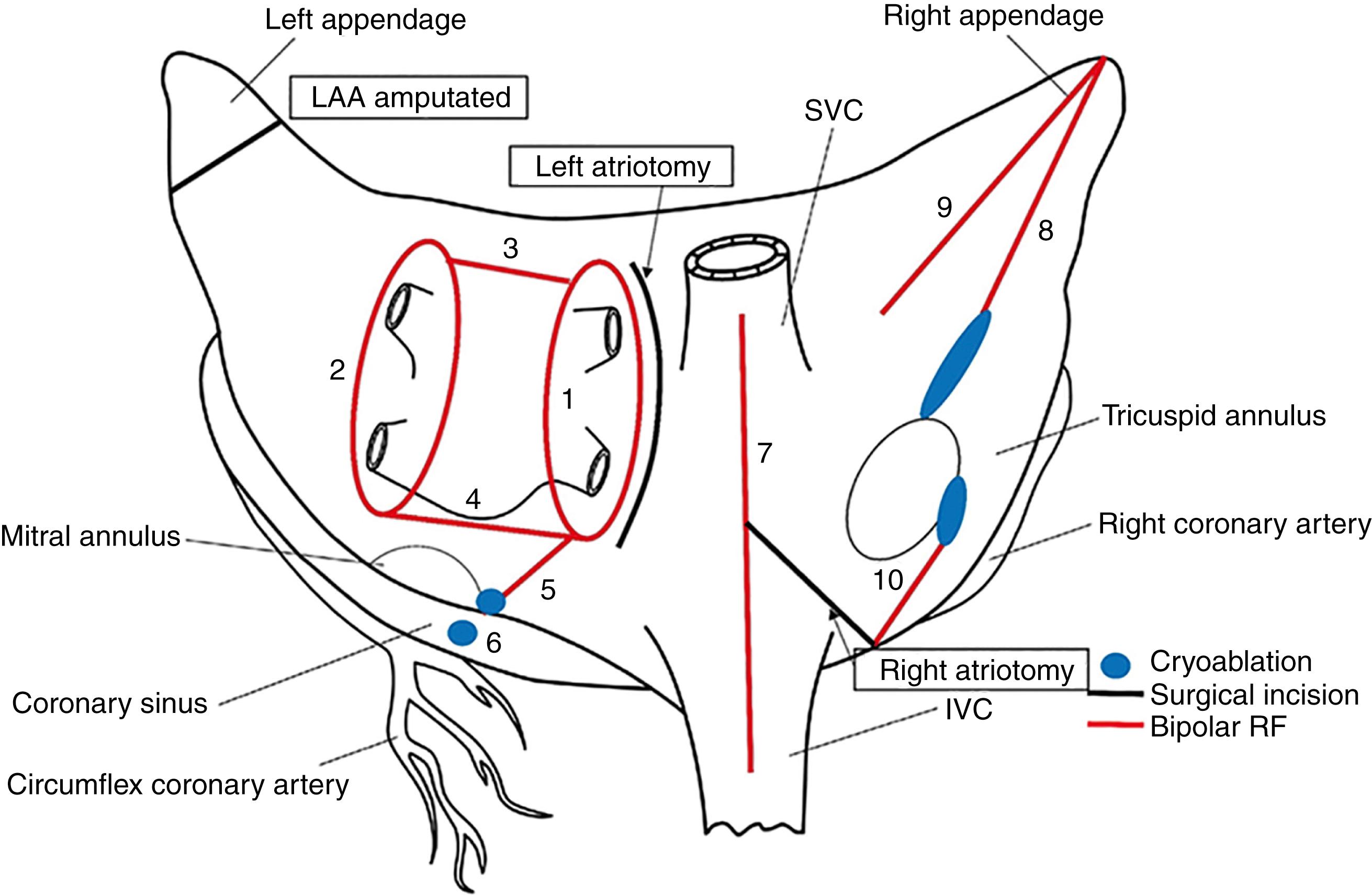
The Cox-Maze procedure has evolved with ongoing clinical experience. The early versions of the Cox-Maze procedure were complicated by late chronotropic incompetence and a high incidence of postoperative pacemaker requirement. The third iteration of this cut-and-sew technique, the Cox-Maze III procedure, achieved excellent results in a long-term study, with 97% of the patients at late follow-up free from symptomatic AF. Nevertheless, despite its clinical success, the Cox-Maze III procedure was not widely adopted because of its technical complexity and prolonged cardiopulmonary bypass times. During the last two decades, the intricate incisions of the cut-and-sew technique have been replaced by surgical energy sources capable of performing tissue ablations. In 2002, our group introduced the Cox-Maze IV operation, the current procedure of choice for the surgical treatment of AF; it uses bipolar radiofrequency (RF) and cryoablation technology to reliably create transmural lesions in a technically simpler, faster, and less invasive manner compared with the Cox-Maze III procedure ( Fig. 135.2 ).
These ablation-based procedures have resulted in a continued increase in the number of operations performed annually for AF. An analysis of the STS Adult Cardiac Surgery Database by Ad et al. showed an increase in the volume of concomitant AF ablation from 8461 in 2005 to 11,363 in 2010. A more recent analysis by Badhwar and colleagues found further growth in the number of patients with AF undergoing surgical ablation from 2011 to 2014. In this study, 48% of AF patients undergoing cardiac surgery had concomitant surgical ablation, most of whom were referred for mitral valve operations. In addition, the volume of stand-alone ablation procedures per year also increased from 552 in 2005 to 1014 in 2010. A more recent analysis confirmed this increase in volume of stand-alone ablation procedures, with a 7% growth per year between 2011 and 2017.
The surgical treatment of AF has evolved over the past two decades thanks to the advent of ablation technology and the introduction of less invasive surgical approaches. Current devices reliably produce ablation lines that replace the incisions of the traditional surgical procedures, thereby simplifying and shortening surgical ablation. Various energy sources have been used to create ablation lines, including RF energy, cryoablation, and microwave, and high-frequency ultrasound energies. Devices employing these energies have been used with varying success and therefore require a detailed understanding before clinical use. ,
An ablation device must meet certain criteria to be effective in terminating AF. First, it must reliably produce bidirectional conduction block across the line of ablation. This requires the creation of a transmural lesion because even small gaps in the ablation line can conduct both sinus and fibrillatory wavefronts. Second, the ablation device must be safe. Thus the energy source must have a well-defined dose-response curve to ensure adequate ablation but also limit excessive energy delivery and collateral damage to surrounding structures, such as the coronary sinus, heart valves, and coronary arteries. Third, the ablation device should be easy to use, create reproducible ablation lines, and be able to be implemented in minimally invasive surgical approaches. For the treatment of lone AF, devices that are able to create transmural lesions on the beating heart without the need for cardiopulmonary bypass are desirable. The failure to reliably create linear epicardial lesions on the beating heart is the biggest shortcoming of unipolar energy sources and has resulted in the development of hybrid procedures to allow for less invasive surgical options. Currently, no single device has properly met all these criteria.
Microwave, laser, and high-frequency ultrasound (HIFU) technologies have thus far proven unreliable for the creation of transmural lesions and have been removed from the market. As such, this chapter will focus on cryothermy and RF energy, the only two ablation technologies now in use.
Cryoablation devices consist of a hollow shaft leading to a conductive metal probe, as well as an integrated thermocouple for temperature recording. Cryogens (nitrous oxide and argon are used clinically) are pumped through a hollow shaft under a high pressure to a distal closed electrode tip. Rapid expansion from the liquid to gas phase absorbs energy, producing cooling in a process known as the Joule-Thompson effect. The resultant gas is aspirated via a vacuum tip and returned through a separate circuit.
Cryoablation is one of the safest energy sources because it preserves fibrous tissue and collagen, maintaining tissue integrity. The technology uses thermal conduction to create direct physical injury, causing sublethal cellular stress response and molecular-based cell death by freezing. Intracellular and extracellular ice crystals form, disrupting the cell membrane. Ice formation occurs along the nonuniform surface of the cells and acts as an insulator to slow heat loss. The ice also causes cryoadhesion between the probe and the tissue, which ensures tissue contact and an area of continuous heat extraction from the tissue. The size and depth of the lesion depend on the probe temperature, probe size, duration and number of freeze cycles, specific heat of the cryogens, and thermal conductivity and temperature of the tissue.
There are currently two commercially available sources of cryothermal energy being used in cardiac surgery: nitrous oxide and argon. At 1 atm pressure, nitrous oxide is capable of achieving a temperature of −89.5°C, whereas argon has a minimum temperature of −185.7°C. The goal of cryoablation is to cause irreversible cell injury while maintaining the extracellular matrix; a temperature of −40°C is required to be certain of intracellular ice formation. , Late apoptosis expands the area of cell death. The nitrous oxide technology has been extensively used and is generally safe and efficacious, except around the coronary arteries, where studies have shown late intimal hyperplasia after cryoablation. Disadvantages of cryoablation include the extended duration required for each individual ablation (2–3 minutes) and the difficulty encountered when trying to achieve transmural lesions by applying epicardial cryoablation to the beating heart. The latter shortfall is, in part, a result of dissipation of thermal energy through convective warming from the circulating endocardial blood, which creates a heat-sink effect. Finally, there is a risk for thromboembolism if blood freezes and coagulates during epicardial ablation on the beating heart. This problem can be overcome by placing the probe endocardially and freezing outward; however, this requires cardiopulmonary bypass. Finally, although injury to the coronary arteries and the esophagus are a concern, experience has shown cryothermal energy does not cause permanent damage to valvular tissue or the coronary sinus.
Malleable disposable cryoprobes have been developed that facilitate minimally invasive application and allow for the creation of longer lesions of varied shapes. The ability of one such cryoprobe to reliably produce transmural lesions has been shown to be equivalent to that of a rigid cryoprobe in in vitro and in vivo studies. , Most manufacturers recommend a 2-minute ablation time for endocardial ablations.
RF technology was one of the first energy sources used in the electrophysiology (EP) laboratory and the operating room for the treatment of AF. RF energy deploys an alternating current with a frequency in the range of 300 kHz to 3 MHz. The high frequency prevents rapid myocardial depolarization and induction of ventricular fibrillation (VF) yet is sufficiently low energy to prevent tissue vaporization and perforation. The lesion size depends on the electrode–tissue contact area, interface temperature, current and voltage (power), topical cooling, and tissue resistance. The tissue immediately adjacent to the probes is directly heated by a process called resistive heating , whereas deeper tissue layers are heated via passive conduction. The heat causes destruction of the myocardial cells through protein denaturation and cellular desiccation. Tissue temperatures of approximately 50°C to 60°C result in coagulation necrosis, followed by destruction of the collagen matrix and replacement with a mature fibrous scar within 1 to 2 months. The depth of the lesion and failure to create a transmural lesion can be limited by char formation, which occurs at temperatures greater than 100ºC, as well as epicardial fat, myocardial and endocardial blood flow, and tissue thickness.
RF energy devices currently used in clinical practice employ either a unipolar or bipolar electrode configuration. Some RF devices use continuous saline irrigation to prevent overheating of the electrodes, to avoid char formation, and to maintain a low-impedance path for energy to penetrate deeper into the tissue. Certain devices intended for epicardial ablation, particularly those intended for use via a minimally invasive approach, employ suction for stabilizing the electrode, in an attempt to promote better tissue contact.
There have been a number of unipolar RF devices developed for ablation. Unipolar RF devices disperse RF energy between an electrode tip on the tissue and a passive electrode, which is typically a grounding pad. Most currently available unipolar devices are irrigated or perfused to facilitate lesion formation. Nonirrigated unipolar RF devices have been shown to create transmural lesions on the arrested heart in animal models after long ablation times of 60 to 120 seconds, but unfortunately this success has not been translated into clinical practice. During mitral valve surgery, only 20% of 2-minute endocardial applications produced transmural lesions. Epicardial ablation on the beating heart has been even more problematic. Animal studies have consistently shown that epicardial application of unipolar RF is incapable of reliably creating transmural lesions on the beating heart, and only 7% of epicardial applications in humans produced transmural lesions. This problem was felt to be caused by the heat-sink effect of circulating blood. In addition, several complications have been described as a result heating adjacent tissue, including coronary artery and esophageal injury. , As a consequence, the use of epicardial unipolar RF ablation outside clinical trials is not recommended.
Bipolar RF technology was developed to overcome the shortcomings of unipolar RF ablation. These devices were developed with two different basic designs. The first is a surface bipolar RF device that contains electrodes side-by-side and applies energy to either the endocardial or epicardial surface. The second is the bipolar RF clamp that contains electrodes embedded in its jaws, offering the advantage of applying high-density current to both the endocardial and epicardial surfaces simultaneously. These clamp devices have become the most commonly used surgical ablation devices. An example is shown in Fig. 135.3 .
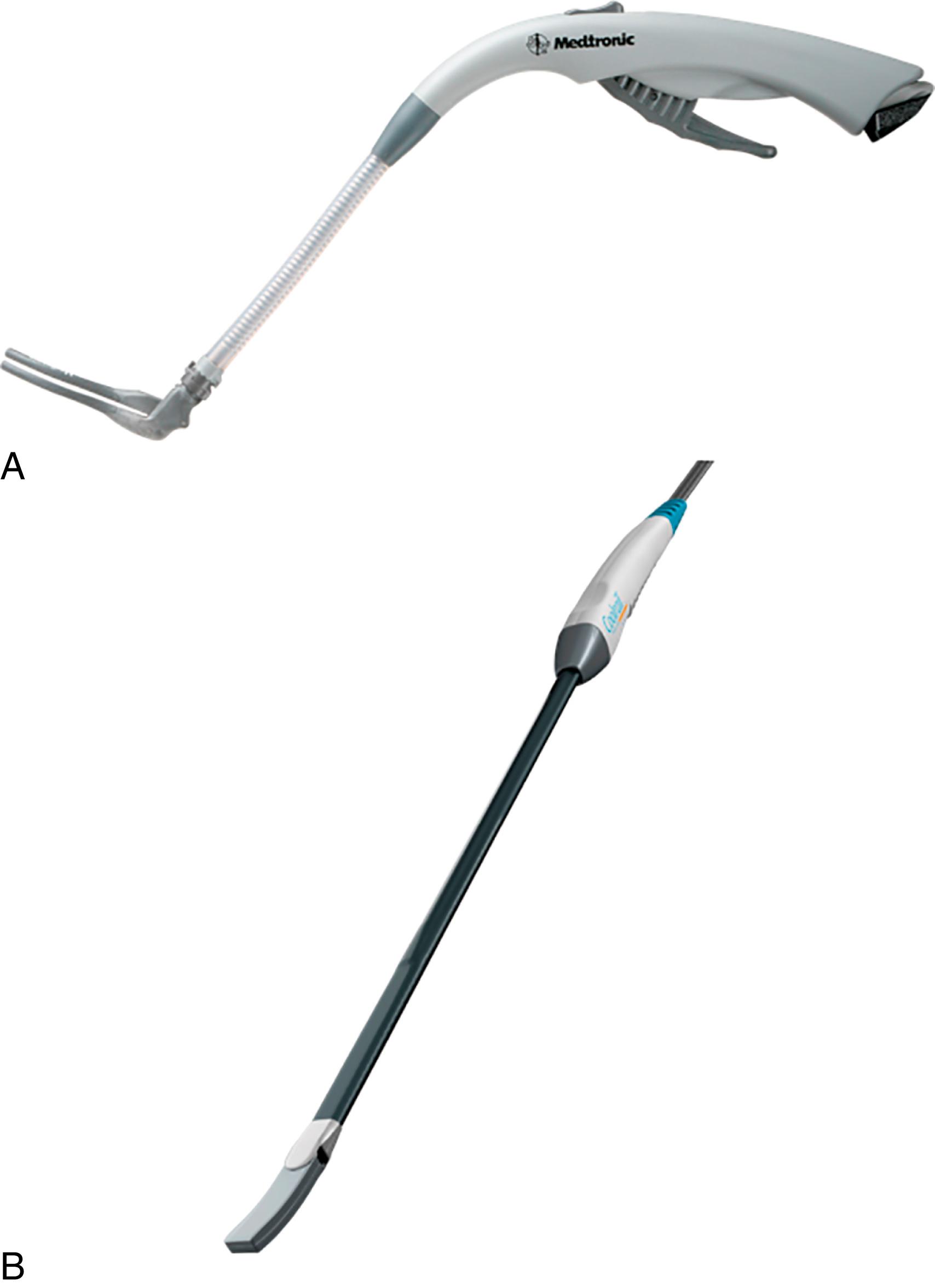
Unfortunately, there has been concern regarding the transmurality of lesions created with surface bipolar RF devices. Animal studies in our laboratory have shown that despite success in creating acute conduction block, none of the lesions produced with one surface device exhibited conduction block at 4 weeks, even though 76% of histologic sections were transmural. A suction-assisted device was tested by two independent groups in a porcine model; one study showed a 96% rate of transmural lesions, and the other showed a similar rate of transmurality on a per-section basis but only a 68% rate of transmurality for the entire lesion.
In comparison to the surface bipolar devices, the bipolar RF clamp has been much more effective at creating transmural lesions. , , Bipolar ablation has been shown to be capable of creating precise transmural lesions on the beating heart in animals, with application times typically between 10 and 20 seconds. , Bipolar clamps also have been shown to not damage commonly used polypropylene suture material, although in our clinical experience, suture disruption has been occasionally observed. Presently available devices employ algorithms to predict lesion transmurality from measured tissue impedance and conductance and to optimize safe energy delivery to the tissue. In our clinical experience, it was observed that the devices would occasionally fail to achieve conduction block with a single ablation, which was never observed in animal models. In a recent study, a new model was developed using excised human hearts turned down for transplantation. Although 89% of histologic sections were transmural, only 65% of single ablations resulted in a completely transmural ablation line. This was usually associated with areas of excessive epicardial fat. If two ablation applications are performed in succession without unclamping, however, 100% of lesions were transmural along their entire length. One drawback of bipolar RF clamps is the requirement for the tissue to be clamped. This has limited potential lesion sets, particularly on the beating heart, and requires the use of adjunctive unipolar or cryothermal technology to create a complete Cox-Maze IV lesion set. Careful attention to surgical technique, meticulous testing for entrance and/or exit block when possible, and improvements in device technology are still needed.
Bipolar devices have been shown to be safer than unipolar devices. Although relatively infrequent, reported clinical complications from unipolar RF devices have included coronary artery injuries, cerebrovascular accidents, and esophageal injury leading to atrioesophageal fistula. Bipolar RF technology has virtually eliminated complications from collateral damage by applying the high-density current between the jaws of the clamp. No device-related injuries have been reported in the literature with the use of bipolar RF clamps.
In summary, each ablation technology has its own advantages and disadvantages. Although efforts have been made to indirectly compare the merits of different ablation devices, there are no randomized controlled trial data. Situations exist where the use of each of these devices is appropriate, but it is necessary to understand the advantages and disadvantages of each to ensure that the devices are applied correctly. Failure to do so will result in suboptimal clinical outcomes.
The Cox-Maze procedure is indicated for both patients with medically refractory, lone AF and for patients undergoing cardiac surgery who have concomitant AF that would benefit from treatment. The latter group is sizable and represents the majority of patients in most clinical experiences. In a review of our experience at Washington University from 1986 to 2005, the incidence of preoperative AF was 33% in patients referred for mitral valve procedures and between 21% and 38% in patients referred for combined valvular/coronary surgery, respectively. Nevertheless, this has been an undertreated cohort of patients. A review of the STS Adult Cardiac Surgery Database by Ad et al. from 2005 to 2010 showed that only 40.6% of patients with preoperative AF received surgical ablation. A higher proportion of patients with preoperative AF referred for mitral valve repair (62%) received concomitant surgical AF ablation compared with only 28% of patients with preoperative AF undergoing coronary artery bypass grafting (CABG). The indications for surgical ablation for AF have been defined in recent guidelines and consensus statements. Surgical ablation for AF is indicated for the following: (1) restoration of sinus rhythm at the time of concomitant mitral operations (Class I, Level A); (2) restoration of sinus rhythm at the time of concomitant isolated aortic valve replacement, isolated coronary artery bypass graft surgery, and aortic valve replacement plus coronary artery bypass graft operations (Class 1, Level B nonrandomized); and restoration of sinus rhythm in symptomatic patients with documented AF in the absence of structural heart disease that is refractory to class I/III AADs or catheter-based therapy (Class IIA, Level B randomized).
At our institution, there are additional relative indications for surgical ablation of AF. Patients with AF who have developed a contraindication to long-term anticoagulation and have a high risk for cerebrovascular thromboembolic events may undergo surgical ablation with exclusion or excision of the left atrial appendage (LAA). This includes patients with a CHADS 2 score less than or equal to 2 who develop a contraindication to long-term anticoagulation. The annual risk for stroke in patients with a CHA 2 DS 2 -VASc score greater than 2 is between 3% and 15%, whereas the annual risk for stroke after a Cox-Maze procedure in 433 patients followed at our institution for a mean of 6.6 years (±5 years) was only 0.2%. In the latter study, no association was found between postoperative neurologic events and either the CHADS 2 score or warfarin use. Although these benefits have not been demonstrated in prospective randomized trials, they are thought to be a result of both successful restoration of sinus rhythm and exclusion of the LAA, which is considered a major source of thromboemboli in patients with AF.
Surgical treatment for AF with management of the LAA should also be considered for patients who have suffered cerebrovascular events while on therapeutic anticoagulation because these patients are at high risk for further embolic events. Although warfarin is capable of reducing the risk for ischemic and hemorrhagic strokes by more than 60% in patients with AF, it does not completely eliminate these serious complications. At our institution, 20% of patients who underwent the Cox-Maze III procedure had experienced at least one cerebral thromboembolic event preoperatively. Furthermore, studies have shown that adding the Cox-Maze procedure in patients undergoing concomitant valve surgery can decrease the late risk for cardiac- and stroke-related deaths. A series from Japan demonstrated a 10% increase in the incidence of stroke at an 8-year follow-up for patients with chronic AF who underwent mitral valve replacement alone compared with similar patients who had mitral valve replacement with a concomitant Cox-Maze procedure. In addition, our group has demonstrated a 20% late survival benefit for cardiac surgery patients with AF who underwent a concomitant procedure to treat the AF over those who did not. Current European guidelines note LAA exclusion may be considered for stroke prevention in patients with AF and a contraindication to long-term anticoagulation. If LAA exclusion is performed, we recommend that the LAA be excised or that a dedicated LAA exclusion device be used.
The Cox-Maze III procedure is rarely performed at the present time because most centers have replaced the surgical incisions described in the original “cut-and-sew” procedure with lines of ablation created by a variety of different energy sources. At our institution, we have successfully used bipolar RF energy and cryoablation to replace most of the surgical incisions of the Cox-Maze III procedure in an operation termed Cox-Maze IV (see Fig. 135.2 ).
The Cox-Maze IV procedure is performed using cardiopulmonary bypass with either a median sternotomy or a less-invasive right minithoracotomy. The latter is our procedure of choice in patients referred for a stand-alone Cox-Maze procedure or in conjunction with a mitral valve procedure. All patients undergo intraoperative transesophageal echocardiography. Both the right and left pulmonary veins (PVs) are bluntly dissected, as is the intraatrial septum. After PV dissection, if the patient is in AF, attempts should be made to restore sinus rhythm. An IV bolus of amiodarone (150 mg) is given, and cardioversion is performed after excluding the presence of an LAA clot. Pacing thresholds are measured from each PV. The PVs are then isolated using a bipolar RF device, ablating a large cuff of surrounding atrial tissue ( Fig. 135.4 ). RF energy is delivered until the algorithm confirms transmurality. An additional ablation is performed before unclamping the device, and then a second and third set of ablations are performed a few millimeters proximally or distally. The adequacy of electrical isolation is demonstrated by confirming exit block by pacing from each PV in patients who have undergone a full sternotomy and from the right PVs in minithoracotomy patients.
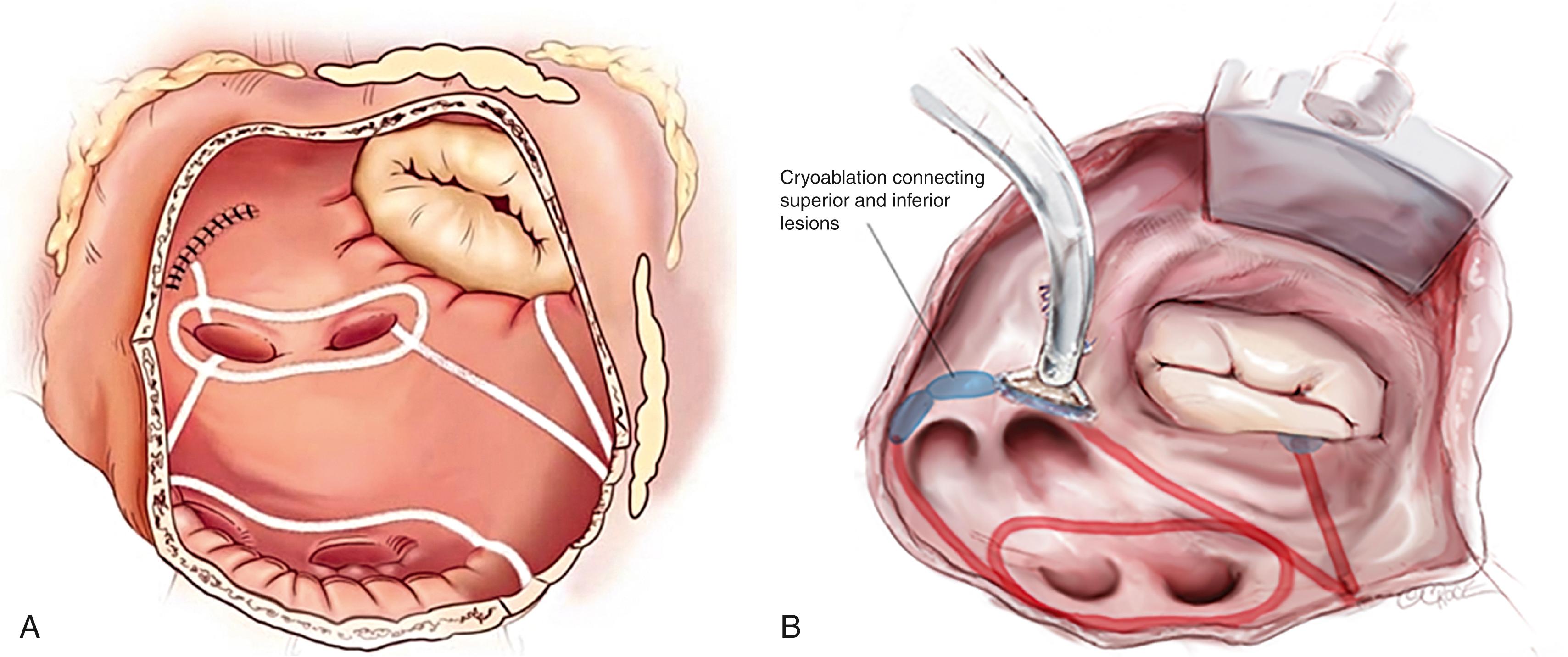
The right atrial lesion set is typically performed on the beating heart during cardiopulmonary bypass through three small purse-string sutures in the minithoracotomy patients and using a small right atriotomy in patients undergoing a median sternotomy ( Fig. 135.5 ). The right atrial free wall and intercaval ablations are performed using a bipolar RF clamp, followed by the two endocardial tricuspid annulus ablations, which are created using a linear cryoprobe. All cryoablations using nitrous oxide devices are performed for 3 minutes at −60°C. With the argon device, a 2-minute ablation application has been sufficient.
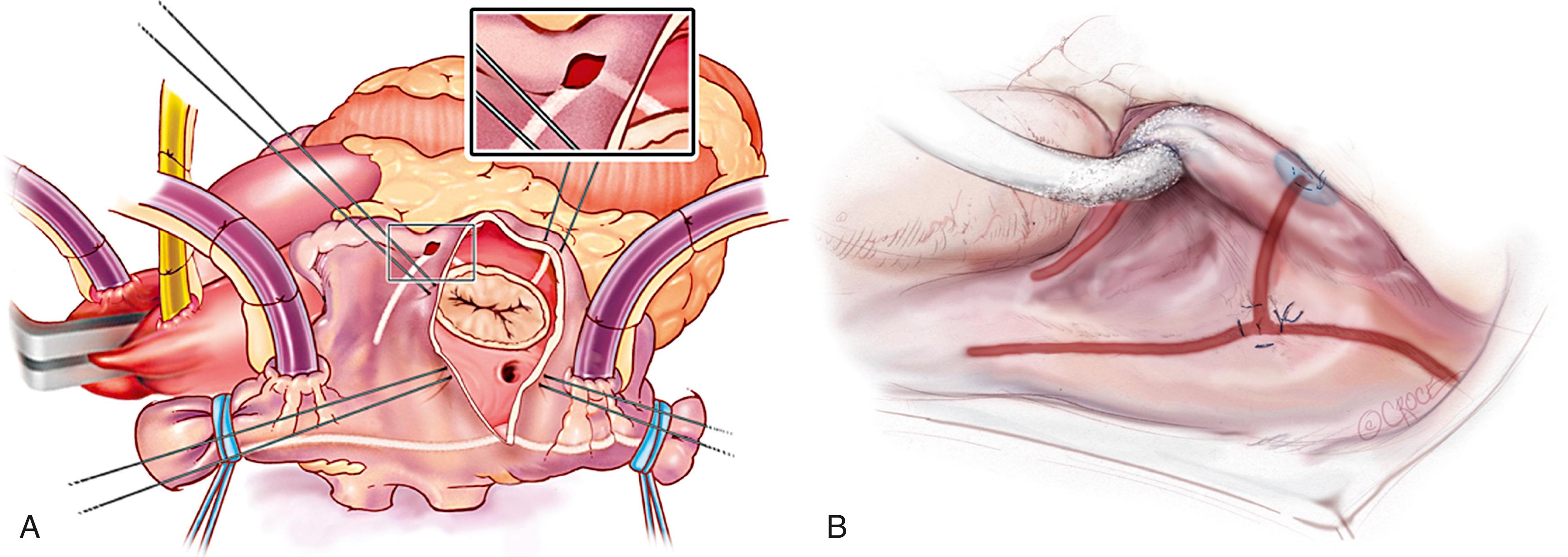
The heart is then arrested by cold cardioplegia. The LAA is amputated or oversewn, and the bipolar RF ablation is performed through the appendage site toward either left PV. The remaining ablation lines are then created using the bipolar clamp through a standard left atriotomy (see Fig. 135.4 ). Connecting lesions into the left superior and inferior PVs effectively isolates the entire posterior left atrium, and a linear line of ablation is created toward the mitral annulus. Cryoablation is then used to connect this ablation line to the mitral annulus, completing the left atrial isthmus line. The coronary sinus is ablated using an epicardial cryoprobe. In patients undergoing a right minithoracotomy, cryoablation is more extensively used to complete the posterior left atrial isolation.
Some groups have added ganglionated plexus (GP) ablation to the Cox-Maze IV procedure; however, there has been no clinical evidence to support the use of GP ablation. In fact, a randomized trial of patients undergoing PV isolation (PVI) with or without GP ablation showed no difference in AF recurrence at 1 year but found a higher rate of major complications in patients undergoing GP ablation. Moreover, in animal studies in our laboratory, evidence of early reinnervation at 1 month after GP ablation suggested that permanent denervation of the atrium is not possible with this technology.
Postoperatively, the epicardial pacing wires sewn to the right atrium and ventricle before closure of the chest are used for pacing as needed. Junctional rhythms are common after the Cox-Maze IV. In the event that heart block develops, the pacer can be changed to AV sequential pacing to optimize hemodynamics. Patients are started on antiarrhythmic medications once they are in normal sinus rhythm. Patients with persistent bradycardia because of a junctional rhythm are followed for 5 to 7 days to allow for sinus node recovery. If they remain in symptomatic bradycardia, a dual-chamber pacemaker is implanted. In our experience, 5% of patients require permanent pacemaker placement because of persistent heart block or sinus node dysfunction after a lone Cox-Maze IV, which increases in the elderly population and in patients undergoing concomitant valvular surgery or septal myectomy for hypertrophic cardiomyopathy. , , Finally, it should be emphasized that the Cox-Maze procedure cannot cause complete heart block (CHB) because there are no ablations in the vicinity of the AV node; thus CHB is the result of the concomitant surgery performed, not the Cox-Maze procedure.
Atrial tachyarrhythmias (ATAs) may occur postoperatively but usually resolve within the first month after the operation. Patients with persistent ATAs receive pharmacologic treatment with class 1 or 3 AADs, as well as cardioversion if necessary. The AADs are usually discontinued at 2 months in patients in sinus rhythm. All patients receiving Cox-Maze IV and without contraindications are started on warfarin or a direct anticoagulant agent postoperatively, and this is continued for 3 to 6 months. Anticoagulation is discontinued when the patient is demonstrated to be free of recurrent ATAs off antiarrhythmic medications, as documented on prolonged monitoring (24-hour or greater Holter recording), who are also shown to have no atrial stasis or thrombus on echocardiography. In patients who have had preoperative embolic neurologic events, our data support lifelong anticoagulation after surgery; this is our current practice.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here